Abstract
Objective
This study examined amyloid-β (Aβ) deposition in 190 non-demented subjects aged 82 and older to determine the proportion of Aβ-positive scans and associations with cognition, APOE status, brain volume, and Ginko biloba (Gb) treatment.
Methods
Subjects who agreed to participate had a brain MRI and positron emission tomography scan with 11C-labeled Pittsburgh compound B (PiB) following completion of a Gb treatment clinical trial. The youngest subject in this imaging study was 82, and the mean age of the subjects was 85.5 at the time of the scans;152 (80%) were cognitively normal and 38 (20%) were diagnosed with mild cognitive impairment (MCI)at the time of the PiB study.
Results
A high proportion of the cognitively normal subjects (51%) and MCI subjects (68%) were PiB-positive. The APOE*4 allele was more prevalent in PiB-positive than in PiB-negative subjects (30% vs 6%). Measures of memory, language and attentional functions were worse in PiB-positive than in PiB-negative subjects, when both normal and MCI cases were analyzed together; however no significant associations were observed within either normal or MCI subject groups alone. There was no relationship between Gb treatment and Aβ deposition as determined by PiB.
Interpretation
The data revealed a 55% prevalence of PiB-positivity in non-demented subjects age >80 and 85% PiB-positivity in the APOE*4 non-demented elderly subjects. The findings also showed that long-term exposure to Gb did not affect the prevalence of cerebral Aβ deposition.
Introduction
Alzheimer's disease (AD) is the most frequent form of dementia in elderly subjects, with estimates of dementia prevalence of 7-8% of the population age <75 and 45% after age 85.1 Although the exact causes of late-onset AD are unknown, altered amyloid-beta (Aβ) metabolism and clearance, and subsequent deposition of Aβ protein in brain likely play a central role in AD pathophysiology. Multiple neuropathological2-4 and neuroimaging5-7 studies have shown the presence of Aβ deposits in cognitively normal subjects, which may represent a pre-dementia state. Studies conducted in memory disorder clinics have shown that Aβ deposition, as measured with carbon-11-labeled Pittsburgh compound B (PiB), is present in approximately 25% of cognitively normal subjects over age 605-9, with higher rates in those age >80.6 Both referral clinic- and community-based studies have shown a higher proportion of Aβ deposition in cognitively normal subjects carrying the apolipoprotein E4 (APOE*4) allele than in non-carriers.7, 10, 11 After aging,the APOE*4 allele is the most important predictor of incident AD.12
The present study extended previous observations by using PiB in a group of 190 non-demented individuals greater than 80 years of age who had been followed for up to 7 years as part of a larger study of the effect of Ginkgo biloba(Gb) on prevention of dementia.13 Specifically, we aimed to determine the proportion of cognitively normal and MCI subjects with elevated Aβ deposition in this elderly group and its relationship to APOE*4 status, cognitive measures, and magnetic resonance imaging (MRI) cerebral volumes.
Subjects/Materials and Methods
Participants
The present study was conducted in a subgroup of subjects who had participated in the Ginkgo Evaluation of Memory study (GEMS) from 2000 to 2008 in Pittsburgh. GEMS was a double-blind, multi-site, placebo-controlled, randomized clinical trial of 240 mg daily dose (120 mg twice daily) of Gb in 3,069 community-dwelling participants aged 72-96 years at study entry.13 Exclusion criteria were reported in detail elsewhere13 and included prevalent dementia, current cholinesterase inhibitor or other psychotropic medication use, history of bleeding disorders, severe depression, abnormal clinical laboratory screening, and disease-limited life expectancy less than 5 years. The median follow-up time from randomization was 6.1 years. A wide array of cognitive, genetic, functional, proxy-reported and medical history variables were collected.14 The primary study outcome, that Gb would slow or delay the development of incident dementia, was negative.13
In 2009, approximately one year following GEMS closeout, 197 participants from the Pittsburgh clinical site were recruited into the GEMS Imaging Sub-Study. The inclusion criterion was completion of the GEMS protocol. Exclusion criteria were dementia at GEMS close-out or contraindications for neuroimaging. Compared to all 671 Pittsburgh site participants who completed the GEMS protocol and did not reach a dementia endpoint, the Imaging Sub-Study participants were slightly younger but otherwise comparable in sex, race, education, APOE*4 status, estimated premorbid IQ and estimated income by zip code (p >0.05). Three subjects were excluded for technical difficulties with PET scanning, three other subjects were excluded for developing dementia, and one subject could not complete cognitive testing. Thus, 190 of the 197 initial participants were entered in the present study.
Cognitive Assessment and Adjudication
At the time of PiB scanning, the participants were assessed with a subset of the GEMS neuropsychological battery.15 Cognitive adjudication was completed blind to neuroimaging results by the GEMS Cognitive Diagnostic Center14, taking into account historical serial cognitive assessments from the parent study. Criteria for MCI included 2 - 3 tests impaired at cutoffs of 1.5 standard deviations (SD) below age-education adjusted means.
Imaging
MRI scanning was performed using a GE Signa 1.5 T scanner and a standard head coil using methods described previously.16 A T1-weighted volumetric spoiled gradient recalled (SPGR) sequence was acquired (0.937 × 0.937 mm) in the sagittal (n=177, slice thickness=1.2mm/0mm interslice) or coronal (n=14, slice thickness=1.5mm/0mm interslice) planes. Voxel-based morphometry (VBM) was performed with the sagittal SPGR MRI acquisition protocol.
The MRI data were normalized to the ICBM 152 template (Montreal Neurological Institute, Montreal, Canada) and tissue priors using Statistical Parametric Mapping 8 (SPM8) software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Unified segmentation was not successful for 6 subjects and 13 subjects had incompatible MRI scans, and VBM was performed for 171 subjects. This subgroup was composed of 136 normal cognition (NC) (71 PiB-positive) and 35 MCI (24 PiB-positive) subjects. The resulting modulated gray matter images were smoothed (8 mm Gaussian filter).
