Abstract
TGFβ1 is an important suppressive mediator of inflammation but can also drive fibrosis and remodeling in the lung. In response to intratracheal LPS, neutrophils migrate into the lung and TGFβ1 has been suggested to protect against the ensuing injury. However, the mechanisms for this protective role remain unknown. Using a model of acute lung injury (ALI), we demonstrate that TGFβ1 decreases neutrophil numbers during the onset of injury. This was due to increased apoptosis, rather than less migration. We demonstrate that TGFβ1 does not directly regulate neutrophil apoptosis, but instead functions through IL-6 to promote neutrophil clearance. Recombinant IL-6 is sufficient to promote neutrophil apoptosis and reduce neutrophilia in brochoalveolar lavage fluid while IL-6 is rapidly elevated following LPS-induced injury. Mast cells are a critical source of the IL-6, as mast cell deficient mice exhibit increased neutrophil numbers that is reduced by reconstitution with WT, but not IL-6−/−, mast cells. While IL-6 diminishes neutrophilia in mast cell-deficient mice, TGFβ1 is ineffective, suggesting that these effects were mast cell dependent. Taken together, our findings establish a novel pathway through which TGFβ1, likely derived from resident Tregs, controls the severity and magnitude of early innate inflammation by promoting IL-6 from mast cells.
Introduction
Acute lung injury (ALI), or acute respiratory distress syndrome (ARDS) in its most severe form, is an edematous inflammatory response in the lung that occurs during direct (e.g. pneumonia) or indirect (e.g. sepsis) insult. Approximately 40% of ALI patients die, despite intensive clinical care (1, 2). Immunologically, a hallmark of most ALI responses is an acute phase which consists of the rapid recruitment and activation of neutrophils to the lung (3). This is followed by a resolution phase, whereby neutrophils enter apoptosis and are cleared by infiltrating mononuclear cells (4). In animal models, failure in this apoptosis, due to deficiency in either TNF-related apoptosis-inducing ligand (TRAIL) or caspase-1, has been shown to lead to an enhanced inflammatory response (5, 6), and strategies to pharmacologically induce apoptosis promote enhanced resolution of ALI responses (7). Neutrophils from patients with ARDS have been shown to exhibit lowered levels of apoptosis than normal (8), while neutrophil numbers were lower in patients who survived ARDS versus those who did not (9). However, the processes that regulate the numbers of neutrophils or induce their apoptotic clearance remain largely unknown.
TGFβ plays a well-established role in promoting lung fibrosis and remodeling in many inflammatory disorders, including asthma (10) and chronic obstructive pulmonary disease (11). Here, TGFβ1 promotes epithelial-mesenchymal transition of alveolar epithelial cells via a functional complex of its receptor with α3 integrin that initiates β-catenin activation (12). In contrast to this pathogenic effect of TGFβ1 on the chronic responses of the lung, TGFβ1 can also exert potent immunoregulatory influences during the onset and progression of inflammation, but the mechanisms behind this role are largely unclear. In the context of ALI, TGFβ1 has been shown to actually diminish the magnitude of the early acute response upon intranasal lipopolysaccharide (LPS) challenge (13). More recently, TGFβ-expressing Foxp3+regulatory T cells have also been shown to augment the later resolution phase of ALI (14). However, while ALI-associated inflammation is highly neutrophilic, the direct effect of TGFβ1 on neutrophils themselves appears to be relatively modest in nature. Depending on the experimental approach taken, TGFβ1 has been reported to both directly stimulate and inhibit neutrophil migration and degranulation (15-17), but these effects were mild when compared to other stimuli. Recent work has shown that blocking TGFβ-activated kinase 1 (TAK1) reduced the production of neutrophil-derived cytokines and resistant to apoptosis (18), suggesting that TGFβ-driven pathways might actually enhance neutrophil functions and survival. Consequently, the mechanisms through which TGFβ diminishes ALI responses are undefined.
We have recently demonstrated that recombinant TGFβ1 and TGFβ-expressing Tregs modulate the responsiveness of mast cells to inflammatory stimuli, including LPS (19). In addition to their established role in allergy, mast cells are being increasingly recognized for their roles in innate immune responses and in shaping the nature of the ensuing inflammatory response (20). Indeed, our study demonstrated that Tregs and TGFβ1 actively increased mast cell-derived IL-6 and that this enhanced the development of Th17 cells and intestinal homeostasis in a food allergy model(19). In vitro production of IL-6 by mast cells occurs rapidly upon LPS-driven activation and is independent of the degranulation response that underlies the established role of mast cells in IgE-mediated immediate hypersensitivity (21). While the physiological roles of mast cell-derived IL-6 remain largely unknown, recent studies have shown that it is important in protection against Klebsiella pneumonia infection (22) and in limiting tumor growth (23), implying a role in innate immune responses.
In a similar way to TGFβ1, IL-6 is most commonly studied for its pro-inflammatory activities and role in chronic inflammatory diseases, such as rheumatoid arthritis (24). However, during the initiation of inflammation, a relatively small number of cells, including neutrophils, are actually capable of directly responding to IL-6 due to the restricted expression of membrane-bound IL-6R. Instead, most cells are stimulated via trans-signaling by IL-6 in complex with soluble IL-6R (sIL-6R) via binding to gp130, the common IL- 6 family signaling receptor subunit (25). Neutrophils express high levels of IL-6R, and the liberation of sIL-6R upon cell death has been shown to be essential in regulating chemokine production from other cells and the subsequent recruitment of mononuclear cells during peritoneal infection (26). Regulation of neutrophil numbers themselves by IL-6 during acute peritoneal inflammation has been shown to be dependent on STAT3 signaling from gp130 (27), although this study did not determine if the alteration of neutrophil frequency was due to changes in recruitment or survival. Interestingly, while IL-6 trans-signaling is anti-apoptotic for many cells, direct IL-6 signaling on neutrophils has been shown to promote their apoptotic death(28), although the mechanistic differences in this unique response remain unclear.
