Abstract

Aryl thiolates have unique reactive, redox, electronic, and spectroscopic properties. A practical approach to synthesize peptides containing thiophenylalanine has been developed via a novel Cu(I)-mediated cross-coupling reaction between thiolacetic acid and iodophenylalanine-containing peptides on solid phase. This approach is compatible with all canonical proteinogenic functional groups, providing general access to aryl thiolates in peptides. Peptides containing thiophenylalanine (pKa 6.4) were readily elaborated to contain methyl, allyl, and nitrobenzyl thioethers; disulfides; sulfoxides; sulfones; or sulfonates.
Cysteine residues play critical roles in protein folding, structure, catalysis, response to oxidative stress, metal binding, modification, and cell signaling.1 These uses are dependent on the redox properties, acidity, and nucleophilicity of thiols and thiolates. The multiple oxidation states accessible to thiols are critical in their versatility in function. Aryl thiols may perform in similar roles, but with reduced pKa and electronically tunable properties.2 Considering the special role of tyrosine in biomolecular recognition, thiophenylalanine represents an intriguing hybrid of tyrosine and cysteine residues.3
Thiophenylalanine and related amino acids have diverse potential applications in fields including medicinal chemistry, enzymology, protein design, materials science, and nanotechnology. However, there are very few published examples of thiophenylalanine-containing peptides. The first such description, applied by Escher in angiotensin II analogues, employed sulfonylation of phenylalanine, followed by tin reduction, thiol protection, and amine protection to generate the Boc amino acids.4 DeGrado extended this approach to prepare Fmoc-thiophenylalanine and employed its disulfide in a peptide as a light-based switch of protein folding.5 In an alternative synthetic approach, Still used diazonium chemistry to access thiophenylalanine-containing peptides that were subsequently photocoupled to prepare thioether analogues of diaryl ether antibiotics.6 Recently, Hecht incorporated thiophenylalanine in place of an active site tyrosine of topoisomerase I to examine the dependence of catalysis on the nucleophilic phenolate.7
In all of these cases, significant effort was required to synthesize protected thiophenylalanine. The development of a practical synthetic approach to thiophenylalanine, ideally employing commercially available amino acids without separate, additional purification steps, could greatly expand the applications of this amino acid. In view of the recent advances in metal-catalyzed crosscoupling reactions between aryl halides and thiols,8,9 we sought to develop an alternative approach to protected thiophenylalanine employing commercially available 4-iodophenylalanine and modification of a peptide via reaction on solid phase.
Copper catalysts have been most broadly employed for cross-coupling reactions with thiols, typically using aryl iodides. Therefore, we examined reaction conditions on solid phase within the protected model peptide Ac-T(4-I-Phe)PN-NH2, which we have previously used to understand aromatic electronic effects in peptides.10
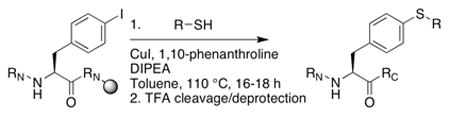
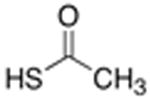
Initial efforts to employ t-BuSH proceeded without success.11 We next examined cross–coupling with thiolacetic acid (Table 1), which could be subjected to thiolysis or hydrolysis in solution or on solid phase to yield the free thiophenylalanine-containing peptide.9 The conditions of Sawada et al., using 1,10-phenanthroline as a ligand and DIPEA as a base in toluene or t-amyl alcohol at 90–110 °C, resulted in good conversion on solid phase to a mixture of cross-coupled thioester and disulfide products. This mixture could be cleanly converted to the thiophenylalanine-containing peptide during standard cleavage/deprotection/purification steps (Figure 1). At 110 °C in toluene, iodophenylalanine underwent nearly complete conversion to cross-coupled products in 16 hours. If the thioester product was desired, maximum yield was obtained by running the reaction at 100 °C, which decreased thiolysis and disulfide formation. The scope of this reaction with different thiols was briefly examined (Table 2). The reaction proceeded readily with thiophenol to generate the diaryl thioether.6 Alkyl thiols reacted poorly under these conditions.
