Abstract
Background
The excellent sensitivity and specificity of commercially available nucleic acid amplification tests (NAATs) for the identification of Neisseria gonorrhoeae have been demonstrated. This study evaluated the performance of the BD ProbeTec™ N. gonorrhoeae Qx (GCQ) Amplified DNA Assay on the BD Viper™ System with XTR™ Technology in a multicenter study.
Methods
Specimens were collected at 7 geographically diverse clinical sites from 1846 women and men attending sexually transmitted disease, family planning, and obstetrics and gynecology clinics. There were 1768 evaluable participants, 994 women and 774 men. GCQ results from female endocervical, self-collected vaginal, male urethral swab specimens, and male and female neat (unpreserved) urine specimens, as well as those obtained using the urine preservative transport (UPT) tube for the GCQ assay were compared with patient infected status (PIS). For each participant, PIS was determined based on the combined results from the reference assays Aptima Combo 2® (AC2) and BD ProbeTec™ ET GC Amplified DNA Assay (PT).
Results
The sensitivity versus PIS for endocervical, vaginal, and female UPT urine, and female neat urine samples was 98.5%, 100.0%, 98.5%, and 96.9%, respectively; the specificity was 99.7%, 99.1%, 99.7%, and 99.5%, respectively. The sensitivity versus PIS for male urethral swabs and both male UPT and neat urine was 100.0%, with specificities of 99.1% for the urethral swab and UPT urine and 98.9% for the neat urine. The overall GCQ assay performance was not statistically different from that of AC2 or PT.
Conclusions
The GCQ assay demonstrated performance characteristics comparable with other commercially available nucleic acid–based tests such as AC2 and PT. Vaginal swabs, endocervical swabs, urethral swabs, and urine specimens may all be used for gonorrhea screening.
Since peaking in 1978 at over a million cases, reported gonorrhea rates in the United States have declined. Nonetheless, infections caused by Neisseria gonorrhoeae remain the second most common infection reported to the Centers for Disease Control and Prevention. In 2009, 301,174 cases were reported which, due to underreporting represent a likely incidence of more than 600,000 cases.1 However, available data suggest that progress in efforts to control gonorrhea is slowing. In the decade between 1989 and 1999, reported US gonorrhea rates declined 48% from 690,042 cases to 360,831, whereas over the subsequent decade the rates declined only 17%, to 301,174 cases. Slowing declines in US gonorrhea rates as well as progressive development of antimicrobial resistance suggest that further emphasis on gonorrhea control is needed.
The starting point for control of any sexually transmitted disease (STD) is detection of infection to guide therapy for infected persons and their sexual partners. Nucleic acid amplification tests (NAATs) are more sensitive than traditional culture, permit more acceptable specimen collection for testing using voided urine or self-obtained vaginal swabs,2–9 and are less susceptible to test performance problems due to specimen transport issues. Use of NAATs for diagnosis of gonococcal and chlamydial infections continues to evolve as manufacturers have developed tests which are both more accurate than earlier NAATs for STD diagnosis and which are technically easier to perform within the laboratory. While these improvements are well described for Chlamydia trachomatis assays, there are fewer studies of newer NAATs for diagnosis of N. gonorrhoeae infections. We report on the findings of a large, multicenter trial evaluating the recently improved BD N. gonorrhoeae Qx Amplified DNA Assay (GCQ) that can be run simultaneously with the chlamydia assay using a variety of specimen types as performed on a fully automated platform (Viper).
METHODS
Study Population
Participants were enrolled from 7 geographically diverse sites that included both traditionally high- and low-risk populations (Table S1, Supplemental Digital Content, online only, available at: http://links.lww.com/OLQ/A28). Consenting men and women between the ages of 16 to 64 years who presented with urogenital symptoms or were being screened for chlamydia (CT) and gonorrhea (GC) were enrolled between November 26, 2007 and March 21, 2008. Asymptomatic male enrollment continued until November 20, 2008.
After exclusions for protocol nonadherence and unavailable specimens, there were 994 women with evaluable results. Sixty-five women were excluded for inclusion/exclusion criteria violations (16), transport/storage errors (8), and for protocol deviations (41) in specimen collection or aliquoting. The number of men included after exclusion for nonadherence to protocol or unavailable specimens was 774. Thirteen men were excluded for inclusion/exclusion criteria violations (4), transport/storage errors (2), and specimen collection errors (7). Protocol violations resulting in exclusion included urination within an hour of specimen collection, taking antibiotics in the past 21 days, prior study enrollment, failure to provide informed consent or if they were below the age requirement approved by each site’s Institutional Review Board. Three sites performed GCQ masked-testing for specimens collected at their own site and/or specimens that were referred from the other collection sites (Table S1, online only). Reference method testing was performed at 5 of the 7 clinical centers, and 2 sites served as specimen collection sites and performed no testing. One additional site served as a specimen testing site only and performed no collection.
