Abstract
Purpose of review
The capacity of the liver to regenerate and maintain a constant size despite injury is unique. However, the exact mechanisms are not completely clear. Cell transplantation has been proposed as an alternative treatment of liver diseases. Recent progress has been reported on the generation of stem/progenitor cells that may differentiate towards the hepatic lineage. However, it is currently difficult to determine which of the stem/progenitor cell populations are the best for therapy of a given disease.
Recent findings
The limited access to donor human hepatocytes has opened a great interest on the generation of hepatocyte-like cells. Several potential cell sources have been identified. However, general standardization of the methods to evaluate these cells is particularly important for the promise of stem/progenitor-derived hepatocyte-based therapies. Moreover, innovations aimed at improving hepatocyte delivery, survival and engraftment have recently opened the field of organ engineering that may improve the perspective of liver repopulation.
Summary
Here we review current evidence reported from the perspective of potential clinical applications of different hepatic cell sources with repopulation capacities and the future perspectives and tools that can facilitate the translation of laboratory work into clinical success.
Keywords: Hepatocyte transplantation, xenogenic hepatocytes, stem cell-derived hepatocytes, liver tissue engineering
INTRODUCTION
Since the development of techniques for the isolation of individual cells from the liver, transplantation of isolated hepatocytes has been considered a potential therapy for the treatment of liver disorders. Over the last several decades, laboratories have progressively shown that primary hepatocytes can engraft in the liver, spleen, peritoneal cavity and other extra hepatic sites, and can function following transplantation to correct liver-based errors of metabolism and prolong the survival of animals with liver failure.
Liver cell transplantation (LCT) presents distinct advantages over orthotopic liver transplantation (OLT) for organ replacement therapy: LCT is technically simpler than OLT, requiring only injection/infusion of a cell suspension; hepatocytes can be cryopreserved for future use; and hepatocytes obtained from one donor can be used for multiple patients. Yet, major obstacles to the broad clinical use of LCT include the competition with OLT for the few suitable donor livers, long-term cell engraftment and function, effective and adequate immunosuppressant protocols and the fact that primary hepatocytes cannot be readily expanded in vitro.
As the host liver and its vasculature remain intact, the consequences of graft loss would be relatively small. LCT could effectively replace whole organ transplantation for the treatment of many inherited disorders of metabolism if an enhanced and facilitated donor cells repopulation could be achieved, hence transplantation of a relatively small number of liver cells, representing only a fraction of the liver mass, could be used to avoid removing an otherwise normally functioning native liver.
A number of studies have been published demonstrating that stem/progenitor cells can be differentiated towards “hepatocyte-like cells”, a term that has been used to described cells generated in vitro that show some characteristics of mature hepatocytes but still are not fully mature and/or characterized. However, it is currently impractical to determine which is the most suitable stem/progenitor population to repopulate the liver. At this point, the differences in the starting cell population, differentiation protocols, and most importantly the assays performed to assess differentiation and endpoints of preclinical transplantation studies have limited the criteria standardization of the term and minimal characterization of stem/progenitor cell populations that are the best for the therapy of a given liver disease.
CURRENT CLINICAL LIVER CELL TRANSPLANTATION
So far, human LCT has been attempted in patients with acute liver failure, in chronic liver disease with cirrhosis, and in children with liver-based metabolic disease. To date, data concerning the efficacy of LCT for hepatic failure in humans has been difficult to interpret, and although clinical experiences lack the impressive stimulation of regeneration found in animal experiments, LCT remains as an alternative experimental treatment to bridge patients to orthotopic transplantation or auxiliary partial orthotopic liver transplantation (APOLT) with the purpose to reduce the risk related to liver transplantation in patients with clinical complications.
Human Liver Cell Transplantation in Metabolic Liver Disease
Allogenic LCT have been reported into patients to correct a variety of metabolic disorders including ornithine transcarbamylase (OTC) deficiency [1-6], alpha-1-antitrypsin deficiency [7,8], glycogen storage disease type 1a [9], infantile Réfsum’s disease [10], factor VII deficiency [11], bile salt export protein deficiency (PFIC2) [8], and Crigler-Najjar syndrome type 1 [12-15]. Recently, the efficacy of LCT as a bridge to subsequent auxiliary partial orthotopic liver transplantation (APOLT) was recently demonstrated in a newborn with OTC deficiency [4]. This report demonstrated that LCT should be considered effective as a bridge to liver transplantation in OTC to improve growth and neurodevelopmental outcomes.