Based on our recent study demonstrating the priming influence of TGFβ1 on mast cell production of IL-6, including in response to LPS (19), we hypothesized that TGFβ1 might actually diminish neutrophilic inflammation during ALI by altering the IL-6 production by mast cells. Presented here, our data demonstrates that TGFβ1 dose-dependently reduces the numbers of neutrophils observed after LPS administration and that this is associated with a significant increase in neutrophil apoptosis. Importantly, our data demonstrates that LPS-induced ALI is regulated by mast cell-derived IL-6, and that TGFβ1 functions indirectly via this mechanism to enhance neutrophilic apoptosis and limit the severity of the early neutrophilic inflammation. Consequently, mast cells and IL-6 represent a novel mechanism through which the magnitude of the developing inflammatory response is controlled in the lung and may be a pathway for therapeutic intervention in the treatment of acute lung injury.
Materials and Methods
Mice
C57BL/6 mice were purchased from Taconic Farms, Cambridge City, IN. IL-6−/−, WBB6F1KitW/W-v (W/Wv) and their littermate controls, WBB6F1 (WBB6) were purchased from Jackson Laboratories, Bar Harbor, ME. KitW-sh (Sash) mice were bred and housed under specific-pathogen free conditions at Northwestern University. All experiments were approved by the Northwestern University Animal Care and Use Committee.
LPS-induced lung injury
Mice were lightly anesthetized with isofluorane (Abbott Laboratories). Intratracheal delivery of 10μg of Escherichia coli LPS (0127:B8 Sigma L4516) with or without recombinant IL-6 or TGFβ1 (R&D Systems), at a final volume of 100μl in sterile 1×PBS, was performed. Sterile PBS was administered as a control.
BAL fluid analysis
BAL was performed by lavage through the trachea with 1ml of BAL fluid (1×PBS, 10%FCS, 1mM EDTA). Total cell counts were performed using a hemacytometer. Differential cell counts were assessed as previously described (29). Briefly, total cell counts were performed using a hemacytometer. Differential cell counts were assessed by cytologic preparation (Cytospin; Shandon Scientific) and stained with Diff-Quik stain (Siemens). Differential cell numbers were calculated based on percent of differential cells of total cells. BAL fluid was centrifuged at 10,000g for 5 minutes and supernatants stored at -80°C for analysis while cells were stained for analysis by flow cytometry. BAL fluid was analyzed for IL-6 by sandwich ELISA (BD Biosciences), and KC, MIP2α and sIL-6R by DuoSet Sandwich ELISA (R&D Systems) following manufacturer's instructions.
Flow cytometry
BAL cells were counted, resuspended at 1×106 cells/ml and 100μl was stained with APC-conjugated anti-Gr-1 (BD Biosciences). Samples were then washed and stained with FITC-labeled AnnexinV (Invitrogen) following manufacturer's recommended protocol. Briefly, AnnexinV was added to cells in AnnexinV binding buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) and incubated for 15 minutes at room temperature. Pacific Blue-Sytox (Invitrogen) was added at 1μl per sample, 5 minutes prior to flow cytometric analysis. Samples were gated on Gr-1+ cells and analysed using FlowJo software.
BMMC generation and reconstitution
Bone marrow was isolated from the femurs of female, age-matched C57BL/6 or IL-6−/−mice and cultured as previously described (19). After 4 weeks, BMMCs were analysed for surface expression of FcεRI and c-kit by flow cytometry. Cultures of greater than 95% FcεRI+ and c-kit+ BMMCs were used to reconstitute 6-week old W/Wv mice by intravenous injection of 5×106 WT or IL-6−/− BMMC. 8 weeks after reconstitution, mast cell reconstitution was confirmed as previously described (30) and hematocrit levels measured to ensure anemia was unaltered by reconstitution.
Results
TGFβ1 promotes neutrophil apoptosis during LPS-induced lung injury
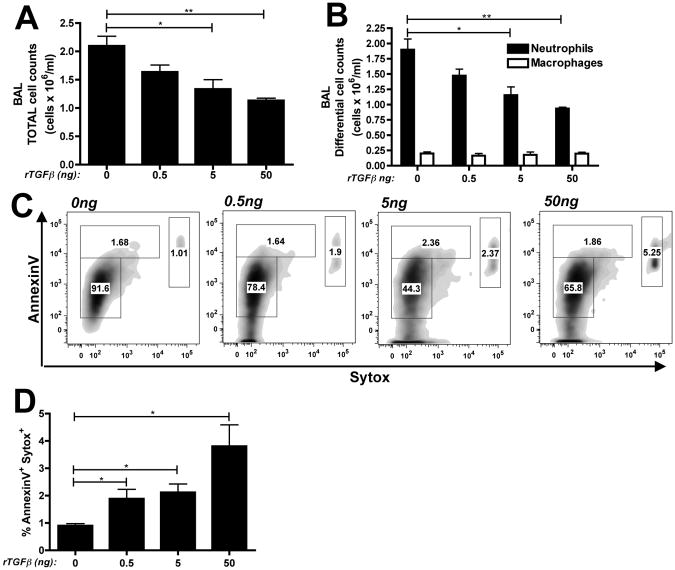
To determine whether rTGFβ1 was sufficient to alter neutrophilia during the onset of ALI responses, C57BL/6 mice (WT) received 10μg LPS via intratracheal injection in combination with rTGFβ1 (0.5, 5 or 50ng). 24 hours later, bronchoalveolar lavage (BAL) was performed and samples were analyzed for cellular infiltration. While rTGFβ1 alone did not elicit any cellular infiltration in the BAL (data not shown), rTGFβ1 dose dependently reduced the total numbers of cells (Figure 1A). The cellular infiltration in all groups remained dominated by neutrophils (∼85%) (Figure S1A), suggesting that TGFβ1 did not alter the cellular composition of the inflammatory response. However, there was a significant decrease in the total numbers of neutrophils in the BAL fluid while macrophage, the only other cell seen, was unaltered (Figure 1B). The reduction in BAL neutrophils by TGFβ1 was most likely not due to reduced neutrophil chemoattraction since levels of KC and MIP2α, two critical neutrophil chemoattractants in the lung (31, 32), were unaltered in the BAL fluid (Figure S1B and S1C). Since neutrophil apoptosis is a key step in the late resolution of ALI inflammation, we questioned whether neutrophil apoptosis might also be occurring within this early initiation period. As represented in Figure 1C, we observed a dose-dependent increase in late apoptotic/dead neutrophils (Gr-1+/AnnexinV+/Sytox+) within the BAL fluid in response to rTGFβ124 hours after treatment that was statistically different from untreated mice over multiple animals and experiments (Figure 1D).