Table 1.
Reaction development.
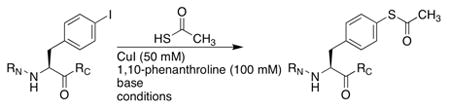
| |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| entry | phase | base | solvent | temp, | °C time, h | 4-SAc-Phe | total cross-coupled productsa |
| 1 | Solution | 0.5 M Na2CO3 | t-amyl-OH | 90 | 6 | 44% | 59% |
| 2 | Solution | 0.1 M DIPEA | t-amyl-OH | 90 | 6 | 15% | 26% |
| 3 | Solution | 0.5 M DIPEA | t-amyl-OH | 90 | 6 | 11% | 60% |
| 4 | Solution | 1 M DIPEA | t-amyl-OH | 90 | 6 | 22% | 58% |
| 5 | Solution | 1 M DIPEA | t-amyl-OH | 90 | 11 | 29% | 65% |
| 6 | Solid | 1 M DIPEA | t-amyl-OH | 90 | 6 | 40% | 45% |
| 7 | Solid | 1 M DIPEA | t-amyl-OH | 100 | 6 | 59% | 69% |
| 8 | Solid | 1 M DIPEA | t-amyl-OH | 100 | 24 | 53% | 90% |
| 9 | Solid | 1 M DIPEA | t-amyl-OH | 110 | 16 | 0% | 99% |
| 10 | Solid | 1 M DIPEA | toluene | 100 | 18 | 57% | 67% |
| 11 | Solid | 1 M DIPEA | toluene | 110 | 16 | 22% | 99% |
All cross-coupled products, including overacetylated side products in solution phase reactions, or disulfide products in solid phase reactions. RN = Ac-Thr(tBu)-(solid) or Ac-Thr-(solution). RC = -Pro-Asn(Trt)-NHRink (solid) or -Pro-Asn-NH2 (solution). Solid phase reactions were followed by TFA cleavage/deprotection.
Figure 1.
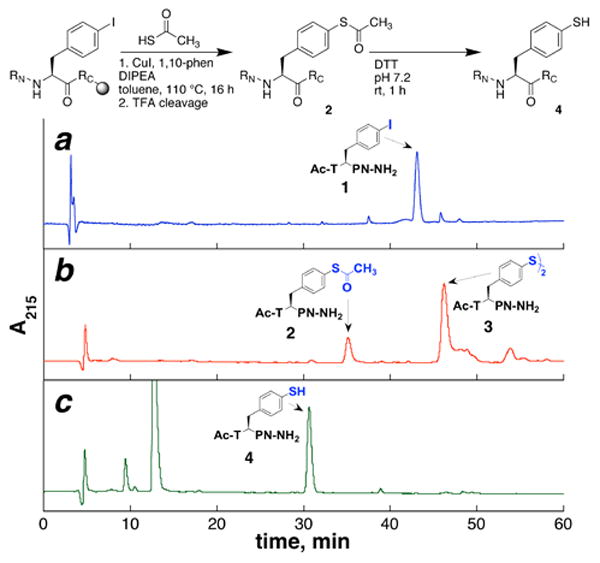
HPLC chromatograms of (a) Ac-T(4-I-Phe)PN-NH2 peptide starting material (1); (b) peptide after cross-coupling reaction and peptide cleavage and deprotection, showing a mixture of thioacetyl (2) and disulfide (3) products; and (c) the result of treatment of the solution in (b) with DTT to effect thiolysis and reduction of disulfide, showing clean overall conversion to the 4-thiophenylalanine-containing product (4).
Table 2.
