Abstract
Early achievements in biomedical approaches for HIV prevention included physical barriers (condoms), clean injection equipment (both for medical use and for injection drug users), blood and blood product safety, and prevention of mother to child transmission. In recent years, antiretroviral drugs to reduce risk of transmission (when the infected person takes the medicines; treatment as prevention or TasP) or reduce risk of acquisition (when the seronegative person takes them; pre-exposure prophylaxis or PrEP) have proven efficacious. Circumcision of men has also been a major tool relevant for higher prevalence regions such as sub-Saharan Africa. Well-established prevention strategies in the control of sexually transmitted diseases and tuberculosis are highly relevant for HIV (i.e., screening, linkage to care, early treatment, and contact tracing). Unfortunately, only slow progress is being made in some available HIV prevention strategies such as family planning for HIV-infected women who do not want more children and prevention mother-to-child HIV transmission. Current studies seek to integrate strategies into approaches that combine biomedical, behavioral, and structural methods to achieve prevention synergies. This review identifies the major biomedical approaches demonstrated to be efficacious that are now available. We also highlight the need for behavioral risk reduction and adherence as essential components of any biomedical approach.
Keywords: Condoms, PMTCT, TasP, PrEP, PEP, male circumcision, vaccines, complimentary strategies
Introduction
Condom barriers, blood and needle safety, and the prevention of mother to child transmission of HIV (PMTCT) were the first biomedical strategies to control HIV that did not focus on behavioral risk reduction alone. In the early 1990s, the hopes for an HIV vaccine led to capacity-building in HIV prevention studies and a boost in large scale trials for prevention1,2. The use of antiretroviral therapy (ART) in a pregnant woman for prevention of infection to her infant was a prescient test of concept for the use of ART as prevention (TasP). The utility of TasP for preventing sexual transmission was demonstrated by the high efficacy demonstrated by the HIV Prevention Trials Network HPTN 052 protocol. Since 2005, compelling biomedical prevention strategies have been added to the armamentarium for HIV prevention, notably male circumcision and ART for prevention of sexual transmission. Efficacy of combining several of these biomedical techniques into a synergistic (additive or multiplicative) approach remains to be determined.
The history of disease control and prevention is replete with examples of effective tools that are available for use, but are underutilized in the field or the clinic. HIV/AIDS prevention is a prominent case in point, a challenge that the National HIV/AIDS Strategy for HIV in the US seeks to address3. Both journalists and scientists have highlighted the disappointing missed opportunities in the HIV epidemic4-13. Combination prevention approaches are now available that combine multiple efficacious strategies to block transmission, but all must include behavioral components to avoid risk compensation--the increased risk taking behavior that may accompany prevention approaches that clients perceive to be more effective than they really are14. All three early approaches (condoms, clean needles/syringes, and PMTCT) also required structural reform and technical capacity-building to enable widespread dissemination of the interventions. Widespread condom and needle distribution confronted political opposition that inhibited program scale-up in many venues. Even blood safety measures were resisted in the pre-HIV screening era by many blood banking authorities for economic reasons.
In this paper, we will review key biomedical tools for the prevention of HIV transmission (Table 1) and what the prospects and obstacles are for their further utilization in global HIV control. A recurring theme is that we have technologies that can reduce the epidemic, but we have not deployed them widely or consistently15-20. Other papers in this JAIDS supplement will address behavioral interventions per se, so we will restrict our discussion to the issues arising in translation of biomedical tools. We acknowledge the subjective nature of a manuscript such as this one; it is challenging to predict how these tools will have an impact on the global pandemic. However, we believe speculations regarding the scale-up and application of biomedical prevention can be well-informed based on present knowledge of disease control and prevention for HIV/sexually transmitted infections (STI), tuberculosis, and blood-borne infections such as hepatitis B and C.
Table 1.
