Abstract
Non-melanoma skin cancers (NMSCs) one of the most common neoplasms causes serious morbidity and mortality. Therefore, identification of non-toxic phytochemicals for prevention/treatment of NMSCs is highly desirable. Fisetin (3,3′,4′,7-tetrahydroxyflavone), a dietary flavonoid, present in fruits and vegetables possesses anti-oxidant and anti-proliferative properties. The aim of this study was to investigate the chemotherapeutic potential of fisetin in cultured human epidermoid carcinoma A431 cells. Treatment of A431 cells with fistein (5-80 μM) resulted in a significant decrease in cell viability in a dose- and time-dependent manner. Employing clonogenic assay, we found that fisetin treatment significantly reduced colony formation in A431 cells. Fisetin treatment of A431 cells resulted in G2/M arrest and induction of apoptosis. Furthermore, treatment of A431 cells with fisetin resulted in (i) decreased expression of anti-apoptotic proteins (Bcl2, Bcl-xL and Mcl-1), (ii) increased expression of pro-apoptotic proteins (Bax, Bak and Bad), (iii) disruption of mitochondrial potential, (iv) release of cytchrome c and Smac/DIABLO from mitochondria, (v) activation of caspases, and (vi) cleavage of PARP protein. Pretreatment of A431 cells with the pan-caspase inhibitor (Z-VAD-FMK) blocked fisetin-induced cleavage of caspases and PARP. Taken together, these data provide evidence that fisetin possesses chemotherapeutic potential against human epidermoid carcinoma A431 cells. Overall, these results suggest that fisetin could be developed as a novel therapeutic agent for the management of NMSCs.
Keywords: Skin cancer, Fisetin, Apoptosis, Bcl2 family proteins, Cytochrome c, Cell cycle, Smac/DIABLO, Caspases
Introduction
The non-melanoma skin cancers (NMSCs), comprising of the basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs), are the most frequently diagnosed cutaneous malignancies. Studies have implicated that solar ultraviolet (UV) radiation is the major etiological factor in the development of cutaneous malignancy, including the NMSCs (1-3). The risk of skin malignancies is increasing with continuous increases in life expectancy, changing dietary habits, lifestyle and particularly environment conditions. Induction of apoptosis is considered as one of the possible mechanisms of inhibition of cancer development, and many clinically chemopreventive/chemotherapeutic agents primarily act by inducing apoptosis or blockade of the carcinogenic process (4-7). There has been a considerable interest in the development of apoptosis inducing agents for treatment of skin disorders, including NMSCs. Therefore, use of non-toxic dietary phytochemicals as chemopreventive and/or chemotherapeutic agents due to their putative ability to suppress cancers has drawn a great attention (8-10).
Fisetin, 3,7,3′,4′-tetrahydroxyflavone, (Fig. 1a) is a naturally occurring flavonoid commonly found in various vegetables and fruits such as onion, cucumber, apple, persimmon and strawberry (11). Fisetin appear to have no toxicity in mice and this is a notable advantage over the selective drugs that are used for treatment of cancers because of toxicity concerns (12,13). Studies on the absorption and metabolism of fisetin are reported in experimental animals. These studies showed that fisetin was readily absorbed and its level was detected in the serum of mice (14,15). Fisetin is known to exhibit a wide variety of biological functions including anti-oxidant, anti-inflammatory and anti-proliferative properties (13, 16-19). Fisetin induces apoptosis in colon cancer cells by inhibiting COX-2 and Wnt/EGFR/NF-κB signaling pathways (20). It also induces apoptosis in human cervical cancer cells through activation of ERK1/2 signaling pathways (21). In addition, fisetin showed growth inhibitory potential against lung cancer cells by inhibiting PI3K/Akt and mTOR signaling (19). Recently, we have shown that fisetin inhibited human melanoma cell growth by suppression of Wnt/β-catenin signaling and decreased Mitf levels (13). Therefore, in an effort to develop an effective chemotherapeutic agent for NMSCs, we attempted to examine the effects of fisetin on human epidermoid carcinoma cells. Here, we show that fisetin inhibits growth, induces G2/M arrest and apoptosis of human epidermoid carcinoma A431 cells by disruption of mitochondrial membrane potential and activation of caspases. Our study also provides insight into the mechanism by which fisetin induces apoptosis in these cells.