Thiol scope of cross-coupling reactions on solid phase.
| ||||
|---|---|---|---|---|
|
| ||||
| entry | thiol (R-SH) | product | 4-SR-Phe product | total cross-coupled productsa |
| 1 |

|
2 | 22% | 99% |
| 2 |
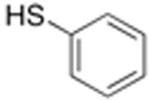
|
5 | 90% | 90% |
| 3 |
|
6 | 2% | 2% |
| 4 | HS-(CH2)7-CH3 | 0% | 0% | |
| 5 |
|
7 | 21 %b | 45%b |
| 6 |
|
2 | 57%b | 67%b |
Includes disulfide products;
at 100 °C. RN = Ac-Thr(tBu)- (solid) or Ac-Thr- (solution). RC = -Pro-Asn(Trt)-NHRink (solid) or -Pro-Asn-NH2 (solution).
To further examine the scope of this reaction, a series of peptides, which included all canonical amino acid functional groups, was synthesized and examined for cross-coupling under the optimized reaction conditions (Figure 2). The peptides included model peptides as well as the trp cage miniprotein, with Tyr3 replaced by 4-I-Phe.12 Reaction on the trp cage provides a critical test to confirm that the reaction conditions do not modify peptide structure in a subtle manner that would not be identified by HPLC or MS.
Figure 2.
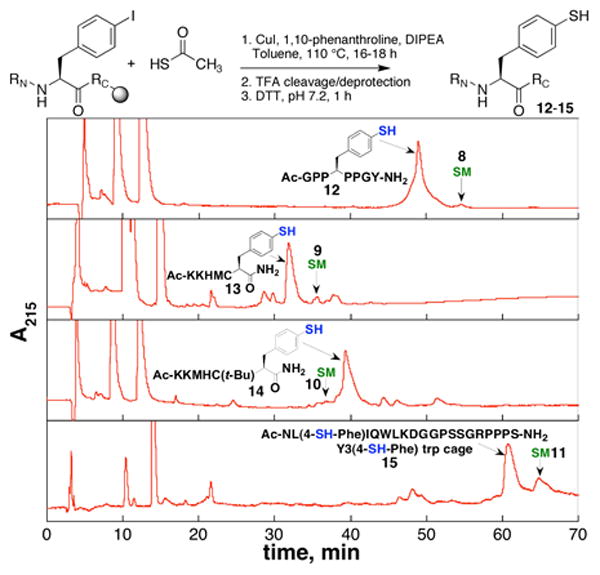
Crude HPLC chromatograms of cross-coupling reactions with different peptides. S.M. = residual starting material peptide.13
Analysis by HPLC indicated clean reactions and high conversions to cross-coupled product in all cases (Figure 2). Trityl-protected cysteine residues exhibited evidence of Cys deprotection and acetylation under reaction conditions. After workup with DTT, both thiophenylalanine and Cys were generated cleanly as free thiols. Alternatively, to allow differentiation of Cys and thiophenylalanine, the peptide with t-butyl-protected Cys was subjected to reaction conditions, cleanly generating the peptide with free aryl thiol and protected Cys.
Analysis of the thiophenylalanine-containing trp cage peptide by circular dichroism (Figure 3) indicated that its structure and stability were similar to that of the native tyrosine-containing peptide. In total, these data indicate that the cross-coupling reaction may be employed to modify complex peptides with diverse amino acid substitutions and implies generality in the application of this reaction to synthesize peptides containing aryl thiols.
Figure 3.
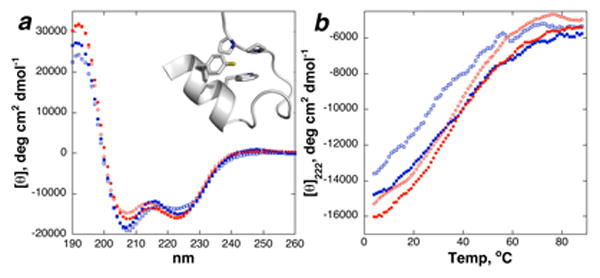
Circular dichroism of native (16, red circles) and Y3(4-SH-Phe) (15, blue squares) trp cage at pH 4.0 (closed) and 7.0 or 8.5 (open). (a) CD data at 4 °C. (b) Thermal denaturation.