The PiB data were acquired for 20 minutes (4 × 5 minute frames) beginning 50 minutes after injection of 15±1.5 mCi of PiB on a Siemens/CTI ECAT HR+ scanner in 3D imaging mode (63 planes with slice width 2.4 mm) equipped with a Neuro-Insert and reconstructed using filtered back-projection (Fourier re-binning and 2D back-projection with Hann filter kernel FWHM = 3 mm). Post-injection transmission scans were acquired using 68Ge/68Ga rods, and data were corrected for photon attenuation, scatter, and radioactive decay. The final reconstructed PET image resolution was about 6 mm (transverse and axial).
APOE Genotyping
APOE genotyping was performed on isolated DNA from blood as described previously.17
Data Analysis
The procedure for co-registration of the MRI and PiB PET images has been described.18 Regions-of-interest (ROIs) were hand-drawn on a template that was a high-resolution MR image of a single elderly MCI subject.19 The ROIs included five primary cortical areas [i.e., anterior cingulate (ACG), frontal cortex (FRC), lateral temporal cortex (LTC), parietal cortex (PAR), precuneus cortex (PRC) which comprised the Global-5 composite region], as well as medial temporal cortex (MTC), anterior ventral striatum (AVS), occipital cortex (OCC), occipital pole (OCP), sensorimotor cortex (SMC), thalamus (THL), subcortical white matter (SWM), pons (PON), and cerebellum (CER)]. PiB retention was measured using the standardized uptake value ratio (SUVR) over the 50-70 min scan (or SUV: scaled to injected dose and body mass) that is then normalized by the SUV of the CER reference region.
All statistical analyses were performed using SAS (version 9.2; SAS Institute Inc., Cary, NC, USA). All two-sample comparisons were evaluated using a Wilcoxon nonparametric test. In settings where the sample size was below 25 in any group, exact methods were used for the computation of the significance level. For the analysis of the neuropsychological outcomes, the significance levels for the two sample comparisons were computed from a linear regression model including age, gender, and education. Each model was evaluated using regression diagnostics to identify potentially influential observations. When a Cook's D value greater than 0.2 was observed, the corresponding model was recomputed with the observation removed from the data set. These instances are denoted in the tables and text.
A two-way ANOVA model was performed with diagnosis (NC and MCI) and PiB status (PiB-negative and PiB-positive) as grouping factors to determine voxel-wise gray matter differences. The interaction effect between diagnosis and PiB status, and the main effects were examined using appropriate contrasts. Analysis of covariance was also performed in SPM8 to determine voxel-wise gray matter differences between groups: PIB-negative NC > PIB-positive MCI and PIB-negative NC > PIB-negative MCI. These models included age and gender as covariates and were applied to gray matter maps with intensity threshold masking of 0.3. Thresholds of 0, 0.2 and 0.3 were examined but the latter was chosen because this provided a good compromise between inclusion of gray matter and exclusion of background instabilities. Significance levels were set to p<0.025, FDR corrected.
Definition of PiB-positive Scan
PiB scans were conducted in a separate group of 62 younger controls (69.4 ± 11.5 yrs; range 35 to 89) using the methods described in this study. An iterative-outlier method defined subjects as PiB-positive if the Global-5 composite region SUVR was ≥1.57.8
Result
Effect of Gb Intervention on Aβ Deposition
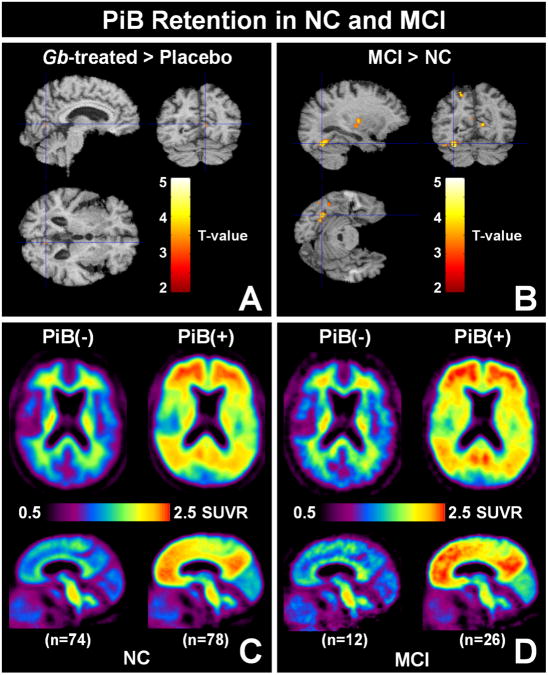
Ninety-five (50%) of the GEMS Imaging Sub-Study participants had been randomized to the Gb intervention arm of the parent GEMS and 95 to the placebo arm. No group differences were found in demographics, mean Global-5 SUVR values, or proportion of PiB-positive cases. There also was no difference in PiB retention on a voxel-wise basis between treatment groups (Figure 2A). Therefore the two groups were combined for further analyses.
Figure 2.
(A & B) Results of voxel-wise analysis of PiB retention. The t-maps show the three orthogonal views of the slice containing the point of peak significance overlaid on the MCI template. Both A & B correspond to k=50 contiguous voxels; p<0.001 uncorrected. No significant voxels are detected at p<0.001 with FDR correction. (A) Comparison of PiB retention in Gb-treated (n=95) and placebo-treated subjects (n=95); Contrast: Gb-treated > placebo-treated shown, no significant voxels for Gb-treated < placebo-treated. (B) Comparison of PiB retention in all controls (n=152) with all MCI subjects (n=38); Contrast: MCI > controls; no significant voxels for controls > MCI. (C & D) The topography of PiB retention in control (C) and MCI subjects (D). The average PiB retention (SUVR) is shown in an axial plane (top) and a sagittal plane (bottom) in PiB-negative (left) and PiB-positive subjects (right).