Figure 1. rTGFβ1 is sufficient to promote neutrophil apoptosis in LPS-induced lung injury.
WT mice received 0, 0.5, 5 or 50ng rTGFβ1 with 10μg LPS intratracheally. (A) Total and (B) differential cell counts (black bars = neutrophils, white bars = macrophages) were performed on BAL samples 24 hours post-treatment. (C) BAL cells were gated on Gr-1+ and analysed for percentage neutrophil death by flow cytometry. Representative flow plots with (D) quantification of dead neutrophils. n=4-5 mice per group from 2 independent experiments. Data is represented as mean ± SEM.
Reduction of lung neutrophilia by TGFβ1 is IL-6 dependent
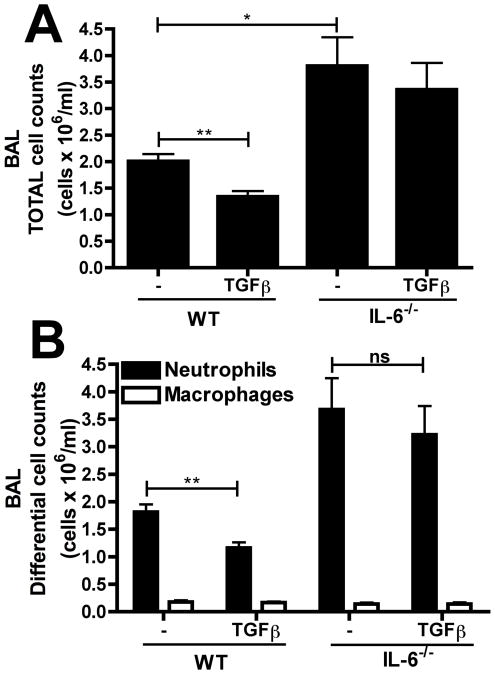
Since IL-6 has been implicated in neutrophil apoptosis and IL-6−/− mice have a significantly exacerbated inflammatory response in the LPS-induced ALI model (33), we postulated that TGFβ1 might alter ALI via an IL-6 dependent mechanism. To test this, WT and IL-6−/− mice received LPS (10μg) with or without 5ng rTGFβ1 and the inhibitory influence of rTGFβ1 was determined. As previously seen, the total inflammatory cell numbers (Figure 2A) and neutrophil numbers (Figure 2B) in WT mice were significantly reduced by rTGFβ1 treatment. In contrast, IL-6−/−mice, which exhibited a heightened responsiveness to LPS alone, were unaffected by rTGFβ1 treatment, suggesting that IL-6 is required for the protective effects of rTGFβ1.
Figure 2. TGFβ1 functions through an IL-6 dependent mechanism to promote neutrophil clearance.
WT or IL-6−/− mice were challenged with 10μg LPS alone or LPS with 5ng rTGFβ1. 24 hours post-challenge, (A) total and (B) differential cell counts were performed on BAL samples. n=3-7 mice per group from 2 independent experiments. Data is represented as mean ± SEM.
IL-6 is sufficient to promote neutrophil apoptosis
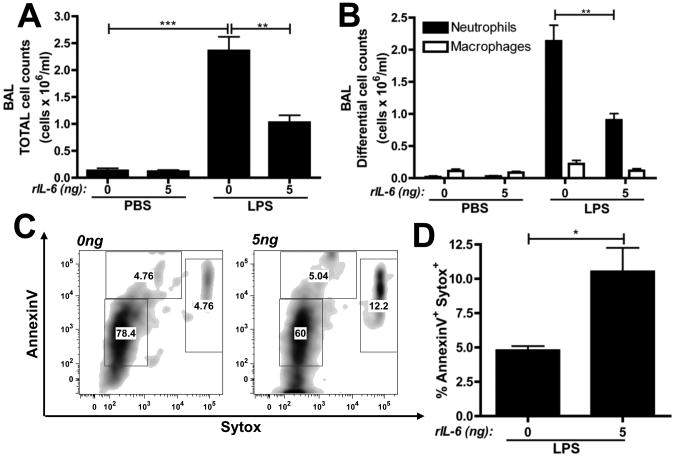
To determine if IL-6 was sufficient to promote neutrophil apoptosis during LPS-induced lung injury, WT mice were administered intratracheal rIL-6 with or without concurrent LPS treatment. As previously demonstrated (34), the intratracheal administration of rIL-6 alone did not elicit any cellular infiltration. However, mice receiving rIL-6 plus LPS exhibited significantly reduced total and neutrophil cell counts than those receiving LPS alone (Figure 3A and 3B). Furthermore, analysis of the BAL cells for neutrophil apoptosis demonstrated that rIL-6 led to a significant increase in late apoptotic neutrophils within the BAL (Figure 3C and 3D).
Figure 3. rIL-6 is sufficient to promote neutrophil apoptosis in LPS-induced lung injury.