Biomedical approaches to HIV prevention and strength of evidence RCT=Randomized clinical trial; EPID=Epidemiologic evidence; ECOL=Ecological associations; OR=Outcomes research; PrEP=pre-exposure prophylaxis; PEP=post-exposure prophylaxis; STI=sexually transmitted infections
| Biomedical HIV prevention strategy | Highest Level of Evidence |
|---|---|
| Antiretroviral treatment to reduce infectiousness of HIV-infected persons | |
| --in sexual relations | RCT, >95% efficacy |
| --from mothers to infants | RCT, >98% efficacy |
| --among injection drug users | EPID |
| Antiretroviral prophylaxis to reduce susceptibility of vulnerable HIV-uninfected persons | |
| --oral PrEP in men | RCT, 44-68% efficacy |
| --oral PrEP in women | RCT (inconsistent) |
| --rectal microbicides (topical PrEP) for men/women | Animal models |
| --vaginal microbicides (topical PrEP) for women | RCT (inconsistent) |
| --PEP for needle stick injuries | EPID |
| --PEP for sexual exposure, including rape | EPID |
| --PEP for infants born to mothers not receiving ART | RCT |
| Medical male circumcision to reduce susceptibility | |
| --Voluntary medical male circumcision in adults | RCT |
| --Infant circumcision | EPID and ECOL |
| Medical male circumcision to reduce infectiousness | |
| --i.e., reducing HIV transmission risk from an HIV+ man | RCT, 38-68% efficacy |
| HIV vaccines to reduce susceptibility (preventive vaccines) | |
| -- ALVAC-HIV® [vCP1521] prime plus AIDSVAX B/E® boost* | RCT |
| --Other vaccines | Animal models |
| HIV vaccines to reduce transmissibility (therapeutic vaccines) | |
| --i.e., vaccine given to HIV+ person to reduce viral load | Animal models |
| Treatment of co-infections to reduce HIV viral load and presumed transmission risk | |
| --e.g., tuberculosis, helminthes, STI | Animal models, EPID |
| Clean needles and syringes for injection | |
| --Needle/syringe exchange programs | EPID, OR |
| --Medical injections | EPID, OR |
| State-of-the-art blood banking | |
| -- Sensitive HIV tests to screening blood/blood products | EPID, OR |
| --Non-use of donations from higher risk sub-populations | EPID, OR |
| Physical barriers to virus-cell contact** | |
| Male condoms | EPID, OR |
| Female condoms | EPID, OR (inconsistent) |
| Prevention of unwanted pregnancy to reduce pediatric HIV infections | |
| Contraception: e.g., hormonal, intrauterine device, barrier | EPID |
4 injected priming doses of recombinant canarypox vector vaccine (ALVAC-HIV® [vCP1521]) followed by 2 injected booster doses with recombinant glycoprotein 120 subunit (AIDSVAX B/E®); details of vaccines are in the online manuscript supplement: http://www.nejm.org/doi/suppl/10.1056/NEJMoa0908492/suppl_file/nejm_rerks-ngarm_2209sa1.pdf, accessed May 12, 2012
Not listed are other techniques that are theoretically beneficial, but have not proven efficacious, e.g., vaginal diaphragm, or have not been tested, e.g., cervical cap
NOTE: Beyond the scope of this table are behavioral approaches towards abstinence, delayed sexual debut, risk reduction among seropositive persons, partner fidelity, including reducing the number of partners, partner selection, including serosorting for persons to have sex only with others with the same serostatus, exclusive breastfeeding for seropositive mothers and uninfected infants, community mobilization for stigma reduction and changes in behavioral and social norms, and altered health care worker practices such as avoiding unnecessary blood/blood product use. Similarly, structural changes are beyond our table's scope, including enforced 100% condom use policies in brothels, behavioral economic approaches such as contingency case transfers to maintain desired behaviors, and adherence to prescribed risk reduction or therapeutic strategies. The authors wish to emphasize the importance of these approaches, but we do not categorize them as biomedical interventions, the topic of this paper.
Antiretroviral treatment (ART) to reduce infectiousness of HIV-infected persons
Sexual transmission
TasP is founded on evidence that persons who are HIV-infected and are on combination antiretroviral therapy (cART) are less likely to transmit the virus sexually than those not on cART. Higher viral load has been associated with higher HIV transmission risk in observational studies21-30. Use of cART has been associated with reduced risk for sexual transmission. In 2011, the HIV Prevention Trials Network (HPTN) 052 trial demonstrated definitively the huge benefit of cART use in persons with higher CD4+ T-lymphocyte counts in protecting their sexual partners (hazard ratio of 96% protective efficacy; 95% confidence interval [95%CI] of 73 to 99%)31-33. As this benefit was apparent when cART was offered to persons who would not have qualified for HIV treatment for their own sakes (as per 2009 WHO treatment guidelines), WHO issued new guidelines in 2012 suggesting that where possible, more persons should be treated with cART than had been recommended heretofore34. While expanding the eligibility of cART with the aim of TasP represents a significant opportunity to prevent new infection, issues of drug resistance, disinhibition, and logistics complexities must be addressed for this approach to be effective in non-clinical trial conditions35.