Subject Characteristics
Demographics, genetic status, and cognitive scores are shown in Table 1; APOE genotype was available for 176 of the 190 cases entered in the analysis. The cohort was composed of NC (n=152; 80%) and MCI (n=38; 20%) at the time of PiB scan. The youngest subject was 82, and the average group age was not different between NC (mean 85.44, SD 2.83) and MCI (mean 85.87, SD 2.78) groups. Over half (55%) of these very elderly subjects were PiB-positive (see Table 1). The proportion of PiB-positive subjects was higher in the MCI group (68%) than in the NC group (51%). However, this difference did not reach statistical significance (p=0.058).
Table 1. Demographic and clinical characteristics by PiB status for all participants.
| All Subjects | Normal Cognition | MCI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PiB-negative (n=86) | PiB-positive (n=104) | P | PiB-negative (n=74) | PiB-positive (n=78) | P | PiB-negative (n=12) | PiB-positive (n=26) | P | |
| Age, mean (SD), y | 85.2 (2.5) | 85.8 (3.0) | .3711 | 85.5 (2.6) | 85.4 (3.1) | .5964 | 83.8 (1.2) | 86.8 (2.8) | .0012 |
| Sex (n, % male) | 54 (62.8%) | 59 (56.7%) | .3971 | 44 (59.5%) | 44 (56.4%) | .7035 | 10 (83.3%) | 15 (57.7%) | .1578 |
| Race | |||||||||
| white | 83 (96.5%) | 101 (97.1%) | .8128 | 73 (98.7%) | 77 (98.7%) | .9701 | 10 (83.3%) | 24 (92.3%) | .5773 |
| Education, mean (SD), y | 14.6 (2.7) | 14.7 (2.5) | .9452 | 14.8 (2.7) | 14.8 (2.6) | .8955 | 13.8 (3.1) | 14.2 (2.3) | .5082 |
| APOE*4 allele carrier (n/available, %) | 5/81 (6.2%) | 29/95 (30.5%) | <.0001 | 5/69 (7.2%) | 22/73 (30.1%) | .0005 | 0/12 (0%) | 7/22 (31.8%) | .0356 |
| APOE*2 allele carrier (n/available, %) | 14/81 (17.3%) | 7/95 (7.4%) | .0608 | 11/69 (15.9%) | 6/73 (8.2%) | .1989 | 3/12 (25%) | 1/22 (4.6%) | .2733 |
| MMSE score, mean (SD) | 27.8 (2.1) | 27.4 (2.1) | .1700 | 28.2 (1.7) | 27.9 (1.6) | .1981 | 25.3 (2.8) | 26.0 (2.6) | .5878 |
There were no differences between PiB-positive and PiB-negative subjects as a function of age, gender, race, education level or MMSE score except the PiB-positive MCI subjects were significantly older than MCI PiB-negative subjects (Table 1). PiB-positive subjects were significantly more likely to be APOE*4 allele carriers, when analyzed across all subjects as well as in both the NC and MCI groups. A positive APOE*4 status was similar in MCI and NC subjects. In contrast, PiB-positivity in the three groups was not significantly effected by APOE*2 status (Table 1). In addition, 85% of all APOE*4 carrier subjects were PiB-positive, while 46% of all APOE*4 non-carriers were PiB-positive.
When comparing the cognitive test scores for all subjects (n=190), those who were PiB-positive had worse scores on animal fluency (p=0.0496) and Trail Making A (p=0.046) tests than those who were PiB negative (Table 2). There were no significant differences in neuropsychological test performance between the PiB-positive and PiB-negative subsets within each of the NC and MCI groups separately (Table 2). Of note, regardless of significance level, all mean test scores were in the predicted direction (worse performance by PiB-positive subjects), except for letter fluency.
Table 2. Concurrent neuropsychological test performance (mean, SD) by PiB status.
† Models adjusted for age, education, sex, race and CESD depression score.
| All Subjects | Normal Cognition | MCI | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| PiB-negative (n=86) | PiB-positive (n=104) | p | PiB-negative (n=74) | PiB-positive (n=78) | p | PiB-negative (n=12) | PiB-positive (n=26) | p | |
| Memory | |||||||||
| CVLT sum of learning trials (range, 0-80) | 44.9 (11.6) | 41.5 (12.1) | .074 | 47.3 (10.5) | 44.1 (11.4) | .094 | 30.7 (6.9) | 33.6 (10.8) | .094 |
| CVLT delayed recall (range, 0-16) | 8.5 (3.7) | 8.0 (3.7) | .365 | 9.2 (3.1) | 9.1 (3.2) | .679 | 3.8 (3.2) | 4.7 (3.4) | .221 |
| Rey figure immediate recall (range, 0-24) | 16.7 (3.8) | 15.6 (4.0) | .077 | 17.1 (3.7) | 16.8 (3.4) | .405 | 14.0 (3.9) | 12.0 (3.3) | .669 |
| Rey figure delayed recall (range, 0-24) | 16.2 (4.1) | 15.8 (4.1) | .736 | 16.6 (3.8) | 16.9 (3.3) | .560 | 13.5 (4.8) | 12.3 (4.0) | .734 |
|
| |||||||||
| Visuospatial construction | |||||||||
| Rey figure copy (range, 0-24) | 20.7 (2.4) | 20.2 (2.2) | .131 | 20.9 (2.2) | 20.8 (1.7) | .683 | 19.8 (3.1) | 18.5 (2.7) | .509 |
|
| |||||||||