WT mice were challenged with 5ng rIL-6 in combination with PBS or 10μg LPS via i.t. administration. (A) Total and (B) differential cell counts cell counts were performed on BAL samples, 24 hours post-challenge. (C) Representative flow plots of BAL cells (gating on Gr-1+ cells) with (D) quantification of dead neutrophils. n=5 mice per group from 2 independent experiments. Data is represented as mean ± SEM.
IL-6 production precedes neutrophil infiltration and apoptosis in LPS-induced lung injury
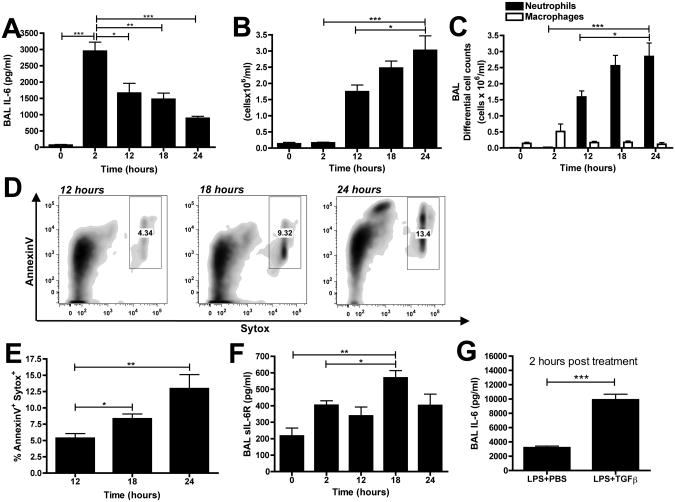
IL-6 production has been shown to occur during the resolution of acute inflammation and in the key transition from innate to adaptive immunity (26, 33) but whether IL-6 is actively produced during the earlier initiation period of neutrophilic inflammation is unknown. Therefore, we examined levels of IL-6 in the lung at 2, 12, 18, and 24 hours after challenge with 10μg LPS. Interestingly, IL-6 levels in the BAL fluid peaked within 2 hours and declined over time, reaching its lowest level at 24 hours (Figure 4A). In contrast, the total (Figure 4B) and neutrophil (Figure 4C) cell counts began at 12 hours post-challenge and increased with time, suggesting that the inflammatory cells enter into an already IL-6-rich environment in the lung.
Figure 4. IL-6 production peaks at 2 hours following LPS challenge.
WT mice were challenged via intratracheal administration of 10μg LPS and BAL samples were collected at 0, 2, 12, 18 and 24 hours. (A) Total and (B) differential cell counts were performed on BAL samples. (C) BAL supernatants were analysed for IL-6 production by ELISA. (D) Representative flow plots of BAL cells (gating on Gr-1+ cells) with (E) quantification of dead neutrophils. (F) sIL-6R levels were measured in BAL supernatants by ELISA. (G) BAL supernatants were analysed for IL-6 production by ELISA with or without 5ng rTGFβ1. n=6 mice per group from 2 independent experiments. Data is represented as mean ± SEM.
We then examined neutrophil apoptosis in the BAL samples at 12, 18, and 24 hours post-LPS challenge. We observed increases in AnnexinV+/Sytox+ neutrophils over time (Figure 4D and 4E). Apoptotic neutrophils have been shown to shed the sIL-6R (35)and we also observed elevated sIL-6R over time (Figure 4F). Taken together, our data demonstrates that IL-6 production precedes neutrophil infiltration and that neutrophil apoptosis is an early event during ALI responses. Finally, in order to determine if IL-6 levels were specifically altered by rTGFβ1 treatment, we examined IL-6 levels two hours after administration of LPS with or without rTGFβ1. As predicted, rTGFβ1 led to a significant increase in BAL IL-6 levels (Figure 4G).
Neutrophil clearance is regulated by mast cell-derived IL-6
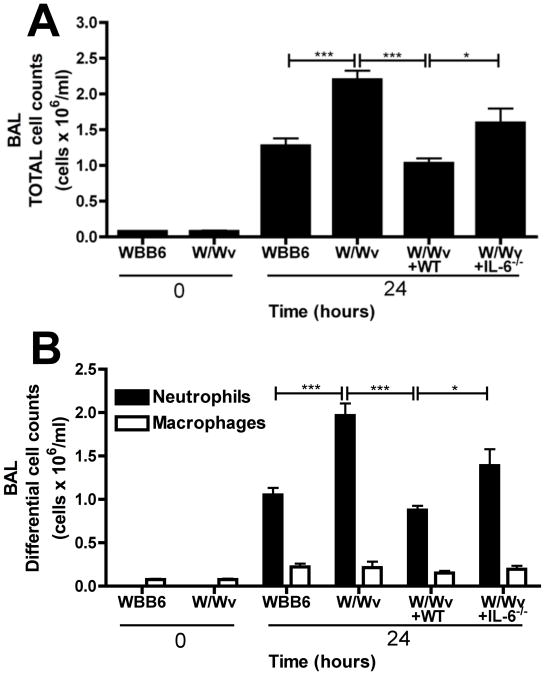
We next asked what role mast cells and, in particular, mast cell-derived IL-6 played in LPS-induced lung injury. Despite possessing an intrinsic neutropenia (36), mast cell deficient W/Wv mice displayed significantly greater total and neutrophil cell counts 24 hours after intratracheal LPS than their littermate controls (WBB6) (Figure 5A and 5B). Reconstituting W/Wv mice with wildtype bone marrow-derived mast cells (W/Wv+WT) reduced the exaggerated inflammatory response back to control levels. Though we observed a modest increase in MIP2α levels in W/Wv mice (Figure S2A), there was no difference in KC levels (Figure S2B), suggesting that expression of neutrophil chemoattractants were not significantly affected. However, W/Wv mice exhibited a significantly lower percentage of dead neutrophils, compared to controls (Figure S2C and S2D), suggesting that mast cells regulate the neutrophil cell death processes. To specifically assess the role of mast cell-derived IL-6, W/Wv mice were reconstituted with mast cells derived from IL-6−/− mice (W/Wv+IL-6−/−). W/Wv+IL-6−/− mice exhibited significantly elevated total (Figure 5A) and neutrophil (Figure 5B) cell counts than those seem with reconstitution with WT mast cells and no statistically significant differences were seen when compared to non-reconstituted W/Wv, although there was a trend towards less cells.
