Abstract
Cerebral dopamine homeostasis has been implicated in a wide range of cognitive processes and is of great pathophysiological importance in schizophrenia. A novel approach to study cognitive effects of dopamine is to deplete its cerebral levels with branched chain amino acids (BCAAs) that acutely lower dopamine precursor amino acid availability. Here, we studied the effects of acute dopamine depletion on early and late attentive cortical processing. Auditory event-related potential (ERP) components N2 and P3 were investigated using high-density electroencephalography in 22 healthy male subjects after receiving BCAAs or placebo in a randomized, double-blind, placebo-controlled crossover design. Total free serum prolactin was also determined as a surrogate marker of cerebral dopamine depletion. Acute dopamine depletion increased free plasma prolactin and significantly reduced prefrontal ERP components N2 and P3. Subcomponent analysis of N2 revealed a significant attenuation of early attentive N2b over prefrontal scalp sites. As a proof of concept, these results strongly suggest that BCAAs are acting on basic information processing. Dopaminergic neurotransmission seems to be involved in auditory top-down processing as indexed by prefrontal N2 and P3 reductions during dopamine depletion. In healthy subjects, intact early cortical top-down processing can be acutely dysregulated by ingestion of BCAAs. We discuss the potential impact of these findings on schizophrenia research.
Keywords: Dopamine depletion, Branched chain amino acid (BCAA), Attention, Top-down processing, Event-related potentials (ERP), Schizophrenia
1 Introduction
Alterations of prefrontal dopamine levels have been implicated in several cognitive processes in healthy control subjects as well as in schizophrenia. Originating from the ventral tegmental area and projecting to prefrontal cortex, the mesocortical dopaminergic pathway is thought to modulate prefrontal cognitive domains such as attention, executive control, and working memory (e.g. Goldberg et al., 2003; Malhotra et al., 2002; see also Goldman-Rakic et al., 2000; Seamans and Young, 2004, for reviews).
An important approach to studying cognitive effects of dopamine involves pharmacological challenge; here, we investigate the manipulation of cerebral dopamine levels by nutritional depletion of its precursor amino acids tyrosine and phenylalanine via branched chain amino acids (BCAAs). Rodent experiments have shown that plasma BCAAs displace aromatic amino acids at a transporter located at the blood-brain-barrier (Smith et al., 1987); further, BCAAs are thought to induce protein synthesis and plasma tryptophan is incorporated into these newly synthesized proteins (Moja et al., 1991). Consequently, brain levels of aromatic amino acids decrease, which induces acute depletion of cerebral monoamines including dopamine (see Fernstrom, 2005, for a review).
In human subjects, surrogate markers of central dopamine like serum prolactin level have been reproducibly impacted by oral administration of BCAAs (Scarna et al., 2002; Scarna et al., 2005). Indirect evidence for central efficacy of dopamine depletion via amino acid mixtures in healthy subjects is provided by cognitive studies that have shown influences on behavioral performance in the Stroop task and in affective and non-affective Go/ NoGo tasks using acute tyrosine/ phenylalanine depletion (Scholes et al., 2006; Vrshek-Schallhorn et al., 2006) as well as in the Iowa Gambling Task using BCAAs (Sevy et al., 2006). However, in frequently studied spatial working memory tasks, the consequences of dopamine depletion seem inconclusive, with more recent studies reporting subtle or no effects of acute tyrosine/ phenylalanine depletion per se (Ellis et al., 2005; Mehta et al., 2005). Inconsistencies across studies using nutritional dopamine depletion may be related to the use of different amino acid mixtures, differences in study and/ or task design, and the difficulty of achieving selective depletion of catecholamine precursors without perturbing brain availability of tryptophan. These inconsistencies across studies increase the need for new and profound measurements of effects of nutritional manipulation on cerebral dopamine homoeostasis, e. g. neural processing by means of electrophysiological measures.
Data from pharmacological challenge as well as genetic studies strongly suggest a central role for dopaminergic transmission in attentive neural processing, as has been repeatedly shown with event-related potentials (ERPs). Acute administration of haloperidol or droperidol to healthy subjects attenuated prefrontal PN, a measure of early selective attention, in three independent studies (Ahveninen et al., 2000; Kahkonen et al., 2001; Shelley et al. 1997). Another study demonstrated a reduction of error-related negativity, an early measure of response monitoring, following haloperidol challenge (Zirnheld et al., 2004). After sulpirid challenge, P3 was found to be affected bidirectionally as a function of baseline (Takeshita and Ogura, 1994). Recent studies using dissection of prefrontal P3 according to genetic polymorphisms found significant effects of genotype for genes coding for catechyl-O-methyl-transferase (Gallinat et al., 2003) as well as dopamine D3 (Mulert et al., 2006) and D4 receptor (Krämer et al., 2007), suggesting that prefrontal P3 phenotype is, at least in part, a function of cortical dopaminergic activity. In sum, there seems to be strong evidence for dopaminergic mediation of early attentive ERP components; less conclusive, yet suggestive evidence is available for P3. Our novel approach may help to further elucidate dopamine’s role in attentive ERP generation. The results obtained following dopamine depletion are likely to be less dependent on pharmacodynamic interactions, as may be the case in studies using different dopamine antagonists with differential receptor affinities.