| Executive functions | |||||||||
| Trail Making B, s to completion (sample range, 43-240) | 106.7 (45.4) | 123.3 (51.9) | .059 | 97.5 (35.5) | 108.2 (41.9) | .229 | 163.4 (59.0) | 168.5 (53.4) | .437 |
|
| |||||||||
| Language | |||||||||
| Animal fluency, no. of words generated in 60 s | 15.8 (3.7) | 14.4 (4.0) | .0496 | 15.9 (3.6) | 15.4 (3.6) | .717 | 15.3 (4.5) | 11.2 (3.4) | .098 |
| Letter fluency (sum F & S), average no. of words generated in 60 s | 27.3 (8.6) | 28.3 (8.0) | .336 | 27.9 (8.3) | 28.7 (7.9) | .250 | 23.3 (10.0) | 27.2 (8.1) | .290 |
|
| |||||||||
| Attention | |||||||||
| Trail Making A, s to completion (sample range 20-94) | 42.3 (15.0) | 48.5 (17.8) | .046 | 42.2 (15.2) | 45.3 (16.6) | .388 | 42.8 (14.3) | 58.2 (18.3) | .095 |
Abbreviations: CVLT, California Verbal Learning Test
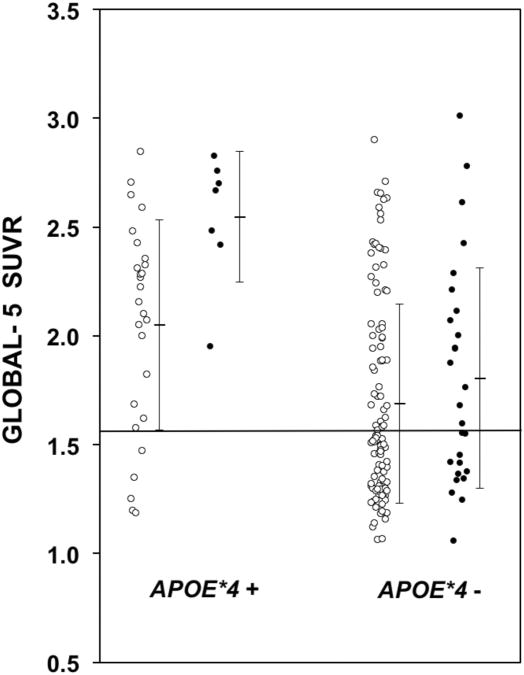
The MCI group showed higher PiB retention than NC in the following brain areas: Global-5, anterior cingulate, frontal cortex, lateral temporal cortex, parietal cortex, precuneus, anterior ventral striatum, occipital cortex, and sensorimotor cortex (Table 3a). APOE*4 carriers showed significantly higher levels of PiB retention in the brain areas typically found to have increased Aβ-deposition in AD 20: anterior cingulate, frontal cortex, lateral temporal cortex, parietal cortex, precuneus and anterior ventral striatum (Table 3b). Similar findings were observed when the NC and MCI groups were examined separately, except the occipital cortex showed significantly higher PiB retention in the APOE*4 carriers of the MCI group. Figure 1 shows a scatter plot of the Global-5 SUVR values of all subjects, highlighting the higher average values for the NC and MCI APOE*4 carriers relative to non-carriers.
Table 3. a. SUVR values by diagnosis.
| Region | All | Subjects | |
|---|---|---|---|
| NC (n=152) | MCI (n=38) | P | |
| Global-5 | 1.75 (0.48) | 1.97 (0.54) | .0251 |
| ACG | 1.71 (0.59) | 1.95 (0.64) | .0448 |
| FRC | 1.84 (0.53) | 2.06 (0.58) | .0380 |
| LTC | 1.72 (0.39) | 1.91 (0.49) | .0221 |
| PAR | 1.74 (0.45) | 1.93 (0.49) | .0418 |
| PRC | 1.75 (0.52) | 1.99 (0.58) | .0177 |
| AVS | 1.59 (0.47) | 1.82 (0.52) | . 0191 |
| MTC | 1.18 (0.16) | 1.19 (0.15) | .6295 |
| PON | 2.08 (0.30) | 2.00 (0.27) | .0812 |
| OCC | 1.49 (0.23) | 1.60 (0.27) | .0348 |
| OCP | 1.70 (0.22) | 1.75 (0.21) | .4060 |
| SMC | 1.47 (0.28) | 1.59 (0.33) | .0418 |
| SWM | 1.94 (0.33) | 1.93 (0.28) | .5904 |
| THL | 1.44 (0.22) | 1.44 (0.19) | .8653 |
| b. SUVR values by APOE*4 carrier status | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | All Subjects | Normal Cognition | MCI | ||||||
|
| |||||||||
| APOE*4+ (n=34) | APOE*4-(n=142) | P | APOE*4+ (n=27) | APOE*4-(n=115) | P | APOE*4+ (n=7) | APOE*4-(n=27) | P | |
| Global-5 | 2.15 (0.49) | 1.71 (0.46) | <.0001 | 2.05 (0.48) | 1.69 (0.46) | .0014 | 2.54 (0.30) | 1.81 (0.51) | .0013 |
| ACG | 2.22 (0.57) | 1.65 (0.56) | <.0001 | 2.11 (0.56) | 1.62 (0.56) | .0003 | 2.65 (0.40) | 1.76 (0.59) | .0014 |
| FRC | 2.26 (0.55) | 1.80 (0.50) | <.0001 | 2.16 (0.54) | 1.77 (0.50) | .0031 | 2.66 (0.38) | 1.90 (0.54) | .0028 |
| LTC | 2.05 (0.43) | 1.69 (0.38) | <.0001 | 1.94 (0.42) | 1.67 (0.37) | .0040 | 2.48 (0.29) | 1.77 (0.45) | .0004 |
| PAR | 2.08 (0.47) | 1.72 (0.43) | .0001 | 2.00 (0.48) | 1.69 (0.42) | .0041 | 2.40 (0.28) | 1.79 (0.47) | .0011 |
| PRC | 2.15 (0.50) | 1.71 (0.51) | <.0001 | 2.05 (0.51) | 1.69 (0.51) | .0018 | 2.53 (0.24) | 1.81 (0.56) | .0014 |
| AVS | 1.94 (0.51) | 1.57 (0.44) | .0001 | 1.84 (0.49) | 1.54 (0.43) | .0018 | 2.29 (0.46) | 1.69 (0.47) | .0087 |
| MTC | 1.22 (0.15) | 1.18 (0.16) | .1110 | 1.22 (0.15) | 1.18 (0.16) | .1837 | 1.24 (0.14) | 1.18 (0.15) | .4505 |
| PON | 2.03 (0.22) | 2.10 (0.28) | .2200 | 2.06 (0.19) | 2.11 (0.30) | .3124 | 1.92 (0.31) | 2.02 (0.26) | .3537 |
| OCC | 1.58 (0.23) | 1.51 (0.23) | .0424 | 1.52 (0.18) | 1.49 (0.22) | .2700 | 1.80 (0.25) | 1.55 (0.26) | .0332 |
| OCP | 1.77 (0.23) | 1.71 (0.20) | .1421 | 1.74 (0.23) | 1.71 (0.21) | .5544 | 1.88 (0.23) | 1.72 (0.19) | .0264 |
| SMC | 1.67 (0.33) | 1.46 (0.27) | .0006 | 1.60 (0.32) | 1.44 (0.26) | .0130 | 1.91 (0.27) | 1.52 (0.31) | .0060 |
| SWM | 1.90 (0.28) | 1.98 (0.31) | .2144 | 1.92 (0.27) | 1.97 (0.32) | .4011 | 1.80 (0.31) | 1.97 (0.27) | .2981 |
| THL | 1.48 (0.23) | 1.44 (0.21) | .4098 | 1.46 (0.23) | 1.44 (0.21) | .7398 | 1.54 (0.23) | 1.43 (0.18) | .2565 |
Figure 1.