Specimen Collection, Transport, and Storage
For each female participant, a first-catch, as opposed to midstream clean catch, urine sample was collected, followed by a self-collected vaginal swab, and then 3 clinician-obtained endocervical swabs collected with each manufacturer’s collect kit. For each male participant, 2 or 3 urethral swabs (collection center dependent but using the manufacturer’s collection devices) were obtained followed by a first-catch urine collection. Swab collection order was randomized throughout the study to minimize the effect sample order on performance estimates. For female participants, 1 endocervical swab was tested on the reference Aptima Combo 2 (AC2) assay, 1 was tested on the reference PT assay with an amplification control, and the third swab was tested using the GCQ assay. Self-collected vaginal swabs were tested only with the GCQ assay. For the male participants from whom only 2 swabs were collected, the reference method was randomized at the collection stage to either the PT or the AC2 assay and the other swab specimen was always evaluated using the GCQ assay. Endocervical, vaginal, and urethral swabs for the PT and AC2 assays were stored and tested according to their respective manufacturer’s package inserts as described previously.10–12 For the GCQ assay, urethral and endocervical swabs were stored at 2 to 30°C for up to 14 days before testing. Vaginal swabs were stored at 2 to 30°C for up to 7 days before testing. These storage conditions are identical to those described in the PT package insert.
Urine volumes were recorded for all specimens and ranged from 20 to 69 mL with the lower limit set by study design and manufacturer’s package inserts, whereas the upper limit was the maximum volume of the collection cup. Urine samples were divided into aliquots and tested with the urine specimen transport tube with the AC2 assay (UTT), the PT assay (UPT urine), and the GCQ assay with neat and UPT urine. Urine processing, storage and testing were performed in accordance with the respective manufacturer’s package inserts.
GCQ Assay
The details of the GCQ assay are identical to those of the C. trachomatis Qx assay which has been described elsewhere.13 Briefly, the steps of the assay are as follows. Samples are warmed to dissolve mucous and homogenize the specimen matrix. After cooling, the specimens were loaded onto the Viper which then performed all steps necessary for extraction and amplification of target DNA without further user intervention.14 The GCQ assay targets the Pilin gene within the genome of N. gonorrhoeae, which is also the gene targeted by the PT assay. However, the 2 assays amplify and detect different sequences. The target sequences that are amplified/detected for the 2 assays are adjacent but do not overlap. Measurements of fluorescent signal were taken approximately once every minute over the course the amplification process and positive/negative results were determined by comparing the maximum relative fluorescent signal (MaxRFU) obtained from a given specimen to a predetermined threshold.
Determination of Patient Infected Status
The definition of the patient infected status (PIS) for evaluation of GCQ performance was based on the reference swab and urine specimen results obtained using the PT assay (DNA target) and AC2 assay (16S rRNA target) which allowed for 4 reference results, 2 from each system. The definition specified by the Food and Drug Administration for submission considered a participant infected with N. gonorrhoeae if a minimum of 1 positive result was reported by each of the reference NAAT assays (i.e., a positive from both the PT assay and AC2 assay). Sensitivity analyses were performed as part of this study to assess the impact of alternate methods for defining PIS. For assay comparison, the performance characteristics of each reference method were estimated by using a PIS defined by a positive from each of the other 2 assays (i.e., AC2 estimates were derived by comparison with a PIS that required a positive result from both the PT and GCQ assays, PT estimates were derived by comparison with a PIS that required a positive result from both the GCQ and AC2 assays). This method of a “rotating” PIS standard has been suggested as a useful means of evaluating relative assay performance.15
Data Analysis
For analysis, specimens were categorized by gender, presence, or absence of genitourinary symptoms and specimen type. The performance characteristics of the GCQ assay were calculated for each category by comparing assay results with PIS. Exact confidence intervals (95% confidence level) were calculated based on the binomial distribution. Comparison of performance, sensitivity, and specificity estimates were completed using logistic regression with Hochberg adjustment for multiple comparisons. Alpha = 0.05 was used for all analyses.
RESULTS
A total of 1846 participants were enrolled in the study from 7 sites. After excluding ineligible participant specimens, data for 1768 participants (6284 specimens) were available for analysis. There were 994 women (56%) and 774 men (44%). Genitourinary symptoms suggestive of a sexually transmitted infection (burning/pain upon urination, abnormal discharge, coital pain/difficulty/bleeding, testicular, or scrotum pain/swelling) were present in 544 women (55%) and 257 men (33%). Twenty-seven female participants were known to be pregnant. Of the 774 male participants, 95 (12%) had only 2 urethral swabs collected while the remainder provided 3 urethral swabs.