For the current investigation, N2 and P3 were selected as attentive ERP components. N2 (MMN/N2b) is an ERP complex obtained during selective attention when repetitive stimuli are interrupted by infrequent events deviating in stimulus characteristics. The first subcomponent, mismatch negativity (MMN), is elicited irrespective of the amount of attention allocated to the stimuli and is regarded as reflecting involuntary or pre-attentive change detection. The second subcomponent, N2b, overlaps with MMN and is elicited only when attention is allocated to the deviant stimuli irrespective of whether or not a behavioral response is required (Naatanen et al., 1982; Opitz et al., 1999; Ritter et al., 1992). P3 is elicited in a wide range of paradigms involving detection of behaviorally relevant targets and represents a later stage of attentional top-down processing. Neuroanatomically, it has been proposed that both attentive ERP components N2b and P3 share generators in the frontal lobe (Baudena et al., 1995; Crottaz-Herbette and Menon, 2006).
We predicted that prefrontal attentive ERP components, i.e. N2 and P3, would decrease as a consequence of acutely down-regulated dopamine flux in prefrontal cortex. To address this hypothesis, prefrontal cortical activation was studied in healthy subjects employing high-density electroencephalography (EEG) recording during a selective attention task, the auditory oddball paradigm. Differences between a physiological and a putatively dopamine-depleted state were investigated using amplitude determination of prefrontal N2 and P3.
2 Methods
2.1 Subjects
Participants were screened for mental and physical health and were excluded when fulfilling the criteria of psychiatric disorders according to DSM-IV as determined by structured clinical interviews (First et al., 2001). Further reasons for exclusion were medical or neurological disorders, or intake of psychotropic drugs including regular smoking. All subjects had negative urine toxicology before participating in the study. Only male participants were recruited in order to minimize potential variance of dependent measures owing to cyclical hormonal variations in females. The final sample for this investigation consisted of 22 right-handed healthy Caucasian subjects with a mean age of 35.5±8.3 years. Mean body mass index (BMI) was 24.9±5.1 kg/m2. All participants gave written consent after the study protocol was explained in detail. All participants were paid for participation (50 $). This study was approved by the Institutional Review Board of the Zucker Hillside Hospital, North Shore-Long Island Jewish Health System, and was conducted in accordance with the declaration of Helsinki.
2.2 Study design
Acute dopamine depletion was achieved by administration of a BCAA drink containing valine, isoleucine, and leucine (60 g; ratio val: ile: leu= 3: 3: 4). The BCAA mixture is commercially available as a nutritional supplement drink called Tarvil® (SHS North America, Gaithersburg, MD, USA). Its validity as a dopamine antagonist as well as the time course of observed effects on peripheral prolactin levels have been established in a prior investigation from our group (Sevy et al., 2006).
BCAA and an identically flavored placebo mixture were administered in a double-blind, randomized, controlled crossover design. The placebo mixture contained a mix of artificial pineapple flavor, gum, artificial sweeteners, and industrial filler that adds volume and texture, but has no known effects; no amino acids or sugars were involved since it has been found that “balanced” amino acid mixtures lowered tyrosine availability to the brain (Montgomery et al., 2003) and thus might confound potential results.
To eliminate potential carry-over effects of BCAAs (t1/2 approximately 1.5 h; Marchesini et al., 1987), recordings were conducted on two non-consecutive days within one week. Subjects were required to fast 12 h before testing (start at 0:00). At 10:00, a heparinized intravenous was placed for repeated assessments of plasma prolactin levels that were determined at baseline before BCAA or placebo administration and hourly thereafter for 5 h. Following intravenous placement, BCAAs or placebo were administered. At 12:00, subjects underwent a comprehensive battery of cognitive tests, including Iowa Gambling Task, working memory, visual attention, and verbal memory (Sevy et al. 2006). After a short break, ERPs were recorded 3 h after BCAA ingestion at approximately 13:00. This schedule was maintained throughout the study.
2.3 Stimuli and task
We employed an auditory oddball paradigm with pseudo-randomized presentation of standard (261 sine waves of 1000 Hz) and target stimuli (37 sinusoidal tones of 1500 Hz, probability .124). All stimuli were presented binaurally by headphones at a nominal intensity of 75 dB sound pressure level and with a duration of 100 ms including 5 ms rise and fall; stimulus onset asynchrony was 1200 ms. Subjects were seated in a reclined chair and were instructed to visually fixate a cross on a computer monitor and to respond to target stimuli by pressing a button with the right index finger as fast as possible.
2.4 ERP recording and analysis
EEG was collected with 64 Ag/Ag-Cl electrodes according to the extended international 10/20 system using an electrode cap. Additional electrodes were placed at left and rights mastoids, the outer canthus of the left eye, and the tip of the nose. A ground electrode was placed on the forehead; electrode impedances were kept below 5 kΩ. All channels were referenced to the tip of the nose. EEG was recorded with a Neuroscan SynAmps (El Paso, TX, US) and digitized continuously at a sampling rate of 500 Hz. During acquisition, EEG data were band-filtered from 0.05 to 100 Hz.
Offline ERP analysis was performed using Brain Vision Analyzer 1.05 (Brain Products, Munich, Germany). At least 15 artifact-free (<100 μV) and correctly responded (within 200 to 1000ms post target stimulus) sweeps per subject and condition were taken into further analyses. Segments containing 350 ms pre-stimulus and 800 ms post-stimulus periods were averaged per subject and stimulus type. ERP data were then low-pass filtered at 45 Hz (24 dB/octave) and, after baseline correction, re-referenced to linked mastoids. Midline leads Fz, FCz, Cz, and Pz were then further analyzed.