Scatter plot of the Global-5 SUVR values of normal cognition (NC) (○) and mild cognitive impairment (MCI) (●) subjects, highlighting the higher average values for both the NC and MCI APOE*4-positive subjects relative to the APOE*4-negative NC and MCI subjects. The horizontal line at a Global-5 SUVR of 1.57 represents the line defining PiB-positivity.
When the association of APOE*4 with PiB retention was limited to those subjects who were PiB-positive (NC and MCI groups combined), the same general trends as those described above were observed, but the findings were blunted (Supplementary Table 1). Within the PiB-positive group, the APOE*4 allele was associated with significantly higher PiB retention only in the anterior cingulate (p=0.014), the frontal cortex (p=0.048), the precuneus (p=0.048), and the Global-5 composite region (p=0.024).
Association Between PiB Retention and Diagnostic Group
In this very elderly cohort, there was very little difference in PiB retention between subjects with normal cognition and subjects with MCI at the time of the scan. This was true when all controls (n=152) and all MCI subjects (n=38) were compared (Figure 2B), or when either the PiB-positive controls (n=78) were compared to the PiB-positive MCI subjects (n=26) or when the PiB-negative controls (n=74) were compared to the PiB-negative MCI subjects (n=12) (data not shown). The topography of the average PiB retention in the PiB-negative controls (n=74) and PiB-negative MCI subjects (n=12) was typical of that reported in other studies (Figures 2C and 2D) and consisted of non-specific white matter retention5, 8, 21. The topography of the average PiB retention in the PiB-positive controls (n=78) and PiB-positive MCI subjects (n=26) was similar to the topography previously reported in MCI and AD22-24.
Association Between Brain Volume and PiB Retention
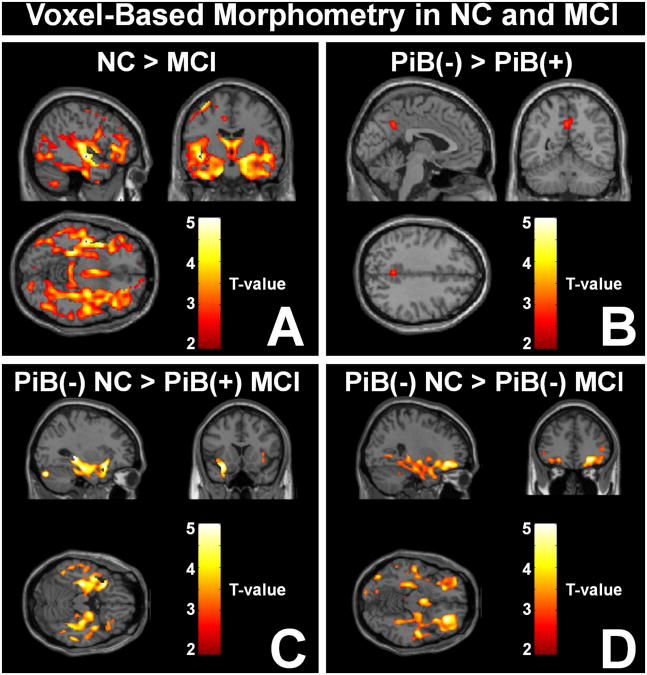
Figure 3 shows the results of VBM analyses of 171 subjects with MRI data available for analysis. Two-way ANOVA did not reveal any significant diagnosis by PiB status interaction effect (p<0.025, FDR corrected). Figure 3A and 3B show the main effect results from the two-way ANOVA model analysis performed with diagnosis (NC, n=136; or MCI, n=35) and PiB status (negative, n=76 negative; or positive, n=95) as grouping factors. As has been shown in previous studies25, the main effect of diagnosis (NC vs. MCI) was observed in the mesial temporal lobes where the MCI subjects showed greater atrophy (see Supplementary Table 2A-D for SPM peak-levels and statistics). Figure 3B shows that there was no significant main effect of PiB status on brain volume across all subjects. Figure 3C shows that PiB-positive MCI subjects (n=24) demonstrated enhanced atrophy relative to PiB-negative NC (n=65) predominantly in the mesial temporal lobes. In contrast, PiB-negative MCI subjects (n=11) differed from PiB-negative NC in a more diffuse manner with the most significant differences observed in the frontal lobes (Fig 3D). No significant differences were observed when PiB-negative and PiB-positive MCI subjects were directly compared (data not shown). Nor was a significant volume difference observed between PiB-positive NC and PiB-positive MCI subjects under the conditions of this analysis (p<0.025 with FDR correction and 100 contiguous voxels).