Gonococcal infection was identified in 6.5% (65/994) women with 58.5% (38/65) of these infections occurring in symptomatic women. The presence or absence of symptoms had no significant effect on gonorrhea prevalence; 6.9% (38) of 544 symptomatic women had gonorrhea, whereas among asymptomatic women gonorrhea prevalence was 6% (27/450). In contrast, the overall prevalence in men was 14.5% (112/774), with the majority of infections 89.3% (100/112) identified from men with symptoms. Across sites, the prevalence of gonococcal infection in the study populations ranged from 1.4% to 19.2% in women and 4.8% to 40.5% in men (Table S1, online only). The effect of collection site was only significant for the female neat urine, with a P value of 0.044. The remaining specimen types across collection sites had P values ranging from 0.0853 to 0.708. For the GCQ assay, there was no statistical difference in performance based on symptomatic status or specimen type for either men or women (P values 0.249–0.8444).
Comparison of All NAATs
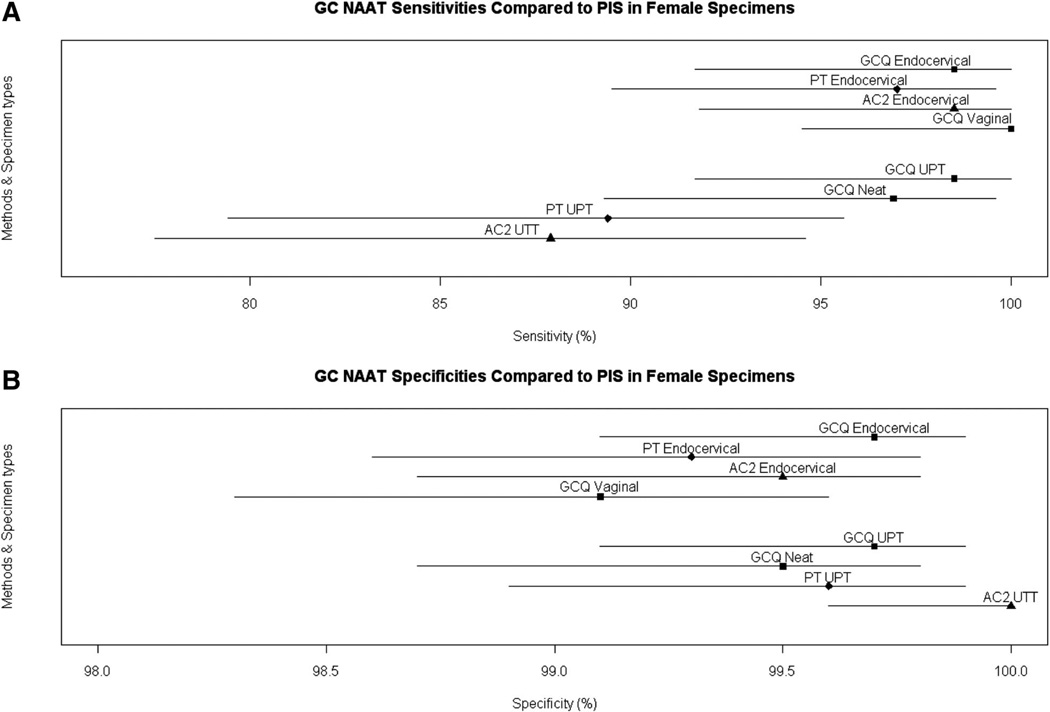
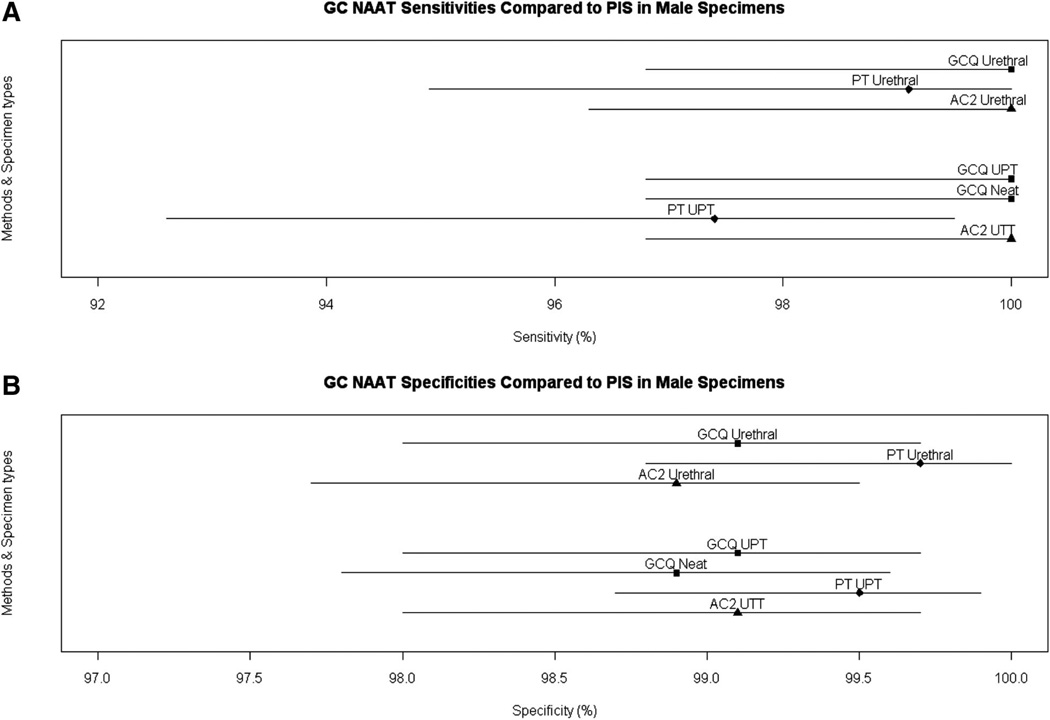
The results obtained with the rotating PIS for the PT and AC2 assays are shown in comparison to the estimates for GCQ stratified by specimen type and symptom status in Table 1. Additional analyses were performed to compare sensitivity and specificity of the GCQ, PT, and AC2 assays. The GCQ assay using female urine had significantly higher sensitivity than either the AC2 and PT assays (P = 0.023 and 0.027, respectively). (Fig. 1A). There were no significant differences in sensitivity for endocervical swabs or in specificity for endocervical swabs, urethral swabs, and male urines (Figs. 1A, 2A). There were no differences in the specificity of any of the assays or sample types (Figs. 1B, 2B).
TABLE 1.
Assay Performance Compared to PIS
| Gender | Specimen Type |
Symptoms | Sensitivity | Specificity | ||||
|---|---|---|---|---|---|---|---|---|
| BD Viper | BD ProbeTec | GP AC2 | BD Viper | BD ProbeTec | GP AC2 | |||
| Female | Endocervical | Symptomatic | 100.0 (38/38) (90.7, 100.0) | 100.0 (38/38) (90.7, 100.0) | 100.0 (38/38) (90.7, 100.0) | 99.8 (503/504) (98.9, 100.0) | 99.4 (501/504) (98.3, 99.9) | 99.4 (500/503) (98.3, 99.9) |
| specimens | Endocervical | Asymptomatic | 96.3 (26/27) (81.0, 99.9) | 92.9 (26/28) (76.5, 99.1) | 96.4 (27/28) (81.7, 99.9) | 99.5 (421/423) (98.3, 99.9) | 99.3 (407/410) (97.9, 99.8) | 99.5 (418/420) (98.3, 99.9) |