N2 (MMN/N2b) was identified at FCz and was assessed as the mean negative difference between ERPs elicited by target minus standard stimuli at 100 to 240 ms post stimulus. At electrodes other than FCz, N2 was assessed as a peak at a comparable latency between 100 and 240 ms; if no clear peak was detectable, this component was chosen at the same latency. MMN and N2b were determined as the first (100 to 200 ms) and second (140-240 ms) negative peak within N2 deflection, respectively. P3 was determined at Pz as the most positive peak between 250 and 600 ms post target stimulus and was assessed as baseline-to-peak amplitude; assessment of P3 at other electrodes followed the procedure for N2.
2.5 Assessment of psychopathology and adverse events
Psychiatric symptoms were assessed using the Hamilton Rating Scale for Depression (Hamilton, 1960), Brief Psychiatric Rating Scale (Woerner et al., 1988), and the Scale for the Assessment of Negative Symptoms (Andreasen, 1989). Extrapyramidal motor symptoms were assessed with Simpson-Angus Scale (Simpson and Angus, 1970). Biological measures included heart rate, blood pressure, and temperature; additionally, subjects were asked to report at any time if they experienced any side effects.
2.6 Statistical Analyses
Statistical calculations were done using SPSS 13.0. The effects of acute dopamine depletion on ERP component amplitudes of N2 (MMN/N2b) and P3 were assessed by separate repeated measures models using condition (placebo vs. BCAA) as the main within-subjects factor and order of treatment (condition on day 1) as the between-subjects factor; age was included as a covariate. Other comparisons (prolactin levels, reaction time, accuracy, N sweeps, MMN, N2b, P3) were performed on the basis of the same repeated measures in a 2×2 design. For analysis of prolactin levels, an additional repeated measures model was computed using all 6 prolactin measures obtained within one study day as within-subjects. All tests were performed as two-tailed tests with a p <.05.
3 Results
3.1 Clinical and behavioral measures
Subjects did not report any side effects nor were there any significant differences between placebo and BCAA condition in any of the rating scales or of the vital signs assessed. There were also no significant differences between treatment arms in terms of accuracy to target stimuli (BCAA 82.06 ± 7.9 % vs. placebo 84.89 ± 6.6 %) or reaction times (RT; BCAA 420.77 ± 89.2 ms vs. placebo 413.07 ± 79.6 ms). No significant interactions of condition × order of treatment were found.
3.2 Peripheral prolactin levels
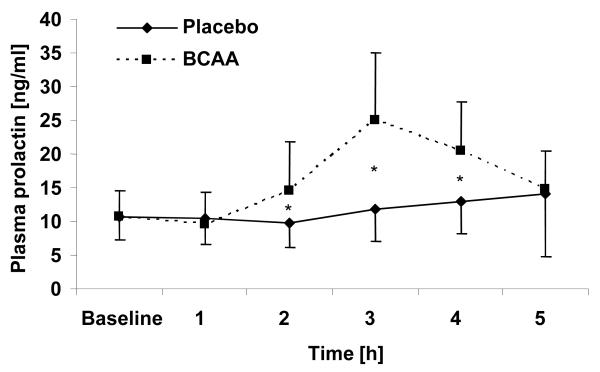
Peripheral serum prolactin levels are shown in Figure 1. There was no significant difference of prolactin levels during challenge with placebo; challenge with BCAA revealed a significant effect on prolactin levels (F(5,16)= 7.881; p= .001). In within-subjects contrasts, no significant differences between acute dopamine depletion and control condition were observed for baseline marker concentration and 5 hours after BCAA administration; however, plasma prolactin level was significantly increased at 2 to 4 hours after BCAA administration (2 h: F(1,20)= 13.835; p= .001; 3 h: F(1,20)= 53.625; p< .0001; 4 h: F(1,20)= 22.077; p< .0001).
Figure 1.
Plasma prolactin levels at baseline and up to 5 hours after administration of placebo or branched-chain amino acids (BCAA). * p< .01.
3.3 Grand average target ERPS: N2 and P3
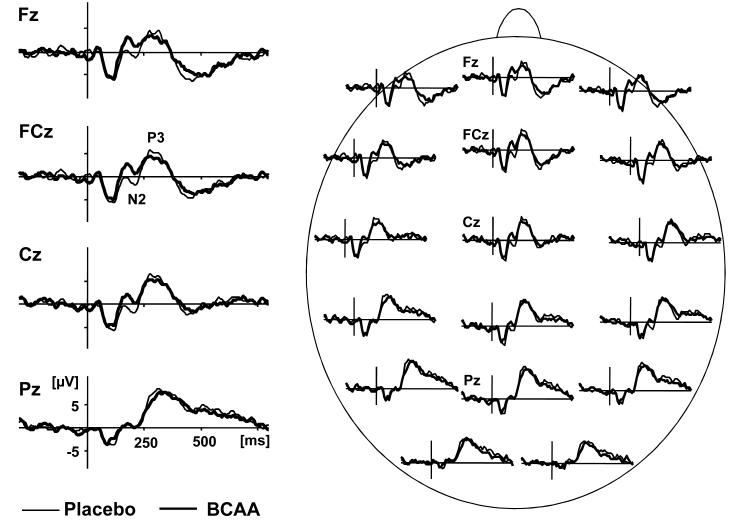
Target-locked ERPs are summarized inTable 1. Mean number of sweeps utilizable for ERP analysis (BCAA 21.95 ± 6.67 vs. placebo 21.50 ± 5.85) and mean artifact ratio (BCAA 33.19 % ± 21.00 vs. placebo 28.21 ± 19.06 %) were not significantly different between groups. Grand average ERP waveforms revealed the classic ERP components N1-P2-N2-P3 in response to target stimuli and indicated a positive shift of prefrontal target ERPs following BCAA ingestion. Critically and in keeping with our prediction, during dopamine depletion, repeated measures MANOVA indicated significant reductions of frontocentral N2 (FCz: F(1,20)= 8.14; p= .0098) and P3 (FCz: F(1,20)= 4.54; p= .046) following BCAA ingestion. There was no interaction of treatment condition × order of treatment neither was there an order effect.