Figure 3.
Results of VBM analyses. The t-maps show the three orthogonal views of the slice containing the point of peak significance overlaid on the MNI template. (A & B) Two-way ANOVA model with diagnosis (control, n=136; or MCI, n=35) and PiB status (negative, n=76; or positive, n=95) as grouping factors to determine voxel-wise gray matter differences. No interaction effect was found between diagnosis and PiB status. (A) Main effect of diagnosis. Contrast: Controls > MCI. (B) Main effect of PiB status. Contrast: PiB-negative > PiB-positive. (C) Comparison of PiB-negative controls (n=65) with PiB-positive MCI subjects (n=24); Contrast: PiB-negative Controls >PiB-positive MCI. (D) Comparison of PiB-negative controls with PiB-negative MCI subjects (n=11); Contrast: PiB-negative Control > PiB-negative MCI. All figures correspond to k=100 contiguous voxels and p<0.025 after FDR correction, except (B) that corresponds to k=100 contiguous voxels and p<0.01 without FDR correction. For the comparison in (B), it is important to note that no significant voxels are detected at p<0.025 with FDR correction.
Discussion
We studied a group of non-demented elderly subjects who had participated in the Gb therapeutic trial and found a number of highly relevant findings in this, the oldest (mean age 85.5) and largest elderly cohort studied with Aβ imaging and cognitive testing. First, the long-term use of Gb treatment, in the most commonly used dose, had no effect on Aβ deposits as indexed by PiB retention (Figure 2A). Second, over half of this elderly cohort had significant PiB retention, as 55% of these elderly subjects were PiB-positive (51% of the cognitively normal subject and 68% of those with MCI), and elevated PiB retention was seen in a regional pattern typical of that previously described in MCI and AD patients (Figures 2C & 2D)22, 26, 27. Third, PiB retention was not extensively greater in the MCI subjects than in the subjects with normal cognition (Figure 2B). Fourth, PiB retention occurred more frequently in subjects carrying the APOE*4 allele. Fifth, semantic fluency and psychomotor speed were associated with PiB retention. Finally, no significant differences in brain volumes were found between PiB-negative and PiB-positive subjects.
GEMS showed that regardless of mechanism, Gb did not decrease the risk of incident dementia.13 The present study showed that Gb did not modify Aβ deposition, and consequently, its use will not affect the outcome of future neuroimaging studies of Aβ deposition. In this very elderly cohort, 51% of cognitively normal subjects and 68% of MCI subjects were found to have elevated PiB retention consistent with having significant brain Aβ deposition. These prevalence percentages are higher than those typically reported in most younger NC cohorts (∼25% across a range of studies7, 8, 19, 22, 28-39) and younger MCI cohorts (∼60% across a range of studies7, 19, 28, 33, 39-43). Our findings that Aβ deposition and its prevalence continues to increase with age are consistent with previous observations that showed that AD pathology can be seen in a high proportion of autopsied NC subjects after age 80+.2, 4, 44 Bennett and colleagues reported that 66% of normal elderly subjects and 68% of those with MCI (age 81 – 85) met the CERAD criteria for possible, probable or definite AD.45 Similarly, Savva and colleagues found an attenuated association between neocortical neuritic plaques and dementia in subjects age 95+, which was due primarily to increased pathology in the cognitively normal subjects. Nevertheless, the primary cause of dementia in this group was AD pathology.46
The dynamics of the association between cognition and Aβ deposition in cross-sectional neuroimaging studies as well as in neuropathological studies are difficult to determine with certainty, since there is no follow-up to determine whether the development of dementia is imminent or whether the subjects will remain cognitively normal despite the presence of significant amounts of Aβ in the brain. We hypothesize that the normal subjects with Aβ deposition on neuroimaging studies will progress to a dementia syndrome.
The previously reported observation of a higher prevalence of the APOE*4 allele among younger PiB-positive subjects10 was observed also in this very elderly cohort. The 5-fold increased prevalence of the APOE*4 allele in PiB-positive individuals with an average age of ∼85 was similar to APOE*4 prevalence among PiB-positive subjects in their 60's and 70's.10 This implies that the APOE*4 effect is still a strong risk factor for Aβ deposition in the mid-80's. The region-specific nature of the APOE*4-associated elevations in PiB retention adds additional support for the clear interaction between APOE*4 and Aβ-deposition.10 It should be noted that the APOE*4 allele carrier frequency in this population (20.6%) is within the range (20-30%) of what is seen in elderly subjects participating of epidemiological studies in the U.S.47, 48
The VBM analyses suggest that this very elderly MCI cohort is no different than younger cohorts in that mesial temporal atrophy is the most prominent structural change compared to age-matched controls.25, 49 PiB-positive MCI appeared to be more associated with mesial temporal atrophy, as are AD50, 51, while PiB-negative MCI appeared to be associated with a more diffuse pattern of atrophy that included frontal lobes. However, this conclusion remains speculative because when directly compared, the atrophy patterns of PiB-positive and PiB-negative MCI did not significantly differ, most likely due to the small samples sizes of these two MCI groups.