Efforts in high access cities such as San Francisco and Vancouver have made progress in expanding TasP36-39. It may be that more than 30 years of work for HIV risk reduction among MSM, with mixed success, has contributed to higher uptake of HIV therapy in these settings40,41. In the US as a whole, however, overall program coverage with testing, linkage to care, and therapy remains disappointing42-46. Several new controlled community randomized studies are being launching to study combination prevention, including implementation of expanded ART coverage, to assess the impact on community HIV transmission. Four groups intend to launch such studies in 2012 or 2013, some with combinations of TasP, medical male circumcision, and strategic and behavioral intervention innovations. The HPTN 071 POPArt study (Richard Hayes, principal investigator [PI]), the Iringa study in Tanzania (David Celentano, PI), the Botswana study (Max Essex, PI), and the ANRS/Africa Center study (Marie-Louise Newell, PI) all intend to address the ART for prevention question within rigorous community randomized studies. There are a number of smaller studies that also intend to address this question, some of which are in the field for preliminary work.
TasP for injection drug users
While much has been achieved among injection drug users in risk reduction using needle and syringe exchange as well as opiate-agonist based heroin addiction therapy, there are still regions of the world that do not implement these measures. It is plausible that TasP could also be implemented effectively in this population, and such studies are in progress.
Prevention of mother-to-child transmission of HIV (PMTCT)
The earliest test-of-concept for the use of antiretroviral drugs to reduce HIV viral load and infectiousness in one person to protect another was in PMTCT. Evidence from many definitive randomized clinical trials, e.g., the AIDS Clinical Trials Group (ACTG) 076 trial, the HPTN's HIVNET 012 trial confirmed that reducing viral loads with ART could reduce transmission markedly to newborn infants. Subsequently, both cART use in HIV-infected pregnant women during the months of breastfeeding and use of pre-exposure prophylaxis (PrEP) with ART in breastfeeding infants were judged safe and effective in reducing transmission in low income settings where replacement feeding is neither safe nor affordable47-52.
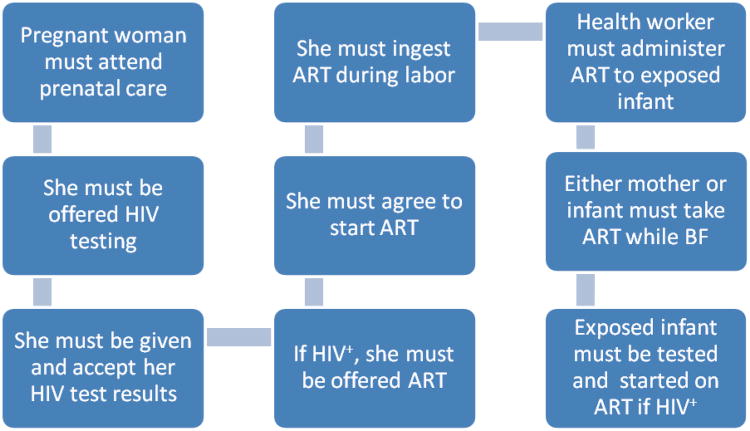
Despite the proven efficacy of antiretroviral prophylaxis or treatment for PMTCT and the high degree of successful implementation in high and middle income countries53-55, the proportion of women and infants receiving all stages of PMTCT in resource limited settings has been disappointing56-64. Failure to engage mothers and/or infants at any step of prevention continuum (Figure 1) can lead to a failure in preventive efficacy during pregnancy or the post-partum periods. Each of these steps are amenable to clinical and community interventions to improve engagement and coverage. In an effort to document barriers to PMTCT uptake, researchers have often focused on patients, although there is movement towards complementary hospital-based quality improvement approaches65-70. Well documented barriers include the lack of PMTCT related care systems capable of delivering quality service57,67,71-73, lack of family or community support65,74,75, stigma66,67, and concern with confidentiality/treatment at the clinical site66. Additional barriers include cost/transportation for travel to the clinic65, cultural pressure to breastfeed (or concern that not-breastfeeding would ‘out’ the mother as being HIV positive)66,76, inadequate alternative food sources for infant feeding64, inadequate knowledge about HIV transmission65,74, and a desire to deliver at home or with a traditional birth attendant65. These and other barriers to full coverage of PMTCT represent some of the challenges of translating the benefits of biomedical knowledge effectively into real-world settings56-58,77-82; other prevention strategies such as TasP and adult circumcision will likely face similar challenges, and will benefit from lessons learned from PMTCT field experience in low-resource settings.