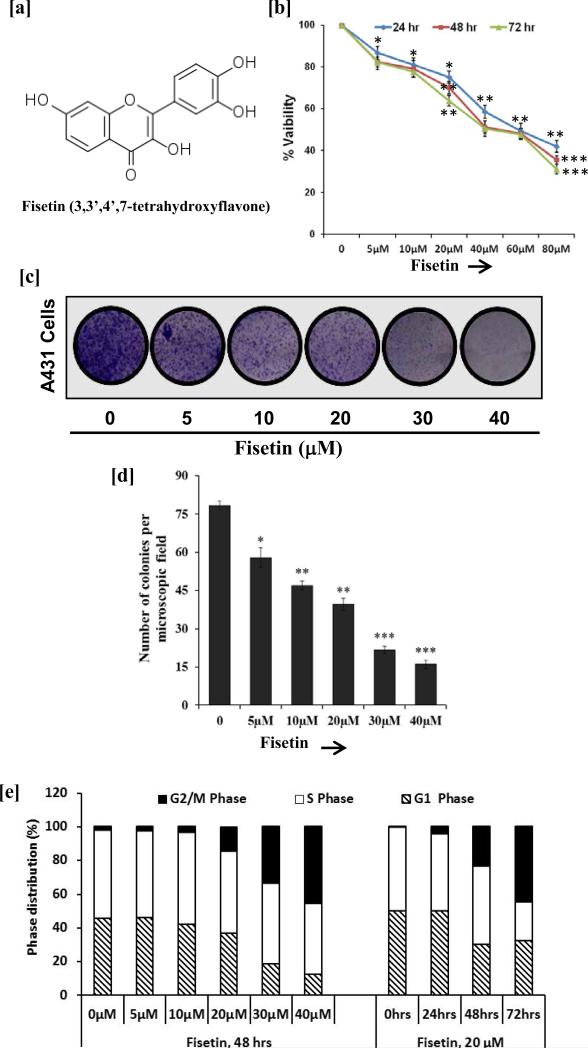
Fig.1. Effect of fisetin treatment on growth, colony formation and cell cycle distribution in A431 cells.
[a] Structure of fisetin. [b] Cell growth, A431 cells were treated with fisetin (5-80 μM) for 24, 48 and 72 h, and their viability was determined by MTT assay. Data shown are mean ± SEM of three separate experiments in which each treatment was repeated in 10 wells. *P<0.05; **P<0.01; ***P < 0.001 vs. control. [c & d] Clonogenic assay, A431 cells were incubated with fisetin (5-40 μM) for 12 h. Following fisetin treatment, cells were plated in 6 well culture plate at a density of 500 cells/well in medium containing 10% FBS. The data shown here are from a representative experiment repeated three times with similar results. *P<0.05; **P<0.01; ***P < 0.001 vs. control. [e] Cell cycle phase distribution, A431 cells were treated with 5-40 μM fisetin for 48 h (dose-dependent study) and 20 μM of fisetin for 24, 48 and 72 h (time-dependent study). Cellular DNA was stained with PI and was analyzed by flow cytometry. The percentage of cells in the G1, S, and G2/M phases were calculated using ModFit LT software. Data shown here are mean percentage of three independent experiments.
Materials and Methods
Materials
Fisetin, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide (MTT), propidium iodide (PI) and rhodamine 123 were purchased from Sigma Chemical (St Louis, MO). The antibodies for caspases (caspsase-3, -7 and -9), PARP, Bak, Bax, Bad, Bcl-2, BclxL, Mcl-1 and COX IV were obtained from Cell Signaling Technology (Danvers, MA). Cytochrome c and Smac/DIABLO antibodies were obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Annexin V/Dead cell apoptosis detection kit was obtained from Life Technologies (Grand Island, NY). Anti-mouse or anti-rabbit secondary antibody horseradish peroxidase conjugate was obtained from Millipore (Temecula, CA). Protein assay kit was obtained from Bio-Rad (Hercules, CA). HyBlot CL and autoradiography films were obtained from Denville Scientific Inc (Metuchen, NJ).
Cell culture and treatment
Human epidermoid carcinoma A431 cells and human squamous carcinoma SCC-13 cells were purchased from ATCC (Manassas, VA) and were cultured and maintained in DMEM supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. Normal human epidermal keratinocytes (NHEK) were obtained from Life Technologies and were maintained in keratinocyte-SFM supplemented medium. Fisetin [dissolved in dimethyl sulfoxide (DMSO)] was used for the treatment of NHEK, A431 and SCC13 cells. The final concentration of DMSO used was 0.1% (v/v) for each treatment. Control cells were treated with equivalent volume of vehicle alone. The cells were maintained under standard cell culture conditions at 37°C and 5% CO2 in a humid environment.
Cell viability
The effect of fisetin on the viability of cells was determined by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide (MTT) assay. NHEK, A431 and SCC13 cells were plated at 1 × 104 cells per well in 200 μl of complete culture medium containing 5–80 μM concentrations of fisetin in 96-well microtiter plates for 24, 48 and 72 h. After incubation for specified times at 37°C in a humidified incubator, 20 μl MTT solution (5 mg/ml in phosphate-buffered saline) was added to each well and incubated for 4 h, after which the plate was centrifuged at 2000 rpm for 10 min at 4°C. The supernatant was discarded and formazan crystals were dissolved in 200 μl of DMSO and absorbance at the wavelength of 540 nm was recorded on a microplate reader. The effect of fisetin on growth inhibition was assessed as percent cell viability where DMSO-treated cells were taken as 100% viable. DMSO at the concentrations used has no effect on cell viability.
Clonogenic assay
For clonogenic assay, A431 cells were incubated with fisetin (5-40 μM) for 12 h. Following fisetin treatment, cells were plated in 6 well culture plate at a density of 500 cells/well in medium containing 10% FBS, and then kept in a humidified incubator at 37 °C and 5% CO2 for 2 weeks. Media was changed every fourth day. Colonies were fixed and stained with 0.05% crystal violet in 10% ethanol and counted.