In these very elderly subjects with no (i.e., controls) or minimal (i.e., MCI) cognitive changes, increased atrophy was not readily apparent in the PiB-positive subjects relative to the PiB-negative subjects (Figure 3B). This could be considered consistent with the hypothesis that Aβ deposition precedes atrophy in the pathophysiological sequence of AD.52 However, even more likely in this very elderly cohort, is the possibility that extensive age-related atrophy was present in both PiB-negative and PiB-positive subjects. That is, brain atrophy was being driven by factors other than or in addition to Aβ deposition. Still, the MCI subjects had greater brain atrophy than the cognitively normal controls (Figure 3A). In contrast, both the controls and the MCI subjects had extensive PiB retention, and the level of PiB retention in MCI subjects was not significantly greater than that in controls (Figure 2B). Taken together, the in vivo evidence of increased atrophy and lack of evidence for increased Aβ deposition in the MCI subjects of this very elderly cohort is consistent with the postmortem findings of Savva et al. 4. Savva et al. suggested that increasing plaque and tangle pathology with increasing age in non-demented subjects tended to obscure differences in these pathologies between demented and non-demented subjects above age 90 years of age, a finding consistent with other studies 53, 54. However, Savva et al. also found a consistent difference in atrophy between demented and non-demented subjects even in subjects above 90 years of age.
As a group, PiB-positive subjects had lower performance on executive and attention tests. The neuropsychological battery was used to define “normal cognition”, and it was difficult to determine statistical differences between NC subjects with and without Aβ deposition. While there were no statistical differences between MCI subjects with and without Aβ deposition, including tests of memory, attention and language functions, trend level differences were observed.
One of the strengths of this study is that the present group of elderly subjects is different from previous cohorts in several important ways. This cohort is much older on average than those included in previous studies. Consequently these subjects are at increased risk of dementia and having Aβ plaque deposits in the brain. This cohort includes both NC subjects as well as subjects with MCI, which allows us to examine two different levels of cognitive function within a group of non-demented subjects. Finally, this cohort had been followed for up to 9 years with detailed annual cognitive and neuropsychiatric evaluations prior to and concurrent with the PiB scan, which strengthened the characterization of the NC and MCI diagnoses.
Taken together, the data from this study extend previous studies of progressive Aβ deposition in a population-based cohort of aging, non-demented subjects. Our data indicate that the increased prevalence of Aβ deposition with age continues at least into the ninth decade of life and that even at these ages, some PiB-positive individuals do not show cognitive symptoms, consistent with the pathological findings of the Religious Orders Study.2 Although these findings support the need for early preventive therapeutic strategies, it is difficult to determine when these interventions should take place. Further longitudinal evaluations of this cohort will provide important information about development of new Aβ deposition in the PiB-negative cases, the risk of dementia in PiB-positive MCI subjects, and outcomes over time in PiB-positive and PiB-negative NC cases. Such information will provide further guidance for intervention trials of PiB-positive and PiB-negative NC and MCI subjects.
Supplementary Material
Acknowledgments
This study was supported by grants P01 AG025204, P50 AG005133, U01 AT000162, AG001039, AG018402, AG020226, MH070729, MH001976, AG025516, and AG030653 from the National Institutes of Health.
GE Healthcare holds a license agreement with the University of Pittsburgh based on the technology described in this manuscript. Drs. Mathis and Klunk are co-inventors of PiB and, as such, have a financial interest in this license agreement. GE Healthcare provided no grant support for this study and had no role in the design or interpretation of results or preparation of the manuscript.
Footnotes
All other authors have no conflicts of interest with this work.
References
- 1.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004 Feb;52(2):195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 2.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006 Jun 27;66(12):1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 3.Morris JC, McKeel DW, Jr, Storandt M, et al. Very mild Alzheimer's disease: informant-based clinical, psychometric, and pathologic distinction from normal aging. Neurology. 1991 Apr;41(4):469–78. doi: 10.1212/wnl.41.4.469. [DOI] [PubMed] [Google Scholar]
- 4.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009 May 28;360(22):2302–9. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 5.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006 Aug 8;67(3):446–52. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 6.Morris JC, Roe CM, Grant EA, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009 Dec;66(12):1469–75. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010 Aug;31(8):1275–83. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008 Nov;65(11):1509–17. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009 May;132(Pt 5):1355–65. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010 Jan;67(1):122–31. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2009 Apr 21;106(16):6820–5. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003 Jan-Feb;22(1):13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 13.DeKosky ST, Williamson JD, Fitzpatrick AL, et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. Jama. 2008 Nov 19;300(19):2253–62. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeKosky ST, Fitzpatrick A, Ives DG, et al. The Ginkgo Evaluation of Memory (GEM) study: design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp Clin Trials. 2006 Jun;27(3):238–53. doi: 10.1016/j.cct.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Snitz BE, O'Meara ES, Carlson MC, et al. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. Jama. 2009 Dec 23;302(24):2663–70. doi: 10.1001/jama.2009.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005 Nov;25(11):1528–47. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 17.Kamboh MI, Aston CE, Hamman RF. The relationship of APOE polymorphism and cholesterol levels in normoglycemic and diabetic subjects in a biethnic population from the San Luis Valley, Colorado. Atherosclerosis. 1995 Jan 20;112(2):145–59. doi: 10.1016/0021-9150(94)05409-c. [DOI] [PubMed] [Google Scholar]