Table 1.
Mean (± SD) amplitudes [μV]) of target ERP components N2 (MMN/N2b) and P3b.
| BCAA | Placebo | F | p | η 2 | |
|---|---|---|---|---|---|
| N2 (MMN/N2b) at Fz | 0.39 ± 3.49 | −1.04 ± 3.01 | 3.89 | .063 | .163 |
| N2 (MMN/N2b) at FCz | −0.27 ± 2.85 | −2.04 ± 2.70 | 8.14 | .0098 | .289 |
| N2 (MMN/N2b) at Cz | −0.91 ± 2.65 | −2.26 ± 2.65 | 5.19 | .034 | .206 |
| N2 (MMN/N2b) at Pz | −0.67 ± 2.55 | −1.02 ± 2.21 | 0.15 | .702 | .007 |
|
| |||||
| P3 at Fz | 2.21 ± 3.34 | 4.51 ± 5.10 | 2.70 | .116 | .118 |
| P3 at FCz | 3.57 ± 4.18 | 6.29 ± 5.07 | 4.54 | .046 | .185 |
| P3 at Cz | 5.63 ± 4.49 | 7.44 ± 4.48 | 1.53 | .231 | .071 |
| P3 at Pz | 10.42 ± 5.54 | 11.68 ± 4.99 | 2.39 | .138 | .107 |
Significant differences (p<.01) are highlighted; BCAA, branched chain amino acid; MMN, mismatch negativity; η2, partial eta-squared.
3.4 Difference ERPs: N2 (MMN/N2b)
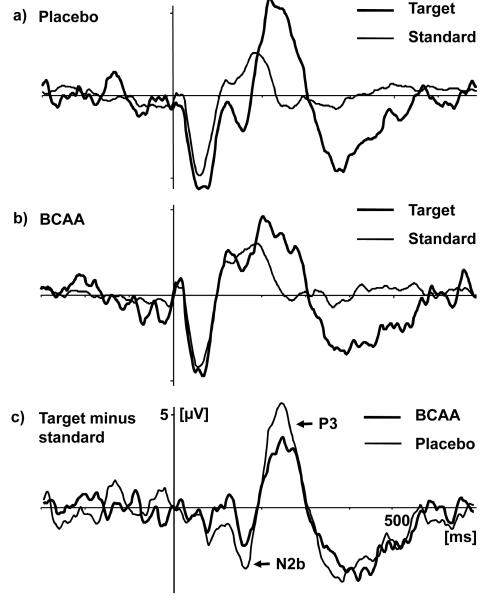
Figure 3 illustrates grand average ERP difference waves that contrast target with standard ERP. MMN was identified with a maximum at Fz, a mean prefrontal latency of 121.45 ± 39.63 ms, and a typical polarity inversion at the left mastoid when referenced to the tip of the nose (data not shown). MMN was followed by a frontocentrally maximal N2b component that peaked at a mean prefrontal latency of 213.64 ± 35.95 ms and lacked polarity reversal at mastoid leads in the nose reference. When dividing N2 into subcomponents, only N2b was found to be significantly attenuated over the prefrontal scalp (FCz: F(1,20)= 9.04; p= .0069; η2= .311) while MMN was insignificantly reduced.
Figure 3.
Grand average ERP for midline electrode FCz. (a) Grand average ERP in response to target and standard stimuli following placebo administration. (b) Grand average ERP in response to target and standard stimuli following BCAA administration. (c) Grand average ERP difference waves (target minus standard ERP) by treatment modality.
4 Discussion
In a randomized, double-blind, placebo-controlled crossover study, diminished attentive cortical processing following BCAA administration was observed. Prefrontal ERP components N2 (MMN/N2b) and P3 were significantly reduced. The changes in attentive ERP amplitudes were accompanied by a significant increase in peripheral prolactin levels. This is the first study to report on attentive ERP attenuation following dopamine depletion with BCAAs.
4.1 Efficacy of nutritional dopamine depletion
BCAA ingestion resulted in an approximately 2.5-fold increase in peripheral prolactin levels. This prolactin elevation in our sample is comparable to or exceeds the effect sizes described in other studies (Gijsman et al., 2002; Harmer et al., 2001; Scarna et al., 2002, 2005; Sevy et al., 2006) suggesting depletion of hypothalamic dopamine via reduced central dopamine precursor availability. Comparable reductions of dopamine precursors have been shown to significantly reduce central catecholamine turnover in experimental animals, which is evidenced by decreased levels of catecholamine metabolites in cerebrospinal fluid (Palmour et al., 1998) as well as decreased cerebral catecholamine synthesis (McTavish et al., 1999).