With respect to long-term engraftment it will be important whether the transplanted hepatocytes will gain a selection advantage over the recipient’s cells. Therefore, it could be reasonable to differentiate between two groups of metabolic liver diseases. A typical representative of group 1 is inherited clotting factor deficiencies. Secretion of clotting factors is important for the organism, but deficiency does not influence survival of hepatocytes. Therefore, a selection advantage of the transplanted hepatocytes cannot be expected. The situation is different for group 2 metabolic liver diseases. A typical example is Wilson’s disease that is caused by a defect in the copper transporting ATPase ATP7B protein. As a consequence of the gene defect copper will accumulate and lead to deterioration of hepatocytes [16]. In this case transplanted wild-type cells may have a selection advantage over the recipient’s hepatocytes. Therefore, higher numbers of transplanted hepatocytes, better cell engraftment and perhaps repeated transplantations may be necessary for the treatment of group 1 metabolic liver diseases. However, clinical data with respect to possible differences in long-term engraft between group 1 and 2 are not yet available.
Human Liver Cell Transplantation in Chronic Liver Failure
Cell therapy of end-stage liver disease is more problematic. Besides loss of functional hepatocytes, abnormalities of the hepatic architecture contribute to the decrease in liver function. In this situation, the benefit of additionally transplanted hepatocytes into the liver without restoring the normal liver architecture may be questionable.
The response to hepatocyte transplantation in humans with end-stage liver disease has not resulted in the same degree of improvement compared to experimental animal studies [7,17]. One explanation may be that the hepatocytes in clinical studies were delivered into the splenic artery and not into the splenic pulp. It has been shown that the route of hepatocyte delivery influences hepatocyte engraftment and function [18].
In the United States, 8 patients with decompensated chronic liver disease have been treated by transplantation with up to 1010 liver cells through the splenic artery. One of the three patients who was a candidate for organ transplantation was successfully transplanted. In 2 patients, scanning for transplanted hepatocytes using uptake of glycosylated albumin with 99mTc indicated the presence of engrafted hepatocytes in the spleen [7]. One patient with hepatitis C virus (HCV) cirrhosis had histologic evidence of transplanted hepatocytes forming cord like structures with normal tight cellular junctions in the spleen by day two postinfusion [19]. In three children, single hepatocyte administrations by transfemoral intra-arterial splenic infusion or transhepatic and transjugular portal venous infusion led to clinically significant ammonia and encephalopathy control for up to 6 weeks, and to successful bridging to liver transplant and full recovery (FR) in two of the three [20]. Recently, a report of a girl of 1 year of age with biliary atresia, as evidenced by a bilirubin of 28.4mg/dL, was treated with hepatic progenitor cell infusion through the hepatic artery [21]. A catheter was manipulated into the main hepatic portal artery and 4 × 106 cells/mL in a total of 3 mL volume of progenitor cells were infused. The total bilirubin and conjugated bilirubin started decreasing during the first month after cell infusion. The conjugated bilirubin was 16.35 mg/dL before cell infusion, decreasing to eightfold after cell infusion. After 2 months of cell infusion, hepatobiliary scintigraphy showed increased liver cell function [21]. The same group conducted autologous transplantation of mesenchymal stem cells (CD34+ cells) in four patients with chronic liver failure, however clear benefit could not be demonstrated from the cell transplantation procedure [22].
Human Liver Cell Transplantation in Acute Liver Failure
Acute liver failure is characterized by rapid deterioration of liver function and a high mortality [23]. Viral hepatitis, idiosyncratic drug reactions, acetaminophen and mushroom ingestion are common causes of acute liver failure. Hepatic encephalopathy, brain edema, coagulopathy, septicemia and multi-organ failure are critical key events [23,24] during the course of the disease.
LCT of acute liver failure should provide rapid support for the failing liver by providing metabolism of liver toxins, the secretion of proteins such as clotting factors or albumin and stabilization of haemodynamic parameters. In several studies, allogeneic primary hepatocytes isolated from cadaver livers were infused into the splenic artery, the portal vein and intraperitoneal cavity [7,20,25-30]. Improvements in ammonia levels encephalopathy scores, and prothrombin time levels were reported.
More than a dozen children, ages 3.5 months to 16 years, have been treated with intraportal and intraperitoneal (one patient) hepatocyte infusions, under conventional immunosuppression, for drug, idiopathic or viral acute liver failure; about 20% patients experienced full recovery without solid organ transplant, and about 30% children were successfully bridged to OLT with full recovery [7,26,27].
Despite the positive results, it should be considered that the evaluation of therapies in acute liver failure might be difficult. This is due to large variations in the course of the disease, multiple etiologies, complex supportive treatment and a spontaneous recovery rate of approximately 20% by successful hepatic self-regeneration [23]. Thus, the inclusion of adequate controls is difficult. In this respect, studies in patients with inherited metabolic diseases are easier to interpret. Although there is some suggestion that the transplanted hepatocytes provided a benefit, convincing evidence for engraftment and function of the transplanted cells has not been obtained. Part of the problem could be that relatively small numbers of liver cells were transplanted in many of the patients.
Unfortunately a large number of issues will need to be addressed before this technology can be applied to the large number of patients who could potentially benefit from it.