DNA cell cycle analysis
A431 cells were treated with fisetin (5-40 μM) for 48 h, and with 20 μM fisetin for 24, 48 and 72 h. At different time-point after treatment cells were harvested and fixed in chilled 70% alcohol overnight, washed twice with PBS, digested with DNase free RNase (10 μg/ml) at 37°C for 1 h and stained with PI (5 μg/ml) for 3 h at 4°C in the dark. Cells were analyzed by FACS Calibur (Becton Dickinson) for cell cycle phase distribution.
Quantification of apoptosis
Apoptotic cells were determined by an Annexin V/Dead cell apoptosis detection kit obtained from Life Technologies. A431 cells were treated with fisetin (5-40 μM) for 48 h, and with 20 μM fisetin for 24, 48 and 72 h. Thereafter, A431 cells (5 × 105 cells/ml) were harvested by centrifugation at 1200 g for 5 min, washed twice with ice-cold PBS, pelleted and resuspended in 400 μl of 1 X Annexin V binding buffer, 4 μl of Annexin V-Alexa Fluor conjugate and 1 μl of PI buffer. The cells were then incubated at room temperature for 15 min in the dark and analyzed by Accuri C6 flow cytometry (Ann Arbor, MI).
Measurement of mitochondrial membrane potential
The change in mitochondrial transmembrane potential (ΔΨmt) as a result of mitochondrial perturbation induced by fisetin treatment was measured after staining with rhodamine-123. A431 cells were treated with fisetin (5-40 μM) for 48 h, and with 20 μM fisetin for 24, 48 and 72 h. Rhodamine-123 (5 μg/ml; stock, 1 mg/ml PBS) was added 1 h before termination of the experiment. Cells were harvested, washed in PBS and centrifuged at 1200 g for 5 min and suspended in PBS. Immediately before analysis, PI (5 μg/ml; stock 1 mg/ml PBS) was added to the samples. The intensity of fluorescence from 10,000 events was analyzed in FL-1 channel by Accuri C6 flow cytometry.
Preparation of cell lysates, mitochondrial fractions and Western blot analysis
After treatment of cells with fisetin, the media was aspirated and the cells were washed with PBS (10 mmol/l, pH 7.45). The cells were then incubated in ice cold lysis buffer (10 mM HEPES (pH 7.9), 100 mM KCl, 10 mM EDTA, 20 mM EGTA, 100 mM DTT, 20 mM PMSF, 0.5% NP-40 with freshly added protease inhibitors leupeptin, aprotinin and benzamidine over ice for 20 min. The cells were scraped and the lysate was collected in a microfuge tube and passed through a 21.5-G needle to break up the cell aggregates. The lysate was cleared by centrifugation at 14,000 g for 10 min at 4°C, and the supernatant (total cell lysate) collected, aliquoted and was used on the day of preparation or immediately stored at –80°C for use at a later time. Mitochondrial lysates were prepared by isolating mitochondria from cells treated with fisetin through differential and density gradient centrifugation, and were lysed with lysis buffer. After centrifugation at 14,000 g for 10 min at 4°C, supernatant was collected as mitochondrial lysate and stored at –80°C. For Western blotting, 25–50 μg protein was resolved over 12% Tris-glycine gels and transferred onto a PVDF membrane. The nonspecific sites on blots were blocked by incubating in blocking buffer (5% nonfat dry milk/0.1% Tween 20 in Tris-Buffered Saline (TBS), pH 7.6) for 1 h at room temperature, incubated with appropriate monoclonal primary antibody in blocking buffer for overnight at 4°C, followed by incubation with anti-mouse or anti-rabbit secondary antibody horse-radish peroxidase conjugate and detected by chemiluminescence and autoradiography.
Statistical analysis
The results are expressed as the mean ± SE. Statistical analysis of all the data was performed by Student's t-test. The P value <0.05 was considered statistically significant.
Results
Fisetin inhibits growth and colony formation of A431 cells
We first studied the inhibitory effect of fisetin on growth of A431 cells by employing MTT assay. Cells were treated with different doses of fisetin (5-80 μM) for different time points (24-72 h). Results of MTT assay showed that fisetin was effective in inhibiting the growth of A431 cells in a dose- and time-dependent manner which ranged from 13-58% (P < 0.05-0.01) after 24 h, 18-65% (P < 0.05-0.001) after 48 h, and 18-69% (P <0.05-0.001) after 72 h of treatment (Fig. 1b). The IC50 was estimated to be 58, 50 and 41 μM at 24, 48 and 72 h respectively (Fig 1b). In addition, treatment of SCC13 with fisetin resulted in a significant cell growth inhibition in a dose-and time dependent manner (data not shown). Furthermore, we examined the effect of fisetin on the growth of NHEK under identical conditions. We found that fisetin had minimal effect on the NHEK at these doses (data not shown). Because the efficacy of therapeutic agents depends on its long term effect on cancer cells, we therefore, examined the effect of fisetin on colony formation of A431 cells by employing clonogenic assay. Fisetin treatment significantly decreased the number of colonies in a dose-dependent manner when compared to untreated cells (Fig. 1c & d).