The UV-Vis spectra of T(4-SH-Phe)PN revealed a strong dependence on pH, with a λmax of 249 nm as the thiol and an intense λmax of 276 nm as the thiolate (ε276 = 17,500) (Figure 4). The pKa of thiophenylalanine was 6.4, similar to other aryl thiols and significantly lower than cysteine (pKa ∼8-8.5). Notably, thiophenylalanine exists predominantly in the anionic form at physiological pH. In addition, thiophenylalanine exhibited weak fluorescence as the thiolate (emission λmax = 405 nm).13
Figure 4.
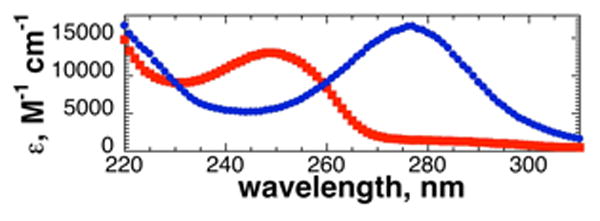
UV-Vis spectra of Ac-T(4-SH-Phe)PN-NH2(4) at pH 4.0 (thiol, red squares) and pH 8.5 (thiolate, blue circles).
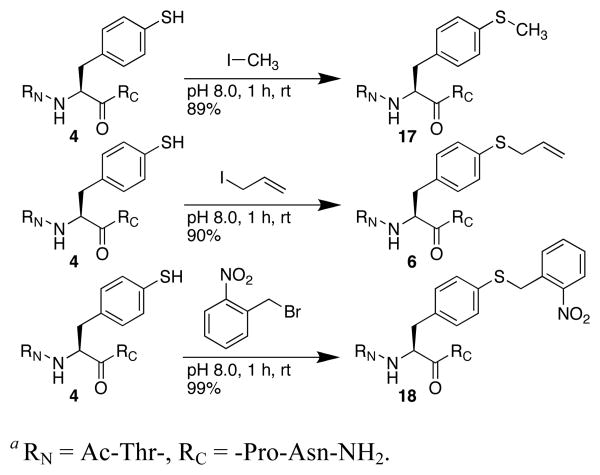
The reactivity of thiols provides the opportunity to modify peptides to incorporate a range of functional groups, which was examined in T(4-SH-Phe)PN peptides. While alkyl thiols were poor substrates in cross-coupling reactions, the aryl thiolate was readily alkylated with methyl iodide or allyl iodide to generate the respective thioethers (Scheme 1). A photocleavable analogue of thiophenylalanine was also prepared by alkylation with 2-nitrobenzyl bromide.14 In addition to reaction in solution, the thioester protecting group could be removed and alkylation effected on solid phase (Scheme 2).13
Scheme 1.
Solution alkylation reactions on 4.a
Scheme 2.
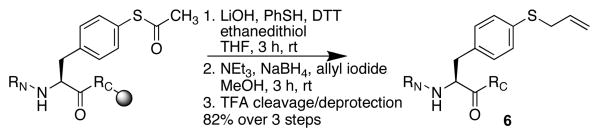
Solid phase alkylation of Ac-TXPN-NH2.
Allyl thioethers have been shown by Davis to be superior substrates to allyl ethers for cross-metathesis reactions.15 Aryl allyl thioethers thus have potential as reactive substrates for bioorthogonal cross-metathesis reactions.15c To explore this possibility, the S-allyl-thiophenylalanine-containing peptide 6 was subjected to Davis' cross-metathesis conditions and found to react with allyl alcohol at room temperature (Scheme 3).13
Scheme 3.