CONDITIONS FOR LIVER REPOPULATION
Although in vitro tests may suggest hepatocyte-like cells characterization, the ultimate proof to demonstrate that differentiation has been achieved is to show that the differentiated cell can repopulate the liver and restore liver function in a preclinical model of liver disease. There is enough evidence suggesting that the injured hepatic microenvironment may induce further maturation of partially differentiated cells [31-35][36].
Following infusion into the portal circulation, or implantation into the spleen, the vast majority of donor liver cells translocates to the liver and accumulates in the hepatic sinusoids, causing transient portal hypertension that resolves in hours [37,38] (Figure 1).
Fig 1. Cell-based therapies for liver repopulation.
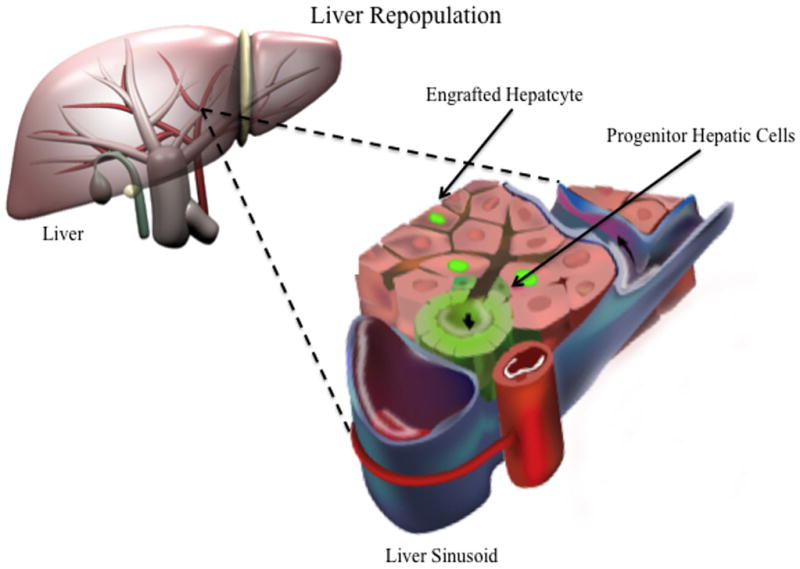
Cells with adult liver repopulating potential are transplanted via portal vein, donor hepatocytes translocates to the liver and accumulates in the hepatic sinusoids. Passage of hepatocytes into the space of Disse requires retraction of the sinusoidal endothelial cells. Shortly after entering the Space of Disse there is a transient disruption of the gap junction and tight junction between adjacent hepatocytes in the vicinity of the transplanted hepatocytes. The transplanted cells then insert themselves between host hepatocytes, with subsequent regeneration of the bile canaliculi and gap junctions.
Investigators have shown that immediately after injection into the spleen or portal vein, transplanted hepatocytes are deposited in the portal region and the sinusoidal spaces. However, the capacity of the sinusoidal spaces limits the number of hepatocytes that can be transplanted. It has been estimated that, at any one time, hepatocytes equivalent to up to 10% of the liver mass can be transplanted into livers with normal architecture without long-term deleterious effects [18,39,40]. Studies indicate that less than 30 percent of transplanted hepatocytes survive in the liver, although the number of surviving cells increases as more hepatocytes are transplanted [37,41,42] (Figure 1).
Passage of hepatocytes into the space of Disse requires retraction of the sinusoidal endothelial cells. Shortly after entering the Space of Disse there is a transient disruption of the gap junction and tight junction between adjacent hepatocytes in the vicinity of the transplanted hepatocytes. The transplanted cells then insert themselves between host hepatocytes. Pharmacologic disruption of endothelial integrity and sinusoidal dilatation appear to improve engraftment [37,38]. Hepatic nonparenchymal cells are major modulators of initial hepatocyte engraftment. First, the hepatic sinusoidal endothelial cells (SEC) pose a physical barrier to the passage of the transplanted hepatocytes into the space of Disse and second, Kupffer cells and probably other inflammation-mediating cells may clear the transplanted cells, reducing the number of hepatocytes available for engraftment.
Now, integration and proliferation of transplanted liver cells requires space, for instance partial hepatectomy or hepatocyte necrosis and/or apoptosis, and that the transplanted cells have a growth advantage over endogenous hepatocytes. Several laboratories have developed strategies to induce preferential proliferation of engrafted hepatocytes. These approaches have led to massive repopulation of transplanted primary hepatocytes. This has been achieved by preparatively treating rodent hosts with retrorsine or lasiocarpine, which are pyrrolizidine alkaloids that induce a long-lasting cell cycle block, and two thirds hepatectomy, which provides a strong mitotic stimulus to hepatocytes. An alternative approach that consists of preparative hepatic irradiation has been suggested, which reduces mitotic capacity of the host hepatocytes. [43,44].