Fisetin induces G2/M arrest in A431 cells
Since fisetin treatment inhibited A431 cells growth and colony formation abilities, therefore we next examined whether growth inhibitory effect of fisetin was mediated through cell cycle arrest. For this purpose, the effect of fisetin on cell cycle progression in A431 cells was determined by flow cytometer. Treatment of A431 cells with fisetin resulted in a significant accumulation of cells in G2/M phase and a reduction in G1 phase of the cell cycle (Fig. 1e, Fig. S1). A significant reduction in S phase cell population was observed at 40 μM dose and when the cells were treated for 72 h with 20 μM fisetin.
Fisetin induces apoptosis of A431 cells
To determine whether blockage of cells in G2/M phase by fisetin induces apoptosis of A431 cells flow cytometric analysis using annexin V/PI was performed. Fisetin (5-40 μM; 48 h) treatment increased the percentage of early apoptotic cell population as shown by increase in annexin V positive cells (8.2-19.2%) in dose-dependent manner when compared with 4.5% early apoptotic cell population of untreated control cells (Fig. S2a). Likewise, an increase in proportion of early apoptotic cells population was 11.0, 14.7 and 19.2% in cells exposed to 20 μM of fisetin for 24, 48 and 72 h respectively as compared to 5.5% of untreated A431 cells (Fig. S2b). Since early apoptotic events or cell death is followed by late apoptotic events, we observed an increase in late apoptotic cells positively stained with both Annexin V Alexa Fluor and PI in fisetin treated A431cells in a dose- and time-dependent manner. Increase in PI stained post-apoptotic cell death was also observed in dose- and time-dependent manner (Fig. S2). Results of Annexin V/PI staining clearly demonstrated that fisetin induces cell death via inducing apoptosis in A431 cells.
Fisetin modulates Bcl2 family proteins in A431 cells
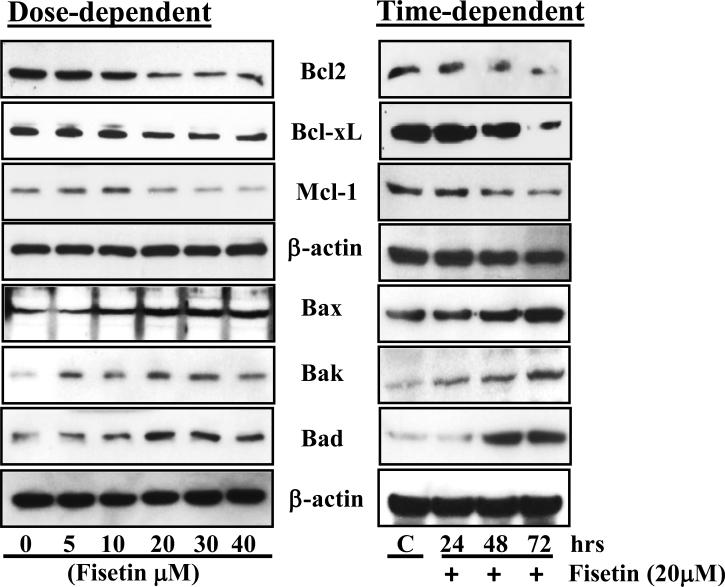
Induction of apoptosis is highly regulated process that involves activation of a series of molecular events governed by Bcl2 family of proteins. Proteins form Bcl2 family can be either pro-apoptotic or anti-apoptotic. Therefore, we determined the effect of fisetin on Bcl2 family proteins. Western blot analysis data demonstrated that fisetin treatment of A431 cells resulted in an increased expression of pro-apoptotic proteins (such as Bax, Bak and Bad), and a decreased expression of anti-apoptotic proteins (such as Bcl2, Bcl-xL and Mcl-1) in a dose- and time-dependent manner (Fig. 2)
Fig. 2. Effect of fisetin treatment on modulation of Bcl2 family proteins in A431 cells.
A431 cells were treated with 5-40 μM fisetin for 48 h (dose-dependent study) and 20 μM of fisetin for 24, 48 and 72 h (time-dependent study) to determine its effect on Bcl2 family proteins. Cells were harvested and cell lysates were prepared and protein was subjected to SDS-PAGE followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are from a representative experiment repeated three times with similar results.