| Endocervical | Total | 98.5 (64/65) (91.7, 100.0) | 97.0 (64/66) (89.5, 99.6) | 98.5 (65/66) (91.8, 100.0) | 99.7 (924/927) (99.1, 99.9) | 99.3 (908/914) (98.6, 99.8) | 99.5 (918/923) (98.7, 99.8) | |
| Vaginal* | Symptomatic | 100.0 (38/38) (90.7, 100.0) | NA | NA | 99.6 (504/506) (98.6, 100.0) | NA | NA | |
| Vaginal | Asymptomatic | 100.0 (27/27) (87.2, 100.0) | NA | NA | 98.6 (416/422) (96.9, 99.5) | NA | NA | |
| Vaginal | Total | 100.0 (65/65) (94.5, 100.0) | NA | NA | 99.1 (920/928) (98.3, 99.6) | NA | NA | |
| Female urine | Symptomatic | 97.4 (37/38) (86.2, 99.9) | 94.7 (36/38) (82.3, 99.4) | 94.7 (36/38) (82.3, 99.4) | 99.8 (504/505) (98.9, 100.0) | 99.6 (501/503) (98.6, 100.0) | 100.0 (505/505) (99.3, 100.0) | |
| Female urine | Asymptomatic | 100.0 (27/27) (87.2, 100.0) | 82.1 (23/28) (63.1, 93.9) | 78.6 (22/28) (59.0, 91.7) | 99.5 (421/423) (98.3, 99.9) | 99.5 (414/416) (98.3, 99.9) | 100.0 (422/422) (99.1, 100.0) | |
| Female urine | Total | 98.5 (64/65) (91.7, 100.0) | 89.4 (59/66) (79.4, 95.6) | 87.9 (58/66) (77.5, 94.6) | 99.7 (925/928) (99.1, 99.9) | 99.6 (915/919) (98.9, 99.9) | 100.0 (927/927) (99.6, 100.0) | |
| Female neat urine† | Symptomatic | 97.4 (37/38) (86.2, 99.9) | NA | NA | 99.6 (503/505) (98.6, 100.0) | NA | NA | |
| Female neat urine | Asymptomatic | 96.3 (26/27) (81.0, 99.9) | NA | NA | 99.3 (420/423) (97.9, 99.9) | NA | NA | |
| Female neat urine | Total | 96.9 (63/65) (89.3, 99.6) | NA | NA | 99.5 (923/928) (98.7, 99.8) | NA | NA | |
| All types | Symptomatic | 98.7 (150/152) (95.3, 99.8) | 97.4 (74/76) (90.8, 99.7) | 97.4 (74/76) (90.8, 99.7) | 99.7 (2014/2020) (99.4, 99.9) | 99.5 (1,002/1,007) (98.8, 99.8) | 99.7 (1,005/1,008) (99.1, 99.9) | |
| All types | Asymptomatic | 98.1 (106/108) (93.5, 99.8) | 87.5 (49/56) (75.9, 94.8) | 87.5 (49/56) (75.9, 94.8) | 99.2 (1,678/1,691) (98.7, 99.6) | 99.4 (821/826) (98.6, 99.8) | 99.8 (840/842) (99.1, 100.0) | |
| All types | Total | 98.5 (256/260) (96.1, 99.6) | 93.2 (123/132) (87.5, 96.8) | 93.2 (123/132) (87.5, 96.8) | 99.5 (3,692/3,711) (99.2, 99.7) | 99.5 (1,823/1,833) (99.0, 99.7) | 99.7 (1,845/1,850) (99.4, 99.9) | |
| Male | Urethral | Symptomatic | 100.0 (100/100) (96.4, 100.0) | 98.9 (94/95) (94.3, 100.0) | 100.0 (87/87) (95.8, 100.0) | 98.7 (155/157) (95.5, 99.8) | 98.6 (141/143) (95.0, 99.8) | 97.9 (142/145) (94.1, 99.6) |