Even though hepatocyte-like cells generated from stem/progenitor cells may yield some levels of engraftment, these levels fall far short of those seen when mature or fetal hepatocytes are engrafted [44,45]. The reasons underlying the poor performance of stem/progenitor derived cells compared with primary liver derived cells when engrafted into the liver are unknown.
The rules governing parenchymal repopulation by hepatocytes are likely to be similar for hepatocyte-like cells generated from stem/progenitor cells. However, a number of differences between mature hepatocytes and stem/progenitor-derived hepatocyte-like cells may explain the differential degree of liver repopulation. For instance, mature cells are of relatively larger size compared to stem/progenitor-derived hepatocyte-like cells, which may cause cell retention within the sinusoids. Signals that govern this process when stem/progenitor cell-derived hepatocytes are grafted remain to be determined. It is also not known whether stem/progenitor cell-derived hepatocytes may have a proliferative disadvantage over mature hepatocytes that have extensive proliferation potential [46], and/or may undergo significantly more apoptosis when grafted.
CELLS WITH LIVER REPOPULATION CAPACITIES
After partial hepatectomy, the excised liver lobes never grow back, but the remaining lobes grow to compensate for the mass of the tissue. Mature cell types mediate reconstitution of the entire liver mass, which is complete within 7 days in rodents. The most important signals are hepatocyte growth factor, interleukin-6, tumor necrosis factor, transforming growth factor, and epidermal growth factor [47]. A small amount of information is known about how liver regeneration is terminated once the appropriate liver mass is restored. However, the exogenous factors (endocrine, paracrine, or autocrine) that sense overall liver size are yet not known, several intracellular signals have been identified. For instance, some evidence suggests that transforming growth factor beta-1 is important in termination of liver regeneration [48]. Recent work strongly implicates the detection of blood bile acid levels by nuclear receptors, including FXR, as a regulator of liver growth [49].
Hepatocytes themselves have high capacity for liver repopulation. Recently, Grompe et al. generated severely immunodeficient, fumarylacetoacetate hydrolase (Fah)-deficient mice. After pretreatment with a urokinase-expressing adenovirus, these animals could be highly engrafted (up to 90%) with human hepatocytes from multiple sources, including liver biopsies. Furthermore, human cells could be serially transplanted from primary donors and repopulate the liver for at least four sequential rounds [46]. Notably, this massive cell repopulation has not been documented in humans.
Liver repopulation by adult stem cells
Liver regeneration capacity results mainly from mitotic division of mature hepatocytes, bile duct, and endothelial cells. But under special circumstances, hepatic progenitors called oval cells are activated to expand and take part in liver regeneration when the proliferative capacity of the mature native hepatocytes is impaired and a stimulus of regeneration is present [34]. Hepatic oval cells express markers associated with immature liver cells, such as alphafetoprotein; mature hepatocytes, such as albumin and hematopoietic stem cell such c-kit, Thy-1, CD34 and Sca-1 [34]. These bi-potential progenitors, isolated from the liver, differentiate into mature hepatocytes after transplantation into the liver [34]. However, currently it is uncertain whether such cells can be maintained and expanded in vitro to be used in lieu of adult hepatocytes for clinical application and its contribution to carcinogenesis is not yet clear. Nonetheless, oval cells isolated from the adult liver represent a promising source for cell-based therapy.
Bone marrow cells are an attractive source for extra-hepatic stem cells. Hepatocytes can, in fact, be generated from hematopoietic stem cells and the mechanism by which hematopoietic cells can directly affect hepatic regeneration includes both transdifferentiation and fusion of a cell of hematopoietic origin with defective hepatocytes [32-34]. Their capacity to integrate into injured livers of animals has been reported to be low and the clinical potential to correct liver disease remains to be determined from either bone-marrow or adipose tissue mesenchymal stem cells (MSCs) [50]. Another mechanism reported to alleviate liver injury using MSCs is the secretion of soluble factors. Recent studies directed by our group have found that injection of conditioned-medium from MSCs or the use of a liver assist device can reduced hepatocytes death and increase hepatocytes replication [51,52]. However, the exact mechanism and the type of liver injury that can be treated by this strategy remains to be determined.
Liver repopulation by liver fetal progenitor cells
Fetal human liver progenitor cells have shown enormous replication and differentiation potential, including the capacity to generate mature hepatocytes after transplantation in immunodeficient animals [53] mainly due to the extended reconstitution of telomerase activity which maintains chromosomal integrity during cell division even after cryopreservation [54]. Oertel et al. have used fetal rat liver cells to show at least 3 distinct subpopulations of hepatoblasts exist between embryonic days 12-14. Based on histochemical markers, one population appeared to be bipotential and the other 2 harbored either unipotent hepatocyte or biliary epithelial cell phenotypes.