4.2 ERPs during dopamine depletion
The observed attenuation of N2 seems to be based on a reduction of its top-down subcomponent N2b, indicating decreased early attentive rather than automatic change detection. Our finding of selective N2b reduction is consistent with other studies investigating the effects of central dopamine antagonism on early attentive ERP components like PN (Ahveninen et al., 2000; Kahkonen et al., 2001; Shelley et al., 1997). However, since PN is usually elicited in dichotic listening tasks these results may not be fully comparable to our findings. ERP data obtained from auditory oddball paradigms in Parkinson’s disease have coherently shown both reduced PN as well as N2; particularly, the grand average curves provided by these studies reveal a remarkable similarity with our findings (Lagopoulos et al., 1998, Fig. 1; Pekkonen et al., 1995, Fig. 2; Vieregge et al., 1994, Fig.7), suggesting a similar deficiency pattern in Parkinson’s disease and in nutritional dopamine depletion, at least on the cortical level. This consistency across studies strongly suggests that early top-down ERP N2b is, at least to a greater extent, dopaminergically mediated and can be disrupted by BCAAs.
The observed effects of BCAAs on P3 at prefrontal sites might reflect effects on overlapping components P3a and P3b. It is widely agreed that P3a is a frontal component, while P3b is maximal at parietal leads; however, P3b is also partially generated in prefrontal cortex. To disentangle P3a and P3b generators, Halgren and co-workers (1998) investigated a three-stimulus oddball paradigm in subjects with intracerebral electrodes. The authors were able to distinguish between P3a generators in anterior cingulate cortex (ACC) and P3b generators confined to ventrolateral prefrontal cortex. In the traditional auditory oddball paradigm that was used here frontal P3 most likely includes both P3a and P3b properties (e.g. Crottaz-Herbette and Menon, 2006; Ritter et al., 1992). We therefore hypothesize that prefrontal P3 reduction might reflect a diminution of P3a; this thought is consistent with good evidence for a central role of dopamine in the neuropharmacology of P3a (e. g. Krämer et al., 2007).
4.3 Potential impact on schizophrenia research
Prefrontal N2 and P3 have been found to be reduced by BCAAs in this study and are also known to be reduced in schizophrenia (e. g. Demiralp et al., 2002; Umbricht et al., 2006; van der Stelt et al., 2005). The neural sources of N2 and P3 have been reported to be located in ACC (Baudena et al., 1995; Halgren et al., 1998; van Veen and Carter, 2002; Woldorff et al., 1999), which is consistent with dysfunctional ACC activations in schizophrenia (e. g. Carter et al., 2001; Dehaene et al., 2003; Neuhaus et al., 2007).
The pattern of ERP deficits following BCAAs points to pathophysiological mechanisms that may be comparable to those in schizophrenia, at least on the cortical level. Thus, BCAAs offer the opportunity to study mechanisms of cortical dopamine dysregulation and may aid further research on the exact mechanisms of cognitive deficits in schizophrenia. Another interesting thought may arise from potential therapeutic benefit of nutritional dopamine manipulation in general; however, given the lack of focality of action, the potential future use of nutritional compounds seems to be limited from the current perspective. Nevertheless, nutritional compounds containing amino acids offer an interesting alternative to challenge studies with pharmaceutical drugs and might one day be integrated into therapeutical concepts for schizophrenia.
4.4 Limitations
Limitations of our study include potential lack of generalization since only male Caucasian subjects have been investigated. Further studies will have to determine whether our findings are replicable in other, less homogenous samples.
Next, comparatively liberal criteria were applied for ERP analysis. The number of sweeps is low and artifact rejection criteria were relatively tolerant. Together with comparatively low accuracy and long reaction times, this shortcoming considerably decreases statistical power and, consequently, results should be regarded as preliminary.
Further, as control condition, we used a placebo mixture that did not contain amino acids and thus our results might be confounded by unspecific effects of amino acid ingestion per se. A superior approach might be provided by the use of nutritionally balanced amino acid mixtures deficient in tyrosine and phenylalanine and a balanced amino acid mixture as control condition (e. g. Leyton et al., 2004; Nagano-Saito et al., 2008). However, it has also been found that balanced amino acid mixtures including tyrosine and phenylalanine lowered tyrosine availability to the brain, presumably due to a lowered ratio of dopamine precursors to large neutral amino acids (Montgomery et al., 2003).
Finally, a major limitation to the present findings may arise from confounding effects of other transmitter systems, notably the serotonergic and noradrenergic systems. However, serotonin increases prolactin release, so a decrease in serotonergic function would have potentially counterbalanced prolactin increase (Attenburrow et al., 2001), which does not seem to be the case in our data; on the other hand, it cannot be ruled out that prolactin increase would have been even greater with a selective dopamine antagonism. Further, it has been found that amino acid mixtures lacking tyrosine and phenylalanine can affect norepinephrine levels in medial prefrontal cortex, at least in preclinical studies (Bongiovanni et al., 2008); our data on unaffected mean RTs, however, argue against a pronounced confounder by noradrenergically mediated effects on generalized arousal processes.
4.5 Conclusion
The present investigation is the first to examine ERPs following BCAA administration in healthy subjects and indicates that BCAAs modulate prefrontal ERP components N2 and P3. A concurrent significant effect of BCAAs on plasma prolactin levels provides evidence that BCAAs act on dopamine homeostasis and suggests a role of dopamine in the pharmacology of neuronal top-down processing. It has to be noted, though, that our results might be confounded by influences from serotonergic and noradrenergic transmitter systems which stresses the need to further elaborate nutritional approaches to studying cognitive functions. Despite this major limitation, the present study emphasizes the utility of dopamine depletion paradigms in future research on attentional functions in general and dopaminergic imbalance in neuropsychiatric disorders like schizophrenia in particular.