| specimens | Urethral | Asymptomatic | 100.0 (12/12) (73.5, 100.0) | 100.0 (12/12) (73.5, 100.0) | 100.0 (11/11) (71.5, 100.0) | 99.2 (492/496) (97.9, 99.8) | 100.0 (480/480) (99.2, 100.0) | 99.2 (469/473) (97.8, 99.8) |
| Urethral | Total | 100.0 (112/112) (96.8, 100.0) | 99.1 (106/107) (94.9, 100.0) | 100.0 (98/98) (96.3, 100.0) | 99.1 (647/653) (98.0, 99.7) | 99.7 (621/623) (98.8, 100.0) | 98.9 (611/618) (97.7, 99.5) | |
| Male urine | Symptomatic | 100.0 (100/100) (96.4, 100.0) | 98.0 (100/102) (93.1, 99.8) | 100.0 (100/100) (96.4, 100.0) | 98.7 (155/157) (95.5, 99.8) | 98.7 (152/154) (95.4, 99.8) | 98.1 (153/156) (94.5, 99.6) | |
| Male urine | Asymptomatic | 100.0 (12/12) (73.5, 100.0) | 92.3 (12/13) (64.0, 99.8) | 100.0 (12/12) (73.5, 100.0) | 99.2 (501/505) (98.0, 99.8) | 99.8 (497/498) (98.9, 100.0) | 99.4 (502/505) (98.3, 99.9) | |
| Male urine | Total | 100.0 (112/112) (96.8, 100.0) | 97.4 (112/115) (92.6, 99.5) | 100.0 (112/112) (96.8, 100.0) | 99.1 (656/662) (98.0, 99.7) | 99.5 (649/652) (98.7, 99.9) | 99.1 (655/661) (98.0, 99.7) | |
| Male neat urine† | Symptomatic | 100.0 (100/100) (96.4, 100.0) | NA | NA | 98.1 (154/157) (94.5, 99.6) | NA | NA | |
| Male neat urine | Asymptomatic | 100.0 (12/12) (73.5, 100.0) | NA | NA | 99.2 (501/505) (98.0, 99.8) | NA | NA | |
| Male neat urine | Total | 100.0 (112/112) (96.8, 100.0) | NA | NA | 98.9 (655/662) (97.8, 99.6) | NA | NA | |
| All types | Symptomatic | 100.0 (300/300) (98.8, 100.0) | 98.5 (194/197) (95.6, 99.7) | 100.0 (187/187) (98.0, 100.0) | 98.5 (464/471) (97.0, 99.4) | 98.7 (293/297) (96.6, 99.6) | 98.0 (295/301) (95.7, 99.3) | |
| All types | Asymptomatic | 100.0 (36/36) (90.3, 100.0) | 96.0 (24/25) (79.6, 99.9) | 100.0 (23/23) (85.2, 100.0) | 99.2 (1,494/1,506) (98.6, 99.6) | 99.9 (977/978) (99.4, 100.0) | 99.3 (971/978) (98.5, 99.7) | |
| All types | Total | 100.0 (336/336) (98.9, 100.0) | 98.2 (218/222) (95.5, 99.5) | 100.0 (210/210) (98.3, 100.0) | 99.0 (1958/1977) (98.5, 99.4) | 99.6 (1,270/1,275) (99.1, 99.9) | 99.0 (1,266/1,279) (98.3, 99.5) | |
| All types | Symptomatic | 99.6 (450/452) (98.4, 99.9) | 98.2 (268/273) (95.8, 99.4) | 99.2 (261/263) (97.3, 99.9) | 99.5 (2,478/2,491) (99.1, 99.7) | 99.3 (1,295/1,304) (98.7, 99.7) | 99.3 (1,300/1,309) (98.7, 99.7) | |
| All types | Asymptomatic | 98.6 (142/144) (95.1, 99.8) | 90.1 (73/81) (81.5, 95.6) | 91.1 (72/79) (82.6, 96.4) | 99.2 (3,172/3,197) (98.8, 99.5) | 99.7 (1,798/1,804) (99.3, 99.9) | 99.5 (1,811/1,820) (99.1, 99.8) | |