The same group has reported that a phenomenon known as cell competition is responsible for the liver repopulation where the transplanted fetal liver cells are capable of repopulating the liver by inducing apoptosis in neighboring host hepatocytes that proliferate more slowly than the transplanted cells [55]. So that if the engrafted hepatocytes possess a greater proliferative capacity than the host hepatocytes, the engrafted cells would grow preferentially in response to mitotic stimuli, progressively competing out the host hepatocytes [16]. Nevertheless, the supply of fetal human tissues is also not unlimited and the possibility of oncogenic perturbations needs further study. Moreover, it will be important to determine whether cells derived from fetal livers before 20 weeks of gestational age, when most elective abortions are performed, would express differentiated hepatocellular function after transplantation [56]. Clinical transplantation of fetal hepatocytes in patients with acute liver failure has been documented but resulted in modest clinical improvement in hepatic coma [27], but further studies need to be performed to corroborate clinical significance.
Liver repopulation by pluripotent stem-derived hepatic cells
At this point, most published embryonic stem cells differentiation protocols that generate hepatocyte-like cells, have reported in general a limited functionality and not complete maturity. Major liver repopulation has been difficult to document. However, it has been reported that by selecting for cells expressing markers such as the asialoglycoprotein receptor, it is possible to isolate the most hepatocyte-like cells in the culture with repopulating capacity [44]. It appears likely that conditions and protocols will be developed for the practical production of embryonic stem cell-derived hepatocytes. Nevertheless, major advances have been difficult to achieve. The development of induced pluripotent stem (iPS) cells from adult somatic tissue (ref) may provide major advantages on the development of hepatocyte-like cells. Confirmation that iPS cells have hepatocyte-lineage differentiation capacity comparable to that of existing differentiated embryonic stem cells needs to be studied. Nevertheless, iPS-derived hepatocytes are a very promising population for future therapeutic transplantation.
FUTURE PERSPECTIVES ON LIVER REPOPULATION
In future studies, clinical investigators, cell biologists and bioengineers will need to investigate together several issues that remain uncertain to date. For instance, the optimal hepatic differentiation level that is required to achieve high levels of liver repopulation and function is unknown. Long-term studies after transplantation of hepatocytes derived from stem/progenitor cells are required to elucidate any adverse effects.
Integration of technologies derived from tissue engineering and liver development would be of great promise. The liver Extracellular Matrix (ECM) presents an ideal scaffold for stem cell differentiation into hepatocytes, as well as cell transplantation. It is known that local environmental factors induce hepatocyte homing, differentiation, and proliferation, and studies indicate that stem cells may differentiate toward mature hepatocytes following transfer into injured liver (ref). It is reasonable to expect that a similar beneficial response will be observed for liver grafts as well. Thus, new techniques for decellularization (ref) of liver matrix present great potential as the scaffold for hepatocyte maturation and transplantation. This process may be further manipulated by sequential delivery of factors involved in the initiation and maturation of stem cells to liver cells, allowing temporal and spatial control over differentiation.
The evidence reviewed here strongly supports the conclusion that the realization of cell transplantation methods would be much enhanced with the engineering of an ideal transplantable scaffold. This scaffold would provide all the necessary microstructure and extracellular cues for cell attachment, differentiation, functioning, vascularization and the necessary space for repopulation with liver cells derived from stem cells. One exciting possibility is the utilization of decellularized liver extracellular matrix as the scaffold for cell transplantation, which could ultimately allow the development of an engineered auxiliary liver grafts for transplantation and open the doors to a new era of organ engineering.
CONCLUSIONS
Whether stem cell-derived liver cells will soon be available to treat liver disease is not known. Although there are many promising laboratory studies, only a handful of disease models have been examined concerning whether stem cells can correct liver disease. Thus, normal liver turnover, regeneration after injury or repopulation following transplantation is mediated differently depending on the precise circumstances and cell type. It is premature to conclude that hepatocytes can be generated from non-hepatic cells in culture that will be clinically useful. Standard criteria will need to be developed to assess the extent to which human stem cell-derived hepatocytes have been produced. To date there are more questions than answers, and several points must be addressed before stem/progenitor cells capable of generating hepatocyte-like cells can be translated into successful clinical therapies.
Acknowledgments
This work was supported by a grant from the NIH, K99DK083556-01 to A.S.G. We thank the support of the American Liver Foundation to A.S.G. and Steven Glynn for his editorial assistance.