Figure 5. Mast cell derived IL-6 promotes neutrophil clearance in LPS-induced lung injury.
Controls (WBB6), mast cell deficient (W/Wv) mice and W/Wv mice reconstituted with WT mast cells (W/Wv+WT) or IL-6−/− mast cells (W/Wv+IL-6−/−) were intratracheally challenged with 10μg LPS. At 24 hours post-treatment, (A) total and (B) differential cell counts were performed on BAL samples. n=5-7 mice per group from 3 independent experiments. Data is represented as mean ± SEM.
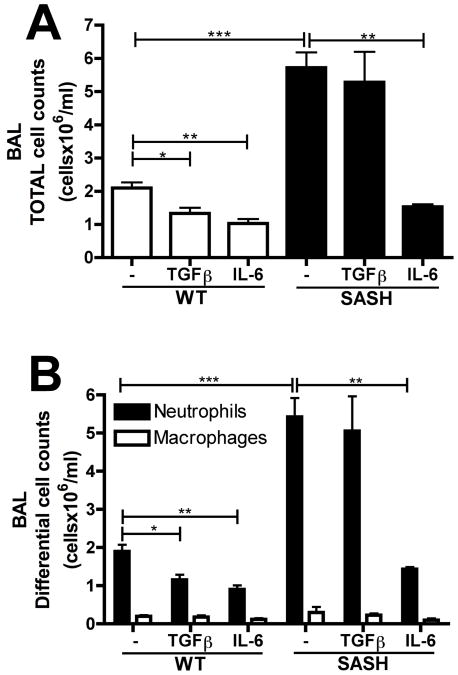
TGFβ1 functions through a mast cell dependent mechanism to promote neutrophil clearance
Since this data suggested a role for mast cells in limiting the early inflammatory response to LPS, we asked whether TGFβ1 might exert its effects via mast cells. For these experiments we chose to utilize Sash mice, another mast cell deficient strain but that lacks the neutropenia seen in W/Wv mice (37). Sashor WT controls were intratracheally challenged with 10μg LPS with or without rTGFβ1. Recombinant IL-6 was also utilized since this would act downstream of the role for mast cells, according to our hypothesis. Similar to the W/Wv strain, Sash mice also exhibited significantly elevated total (Figure 6A) and neutrophil (Figure 6B) cell counts in response to LPS-challenge alone, although the magnitude of elevation was actually greater than that seen with W/Wv mice. The administration of rTGFβ1 led to a significant reduction in total and neutrophil cell counts in the BAL of WT mice but had no effect in Sash mice. Conversely, rIL-6 reduced the inflammatory response in both WT and Sash mice. This data demonstrates that the protective effects of TGFβ1 on lung neutrophilia during ALI functions via a mast cell dependent mechanism.
Figure 6. TGFβ promotes neutrophil clearance through a mast cell dependent mechanism.
Mast cell deficient Kitw-sh (Sash) mice were challenged with 10μg LPS alone, LPS with 5ng rIL-6 or LPS with 5ng rTGFβ1. 24 hours after challenge, (A) total and (B) different cell counts were performed on BAL samples. n= 3-7 mice per group from 3 independent experiments. Data is represented as mean ± SEM.
Regulatory T cells limit neutrophilic inflammation during the early stages of ALI
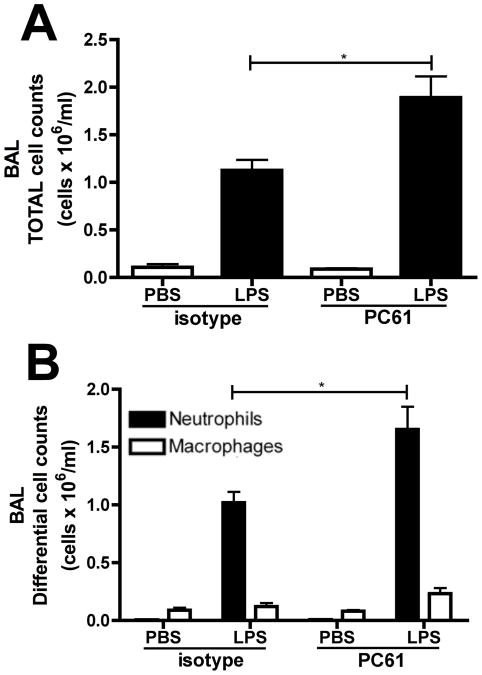
Since our previous work demonstrated that regulatory T cells are capable of providing TGFβ to mast cells and enhancing their ability to generate IL-6 (19), and mast cells and Tregs have been shown to co-localize within tissues (38), we postulated that these cells might also participate in limiting the number of neutrophils. While D'Alessio et al. already demonstrated that Tregs are necessary for the resolution of ALI at 72 hours post LPS challenge (14), we examined the effects of depleting Tregs prior to the initiation of ALI. Treatment with an anti-CD25 antibody (PC61) led to a significant increase in total and neutrophil cell counts within the BAL at 24 hours post LPS challenge (Figure 7A and 7B), suggesting that Tregs are also likely to be important in limiting the early neutrophilic inflammatory response during the initiation of ALI responses.
Figure 7. Tregs are required for early neutrophil clearance in ALI.
Wildtype mice were administered 1mg anti-CD25 mAb (PC61)or isotype control. Mice were then treated with PBS or 10μg LPS intratracheally. (A) Total and (B) differential cell counts were performed on BAL samples 24 hours post-treatment. n=4-6 mice per group from 3 independent experiments. Data represented as mean ± SEM, * p<0.05, **p<0.01, ***p<0.001.