| All types | Total | 99.3 (592/596) (98.3, 99.8) | 96.3 (341/354) (93.8, 98.0) | 97.4 (333/342) (95.1, 98.8) | 99.3 (5,650/5,688) (99.1, 99.5) | 99.5 (3,093/3,108) (99.2, 99.7) | 99.4 (3,111/3,129) (99.1, 99.7) | |
No comparator test results were available for vaginal specimens.
All comparator urine samples were placed in urine transport tube of the manufacturer: this was the only urine sample tested without a urine preservative.
Figure 1.
AC2 UTT is the urine sample in the AC2 transport medium. A, Sensitivity estimate based on rotating standard with 95% CI and (B) specificity estimates with 95% CI.
Figure 2.
AC2 UTT is the urine sample in the AC2 transport medium. A, Sensitivity estimate based on rotating standard with 95% CI and (B) specificity estimates with 95% CI.
Individual Female Specimen Performance
Sensitivity of the GCQ assay versus the PIS for endocervical swabs was 98.5% (64/65) (Table 1). Self-collected vaginal swabs identified 65/65 (100.0%) of all infections defined by the PIS, whereas urine samples detected 63/65 (96.9%) for neat and 64/65 (98.5%) for UPT in infected women. The specificity of the GCQ assay was at least 99.0% for each specimen type with an overall specificity of 99.5% across combined specimen types (Table 1).
For the 65 women identified as infected using the Food and Drug Administration-required PIS to which GCQ was compared, 54 (83.1%) were positive by all tests for every specimen type, whereas the remaining 11 (16.9%) had between 1 and 4 negative results despite being defined as infected (Table S2, Supplemental Digital Content, online only, available at: http://links.lww.com/OLQ/A29). The overall sensitivity for combined female sample types is 98.5% (256/260) and overall specificity 99.5% (3692/3711).
Individual Male Specimen Performance
The sensitivity of the GCQ assay using male urethral swabs versus PIS was 100.0% (112/112) (Table 1). Specificity of the male urethral swab was 99.1% (647/653). Three of the 6 GCQ positive swabs (650/653) with negative PIS were also positive by one of the reference assays or another specimen type (Table S3, Supplemental Digital Content, online only, available at: http://links.lww.com/OLQ/A30). Male neat and UPT urine specimens also both had sensitivity of 100.0% (112/112). The GCQ assay specificity versus PIS for male UPT and unpreserved urine specimens were 99.1% (656/662) and 98.9% (655/662), respectively. Three of the 6 GCQ positive UPT and all 3 GCQ positive neat specimens with negative PIS were positive by another NAAT or another specimen.