Abbreviations
- LCT
Liver cell transplantation
- ES cells
Embryonic stem cells
- OLT
orthotopic liver transplantation
- APOLT
auxiliary partial orthotopic liver transplantation
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
-
*
of special interest
-
**
of outstanding interest
- 1.Strom SC, Fisher RA, Rubinstein WS, Barranger JA, Towbin RB, Charron M, Mieles L, Pisarov LA, Dorko K, Thompson MT, et al. Transplantation of human hepatocytes. Transplant Proc. 1997;29:2103–2106. doi: 10.1016/s0041-1345(97)00252-2. [DOI] [PubMed] [Google Scholar]
- 2.Horslen SP, McCowan TC, Goertzen TC, Warkentin PI, Cai HB, Strom SC, Fox IJ. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics. 2003;111:1262–1267. doi: 10.1542/peds.111.6.1262. [DOI] [PubMed] [Google Scholar]
- 3.Mitry RR, Dhawan A, Hughes RD, Bansal S, Lehec S, Terry C, Heaton ND, Karani JB, Mieli-Vergani G, Rela M. One liver, three recipients: segment IV from split-liver procedures as a source of hepatocytes for cell transplantation. Transplantation. 2004;77:1614–1616. doi: 10.1097/01.tp.0000122224.98318.19. [DOI] [PubMed] [Google Scholar]
- 4*.Puppi J, Tan N, Mitry RR, Hughes RD, Lehec S, Mieli-Vergani G, Karani J, Champion MP, Heaton N, Mohamed R, et al. Hepatocyte transplantation followed by auxiliary liver transplantation--a novel treatment for ornithine transcarbamylase deficiency. Am J Transplant. 2008;8:452–457. doi: 10.1111/j.1600-6143.2007.02058.x. First report of the experience of hepatocyte transplantations as a bridge therapy for auxiliary partial orthotopic liver transplantation. [DOI] [PubMed] [Google Scholar]
- 5.Meyburg J, Hoerster F, Weitz J, Hoffmann GF, Schmidt J. Use of the middle colic vein for liver cell transplantation in infants and small children. Transplant Proc. 2008;40:936–937. doi: 10.1016/j.transproceed.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 6.Meyburg J, Hoffmann GF. Liver cell transplantation for the treatment of inborn errors of metabolism. J Inherit Metab Dis. 2008 doi: 10.1007/s10545-008-0829-6. [DOI] [PubMed] [Google Scholar]
- 7.Strom SC, Chowdhury JR, Fox IJ. Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis. 1999;19:39–48. doi: 10.1055/s-2007-1007096. [DOI] [PubMed] [Google Scholar]
- 8.Horslen SP, Fox IJ. Hepatocyte transplantation. Transplantation. 2004;77:1481–1486. doi: 10.1097/01.tp.0000113809.53415.c2. [DOI] [PubMed] [Google Scholar]
- 9.Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, Giron G, Burlina AB. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317–318. doi: 10.1016/S0140-6736(02)07529-3. [DOI] [PubMed] [Google Scholar]
- 10.Sokal EM, Smets F, Bourgois A, Van Maldergem L, Buts JP, Reding R, Bernard Otte J, Evrard V, Latinne D, Vincent MF, et al. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation. 2003;76:735–738. doi: 10.1097/01.TP.0000077420.81365.53. [DOI] [PubMed] [Google Scholar]
- 11.Dhawan A, Mitry RR, Hughes RD, Lehec S, Terry C, Bansal S, Arya R, Wade JJ, Verma A, Heaton ND, et al. Hepatocyte transplantation for inherited factor VII deficiency. Transplantation. 2004;78:1812–1814. doi: 10.1097/01.tp.0000146386.77076.47. [DOI] [PubMed] [Google Scholar]
- 12.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 13.Hughes RD, Mitry RR, Dhawan A. Hepatocyte transplantation for metabolic liver disease: UK experience. J R Soc Med. 2005;98:341–345. doi: 10.1258/jrsm.98.8.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosino G, Varotto S, Strom SC, Guariso G, Franchin E, Miotto D, Caenazzo L, Basso S, Carraro P, Valente ML, et al. Isolated hepatocyte transplantation for Crigler-Najjar syndrome type 1. Cell Transplant. 2005;14:151–157. doi: 10.3727/000000005783983250. [DOI] [PubMed] [Google Scholar]
- 15*.Khan AA, Parveen N, Mahaboob VS, Rajendraprasad A, Ravindraprakash HR, Venkateswarlu J, Rao P, Pande G, Narusu ML, Khaja MN, et al. Treatment of Crigler-Najjar Syndrome type 1 by hepatic progenitor cell transplantation: a simple procedure for management of hyperbilirubinemia. Transplant Proc. 2008;40:1148–1150. doi: 10.1016/j.transproceed.2008.03.022. First report of fetal hepatocytes transplantation for enzimatic deficiency disease. [DOI] [PubMed] [Google Scholar]
- 16.Harada M, Kawaguchi T, Kumemura H, Terada K, Ninomiya H, Taniguchi E, Hanada S, Baba S, Maeyama M, Koga H, et al. The Wilson disease protein ATP7B resides in the late endosomes with Rab7 and the Niemann-Pick C1 protein. Am J Pathol. 2005;166:499–510. doi: 10.1016/S0002-9440(10)62272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mito M. Hepatocyte transplantation in man. Cell Transplant. 1993;2:65. [Google Scholar]