Discussion
TGFβ1 has long been recognized for its pivotal role in promoting the fibrotic remodeling of the lung during inflammation (39) but its role during the initiation of inflammation was unclear. In this study, we report that TGFβ1 enhances neutrophil apoptosis in a model of acute lung injury. We demonstrate that this is mediated via an indirect pathway that is dependent on IL-6 and mast cells. Indeed, the exaggerated neutrophilic inflammation in two different strains of mast cell deficient mice and in IL-6−/− mice suggests that this mechanism is required for homeostatic regulation over neutrophil numbers in the lung.
The overall concept that IL-6 might serve as a protective factor in inflammation seemed initially contradictory to the well-established pro-inflammatory effects of IL-6 and the therapeutic benefit of anti-IL-6R antibodies in diseases such as rheumatoid arthritis and systemic lupus erythematosus (SLE) (40). However, IL-6 is capable of exerting several inhibitory influences over innate immune responses, including suppression of CXCL1 and CXCL8 release, induction of IL-1 and TNF antagonists and neutrophil apoptosis (28, 41). Interestingly, the -174 G/C polymorphism in the IL-6 promoter, which correlates with enhanced IL-6 levels (42), has been shown to associate with enhanced disease severity in both rheumatoid arthritis (43-46) and SLE (47-49). In sharp contrast, it associates with reduced ARDS, septic shock and multiple organ dysfunction syndrome in patients with pneumococcal community acquired pneumonia (50), illustrating that increased IL-6 may be clinically protective in the context of neutrophilic diseases.
We previously demonstrated that Tregs were capable of enhancing mast cell production of IL-6 upon IgE-mediated activation and that this was mediated via TGFβ1 (19). Interestingly, TGFβ1 primed the mast cell for IL-6 production upon stimulation, rather than drive IL-6 production directly. In Fig S3, we now also show this is also evident with LPS-driven activation in vitro. In the context of mast cell activation, LPS-mediated activation has been previously shown to increase IL-6 production independent of degranulation and preformed mediator release (21). This separation of cytokine production from degranulation may be a critical aspect that facilitates seeing this as a regulatory response by the mast cell versus the established inflammatory potential of mast cell activation. In support of this, histamine, released upon degranulation, has been shown to reduce the suppressive ability of Tregs (51). Consequently, it is intriguing to postulate that the precise role of mast cells and Tregs in regulating inflammation may be highly context specific and influenced by the nature of the stimuli. Supporting this notion, neutrophil recruitment to the lung in response to adenosine, which leads to mast cell degranulation, was significantly lower in W/Wv mice (52), contrasting our findings with LPS stimulation.
Our data showing that Treg depletion leads to elevated neutrophils 24 hours after LPS administration suggests that resident Tregs are regulating the early initiation of inflammation. In allergic airway inflammation, Treg depletion prior to sensitization has been shown to lead to exaggerated responsiveness to airway sensitization (53) while, more recently, tissue-resident Tregs were shown to be critically important in oral tolerance (54). Consequently, it is becoming increasingly evident that tissue-resident Tregs contribute to controlling a variety of local inflammatory responses. In contrast, D'Alessio et al. demonstrated a requirement for newly recruited Tregs in the resolution of LPS-induced ALI (14). This also was mediated via TGFβ1 since a blocking anti-TGFβ1 antibody impaired resolution. Whether the resolution of ALI responses is also dependent on mast cells and IL-6 remains to be determined. However, we saw no changes in the Treg numbers within the lung during the initiation time period that we focused on (data not shown), suggesting tissue-resident cells were the likely population regulating responses at 24 hours.
In considering the biological relevance of the pathway we have defined in this study, there would seem to be two potential benefits to regulating neutrophil apoptosis during the initiation of inflammation. In the first, Tregs may be exerting a controlling influence over the strength of the inflammatory response such that, in the example of a modest infectious stimulus, they prevent over exuberant responses that may lead to tissue damage. In support of this concept, high doses of LPS exhibited higher neutrophilia and overcame the limiting influence of TGFβ1 (data not shown), perhaps one explanation why D'Alessio et al. failed to observe a difference in neutrophilia at 24 hours, since they utilized a relatively high LPS dose compared to that used in our study. Alternatively, the induction of neutrophil apoptosis may be necessary for the dissemination of the soluble IL-6R and, subsequently, the ability of other cells to respond to IL-6 via gp130. Indeed, as we show in Figure 4, the shedding of sIL-6R seems to coincide with the arrival of neutrophils into the lung. Abrogation of IL-6 mediated gp130 responses has been shown to prevent the generation of adaptive immune responses to Toxoplasma gondii infection (55), suggesting that signals via gp130 are important for protective memory responses.
In conclusion, our findings describe a previously unappreciated process through which the magnitude of innate inflammation is regulated in the lung. Surprisingly, this occurs via two cytokines, TGFβ1 and IL-6, which have been extensively characterized for their deleterious effects in chronic inflammation. However, as we propose, the priming influence of TGFβ1 on tissue resident mast cells serves to enhance IL-6 production upon LPS exposure which drives the infiltrating neutrophil into apoptotic clearance.
Supplementary Material
Acknowledgments
The authors thank Miller Scientific Communications for editorial assistance.
Footnotes
This work was supported by NIH R01 AI-076456 (PJB) and predoctoral award AHA#10PRE2630137 from the American Heart Association (KG).