Fisetin disrupts mitochondrial membrane potential and causes release of cytochrome c and Smac/DIABLO from mitochondria
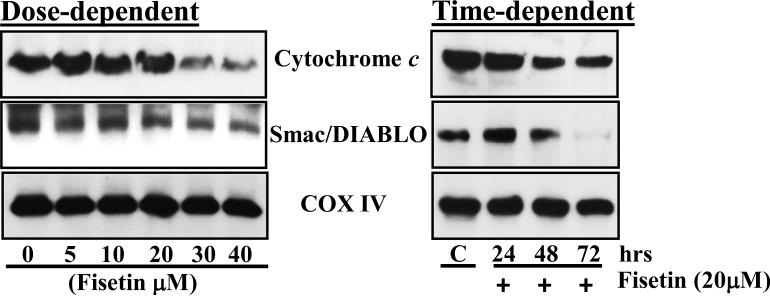
Mitochondria play an essential role in activation and progression of apoptosis. To determine whether fisetin treatment disrupts mitochondrial membrane potential, A431 cells were treated with different doses of fisetin for different time points and stained with rhodamine 123 and analyzed for change in its fluoresence intensity using flow cytometry. A dose-dependent decrease in rhodamine 123 flurescence intensity revealed that 8.8, 29.8, 39.0, 74.2 and 78.6% of A431 cell populations exhibited loss of mitochondrial membrane potential after 48 h exposure with 5, 10, 20, 30 and 40μM fisetin respectively as compared to 1.5% of the cell population in untreated A431 cells (Fig. S3a). Furthermore, a decrease in rhodamine 123 flurescence intensities revealed that 23.6, 32.1% and 41.2% cell populations exhibited loss of mitochondrial membrane potential when A431 cells were treated with 20μM fisetin for 24, 48 and 72 h respectively (Fig. S3b). Decrease in mitochondrial membrane potential also resulted in dose- and time-dependent release of cytochrome c and Smac/DIABLO from mitochondria (Fig. 3).
Fig. 3. Effect of fisetin treatment on release of cytochrome c and Smac/DIABLO from mitochondria in A431 cells.
After treatment with fisetin, A431 cells were harvested, mitochondrial fraction was prepared and expression of cytochrome c and Smac/DIABLO was determined. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for COX IV (mitochondrial fraction). The immunoblots shown here are from a representative experiment repeated three times with similar results.
Fisetin induces cleavage of caspases and PARP in A431 cells
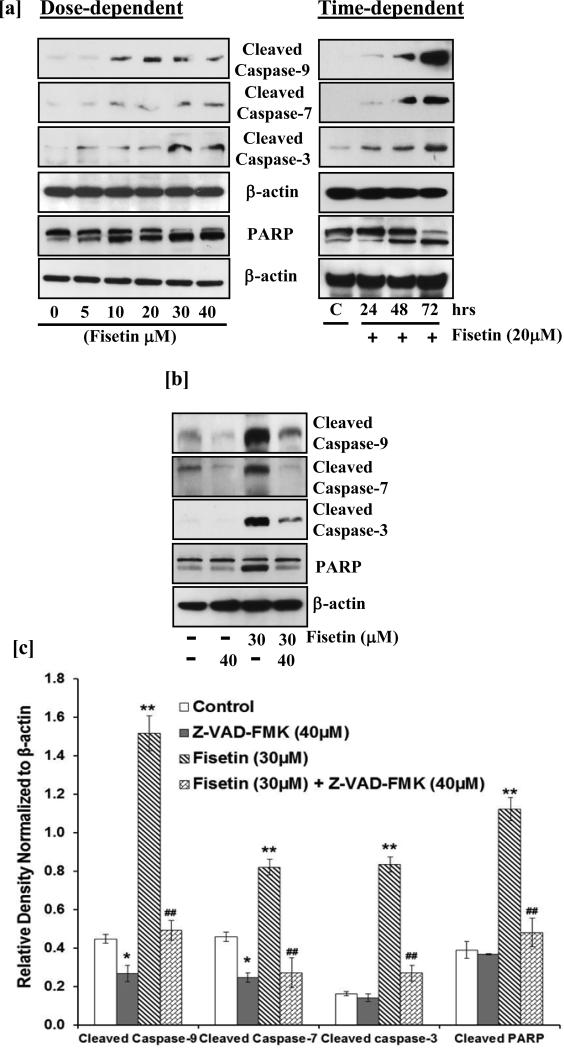
During apoptosis, release of cytochrome c and Smac/DIABLO induces proteolytic cleavage of procaspases to generate active caspases. Next, we examined whether fisetin treatment induced activation of caspases or cleavage of PARP, both of which are hallmarks of apoptosis. Treatment of A431 cells with fisetin resulted in the cleavage of caspase-9, -7 and -3 in a dose- and time-dependent manner as demonstrated by western blot analysis (Fig. 4a). In addition, as shown in Fig. 4a, fisetin treatment of cells increased the cleavage of PARP (116 kDa) into 85 kDa fragment.
Fig.4. Effect of fisetin treatment on cleavage of caspases and PARP in A431 cells.
[a] After treatment with fisetin, A431 cells were harvested, cell lysates were prepared and the expression of cleaved caspases and PARP was determined. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are from a representative experiment repeated three times with similar results. [b & c] A431 cells were treated with Z-VAD-FMK (40 μM; 2 h) followed by incubation with fisetin (30 μM; 48 h). The cells were harvested and cell lysates were prepared followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The relative density of the bands was normalized to β-actin. Data shown are from representative experiments repeated thrice with similar results. *p<0.05, **p<0.01 vs control; ##p<0.01 vs fisetin.