- 18.Nagata H, Ito M, Shirota C, Edge A, McCowan TC, Fox IJ. Route of hepatocyte delivery affects hepatocyte engraftment in the spleen. Transplantation. 2003;76:732–734. doi: 10.1097/01.TP.0000081560.16039.67. [DOI] [PubMed] [Google Scholar]
- 19.Strom SC, Fisher RA, Thompson MT, Sanyal AJ, Cole PE, Ham JM, Posner MP. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63:559–569. doi: 10.1097/00007890-199702270-00014. [DOI] [PubMed] [Google Scholar]
- 20.Soriano HE, Wood RP, Kang DC. Hepatocellular transplantation in children with fulminant liver failure. Hepatology. 1997;30:239A. [Google Scholar]
- 21*.Khan AA, Parveen N, Mahaboob VS, Rajendraprasad A, Ravindraprakash HR, Venkateswarlu J, Rao P, Pande G, Narusu ML, Khaja MN, et al. Management of hyperbilirubinemia in biliary atresia by hepatic progenitor cell transplantation through hepatic artery: a case report. Transplant Proc. 2008;40:1153–1155. doi: 10.1016/j.transproceed.2008.03.110. First report of fetal hepatocytes transplantation through the hepatic artery. [DOI] [PubMed] [Google Scholar]
- 22.Khan AA, Parveen N, Mahaboob VS, Rajendraprasad A, Ravindraprakash HR, Venkateswarlu J, Rao SG, Narusu ML, Khaja MN, Pramila R, et al. Safety and efficacy of autologous bone marrow stem cell transplantation through hepatic artery for the treatment of chronic liver failure: a preliminary study. Transplant Proc. 2008;40:1140–1144. doi: 10.1016/j.transproceed.2008.03.111. [DOI] [PubMed] [Google Scholar]
- 23.Lee WM, Squires RH, Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jalan R. Acute liver failure: current management and future prospects. J Hepatol. 2005;42(Suppl):S115–123. doi: 10.1016/j.jhep.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Bilir BM, Guinette D, Karrer F, Kumpe DA, Krysl J, Stephens J, McGavran L, Ostrowska A, Durham J. Hepatocyte transplantation in acute liver failure. Liver Transpl. 2000;6:32–40. doi: 10.1002/lt.500060113. [DOI] [PubMed] [Google Scholar]
- 26.Sterling RK, Fisher RA. In: Liver Transplantation: Living Donor, Hepatocyte, and Xenotransplantation. Gish R, editor. Philadelphia: WB Sanders; 2001. [DOI] [PubMed] [Google Scholar]
- 27.Habibullah CM, Syed IH, Qamar A, Taher-Uz Z. Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation. 1994;58:951–952. doi: 10.1097/00007890-199410270-00016. [DOI] [PubMed] [Google Scholar]
- 28.Fisher RA, Bu D, Thompson M, Tisnado J, Prasad U, Sterling R, Posner M, Strom S. Defining hepatocellular chimerism in a liver failure patient bridged with hepatocyte infusion. Transplantation. 2000;69:303–307. doi: 10.1097/00007890-200001270-00018. [DOI] [PubMed] [Google Scholar]
- 29.Ott M, Barthold M, Alexandrova K. Clinical applications of human hepatocytes isolated under CGMP conditions. 40th annual meeting of the European Association for the Study of the Liver; 2005. [Google Scholar]
- 30.Schneider A, Attaran M, Meier PN, Strassburg C, Manns MP, Ott M, Barthold M, Arseniev L, Becker T, Panning B. Hepatocyte transplantation in an acute liver failure due to mushroom poisoning. Transplantation. 2006;82:1115–1116. doi: 10.1097/01.tp.0000232451.93703.ab. [DOI] [PubMed] [Google Scholar]
- 31*.Oertel M, Menthena A, Chen YQ, Teisner B, Jensen CH, Shafritz DA. Purification of fetal liver stem/progenitor cells containing all the repopulation potential for normal adult rat liver. Gastroenterology. 2008;134:823–832. doi: 10.1053/j.gastro.2008.01.007. The authors describe subpopulations of fetal liver cells with liver cell repopulation capacities. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 33.Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- 34*.Navarro-Alvarez N, Soto-Gutierrez A, Rivas-Carrillo J, Fox I, Tanaka N, Kobayashi N. Stem cell-derived hepatocytes. Curr Opin Organ Transplant. 2006;11:659–664. A concise review that describes current options to produce hepatic cells for liver repopulation. [Google Scholar]
- 35.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 36.Muraca M, Ferraresso C, Vilei MT, Granato A, Quarta M, Cozzi E, Rugge M, Pauwelyn K, Caruso M, Avital I, et al. Liver repopulation with bone marrow-derived cells improves the metabolic disorder in the Gunn rat. Gut. 2007 doi: 10.1136/gut.2007.127969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Wu YM, Joseph B, Berishvili E, Kumaran V, Gupta S. Hepatocyte transplantation and drug-induced perturbations in liver cell compartments. Hepatology. 2008;47:279–287. doi: 10.1002/hep.21937. An interesting report that presents the transplantation of reporter cells, showing the important insights into homeostatic mechanisms involving liver cell compartments, targeting injury in hepatic endothelial and parenchymal cells. [DOI] [PubMed] [Google Scholar]