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 3.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 5.McGrath EE, Marriott HM, Lawrie A, Francis SE, Sabroe I, Renshaw SA, Dockrell DH, Whyte MK. TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. J Leukoc Biol. 2011;90:855–865. doi: 10.1189/jlb.0211062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowe SJ, Allen L, Ridger VC, Hellewell PG, Whyte MK. Caspase-1-deficient mice have delayed neutrophil apoptosis and a prolonged inflammatory response to lipopolysaccharide-induced acute lung injury. J Immunol. 2002;169:6401–6407. doi: 10.4049/jimmunol.169.11.6401. [DOI] [PubMed] [Google Scholar]
- 7.Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, Caldicott A, Martinez-Losa M, Walker TR, Duffin R, Gray M, Crescenzi E, Martin MC, Brady HJ, Savill JS, Dransfield I, Haslett C. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nature medicine. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 8.Fialkow L, Fochesatto Filho L, Bozzetti MC, Milani AR, Rodrigues Filho EM, Ladniuk RM, Pierozan P, de Moura RM, Prolla JC, Vachon E, Downey GP. Neutrophil apoptosis: a marker of disease severity in sepsis and sepsis-induced acute respiratory distress syndrome. Crit Care. 2006;10:R155. doi: 10.1186/cc5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinberg KP, Milberg JA, Martin TR, Maunder RJ, Cockrill BA, Hudson LD. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. American journal of respiratory and critical care medicine. 1994;150:113–122. doi: 10.1164/ajrccm.150.1.8025736. [DOI] [PubMed] [Google Scholar]
- 10.Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-beta in airway remodeling in asthma. American journal of respiratory cell and molecular biology. 2011;44:127–133. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 11.Morty RE, Konigshoff M, Eickelberg O. Transforming growth factor-beta signaling across ages: from distorted lung development to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:607–613. doi: 10.1513/pats.200908-087RM. [DOI] [PubMed] [Google Scholar]
- 12.Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg JA, Chapman HA. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. The Journal of clinical investigation. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulich TR, Yin S, Guo K, Yi ES, Remick D, del Castillo J. Intratracheal injection of endotoxin and cytokines. II. Interleukin-6 and transforming growth factor beta inhibit acute inflammation. The American journal of pathology. 1991;138:1097–1101. [PMC free article] [PubMed] [Google Scholar]
- 14.D'Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. The Journal of clinical investigation. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balazovich KJ, Fernandez R, Hinkovska-Galcheva V, Suchard SJ, Boxer LA. Transforming growth factor-beta1 stimulates degranulation and oxidant release by adherent human neutrophils. J Leukoc Biol. 1996;60:772–777. doi: 10.1002/jlb.60.6.772. [DOI] [PubMed] [Google Scholar]
- 16.Shen L, Smith JM, Shen Z, Eriksson M, Sentman C, Wira CR. Inhibition of human neutrophil degranulation by transforming growth factor-beta1. Clinical and experimental immunology. 2007;149:155–161. doi: 10.1111/j.1365-2249.2007.03376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghio M, Ottonello L, Contini P, Amelotti M, Mazzei C, Indiveri F, Puppo F, Dallegri F. Transforming growth factor-beta1 in supernatants from stored red blood cells inhibits neutrophil locomotion. Blood. 2003;102:1100–1107. doi: 10.1182/blood.V102.3.1100. [DOI] [PubMed] [Google Scholar]
- 18.Ear T, Fortin CF, Simard FA, McDonald PP. Constitutive association of TGF-beta-activated kinase 1 with the IkappaB kinase complex in the nucleus and cytoplasm of human neutrophils and its impact on downstream processes. J Immunol. 2010;184:3897–3906. doi: 10.4049/jimmunol.0902958. [DOI] [PubMed] [Google Scholar]
- 19.Ganeshan K, Bryce PJ. Regulatory T cells enhance mast cell production of IL-6 via surface-bound TGF-beta. J Immunol. 2012;188:594–603. doi: 10.4049/jimmunol.1102389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palker TJ, Dong G, Leitner WW. Mast cells in innate and adaptive immunity to infection. European journal of immunology. 2010;40:13–18. doi: 10.1002/eji.200990325. [DOI] [PubMed] [Google Scholar]
- 21.Leal-Berumen I, Conlon P, Marshall JS. IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J Immunol. 1994;152:5468–5476. [PubMed] [Google Scholar]
- 22.Sutherland RE, Olsen JS, McKinstry A, Villalta SA, Wolters PJ. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J Immunol. 2008;181:5598–5605. doi: 10.4049/jimmunol.181.8.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldford SA, I, Haidl D, Howatt MA, Leiva CA, Johnston B, Marshall JS. A critical role for mast cells and mast cell-derived IL-6 in TLR2-mediated inhibition of tumor growth. J Immunol. 2010;185:7067–7076. doi: 10.4049/jimmunol.1001137. [DOI] [PubMed] [Google Scholar]
- 24.Nishimoto N, Kishimoto T. Inhibition of IL-6 for the treatment of inflammatory diseases. Curr Opin Pharmacol. 2004;4:386–391. doi: 10.1016/j.coph.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 26.Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, Rose-John S, Fuller GM, Topley N, Jones SA. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 27.Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008;181:2189–2195. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- 28.McLoughlin RM, Witowski J, Robson RL, Wilkinson TS, Hurst SM, Williams AS, Williams JD, Rose-John S, Jones SA, Topley N. Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. The Journal of clinical investigation. 2003;112:598–607. doi: 10.1172/JCI17129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryce PJ, Geha R, Oettgen HC. Desloratadine inhibits allergen-induced airway inflammation and bronchial hyperresponsiveness and alters T-cell responses in murine models of asthma. The Journal of allergy and clinical immunology. 2003;112:149–158. doi: 10.1067/mai.2003.1616. [DOI] [PubMed] [Google Scholar]