Inhibition of fisetin-mediated activation of caspases by pan-caspase inhibitor (Z-VAD-FMK) in A431 cells
Activation of caspases is considered as a downstream event during apoptosis and blocking caspases activity has been shown to eliminate almost all programmed cell death. To confirm that the apoptosis induced by fisetin treatment is caspase-dependent, activation of caspases was blocked in A431 cells by Z-VAD-FMK, a pan caspase inhibitor, prior to fisetin treatment. Western blot analysis data and relative density analysis revealed that Z-VAD-FMK blocked fisetin-induced cleavage of caspase-9, -7 and -3 and PARP (Fig. 4b & c) confirming that the mechanism of fisetin-mediated cell death involves caspases as apoptosis executioners.
Discussion
In the United States more than 1 million new cases of NMSCs are diagnosed annually, which is approximately equivalent to the incidence of malignancies in all organs combined (22). Therefore, there is a need to better understand the consequence of this disease and to prevent and/or treat by mechanism based approaches. Phytochemicals have been shown to have anti-carcinogenic properties due to their anti-proliferative, anti-inflammatory and anti-oxidant properties (1-3,7,8,10). In the present investigation fisetin was explored for its chemotherapeutic potential against human epidermoid carcinoma. Treatment of A431 cells with fisetin resulted in cell growth inhibition without affecting the growth of NHEK under identical conditions. The growth inhibitory potential of fisetin also restricted the colony formation abilities of A431 cells (Fig. 1). These data suggest the chemotherapeutic potential of fisetin against human epidermoid carcinoma cells.
It is well known that uncontrolled cellular growth, which may be a result of defects in cell cycle and apoptotic machinery, is responsible for the development and growth of most of the cancers, including NMSCs (23,24). Apoptosis is regarded as an ideal way of elimination of cells with damaged DNA and is distinct from necrotic cell death (25). Therefore, inhibition of unchecked cell cycle regulation and induction of apoptosis could be a potential strategy for the management of NMSCs. In the present study, flow cytometric analysis revealed that fisetin inhibited cell growth of A431 by inducing cell cycle arrest at G2/M phase and induction of apoptosis (Fig. 1; Fig. S1 & S2). G2/M phase arrest provides an opportunity for cells to either undergo orderly and timely repair of DNA damage and prevents inappropriate mitotic entry or undergo apoptosis. The G2/M phase arrest is one of the most prominent checkpoints of many anticancer agents in cancer cells. Studies have shown that members of Bcl2 family are critical regulators of the apoptotic pathway (26). The proteins of Bcl2 family are either anti-apoptotic or pro-apoptotic (26,27). Studies have demonstrated that the photo-activated Hedyotis corymbosa extracts-induced apoptosis in skin cancer cells that is accompanied by nuclear condensation and changes in apoptosis-related proteins (28). Apigenin induces apoptosis of head and neck squamous cell carcinoma via tumor necrosis factor receptor and Bcl2-mediated pathway (29). We found that treatment of A431 cells with fisetin resulted in increased expression of pro-apoptotic proteins and decreased expression of anti-apoptotic proteins (Fig. 2). The changes in these proteins expression of the Bcl2 family may be responsible for apoptosis.
Mitochondria play a decisive role in the intrinsic apoptotic pathway specifically by releasing different pro-apoptotic proteins, such as cytochrome c and Smac/DIABLO from intermembrane space to cytosol during apoptosis (30-32). The release of these proteins is a consequence of a process called mitochondrial outer membrane permeabilization in which the integrity of the mitochondrial outer membrane is being compromised. It is well established that Bax and Bak proteins, members of pro-apoptotic Bcl2 family are crucial for inducing permeabilization of the outer mitochondrial membrane and thus subsequent release of cytochrome c, Smac/DIABLO and other apoptogenic molecules (31,33). Quercetin treatment of cervical cancer cells resulted in deplorization of mitochondrial membrane potential by increase production of ROS to promote apoptosis (34). A dose- and time-dependent increase in cell populations with low mitochondrial membrane potential, exhibiting loss of mitochondrial integrity was observed in fisetin treated A431 cells indicating that apoptosis induced by fisetin treatment involves mitochondrial disruption (Fig. S3). The shift in mitochondrial membrane potential induced by fisetin treatment was evident by decrease in mitochondrial cytochrome c and Smac/DIABLO levels (Fig. 3). Permeabilization of outer mitochondrial membrane and release of apoptogenic molecules from intermembrane space to cytosol results in activation of downstream caspases (31,35). Caspases act either as initiator or executioner within the apoptotic process. Executioner caspases are activated from their pro-enzymatic form by the action of other caspases within a cascade reaction. Erythroid differentiation regulator 1, a proapoptotic factor, overexpression in human keratinocytes increased UVB-induced apoptosis and also induced activation of caspase 3 (36). The increased expression of cleaved caspase-9, -7 and -3 suggests that fisetin induced apoptosis in A431 cells is mediated through activation of these proteases (Fig. 4). Since activated caspases utilize PARP as their substrate leading to its cleavage, we found that fisetin treatment of A431 cells resulted in cleavage of PARP. Furthmore, fisetin-mediated activation of caspases and PARP cleavage was blocked by a pan caspase inhibitor in A431 cells (Fig.4).