Figure 1.
The prevention of mother-to-child transmission of HIV cascade: steps required to realize the full benefit of testing and linkage to antiretroviral-based antenatal and post-partum care. ART=antiretroviral therapy; dx=diagnosis; BF=breastfeeding
Systems strengthening for implementation of PMTCT and TasP
Widespread health system deficiencies have been identified in a number of low income countries that lead to a low uptake and adherence to ARV prophylaxis56,61,83 and EID84. Although socioeconomic factors are often cited as drivers of poor access to PMTCT or EID85-88, there is increasing attention to the role that strengthening health systems may play in improving access and other program outcomes89-91, and efforts are underway to strength health systems to improve PMTCT-related care92,93. In one study at two district hospitals in rural Mozambique, health systems barriers preventing access to EID were addressed using a conceptual framework for quality improvement adapted from Langley et al92. The process of maternal post-partum care for HIV-infected mothers was analyzed at each hospital; a two phase intervention was designed with the help of nursing staff and patients and evaluated using a before/after intervention study design69,70. Hospital staff were introduced to the Langley model for quality improvement and given the opportunity to participate in the study by describing the process of care, identifying modifiable health system barriers, and designing an intervention aimed at impacting infant retention in EID. The standard process of referral to the EID clinic during maternity discharge was identified as a target for improvement and an intervention was designed that enhanced referral by more tightly linking maternity and EID services through direct accompaniment and by providing privacy for women during counseling. After 2 cycles of intervention, the proportion of mother/infant pairs that succeeded in accessing EID within 3 months of the child's life improved by 55%. This is but one example highlighting the potential benefit of practical, site based innovations to improve retention for HIV prevention from mother to child90,93. Scaling up of quality improvement based strategies is an essential approach to strengthen health systems and make the best use of available resources in developing countries.
Complementary Strategies to PMTCT
Implementing an intervention designed to create and sustain behavior change is another alternative to increasing access to biomedical interventions, but this may be a gradual process of education and culture change94. Attempting to change maternal birthing practices and early childrearing behavior can be especially challenging, as target behavior may conflict with culturally accepted practices and beliefs. Traditionally, interventions to improve uptake have been targeted at pregnant women95-97. Recently, the importance support from husbands in ensuring uptake of PMTCT has been addressed through the engagement men in antenatal HIV counseling65,98 and changes to hospital or clinical systems58,59,68,93,99.
Many HIV-infected women who would like to plan their family size do not have access to contraception; it has been estimated that filling the unmet need for contraception among all the HIV-infected women who need it would result in a huge decline in mother-to-child transmissions100-121. While this is not TasP, it is another PMTCT intervention that depends on capacity building and broadening of the HIV prevention mandate to include other primary health care needs in afflicted communities. Concerns that hormonal contraception use in seronegative women may result in higher risk of acquisition do not apply in the case of seropositive women122,123. Furthermore, there are alternative approaches to hormonal use that are being underutilized, including the intrauterine device124-127.
Antiretroviral prophylaxis to reduce susceptibility of HIV-uninfected persons
Pre-exposure prophylaxis (PrEP)
At a May 10, 2012 meeting, the U.S. Food and Drug Administration's Antiviral Drugs Advisory Committee recommended approval of a drug labeling “efficacy supplement” for the use of Truvada® tablets (each tablet has 200 mg of emtricitabine [FTC] and 300 mg of tenofovir disoproxil fumarate [TDF], made by Gilead Sciences, Inc.) for PrEP, namely oral tablet use for prevention of HIV transmission in HIV-uninfected persons. Oral PrEP has been consistently effective in successive trials in men, ranging from 44-68% efficacy in clinical trials such as iPrEx, Partners PrEP, and TDF-2128-132. In women, the Partners PrEP and TDF-2 studies suggested oral PrEP efficacy, but the FEMPrEP133 and VOICE134,135 trials did not, though the FTC/TDF oral PrEP arm of the VOICE trial continues as of this writing (June 2012). Similarly, use of a topical 1% tenofovir microbicide intravaginally worked to protect against HIV in women in the CAPRISA 004 trial136, but did not work in the VOICE trial137,138. Hence, the evidence is more consistent that oral PrEP works well in men, but is less consistent for oral and for topical PrEP in women (Table 1). Rectal microbicides (also topical PrEP, but designed for anal sex protection) are theoretically useful for women and men practicing anal sex, but they have not yet been tested in Phase 3 clinical efficacy trials. Topical PrEP remains an area of intense current investigation139-144. Opinions differ as to the likely utility of PrEP as a substantial public health tool for HIV prevention, though a female-controlled product could be a valuable additional to women's options145-148.