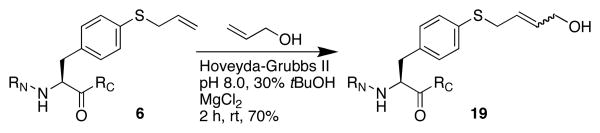
Cross-metathesis on Ac-T(4-SAllyl-Phe)PN-NH2.
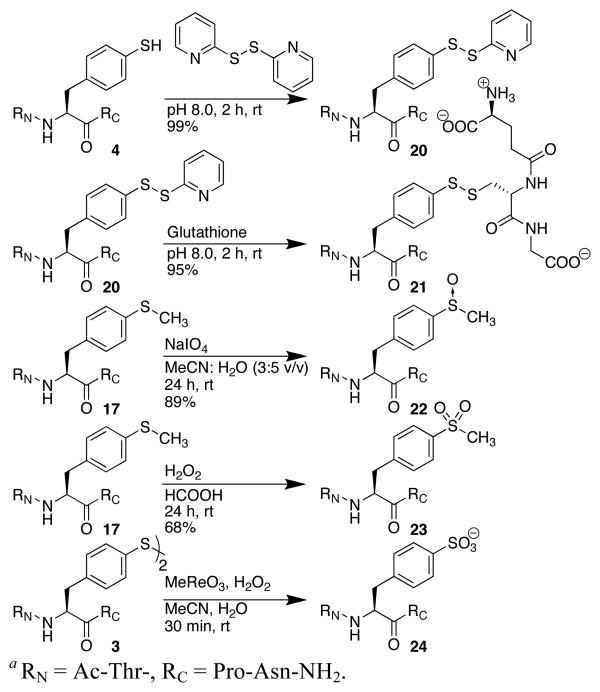
Thiol oxidation state dramatically impacts protein structure and function.1,16 Thiophenylalanine-containing peptides were subjected to a variety of oxidation reactions, including formation of the activated disulfide, glutathionylated mixed disulfide, sulfoxide, or sulfone (Scheme 4). Thiophenylalanine was also converted to the sulfonate, a non-hydrolyzable analogue of sulfotyrosine, using methyltrioxorhenium (MTO).17
Scheme 4.
Solution phase peptide oxidation reactions.a
Aryl electronics can significantly affect protein structure and function.10,18 The derivatives described above represent a continuum from strongly electron-donating to electron-withdrawing groups. In the TXPN (X = aryl) context, the strength of an aromatic-proline CH/π interaction is manifested in stabilization of a cis amide bond, with more electron-donating substituents favoring cis amide bond. All model peptides were examined by NMR spectroscopy and Ktrans/cis quantified to identify the structural effects of aryl substitution (Table 3). The data indicate that thiophenylalanine and its modified variants exhibit a range of electronic properties that might be exploited for electronic control of peptide structure.
Table 3.
Electronic effects of aryl substution on peptide structure (Ktrans/cis of the prolyl amide bond).
| Ac-TXPN-NH2, X= | ktrans/cis | ΔGtrans/cis kcal mol−1 | ΔΔGtrans/cis kcal mol−1 |
|---|---|---|---|
| 4-S−-Phe (4, pH 8.5) | 2.2 | -0.47 | 0.22 |
| 4-S-SPyridyl-Phe (20) | 2.9 | -0.63 | 0.06 |
| 4-SAllyl-Phe (6) | 3.3 | -0.71 | -0.02 |
| 4-SH-Phe (4, pH 4.0) | 3.5 | -0.74 | -0.05 |
| 4-SCH3-Phe (17) | 3.5 | -0.74 | -0.05 |
| 4-SPh-Phe (5) | 3.6 | -0.76 | -0.07 |
| 4-S-SGlutathione-Phe (21) | 3.7 | -0.77 | -0.08 |
| 4-SAc-Phe (2) | 4.3 | -0.86 | -0.17 |
| 4-SO3−-Phe (24) | 4.4 | -0.88 | -0.19 |
| 4-S(2-Nitrobenzyl)-Phe (18) | 4.4 | -0.88 | -0.19 |
| 4-S(O)Me-Phe (22) | 4.7 | -0.92 | -0.23 |
| 4-SO2Me-Phe (23) | 5.9 | -1.05 | -0.36 |
| Phe (F)a | 3.2 | -0.69 | 0.00 |
| Tyr (Y)a | 2.7 | -0.59 | 0.10 |
We have described a practical approach to the synthesis of thiophenylalanine-containing peptides that is compatible with all proteinogenic functional groups. The method employs the commercially available amino acid 4-iodophenylalanine and is readily applied to fully synthesized peptides, providing convenient access to a range of versatile thiol-modified aromatic amino acids.