Figure 2.
Grand average ERP by treatment status in response to target stimuli for selected channels in topographic head montage (right) and for midline electrodes Fz, FCz, Cz, and Pz (left).
Table 2.
Mean (± SD) amplitudes [μV] of target ERP subcomponents MMN and N2b.
| BCAA | Placebo | F | p | η 2 | |
|---|---|---|---|---|---|
| MMN at Fz | −3.56 ± 3.80 | −4.91 ± 2.64 | 1.40 | .250 | .075 |
| MMN at FCz | −3.48 ± 3.55 | −4.72 ± 2.88 | 1.82 | .192 | .083 |
| MMN at Cz | −3.33 ± 2.74 | −4.19 ± 3.35 | 1.63 | .217 | .066 |
| MMN at Pz | −2.02 ± 2.72 | −3.83 ± 2.92 | 2.82 | .117 | .119 |
| N2b at Fz | −3.29 ± 3.84 | −5.17 ± 4.26 | 4.16 | .055 | .172 |
|
| |||||
| N2b at FCz | −4.16 ± 3.70 | −6.57 ± 4.22 | 9.04 | .0069 | .311 |
| N2b at Cz | −4.70 ± 3.92 | −6.44 ± 4.49 | 4.89 | .039 | .197 |
| N2b at Pz | −3.94 ± 4.06 | −3.96 ± 3.53 | 0.03 | .867 | .001 |
Significant differences (p<.01) are highlighted; BCAA, branched chain amino acid; MMN, mismatch negativity; η2, partial eta-squared.
Acknowledgments
The authors wish to thank John Foxe and Daniel Javitt for assistance in event-related potential methodology, Adriana Franco and Denise Coscia for data collection, and Eugene Kats for technical support.
Footnotes
Author disclosure Role of funding source Funding sources had no involvement in study design; data collection; analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Contributors Authors YH, KN, JAB, and AKM designed the study and wrote the protocol. Authors SS, COR, TEG, and AHN undertook the statistical analyses. Author AHN wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest There are no conflicts of interest relating to this manuscript.
References
- 1.Ahveninen J, Kahkonen S, Tiitinen H, Pekkonen E, Huttunen J, Kaakkola S, Ilmoniemi RJ, Jaaskelainen IP. Suppression of transient 40-Hz auditory response by haloperidol suggests modulation of human selective attention by dopamine D2 receptors. Neurosci. Lett. 2000;292(1):29–32. doi: 10.1016/s0304-3940(00)01429-4. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen NC. Scale for the assessment of negative symptoms (SANS): conceptual and theoretical foundations. Br. J. Psychiatry. 1989;155(7 Suppl.):49–58. [PubMed] [Google Scholar]
- 3.Attenburrow MJ, Mitter PR, Whale R, Terao T, Cowen PJ. Low-dose citalopram as 5-HT neuroendocrine probe. Psychopharmacology. 2001;155(3):323–326. doi: 10.1007/s002130100729. [DOI] [PubMed] [Google Scholar]
- 4.Baudena P, Halgren E, Heit G, Clarke JM. Intracerebral potentials to rare target and distractor auditory and visual stimuli. III. Frontal cortex. Electroencephalogr. Clin. Neurophysiol. 1995;94(4):251–264. doi: 10.1016/0013-4694(95)98476-o. [DOI] [PubMed] [Google Scholar]
- 5.Carter CS, MacDonald AW, 3rd, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am. J. Psychiatry. 2001;158(9):1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- 6.Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J. Cogn. Neurosci. 2006;18(5):766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- 7.Demiralp T, Ucok A, Devrim M, Isoglu-Alkac U, Tecer A, Polich J. N2 and P3 components of event-related potential in first-episode schizophrenic patients: scalp topography, medication, and latency effects. Psychiatry Res. 2002;111(2-3):167–179. doi: 10.1016/s0165-1781(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 8.Dehaene S, Artiges E, Naccache L, Martelli C, Viard A, Schürhoff F, Recasens C, Martinot ML, Leboyer M, Martinot JL. Conscious and subliminal conflicts in normal subjects and patients with schizophrenia: the role of the anterior cingulate. Proc. Natl. Acad. Sci. 2003;100(23):13722–13727. doi: 10.1073/pnas.2235214100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis KA, Mehta MA, Wesnes KA, Armstrong S, Nathan PJ. Combined D1/D2 receptor stimulation under conditions of dopamine depletion impairs spatial working memory performance in humans. Psychopharmacology. 2005;181(4):771–780. doi: 10.1007/s00213-005-0019-2. [DOI] [PubMed] [Google Scholar]
- 10.Fernstrom JD. Branched-chain amino acids and brain function. J. Nutr. 2005;135(6 Suppl.):1539S–1546S. doi: 10.1093/jn/135.6.1539S. [DOI] [PubMed] [Google Scholar]
- 11.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders – non-patient edition. New York State Psychiatric Institute; New York: 2001. [Google Scholar]
- 12.Gallinat J, Bajbouj M, Sander T, Schlattmann P, Xu K, Ferro EF, Goldman D, Winterer G. Association of the G1947A COMT (Val(108/158)Met) gene polymorphism with prefrontal P300 during information processing. Biol. Psychiatry. 2003;54(1):40–48. doi: 10.1016/s0006-3223(02)01973-x. [DOI] [PubMed] [Google Scholar]