In summary our data suggest that treatment of A431 cells with fisetin inhibited growth and induced apoptosis. These effects of fisetin are due to G2/M arrest, modulation in Bcl2 family proteins expression, loss of mitochondrial membrane potential, activation of caspases and cleavage of PARP protein. However, other pathways may also have a role that requires in-depth investigation. Based on these observations, it is plausible to design fisetin-containing emollient or a patch for the prevention and treatment of NMSCs.
Supplementary Material
Figure S1: Effect of fisetin treatment on cell cycle phase distribution in A431 cells. Cell cycle phase distribution, A431 cells were treated with 5-40 μM fisetin for 48 h for dose-dependent effect and 20 μM of fisetin for 24, 48 and 72 h for time-dependent effect. Cellular DNA was stained with PI and was analyzed by flow cytometry. The percentage of cells in the G1, S, and G2/M phases were calculated using ModFit LT software. Data shown here are mean percentage of three independent experiments.
Figure S2 : Effect of fisetin treatment on apoptosis in A431 cells. A431 cells were treated with 5-40 μM fisetin for 48 h (dose-dependent study) and 20 μM of fisetin for 24, 48 and 72 h (time-dependent study) to determine its effect on apoptosis. At indicated time, cells were harvested for apoptosis detection by using Annexin V Alexa Fluor® 488 and PI. The data shown here are from a representative experiment repeated three times with similar results.
Figure S3: Effect of fisetin treatment on disruption of mitochondrial membrane potential in A431 cells. A431 cells were treated with 5-40 μM fisetin for 48 h (dosedependent study) and 20 μM of fisetin for 24, 48 and 72 h (time-dependent study) to determine its effect on mitochondrial membrane potential. Cells were stained with rhodamine 123 and analyzed by flow cytometry. The data shown here are from a representative experiment repeated three times with similar results.
Acknowledgement
This work was supported by NIH Grant AT004966 and UAB Skin Disease Research Center Pilot and Feasibility Grant (P30AR50948). HCP and SS performed the research and analyzed the data, CAE and MA provided intellectual input and analyzed the data, FA designed experiments, analyzed the data, coordinated experiments, supervised the project and wrote the manuscript.
Abbreviation
- NMSCs
Non-melanoma skin cancers
- BCCs
Basal cell carcinomas
- SCCs
Squamous cell carcinomas
- UV
Ultraviolet
- COX-2
Cyclooxygenase-2
- EGFR
Epidermal growth factor receptor
- PI3K
Phosphatidylinositol 3-kinase
- PARP
Poly(ADP-ribose) polymerase
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide
Footnotes
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1.Afaq F. Natural agents: cellular and molecular mechanisms of photoprotection. Arch Biochem Biophys. 2011;508:144–151. doi: 10.1016/j.abb.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signaling. Nat Rev Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 3.Adhami VM, Syed DN, Khan N, et al. Phytochemicals for prevention of solar ultraviolet radiation-induced damages. Photochem Photobiol. 2008;84:489–500. doi: 10.1111/j.1751-1097.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim EH, Deng CX, Sporn MB, et al. CDDO-imidazolide induces DNA damage, G2/M arrest and apoptosis in BRCA1-mutated breast cancer cells. Cancer Prev Res (Phila) 2011;4:425–434. doi: 10.1158/1940-6207.CAPR-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurundkar D, Srivastava RK, Chaudhary SC, et al. Vorinostat, an HDAC inhibitor attenuates epidermoid squamous cell carcinoma growth by dampening mTOR signaling pathway in a human xenograft murine model. Toxicol Appl Pharmacol. 2013;266:233–244. doi: 10.1016/j.taap.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang X, Zhu Y, Han L, et al. CP-31398 restores mutant p53 tumor suppressor function and inhibits UVB-induced skin carcinogenesis in mice. J Clin Invest. 2007;117:3753–3764. doi: 10.1172/JCI32481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bickers DR, Athar M. Novel approaches to chemoprevention of skin cancer. J Dermatol. 2000;27:691–695. doi: 10.1111/j.1346-8138.2000.tb02259.x. [DOI] [PubMed] [Google Scholar]
- 8.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 9.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 11.Arai Y, Watanabe S, Kimira M, et al. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000;130:2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 12.Touil YS, Seguin J, Scherman D, et al. Improved antiangiogenic and antitumour activity of the combination of the natural flavonoid fisetin and cyclophosphamide in Lewis lung carcinoma-bearing mice. Cancer Chemother Pharmacol. 2011;68:445–455. doi: 10.1007/s00280-010-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Syed DN, Afaq F, Maddodi N, et al. Inhibition of human melanoma cell growth by the dietary flavonoid fisetin is associated with disruption of Wnt/β-catenin signaling and decreased Mitf levels. J Invest Dermatol. 2011;131:1291–1299. doi: 10.1038/jid.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shia CS, Tsai SY, Kuo SC, et al. Metabolism and pharmacokinetics of 3,3′,4′,7-tetrahydroxyflavone (fisetin), 5-hydroxyflavone, and 7-hydroxyflavone and antihemolysis effects of fisetin and its serum metabolites. J Agric Food Chem. 2009;57:83–89. doi: 10.1021/jf802378q. [DOI] [PubMed] [Google Scholar]