Post-exposure prophylaxis (PEP)
Occupational exposure to needle stick injuries, surgical instruments, or other substantial medical injuries within the context of caring for an HIV-infected person can expose health care workers (or even sanitation workers) to HIV149-151. Post-exposure prophylaxis with cART is now standard practice for many high risk exposures (with higher volume in persons who are not viral load suppressed, to give one example) and is an option for prophylaxis even in lower risk exposures152,153. Again, implementation issues loom large: successful PEP requires reducing the risk of the needle exposure to begin with, prompt reporting of needle stick injuries, and successful adherence of exposed staff to the cART PEP regimen154.
PEP is also an alternative for inadvertent sexual exposure, as with condom breakage or non-use, or with rape. Randomized clinical trial evidence is lacking for both occupational and non-occupational PEP, but epidemiology suggest cART to be possibly effective for reducing transmission risk155,156. Again, the translation of PEP efficacy to population effectiveness depends on working systems of surveillance, availability of expertise and cART, willingness of the exposed person to uptake the PEP intervention, and their success in adherence to PEP. The HPTN 040 study demonstrated in an RCT that PEP given to infants born to mothers who had not received ART worked to prevent infant infection157-160. Intrapartum transmission occurred in 3.2% (47) of infants studied. Transmission rates were significantly lower in the ZDV + NVP arm 2.2% (11) (95% CI: 1.2 to 4.0, p = 0.045 and the ZDV+NFV+3TC 2.5% (12) (95% CI: 1.4 to 4.3%, p = 0.045) compared to the ZDV arm157-159.
Medical male circumcision to reduce susceptibility
Ecological and epidemiological evidence has suggested that infant or later circumcision might reduce HIV transmission risk161-165. Voluntary medical male circumcision in adults was tested in three RCTs in sub-Saharan Africa whose results were remarkably consistent166-168. Hence, when the cheaper, simpler, and safer infant circumcision had not been performed previously, adult male circumcision is deemed advisable in high HIV prevalence settings. There are obstacles to uptake: cultural acceptability, fear of pain and/or surgical mistakes, and poor understanding about the risks and benefits of circumcision169-183. The decision to circumcise or not are often based more on social acceptability than medical evidence. In sub-Saharan Africa, rites of passage for men often include circumcision (traditionally conducted outside of a clinical setting) presenting an opportunity for health workers to incorporate safe surgical practices into traditional rituals. Education campaigns and improved access to safe surgical services has led to increased uptake among communities where circumcision was uncommon184,185. Western countries are experiencing the opposite trend. The belief that male circumcision is akin to genital mutilation is becoming more widespread. Some researchers and laypeople argue that it leads to long-term psychological trauma in the male infant, impacting everything from mother-son bonding to future sexual relationships, although there is no scientific evidence to support this belief.
Male circumcision of either HIV-infected men or HIV-uninfected men who later seroconvert may reduce their infectiousness to others through reduction of sexually transmitted infections (such as the very common human papillomavirus) among other potential mechanisms186-199. However, if men have sex before their wounds are healed, after surgery, risk could rise as observed in Uganda200. Also, risk compensation is a concern if men increase high risk behavior because they have been circumcised and no longer perceive personal risk201,202. If proper technical procedures, risk reduction counseling, and community consultations are adhered to, male circumcision is a theoretically powerful tool, especially when incorporated into a combination prevention strategy designed to reduce risk through behavioral modification and biological interventions164,203-207.