- 13.Gijsman HJ, Scarna A, Harmer CJ, McTavish SF, Odontiadis J, Cowen PJ, Goodwin GM. A dose-finding study on the effects of branch chain amino acids on surrogate markers of brain dopamine function. Psychopharmacology. 2002;160(2):192–197. doi: 10.1007/s00213-001-0970-5. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch. Gen. Psychiatry. 2003;60(9):889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- 15.Goldman-Rakic PS, Muly EC, III, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res. Rev. 2000;31(2-3):295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 16.Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephal. Clin. Neurophysiol. 1998;106(2):156–164. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harmer CJ, McTavish SF, Clark L, Goodwin GM, Cowen PJ. Tyrosine depletion attenuates dopamine function in healthy volunteers. Psychopharmacology. 2001;154(1):105–111. doi: 10.1007/s002130000613. [DOI] [PubMed] [Google Scholar]
- 19.Kahkonen S, Ahveninen J, Jaaskelainen IP, Kaakkola S, Naatanen R, Huttunen J, Pekkonen E. Effects of haloperidol on selective attention: a combined whole-head MEG and high-resolution EEG study. Neuropsychopharmacology. 2001;25(4):498–504. doi: 10.1016/S0893-133X(01)00255-X. [DOI] [PubMed] [Google Scholar]
- 20.Krämer UM, Cunillera T, Camara E, Marco-Pallares J, Cucurell D, Nager W, Bauer P, Schüle R, Schöls L, Rodriguez-Fornells A, Münte TF. The impact of catechyl-O-methyltransferase and dopamine D4 receptor genotypes on neurophysiological markers of performance monitoring. J. Neurosci. 2007;27(51):14190–14198. doi: 10.1523/JNEUROSCI.4229-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagopoulos J, Clouston P, Barhamali H, Gordon E, Li WM, Lesley J, Morris JG. Late components of the event-related potentials and their topography in Parkinson’s disease. Mov. Disord. 1998;13(2):262–267. doi: 10.1002/mds.870130211. [DOI] [PubMed] [Google Scholar]
- 22.Leyton M, Dagher A, Boileau I, Casey K, Baker GB, Diksic M, Gunn R, Young SN, Benkelfat C. Decreasing amphetamine-induced dopamine release by acute phenylalanine/tyrosine depletion: A PET/ [11C]raclopride study in healthy men. Neuropsychopharmacology. 2004;29(2):427–432. doi: 10.1038/sj.npp.1300328. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance in a test of prefrontal cognition. Am. J. Psychiatry. 2002;159(4):652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- 24.Marchesini G, Bianci GP, Vilstrup H, Checchia GA, Patrono D, Zoli M. Plasma clearances of branched chain amino acids in control subjects and in patients with cirrhosis. J. Hepatol. 1987;4(1):108–117. doi: 10.1016/s0168-8278(87)80017-x. [DOI] [PubMed] [Google Scholar]
- 25.McTavish SF, Cowen PJ, Sharp T. Effect of a tyrosine-free amino acid mixture on regional brain catecholamine synthesis and release. Psychopharmacology. 1999;141(2):182–188. doi: 10.1007/s002130050823. [DOI] [PubMed] [Google Scholar]
- 26.Mehta MA, Gumaste D, Montgomery AJ, McTavish SF, Grasby PM. The effects of acute tyrosine and phenylalanine depletion on spatial working memory and planning in healthy volunteers are predicted by changes in striatal dopamine levels. Psychopharmacology. 2005;180(4):654–663. doi: 10.1007/s00213-004-2128-8. [DOI] [PubMed] [Google Scholar]
- 27.Moja EA, Restani P, Corsini E, Stacchezzini MC, Assereto R, Galli CL. Cycloheximide blocks the fall of plasma and tissue tryptophan levels after tryptophane-free amino acid mixtures. Life Sci. 1991;49(15):1121–1128. doi: 10.1016/0024-3205(91)90600-g. [DOI] [PubMed] [Google Scholar]
- 28.Montgomery AJ, McTavish SF, Cowen PJ, Grasby PM. Reduction of brain dopamine concentration with dietary tyrosine plus phenylalanine depletion: AN [11C]raclopride study. Am. J. Psychiatry. 2003;160(10):1887–1889. doi: 10.1176/appi.ajp.160.10.1887. [DOI] [PubMed] [Google Scholar]
- 29.Mulert C, Juckel G, Giegling I, Pogarell O, Leicht G, Karch S, Mavrogiorgiou P, Holler HJ, Hegerl U, Rujescu D. A Ser9Gly polymorphism in the dopamine D3 receptor gene (DRD3) and event-related P300 potentials. Neuropsychopharmacology. 2006;31(6):1335–1344. doi: 10.1038/sj.npp.1300984. [DOI] [PubMed] [Google Scholar]
- 30.Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J. Neurosci. 2004;28(14):3697–3706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuhaus AH, Koehler S, Opgen-Rhein C, Urbanek C, Hahn E, Dettling M. Selective anterior cingulate cortex deficit during conflict solution in schizophrenia: an event-related potential study. J. Psychiatr. Res. 2007;41(8):635–644. doi: 10.1016/j.jpsychires.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Opitz B, Mecklinger A, von Cramon DY, Kruggel F. Combining electrophysiological and hemodynamic measures of the auditory oddball. Psychophysiology. 1999;36(1):142–147. doi: 10.1017/s0048577299980848. [DOI] [PubMed] [Google Scholar]