Supplementary Material
Acknowledgments
We thank NSF (CAREER, CHE-0547973) and NIH (RR17716) for funding this work.
Footnotes
Supporting Information Available Experimental procedures, HPLC chromatograms, and spectral and characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Leonard SE, Carroll KS. Curr Opin Chem Biol. 2011;15:88–102. doi: 10.1016/j.cbpa.2010.11.012. [DOI] [PubMed] [Google Scholar]; Paulsen CE, Carroll KS. ACS Chem Biol. 2010;5:47–62. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reddie KG, Carroll KS. Curr Opin Chem Biol. 2008;12:746–54. doi: 10.1016/j.cbpa.2008.07.028. [DOI] [PubMed] [Google Scholar]; Leonard SE, Garcia FJ, Goodsell DS, Carroll KS. Angew Chem Int Ed. 2011;50:4423–7. doi: 10.1002/anie.201007871. [DOI] [PubMed] [Google Scholar]; Cline DJ, Thorpe C, Schneider JP. Anal Biochem. 2004;325:144–50. doi: 10.1016/j.ab.2003.10.014. [DOI] [PubMed] [Google Scholar]; Kodali VK, Thorpe C. Antioxid Redox Signaling. 2010;13:1217–30. doi: 10.1089/ars.2010.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]; Berg JM, Godwin HA. Ann Rev Biophys Biomol Struct. 1997;26:357–71. doi: 10.1146/annurev.biophys.26.1.357. [DOI] [PubMed] [Google Scholar]; Adams SR, Campbell RE, Gross LA, Martin BR, Walkup GK, Yao Y, Llopis J, Tsien RY. J Am Chem Soc. 2002;124:6063–76. doi: 10.1021/ja017687n. [DOI] [PubMed] [Google Scholar]; Chalker JM, Bernardes GJL, Lin YA, Davis BG. Chem Asian J. 2009;4:630–40. doi: 10.1002/asia.200800427. [DOI] [PubMed] [Google Scholar]; Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Science. 1994;266:776–9. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 2.Application of aryl thiolates to enhance protein folding rates at pH 6: Gough JD, Williams JRH, Donofrio AE, Lees WJ. J Am Chem Soc. 2002;124:3885–92. doi: 10.1021/ja016938p.
- 3.Koide S, Sidhu SS. ACS Chem Biol. 2009;4:325–34. doi: 10.1021/cb800314v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escher E, Bernier M, Parent P. Helv Chim Acta. 1983;66:1355–65. [Google Scholar]; Guillemette G, Bernier M, Parent P, Leduc R, Escher E. J Med Chem. 1984;27:315–20. doi: 10.1021/jm00369a015. [DOI] [PubMed] [Google Scholar]; Other syntheses of 4-ThioPhe: Johnson TB, Brautlecht CA. J Biol Chem. 1912;12:175–96.; Elliott DF, Harrington C. J Chem Soc. 1949:1374–8. [Google Scholar]; Colescott RL, Herr RR, Dailey JP. J Am Chem Soc. 1957;79:4232–5. [Google Scholar]; Bergel F, Stock JA. J Chem Soc. 1959:90–7. [Google Scholar]; Synthesis of S-t-butyl Boc-ThioPhe via Pd cross-coupling: Rajagopalan S, Radke G, Evans M, Tomich JM. Synth Commun. 1996;26:1431–40.