- 30.Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, Caughey GH. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit(W-sh) sash mice. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2005;35:82–88. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. American journal of respiratory cell and molecular biology. 2005;32:531–539. doi: 10.1165/rcmb.2005-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeyaseelan S, Chu HW, Young SK, Freeman MW, Worthen GS. Distinct roles of pattern recognition receptors CD14 and Toll-like receptor 4 in acute lung injury. Infection and immunity. 2005;73:1754–1763. doi: 10.1128/IAI.73.3.1754-1763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. The Journal of clinical investigation. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanley TP, Foreback JL, Remick DG, Ulich TR, Kunkel SL, Ward PA. Regulatory effects of interleukin-6 in immunoglobulin G immune-complex-induced lung injury. The American journal of pathology. 1997;151:193–203. [PMC free article] [PubMed] [Google Scholar]
- 35.Chalaris A, Rabe B, Paliga K, Lange H, Laskay T, Fielding CA, Jones SA, Rose-John S, Scheller J. Apoptosis is a natural stimulus of IL6R shedding and contributes to the proinflammatory trans-signaling function of neutrophils. Blood. 2007;110:1748–1755. doi: 10.1182/blood-2007-01-067918. [DOI] [PubMed] [Google Scholar]
- 36.Chervenick PA, Boggs DR. Decreased neutrophils and megakaryocytes in anemic mice of genotype W/W. J Cell Physiol. 1969;73:25–30. doi: 10.1002/jcp.1040730104. [DOI] [PubMed] [Google Scholar]
- 37.Zhou JS, Xing W, Friend DS, Austen KF, Katz HR. Mast cell deficiency in Kit(W-sh) mice does not impair antibody-mediated arthritis. The Journal of experimental medicine. 2007;204:2797–2802. doi: 10.1084/jem.20071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, Odom S, Rivera J, Colombo MP, Pucillo CE. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wynn TA. Integrating mechanisms of pulmonary fibrosis. The Journal of experimental medicine. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mima T, Nishimoto N. Clinical value of blocking IL-6 receptor. Curr Opin Rheumatol. 2009;21:224–230. doi: 10.1097/BOR.0b013e3283295fec. [DOI] [PubMed] [Google Scholar]
- 41.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 42.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. The Journal of biological chemistry. 2000;275:18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- 43.Lee YH, Bae SC, Choi SJ, Ji JD, Song GG. The association between interleukin-6 polymorphisms and rheumatoid arthritis: a meta-analysis. Inflammation research: official journal of the European Histamine Research Society … [et al] 2012 doi: 10.1007/s00011-012-0459-1. [DOI] [PubMed] [Google Scholar]
- 44.Panoulas VF, Stavropoulos-Kalinoglou A, Metsios GS, Smith JP, Milionis HJ, Douglas KM, Nightingale P, Kitas GD. Association of interleukin-6 (IL-6)-174G/C gene polymorphism with cardiovascular disease in patients with rheumatoid arthritis: the role of obesity and smoking. Atherosclerosis. 2009;204:178–183. doi: 10.1016/j.atherosclerosis.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 45.Pawlik A, Wrzesniewska J, Florczak M, Gawronska-Szklarz B, Herczynska M. IL-6 promoter polymorphism in patients with rheumatoid arthritis. Scand J Rheumatol. 2005;34:109–113. doi: 10.1080/03009740510026373. [DOI] [PubMed] [Google Scholar]
- 46.Pascual M, Nieto A, Mataran L, Balsa A, Pascual-Salcedo D, Martin J. IL-6 promoter polymorphisms in rheumatoid arthritis. Genes Immun. 2000;1:338–340. doi: 10.1038/sj.gene.6363677. [DOI] [PubMed] [Google Scholar]
- 47.Lee YH, Lee HS, Choi SJ, Ji JD, Song GG. The association between interleukin-6 polymorphisms and systemic lupus erythematosus: a meta-analysis. Lupus. 2012;21:60–67. doi: 10.1177/0961203311422711. [DOI] [PubMed] [Google Scholar]
- 48.Santos MJ, Fernandes D, Capela S, da Silva JC, Fonseca JE. Interleukin-6 promoter polymorphism -174 G/C is associated with nephritis in Portuguese Caucasian systemic lupus erythematosus patients. Clinical rheumatology. 2011;30:409–413. doi: 10.1007/s10067-010-1640-y. [DOI] [PubMed] [Google Scholar]
- 49.Schotte H, Schluter B, Rust S, Assmann G, Domschke W, Gaubitz M. Interleukin-6 promoter polymorphism (--174 G/C) in Caucasian German patients with systemic lupus erythematosus. Rheumatology (Oxford) 2001;40:393–400. doi: 10.1093/rheumatology/40.4.393. [DOI] [PubMed] [Google Scholar]
- 50.Martin-Loeches I, Sole-Violan J, Rodriguez de Castro F, Garcia-Laorden MI, Borderias L, Blanquer J, Rajas O, Briones ML, Aspa J, Herrera-Ramos E, Marcos-Ramos JA, Sologuren I, Gonzalez-Quevedo N, Ferrer-Aguero JM, Noda J, Rodriguez-Gallego C. Variants at the promoter of the interleukin-6 gene are associated with severity and outcome of pneumococcal community-acquired pneumonia. Intensive Care Med. 2012;38:256–262. doi: 10.1007/s00134-011-2406-y. [DOI] [PubMed] [Google Scholar]
- 51.Forward NA, Furlong SJ, Yang Y, Lin TJ, Hoskin DW. Mast cells down-regulate CD4+CD25+ T regulatory cell suppressor function via histamine H1 receptor interaction. J Immunol. 2009;183:3014–3022. doi: 10.4049/jimmunol.0802509. [DOI] [PubMed] [Google Scholar]
- 52.Tilley SL, Tsai M, Williams CM, Wang ZS, Erikson CJ, Galli SJ, Koller BH. Identification of A3 receptor- and mast cell-dependent and -independent components of adenosine-mediated airway responsiveness in mice. J Immunol. 2003;171:331–337. doi: 10.4049/jimmunol.171.1.331. [DOI] [PubMed] [Google Scholar]
- 53.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. The Journal of experimental medicine. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 55.Silver JS, Stumhofer JS, Passos S, Ernst M, Hunter CA. IL-6 mediates the susceptibility of glycoprotein 130 hypermorphs to Toxoplasma gondii. J Immunol. 2011;187:350–360. doi: 10.4049/jimmunol.1004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.