Urine Volume
The urine specimen volume requested from participants (men and women) was between 20 and 25 mL, but we processed specimens with volumes of up to 69 mL, which was the limit of the urine collection cup. Based on a previous report of the effect of urine volume on assay sensitivity,16 an analysis was conducted to determine the impact of urine volume on the GCQ assay. Urine volume was not associated with false-negative results for either the neat or UPT urine specimen types: P values of 0.5627 and 0.7182 for neat and UPT urine, respectively (Table S4, Supplemental Digital Content, online only, available at: http://links.lww.com/OLQ/A31).
Inhibitory Samples
There were 21 initial indeterminate urine results (with failed amplification controls) from the PT of which 9 resolved as negative by repeat testing and 12 remained as indeterminate (shown as “I” in Tables S2 and S3, online only). All 21 specimens were negative by GCQ and AC2.
DISCUSSION
The GCQ assay for detection of gonococcal infection performed well for all specimens tested in this large multicenter study with sensitivities which were at least comparable with those of 2 other currently approved assays for gonorrhea detection. Using the PIS as the standard for comparison, in women vaginal, urine, and endocervical specimens tested using the CGQ detected 100%, 98.5%, and 98.5% of gonococcal infections, respectively. For men, the sensitivity of the GCQ was 100% for both urethral swab and urine specimens. In addition, the specificity of the GCQ assay was high, an important attribute in many locations as a result of declining national gonorrhea prevalence. For laboratorians, the Viper testing instrument, with its “walk away” performance, provides the potential to enhance specimen throughput while providing standardized, reliable test results. The instrument can be used with batch sizes of 48 or 96, thus meeting the needs of medium and high throughput laboratories. When used with the Viper instrument, this test provides relatively rapid (approximately 3.5 hours), reliable results for gonorrhea screening for 94 patient samples.14
Assessment of test performance can be challenging. In part because multiple anatomical sites may be infected, it is clear that no single specimen will be uniformly positive for all patients with urogenital gonococcal infection. For this study, we incorporated 2 widely used assays that used molecular targets which were both different from each other and from the target used in the GCQ assay to establish a PIS for evaluation of test performance. Using the PIS to determine what proportion of persons with urogenital infections are detected with any specific specimen type is useful in consideration of what tests and specimen types should be used for screening for gonococcal infections. In addition, in studies in which more than 2 different types of assays are used to evaluate performance of newer tests, another possible benefit of such an approach is the opportunity to compare each of the assays used in a study in terms of their performance relative to one another using a rotating standard previously suggested by Martin et al.15 for comparison of multiple assays. Using this approach to supplement our PIS directed assessment; we found that the GCQ assay performed as well or better than the PT or AC2 assays. In addition, for female samples, which tend to be more complicated to analyze, latent class analysis was performed to obtain an unbiased estimate of sensitivity and specificity (data not shown). These analyses estimated 100% sensitivity (both sample types) and 99.5% to 99.8% specificity, for swab and urines samples from women, respectively.
Several observations made in this study have direct relevance for clinical practice beyond the laboratory performance of the GCQ assay. We found that for at-risk women urogenital symptoms were unrelated to the prevalence of N. gonorrhoeae suggesting that decisions on testing practices should be determined by factors other than symptoms. This finding is consistent with the Centers for Disease Control and Prevention guidelines for STD diagnostics,17 which recognize that NAATs perform equally well for screening and diagnostic purposes. This observation, combined with the ability to collect reliable specimens from patients of either gender without the need for genital examination or potentially uncomfortable swab specimens (i.e., voided urine or, for women, self-collected vaginal swabs), provides opportunities to simplify and therefore facilitate accurate screening for gonococcal infection.
While the ability to use voided urine for gonorrhea screening is an attribute of NAATs for gonorrhea diagnosis, on occasion patients may collect more than the requested volume of urine. Our analyses demonstrate that the GCQ assay performs as well for N. gonorrhoeae detection when specimens exceed the recommended amount, filling the entire collection cup, as when the recommended volume of urine (20 – 60 mL) is provided. Thus, the GCQ assay has the potential to obviate the need to qualify test results derived from patients who provide more than the recommended volume of urine for testing.
In summary, the GCQ assay for detection of N. gonorrhoeae infections in both men and women provides highly reliable diagnosis of symptomatic or asymptomatic infection. The Viper instrument for performing these assays represents a further advantage for this testing system, providing walk away NAAT testing, an appealing attribute for busy laboratories where staff are increasingly asked to look for ways to enhance specimen throughput and turnaround time.