- 5.Lu HSM, Volk M, Kholodenko Y, Gooding E, Hochstrasser RM, DeGrado WF. J Am Chem Soc. 1997;119:7173–80. [Google Scholar]
- 6.Hobbs DW, Still WC. Tetrahedron Lett. 1987;28:2805–8. [Google Scholar]; Hobbs DW, Still WC. Tetrahedron Lett. 1989;30:5405–8. [Google Scholar]; related approaches to aryl thioethers in peptides: West CW, Rich DH. Org Lett. 1999;1:1819–22. doi: 10.1021/ol991084n.; Moreau X, Campagne JM. J Organomet Chem. 2003;687:322–6. [Google Scholar]
- 7.Gao R, Zhang Y, Choudhury AK, Dedkova LM, Hecht SM. J Am Chem Soc. 2005;127:3321–31. doi: 10.1021/ja044182z. [DOI] [PubMed] [Google Scholar]; Chen S, Zhang Y, Hecht SM. Biochemistry. 2011;50:9340–51. doi: 10.1021/bi201291p. [DOI] [PubMed] [Google Scholar]
- 8.Kondo T, Mitsudo T. Chem Rev. 2000;100:3205–20. doi: 10.1021/cr9902749. [DOI] [PubMed] [Google Scholar]; Ley SV, Thomas AW. Angew Chem Int Ed. 2003;42:5400–49. doi: 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]; Beletskaya IP, Ananikov VP. Chem Rev. 2011;111:1596–636. doi: 10.1021/cr100347k. [DOI] [PubMed] [Google Scholar]; Kwong FY, Buchwald SL. Org Lett. 2002;4:3517–20. doi: 10.1021/ol0266673. [DOI] [PubMed] [Google Scholar]; Fernandez-Rodriguez MA, Shen QL, Hartwig JF. J Am Chem Soc. 2006;128:2180–1. doi: 10.1021/ja0580340. [DOI] [PubMed] [Google Scholar]; Correa A, Carril M, Bolm C. Angew Chem Int Ed. 2008;47:2880–3. doi: 10.1002/anie.200705668. [DOI] [PubMed] [Google Scholar]; Alvaro E, Hartwig JF. J Am Chem Soc. 2009;131:7858–68. doi: 10.1021/ja901793w. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bichler P, Love JA. Top Organomet Chem. 2010;31:39–64. [Google Scholar]
- 9.Sawada N, Itoh T, Yasuda N. Tetrahedron Letters. 2006;47:6595–7. [Google Scholar]; Pd catalyzed: Lai C, Backes BJ. Tetrahedron Lett. 2007;48:3033–7.; van den Hoogenband A, Lange JHM, Bronger RPJ, Stoit AR, Terpstra JW. Tetrahedron Lett. 2010;51:6877–81. [Google Scholar]
- 10.Thomas KM, Naduthambi D, Tririya G, Zondlo NJ. Org Lett. 2005;7:2397–400. doi: 10.1021/ol0506720. [DOI] [PubMed] [Google Scholar]; Meng HY, Thomas KM, Lee AE, Zondlo NJ. Biopolymers. 2006;84:192–204. doi: 10.1002/bip.20382. [DOI] [PubMed] [Google Scholar]; Thomas KM, Naduthambi D, Zondlo NJ. J Am Chem Soc. 2006;128:2216–7. doi: 10.1021/ja057901y. [DOI] [PubMed] [Google Scholar]
- 11.Pd-mediated cross-coupling reactions with tBuSH: refs 4g and 8e.