- 18.Rosario BL, Weissfeld LA, Laymon CM, et al. Inter-rater reliability of manual and automated region-of-interest delineation for PiB PET. Neuroimage. 2011 Apr 1;55(3):933–41. doi: 10.1016/j.neuroimage.2010.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagust WJ, Bandy D, Chen K, et al. The Alzheimer's Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement. 2010 May;6(3):221–9. doi: 10.1016/j.jalz.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002 Jun 25;58(12):1791–800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 21.Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain. 2007 Nov;130(Pt 11):2837–44. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 22.Kemppainen NM, Aalto S, Wilson IA, et al. Voxel-based analysis of PET amyloid ligand [11C]PIB uptake in Alzheimer disease. Neurology. 2006 Nov 14;67(9):1575–80. doi: 10.1212/01.wnl.0000240117.55680.0a. [DOI] [PubMed] [Google Scholar]
- 23.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004 Mar;55(3):306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 24.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007 May 15;68(20):1718–25. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 25.Whitwell JL, Przybelski SA, Weigand SD, et al. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer's disease. Brain. 2007 Jul;130(Pt 7):1777–86. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziolko SK, Weissfeld LA, Klunk WE, et al. Evaluation of voxel-based methods for the statistical analysis of PIB PET amyloid imaging studies in Alzheimer's disease. Neuroimage. 2006 Oct 15;33(1):94–102. doi: 10.1016/j.neuroimage.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 27.Drzezga A, Grimmer T, Henriksen G, et al. Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer's disease. Neuroimage. 2008 Jan 15;39(2):619–33. doi: 10.1016/j.neuroimage.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Devanand DP, Mikhno A, Pelton GH, et al. Pittsburgh compound B (11C-PIB) and fluorodeoxyglucose (18 F-FDG) PET in patients with Alzheimer disease, mild cognitive impairment, and healthy controls. J Geriatr Psychiatry Neurol. 2010 Sep;23(3):185–98. doi: 10.1177/0891988710363715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drzezga A, Grimmer T, Henriksen G, et al. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology. 2009 Apr 28;72(17):1487–94. doi: 10.1212/WNL.0b013e3181a2e8d0. [DOI] [PubMed] [Google Scholar]
- 30.Edison P, Archer HA, Gerhard A, et al. Microglia, amyloid, and cognition in Alzheimer's disease: An [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis. 2008 Dec;32(3):412–9. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Forsberg A, Almkvist O, Engler H, Wall A, Langstrom B, Nordberg A. High PIB retention in Alzheimer's disease is an early event with complex relationship with CSF biomarkers and functional parameters. Curr Alzheimer Res. 2010 Feb;7(1):56–66. doi: 10.2174/156720510790274446. [DOI] [PubMed] [Google Scholar]
- 32.Hedden T, Van Dijk KR, Becker JA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009 Oct 7;29(40):12686–94. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowe VJ, Kemp BJ, Jack CR, Jr, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med. 2009 Jun;50(6):878–86. doi: 10.2967/jnumed.108.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maetzler W, Liepelt I, Reimold M, et al. Cortical PIB binding in Lewy body disease is associated with Alzheimer-like characteristics. Neurobiol Dis. 2009 Apr;34(1):107–12. doi: 10.1016/j.nbd.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Rabinovici GD, Furst AJ, Alkalay A, et al. Increased metabolic vulnerability in early-onset Alzheimer's disease is not related to amyloid burden. Brain. 2010 Feb;133(Pt 2):512–28. doi: 10.1093/brain/awp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roe CM, Mintun MA, Ghoshal N, et al. Alzheimer disease identification using amyloid imaging and reserve variables: proof of concept. Neurology. 2010 Jul 6;75(1):42–8. doi: 10.1212/WNL.0b013e3181e620f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin J, Lee SY, Kim SH, Kim YB, Cho SJ. Multitracer PET imaging of amyloid plaques and neurofibrillary tangles in Alzheimer's disease. Neuroimage. 2008 Nov 1;43(2):236–44. doi: 10.1016/j.neuroimage.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 38.Tolboom N, van der Flier WM, Boverhoff J, et al. Molecular imaging in the diagnosis of Alzheimer's disease: visual assessment of [11C]PIB and [18F]FDDNP PET images. J Neurol Neurosurg Psychiatry. 2010 Aug;81(8):882–4. doi: 10.1136/jnnp.2009.194779. [DOI] [PubMed] [Google Scholar]
- 39.Wolk DA, Price JC, Saxton JA, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009 May;65(5):557–68. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008 Oct;29(10):1456–65. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 41.Koivunen J, Pirttila T, Kemppainen N, et al. PET amyloid ligand [11C]PIB uptake and cerebrospinal fluid beta-amyloid in mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;26(4):378–83. doi: 10.1159/000163927. [DOI] [PubMed] [Google Scholar]
- 42.Okello A, Koivunen J, Edison P, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009 Sep 8;73(10):754–60. doi: 10.1212/WNL.0b013e3181b23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tolboom N, van der Flier WM, Yaqub M, et al. Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med. 2009 Sep;50(9):1464–70. doi: 10.2967/jnumed.109.064360. [DOI] [PubMed] [Google Scholar]
- 44.Haroutunian V, Schnaider-Beeri M, Schmeidler J, et al. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol. 2008 Sep;65(9):1211–7. doi: 10.1001/archneur.65.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005 Mar 8;64(5):834–41. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 46.Robinson JL, Geser F, Corrada MM, et al. Neocortical and hippocampal amyloid-beta and tau measures associate with dementia in the oldest-old. Brain. 2011 Dec;134(Pt 12):3708–15. doi: 10.1093/brain/awr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuller LH, Shemanski L, Manolio T, et al. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke. 1998 Feb;29(2):388–98. doi: 10.1161/01.str.29.2.388. [DOI] [PubMed] [Google Scholar]
- 48.Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. Jama. 1998 Mar 11;279(10):751–5. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 49.de Leon MJ, Mosconi L, Blennow K, et al. Imaging and CSF studies in the preclinical diagnosis of Alzheimer's disease. Ann N Y Acad Sci. 2007 Feb;1097:114–45. doi: 10.1196/annals.1379.012. [DOI] [PubMed] [Google Scholar]
- 50.de Leon MJ, Golomb J, George AE, et al. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. AJNR Am J Neuroradiol. 1993 Jul-Aug;14(4):897–906. [PMC free article] [PubMed] [Google Scholar]
- 51.Jack CR, Jr, Petersen RC, Xu Y, et al. Rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology. 1998 Oct;51(4):993–9. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010 Jan;9(1):119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawas CH, Corrada MM. Alzheimer's and dementia in the oldest-old: a century of challenges. Curr Alzheimer Res. 2006 Dec;3(5):411–9. doi: 10.2174/156720506779025233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Middleton LE, Grinberg LT, Miller B, Kawas C, Yaffe K. Neuropathologic features associated with Alzheimer disease diagnosis: age matters. Neurology. 2011 Nov 8;77(19):1737–44. doi: 10.1212/WNL.0b013e318236f0cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.