- 15.Touil YS, Auzeil N, Boulinguez F, et al. Fisetin disposition and metabolism in mice: Identification of geraldol as an active metabolite. Biochem Pharmacol. 2011;82:1731–1739. doi: 10.1016/j.bcp.2011.07.097. [DOI] [PubMed] [Google Scholar]
- 16.Hanneken FF, Lin JJ, Maher P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Invest Ophthalmol Vis Sci. 2006;47:3164–3177. doi: 10.1167/iovs.04-1369. [DOI] [PubMed] [Google Scholar]
- 17.Higa S, Hirano T, Kotani M, et al. Fisetin, a flavonol, inhibits TH2-type cytokine production by activated human basophils. J Allergy Clin Immunol. 2003;111:1299–1306. doi: 10.1067/mai.2003.1456. [DOI] [PubMed] [Google Scholar]
- 18.Haddad AQ, Venkateswaran V, Viswanathan L, et al. Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2006;9:68–76. doi: 10.1038/sj.pcan.4500845. [DOI] [PubMed] [Google Scholar]
- 19.Khan N, Afaq F, Khusro FH, et al. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int J Cancer. 2012;130:1695–1705. doi: 10.1002/ijc.26178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suh Y, Afaq F, Johnson JJ, et al. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis. 2009;30:300–307. doi: 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ying TH, Yang SF, Tsai SJ, et al. Fisetin induces apoptosis in human cervical cancer HeLa cells through ERK1/2-mediated activation of caspase-8-/-caspase-3-dependent pathway. Arch Toxicol. 2012;86:263–273. doi: 10.1007/s00204-011-0754-6. [DOI] [PubMed] [Google Scholar]
- 22.Housman TS, Feldman SR, Williford PM, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003;48:425–429. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 23.Vazquez A, Bond EE, Levine AJ, et al. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov. 2008;7:979–987. doi: 10.1038/nrd2656. [DOI] [PubMed] [Google Scholar]
- 24.Afaq F, Adhami VM, Mukhtar H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat Res. 2005;571:153–173. doi: 10.1016/j.mrfmmm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Nickoloff, Qin JZ, Chaturvedi V, et al. Denning, Life and death signaling pathways contributing to skin cancer. J Investig Dermatol Symp Proc. 2002;7:27–35. doi: 10.1046/j.1523-1747.2002.19633.x. [DOI] [PubMed] [Google Scholar]
- 26.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 27.Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 28.You BJ, Wu YC, Wu CY, et al. Proteomics displays cytoskeletal proteins and chaperones involvement in Hedyotis corymbosa-induced photokilling in skin cancer cells. Exp Dermatol. 2011;20:653–658. doi: 10.1111/j.1600-0625.2011.01290.x. [DOI] [PubMed] [Google Scholar]
- 29.Chan LP, Chou TH, Ding HY, et al. Apigenin induces apoptosis via tumor necrosis factor receptor- and Bcl-2-mediated pathway and enhances susceptibility of head and neck squamous cell carcinoma to 5-fluorouracil and cisplatin. Biochim Biophys Acta. 2012;1820:1081–1091. doi: 10.1016/j.bbagen.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Youle RJ. The role of Mitochondria in Apoptosis. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ow YP, Green DR, Hao Z, et al. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol. 2008;9:532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- 32.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 33.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 34.Bishayee K, Ghosh S, Mukherjee A, et al. Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: signal cascade and drug-DNA interaction. Cell Prolif. 2013;46:153–163. doi: 10.1111/cpr.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinou JC, Youle RJ. Mitochondria in Apoptosis: Bcl-2 Family Members and Mitochondrial Dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HJ, Song SB, Yang Y, et al. Erythroid differentiation regulator 1 (Erdr1) is a proapototic factor in human keratinocytes. Exp Dermatol. 2011;20:920–925. doi: 10.1111/j.1600-0625.2011.01354.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Effect of fisetin treatment on cell cycle phase distribution in A431 cells. Cell cycle phase distribution, A431 cells were treated with 5-40 μM fisetin for 48 h for dose-dependent effect and 20 μM of fisetin for 24, 48 and 72 h for time-dependent effect. Cellular DNA was stained with PI and was analyzed by flow cytometry. The percentage of cells in the G1, S, and G2/M phases were calculated using ModFit LT software. Data shown here are mean percentage of three independent experiments.
Figure S2 : Effect of fisetin treatment on apoptosis in A431 cells. A431 cells were treated with 5-40 μM fisetin for 48 h (dose-dependent study) and 20 μM of fisetin for 24, 48 and 72 h (time-dependent study) to determine its effect on apoptosis. At indicated time, cells were harvested for apoptosis detection by using Annexin V Alexa Fluor® 488 and PI. The data shown here are from a representative experiment repeated three times with similar results.
Figure S3: Effect of fisetin treatment on disruption of mitochondrial membrane potential in A431 cells. A431 cells were treated with 5-40 μM fisetin for 48 h (dosedependent study) and 20 μM of fisetin for 24, 48 and 72 h (time-dependent study) to determine its effect on mitochondrial membrane potential. Cells were stained with rhodamine 123 and analyzed by flow cytometry. The data shown here are from a representative experiment repeated three times with similar results.