- 12.Neidigh JW, Fesinmeyer RM, Andersen NH. Nature Struct Biol. 2002;9:425–30. doi: 10.1038/nsb798. [DOI] [PubMed] [Google Scholar]; Naduthambi D, Zondlo NJ. J Am Chem Soc. 2006;128:12430–1. doi: 10.1021/ja0648458. [DOI] [PubMed] [Google Scholar]; Williams DV, Barua B, Andersen NH. Org Biomol Chem. 2008;6:4287–9. doi: 10.1039/b814314e. [DOI] [PubMed] [Google Scholar]; Barua B, Lin JC, Williams VD, Kummler P, Neidigh JW, Andersen NH. Protein Eng., Des Sel. 2008;21:171–85. doi: 10.1093/protein/gzm082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.See the Supporting Information for details.
- 14.Pelliccioli AP, Wirz J. Photochem Photobiol Sci. 2002;1:441–58. doi: 10.1039/b200777k. [DOI] [PubMed] [Google Scholar]
- 15.Lin YA, Chalker JM, Floyd N, Bernardes GJL, Davis BG. J Am Chem Soc. 2008;130:9642–3. doi: 10.1021/ja8026168. [DOI] [PubMed] [Google Scholar]; Lin YA, Chalker JM, Davis BG. J Am Chem Soc. 2010;132:16805–11. doi: 10.1021/ja104994d. [DOI] [PubMed] [Google Scholar]; Davis has speculated about the potential of S-allyl-thiophenylalanine for cross-methathesis reactions: Lin YYA, Chalker JM, Davis BG. ChemBioChem. 2009;10:959–69. doi: 10.1002/cbic.200900002.
- 16.Biswas S, Chida AS, Rahman I. Biochem Pharmacol. 2006;71:551–64. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]; Schenck HL, Dado GP, Gellman SH. J Am Chem Soc. 1996;118:12487–94. [Google Scholar]; Jiang LY, Burgess K. J Am Chem Soc. 2002;124:9028–9. doi: 10.1021/ja0171411. [DOI] [PubMed] [Google Scholar]
- 17.Ballistreri FP, Tomaselli GA, Toscano RM. Tetrahedron Lett. 2008;49:3291–3. [Google Scholar]; Romão CC, Kühn FE, Herrmann WA. Chem Rev. 1997;97:3197–246. doi: 10.1021/cr9703212. [DOI] [PubMed] [Google Scholar]; Oxidation of Met, Cys, and Trp residues with MTO: Lazzaro F, Crucianelli M, De Angelis F, Neri V, Saladino R. Tetrahedron Lett. 2004;45:9237–40.; Huang KP, Huang FL, Shetty PK, Yergey AL. Biochemistry. 2007;46:1961–71. doi: 10.1021/bi061955i. [DOI] [PubMed] [Google Scholar]; Sulfonated Phe as a sulfotyrosine mimic: Cebrat M, Lisowski M, Siemion IZ, Zimecki M, Wieczorek Z. J Pept Res. 1997;49:415–20. doi: 10.1111/j.1399-3011.1997.tb00893.x.; Bahyrycz A, Matsubayashi Y, Ogawa M, Sakagami Y, Konopinska D. J Pept Sci. 2004;10:462–9. doi: 10.1002/psc.492. [DOI] [PubMed] [Google Scholar]
- 18.Ma JC, Dougherty DA. Chem Rev. 1997;97:1303–24. doi: 10.1021/cr9603744. [DOI] [PubMed] [Google Scholar]; Hunter CA, Lawson KR, Perkins J, Urch CJ. J Chem Soc Perkin. 2001;2:651–69. [Google Scholar]; Meyer EA, Castellano RK, Diederich F. Angew Chem Int Ed. 2003;42:1210–50. doi: 10.1002/anie.200390319. [DOI] [PubMed] [Google Scholar]; Waters ML. Biopolymers. 2004;76:435–45. doi: 10.1002/bip.20144. [DOI] [PubMed] [Google Scholar]; Pagel K, Koksch B. Curr Opin Chem Biol. 2008;12:730–9. doi: 10.1016/j.cbpa.2008.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.