Supplementary Material
Acknowledgments
Supported by BD Diagnostics, Sparks, MD.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2009. Atlanta, GA: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 2.Shafer MA, Moncada J, Boyer CB, et al. Comparing first-void urine specimens, self-collected vaginal swabs, and endocervical specimens to detect Chlamydia trachomatis and Neisseria gonorrhoeae by a nucleic acid amplification test. J Clin Microbiol. 2003;41:4395–4399. doi: 10.1128/JCM.41.9.4395-4399.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsieh YH, Howell MR, Gaydos JC, et al. Preference among female Army recruits for use of self-administered vaginal swabs or urine to screen for Chlamydia trachomatis genital infections. Sex Transm Dis. 2003;30:769–773. doi: 10.1097/01.OLQ.0000079048.11771.46. [DOI] [PubMed] [Google Scholar]
- 4.Schachter J, McCormack WM, Chernesky MA, et al. Vaginal swabs are appropriate specimens for diagnosis of genital tract infection with Chlamydia trachomatis. J Clin Microbiol. 2003;41:3784–3789. doi: 10.1128/JCM.41.8.3784-3789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chernesky MA, Hook EW, Martin DH, et al. Women find it easy and prefer to collect their own swabs to diagnose Chlamydia trachomatis and Neisseria gonorrhoeae infectitons. Sex Transm Dis. 2005;32:729–733. doi: 10.1097/01.olq.0000190057.61633.8d. [DOI] [PubMed] [Google Scholar]
- 6.Gaydos CA, Crotchfelt KA, Shah N, et al. Evaluation of dry and wet transported intravaginal swabs in detection of Chlamydia trachomatis and Neisseria gonorrhoeae infections in female soldiers by PCR. J Clin Microbiol. 2002;40:758–761. doi: 10.1128/JCM.40.3.758-761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rompalo AM, Gaydos CA, Shah N, et al. Evaluation of use of a single intravaginal swab to detect multiple sexually transmitted diseases in active-duty military women. Clin Infect Dis. 2001;33:1455–1461. doi: 10.1086/322588. [DOI] [PubMed] [Google Scholar]
- 8.Knox J, Tabrizi SN, Miller P, et al. Evaluation of self-collected samples in contrast to practitioner-collected samples for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by polymerase chain reaction among women living in remote areas. Sex Transm Dis. 2002;29:647–654. doi: 10.1097/00007435-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Polaneczky M, Quigley C, Pollock L, et al. Use of self-collected vaginal specimens for detection of Chlamydia trachomatis infection. Obstet Gynecol. 1998;91:375–378. doi: 10.1016/s0029-7844(97)00674-1. [DOI] [PubMed] [Google Scholar]
- 10.Chernesky MA, Martin DH, Hook EW, III, et al. Ability of new APTIMA CT and APTIMA GC assays to detect Chlamydia trachomatis and Neisseria gonorrhoeae in male urine and urethral swabs. J Clin Microbiol. 2005;43:127–131. doi: 10.1128/JCM.43.1.127-131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaydos CA, Quinn TC, Willis D, et al. Performance of the APTIMA Combo 2 assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in female urine and endocervical swab specimens. J Clin Microbiol. 2003;41:304–309. doi: 10.1128/JCM.41.1.304-309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Der Pol B, Ferrero DV, Buck-Barrington K, et al. Multicenter evaluation of the BD ProbeTec ET system for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens, female endocervical swabs, and male urethral swabs. J Clin Microbiol. 2001;39:1008–1016. doi: 10.1128/JCM.39.3.1008-1016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor SN, Van Der Pol B, Lillis R, et al. Clinical Evaluation of the BD ProbeTec™ Chlamydia trachomatis Qx Amplified DNA Assay on the BD Viper™ System With XTR™ Technology. Sex Transm Dis. 2011;38:603–609. doi: 10.1097/OLQ.0b013e31820a94d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felder RA, Foster M, Lizzi M, et al. Process evaluation of a fully automated molecular diagnostics system. JALA. 2009;14:262–268. [Google Scholar]
- 15.Moncada J, Chow JM, Schachter J. Volume effect on sensitivity of nucleic acid amplification tests for detection of Chlamydia trachomatis in urine specimens from females. J Clin Microbiol. 2003;41:4842–4843. doi: 10.1128/JCM.41.10.4842-4843.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin DH, Nsuami M, Schachter J, et al. Use of multiple nucleic acid amplification tests to define the infected-patient “gold standard” in clinical trials of new diagnostic test for Chlamydia trachomatis infections. J Clin Microbiol. 2004;42:4749–4758. doi: 10.1128/JCM.42.10.4749-4758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Laboratory diagnostic testing for Chlamydia trachomatis and Neisseria gonorrhoeae. [cited 19 July 2010];Am Public Health Laboratories. 2009 updated 2009; Available from: http://www.aphl.org/aphlprograms/infectious/std/documents/ctgclabguidelinesmeetingreport.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.