- 38.Kumaran V, Joseph B, Benten D, Gupta S. Integrin and extracellular matrix interactions regulate engraftment of transplanted hepatocytes in the rat liver. Gastroenterology. 2005;129:1643–1653. doi: 10.1053/j.gastro.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Nagata H, Nishitai R, Shirota C, Zhang JL, Koch CA, Cai J, Awwad M, Schuurman HJ, Christians U, Abe M, et al. Prolonged survival of porcine hepatocytes in cynomolgus monkeys. Gastroenterology. 2007;132:321–329. doi: 10.1053/j.gastro.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Rajvanshi P, Kerr A, Bhargava KK, Burk RD, Gupta S. Efficacy and safety of repeated hepatocyte transplantation for significant liver repopulation in rodents. Gastroenterology. 1996;111:1092–1102. doi: 10.1016/s0016-5085(96)70078-1. [DOI] [PubMed] [Google Scholar]
- 41.Gupta S, Gorla GR, Irani AN. Hepatocyte transplantation: emerging insights into mechanisms of liver repopulation and their relevance to potential therapies. J Hepatol. 1999;30:162–170. doi: 10.1016/s0168-8278(99)80022-1. [DOI] [PubMed] [Google Scholar]
- 42.Benedetti E, Kirby JP, Asolati M, Blanchard J, Ward MG, Williams R, Hewett TA, Fontaine M, Pollak R. Intrasplenic hepatocyte allotransplantation in dalmation dogs with and without cyclosporine immunosuppression. Transplantation. 1997;63:1206–1209. doi: 10.1097/00007890-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 43**.Yamanouchi K, Zhou H, Roy-Chowdhury N, Macaluso F, Liu L, Yamamoto T, Yannam GR, Enke C, Solberg TD, Adelson AB, et al. Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology. 2009;49:258–267. doi: 10.1002/hep.22573. An important report that shows the effects hepatic irradiation as a preparative therapy to augment hepatocyte transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Basma H, Soto-Gutierrez A, Yannam GR, Liu L, Ito R, Yamamoto T, Ellis E, Carson SD, Sato S, Chen Y, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–999. doi: 10.1053/j.gastro.2008.10.047. An elegant work showing for the first time liver repopuation using a subpopulation of liver cells differentiated from human embryonic stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Oertel M, Shafritz DA. Stem cells, cell transplantation and liver repopulation. Biochim Biophys Acta. 2008;1782:61–74. doi: 10.1016/j.bbadis.2007.12.004. A concise review describing current cellular options for liver repopulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, Strom S, Kay MA, Finegold M, Grompe M. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. A short review describing the developmental pathways for pancreatic and hepatic differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duncan AW, Dorrell C, Grompe M. Stem Cells and Liver Regeneration. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 50.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, Okochi H, Ochiya T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 51*.Yagi H, Parekkadan B, Suganuma K, Soto-Gutierrez A, Tompkins R, Tilles A, Yarmush M. Long Term Superior Performance of a Stem Cell/Hepatocyte Device for the Treatment of Acute Liver Failure. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2008.0681. First report that demonstrates the use of mesenchymal stem cells and primary hepatocytes as a cell source for bioartificial liver devices. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634–1643. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- 53.Dan YY, Riehle KJ, Lazaro C, Teoh N, Haque J, Campbell JS, Fausto N. Isolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineages. Proc Natl Acad Sci U S A. 2006;103:9912–9917. doi: 10.1073/pnas.0603824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oertel M, Menthena A, Chen YQ, Shafritz DA. Properties of cryopreserved fetal liver stem/progenitor cells that exhibit long-term repopulation of the normal rat liver. Stem Cells. 2006;24:2244–2251. doi: 10.1634/stemcells.2006-0141. [DOI] [PubMed] [Google Scholar]
- 55.Oertel M, Menthena A, Dabeva MD, Shafritz DA. Cell competition leads to a high level of normal liver reconstitution by transplanted fetal liver stem/progenitor cells. Gastroenterology. 2006;130:507–520. doi: 10.1053/j.gastro.2005.10.049. quiz 590. [DOI] [PubMed] [Google Scholar]
- 56.Fox IJ, Roy-Chowdhury J. Hepatocyte transplantation. J Hepatol. 2004;40:878–886. doi: 10.1016/j.jhep.2004.04.009. [DOI] [PubMed] [Google Scholar]