- 33.Palmour RM, Ervin FR, Baker GB, Young SN. An amino acid mixture deficient in phenylalanine and tyrosine reduces cerebrospinal fluid catecholamine metabolites and alcohol consumption in vervet monkeys. Psychopharmacology. 1998;136(1):1–7. doi: 10.1007/s002130050532. [DOI] [PubMed] [Google Scholar]
- 34.Pekkonen E, Jousmaki V, Reinikainen K, Partanen J. Automatic auditory discrimination is impaired in Parkinson’s disease. Electroencephal. Clin. Neurophysiol. 1995;95(1):47–52. doi: 10.1016/0013-4694(94)00304-4. [DOI] [PubMed] [Google Scholar]
- 35.Ritter W, Paavilainen P, Lavikainen J, Reinikainen K, Alho K, Sams M, Naatanen R. Event-related potentials to repetition and change of auditory stimuli. Electroencephal. Clin. Neurophysiol. 1992;83(5):306–321. doi: 10.1016/0013-4694(92)90090-5. [DOI] [PubMed] [Google Scholar]
- 36.Scarna A, Gijsman HJ, Harmer CJ, Goodwin GM, Cowen PJ. Effects of branch chain amino acids supplemented with tryptophan on tyrosine availability and plasma prolactin. Psychopharmacology. 2002;159(2):222–223. doi: 10.1007/s00213-001-0963-4. [DOI] [PubMed] [Google Scholar]
- 37.Scarna A, McTavsih SF, Cowen PJ, Goodwin GM, Rogers RD. The effects of a branched chain amino acid mixture supplemented with tryptophan on biochemical indices of neurotransmitter function and decision-making. Psychopharmacology. 2005;179(4):761–768. doi: 10.1007/s00213-004-2105-2. [DOI] [PubMed] [Google Scholar]
- 38.Scholes KE, Harrison BJ, O’Neill BV, Leung S, Croft RJ, Pipingas A, Phan KL, Nathan PJ. Acute serotonin and dopamine depletion improves attentional control: findings from the Stroop task. Neuropsychopharmacology. 2007;32(7):1600–1610. doi: 10.1038/sj.npp.1301262. [DOI] [PubMed] [Google Scholar]
- 39.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Progr. Neurobiol. 2004;74(1):1–57. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Sevy S, Hassoun Y, Bechara A, Yechiam E, Napolitano B, Burdick K, Delamn H, Malhotra A. Emotion-based decision-making in healthy subjects: short-term effects of reducing dopamine levels. Psychopharmacology. 2006;188(2):228–235. doi: 10.1007/s00213-006-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shelley AM, Catts SV, Ward PB, Andrews S, Mitchell P, Michie P, McConaghy N. The effect of decreased catecholamine transmission on ERP indices of selective attention. Neuropsychopharmacology. 1997;16(3):202–210. doi: 10.1016/S0893-133X(96)00190-X. [DOI] [PubMed] [Google Scholar]
- 42.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr. Scand. 1970;212(44 Suppl.):11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 43.Smith QR, Momma S, Aoyagi M, Rapoport SI. Kinetics of neutral amino acid transport across the blood-brain barrier. J. Neurochem. 1987;49(5):1651–1658. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 44.Takeshita S, Ogura C. Effect of the dopamine D2 antagonist sulpiride on event-related potentials and its relation to the law of initial value. International J. Psychophysiol. 1994;16(1):99–106. doi: 10.1016/0167-8760(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 45.Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol. Psychiatry. 2006;59(8):762–772. doi: 10.1016/j.biopsych.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 46.Van der Stelt O, Lieberman JA, Belger A. Auditory P300 in high-risk, recent-onset and chronic schizophrenia. Schizophr. Res. 2005;77(2-3):309–320. doi: 10.1016/j.schres.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 47.Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J. Cogn. Neurosci. 2002;14(4):593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- 48.Vieregge P, Verleger R, Wascher E, Stuven F, Kompf D. Auditory selective attention is impaired in Parkinson’s disease--event-related evidence from EEG potentials. Brain Res. Cogn. Brain Res. 1994;2(2):117–129. doi: 10.1016/0926-6410(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 49.Vrshek-Schallhorn S, Wahlstrom D, Benolkin K, White T, Luciana M. Affective bias and response modulation following tyrosine depletion in healthy adults. Neuropsychopharmacology. 2006;31(11):2523–2536. doi: 10.1038/sj.npp.1301172. [DOI] [PubMed] [Google Scholar]
- 50.Woerner MG, Manuzza S, Kane JM. Anchoring the BPRS: an aid to improved reliability. Psychopharmacol. Bull. 1988;24(1):112–117. [PubMed] [Google Scholar]
- 51.Woldorff MG, Matzke M, Zamarripa F, Fox PT. Hemodynamic and electrophysiological study of the role of the anterior cingulate in target-related processing and selection for action. Hum. Brain Mapp. 1999;8(2-3):121–127. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<121::AID-HBM9>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zirnheld PJ, Carroll CA, Kieffaber PD, O’Donnell BF, Shekhar A, Hetrick WP. Haloperidol impairs learning and error-related negativity in humans. J. Cogn. Neurosci. 2004;16(6):1098–1112. doi: 10.1162/0898929041502779. [DOI] [PubMed] [Google Scholar]