Evidence that circumcision will prevent HIV acquisition or transmission among men who have sex with men (MSM) is not consistent and is less likely to be as strong in its association as with heterosexual transmission195,208-211. For example, being circumcised may not help much if one is the receptive partner in MSM sexual relations. Infant circumcision is cheaper, easier, and less risky than adult circumcision. An excellent long-term investment is seeking universal male infant circumcision in high prevalence regions to nurture a new generation of lower risk men169,212-219.
HIV vaccines to reduce susceptibility (preventive vaccines)
In a huge RCT in Thailand (n=16,402), a 4-dose priming live vector canarypox vaccine (ALVAC-HIV® [vCP1521]) vaccine followed by 2-dose gp120 subunit bivalent (AIDSVAX B/E®) booster regimen proved somewhat efficacious in preventing HIV infection220. The modified intention-to-treat analysis excluded seven participants who had acute HIV-1 infection at baseline unbeknownst to the investigators, finding a vaccine efficacy of 31% (95% CI: 1 to 52%)221. The vaccine regimen did not reduce the viral set-point in participants who seroconverted despite being in the vaccine group. Despite this success, neither product is being carried forward into production nor is the trial being confirmed to propose licensure. This illustrates the global economic dynamics of vaccine development; only a more efficacious product is likely to inspire the private sector to license and market the vaccine.
Successful vaccine development is no longer just a theoretical possibility; having succeeded in primate animal models and now in a human RCT, investigators will not rest until a viable and more efficacious product is developed222,223. This suggests a host of challenges from which we can learn from other vaccine experiences. HIV is an STI, so suboptimal coverage with hepatitis B vaccine and human papillomavirus vaccine (the only licensed STI vaccines) in adolescents and adults suggests that HIV vaccine deployment would run into the same problems224-229. We have no good global vaccine infrastructures or routine health care engagement for adult vaccination. There is some reticence to agree to STI vaccines given stigma, including among parents for their children230. Continued challenges in global vaccine coverage, even for childhood vaccines, are to be expected to be relevant for HIV vaccines, once available231. This includes anti-vaccine forces claiming the lack of cost-benefit evidence for vaccines, spurious toxicities attributed falsely to vaccines, and arguments about immunological overload that have no evidence to support them232,233.
One of the principal objections voiced to STI vaccines is that of disinhibition, or risk compensation, the possibility that if someone is protected against an STI, then they might be more likely to engage in risky behavior. In the early hepatitis B virus (HBV) vaccine RCTs, there was a rise in HBV incidence after the first dose of HBV vaccine, attributed to disinhibition among men who had sex with men (MSM) who were in the trial234,235. Hence, this potential risk must be taken seriously and studied alongside prevention technology benefits.
HIV vaccines to reduce transmissibility (therapeutic vaccines)
An effective vaccine given to HIV-infected persons could theoretically reduce the viral load in the infected person by enhancing or complementing natural, imperfect immune responses. Animal models have suggested feasibility of such approaches in idealized experiments, but no human data have been convinced to suggest that any tested products have been effective236.
Treatment of co-infections to reduce HIV viral load and transmission risk
Co-infection with such infectious agents as Mycobacterium tuberculosis, helminthes, herpesviruses, and syphilis can cause immune activation and upmodulate HIV expression27,237-239. Coinfection with Schistosoma haematobium is associated with increased HIV risk, as was seen previously for STIs, perhaps related to the disruption of integumentary integrity and/or local inflammation240-243. Rhesus macaques infected with Schistosoma mansoni more susceptible to HIV and shed more HIV once infected244-247. Treatment or suppression of the co-infections can reduce plasma and presumably genital viral load, as suggested in genital herpesvirus suppression studies248-255. Thus, excellent primary care for HIV-infected persons that involves co-infection treatment or suppression could reduce transmissibility of HIV by reducing HIV viral load and transmissibility.
Clean needles and syringes for injection
Despite global reductions in HIV infection, substance use specifically injection drug use (IDU) continues to be a significant driver of the epidemic256. IDU has been estimated to be responsible for about 9% (3 million) of the 34 million persons of persons living with HIV globally, including about 17% of prevalent HIV cases in the US257,258. The WHO estimates that one out of ten new HIV infections globally is attributed to IDU, and CDC suggests IDU to be associated with 9% of new HIV infections in the US258,259. Eastern Europe and Central Asia continue to incur high rates due to IDU; the numbers infected with HIV in these regions have tripled over the past decade259-261. To stem the impending surge in new cases in regions not yet saturated and/or effectively implemented control measures, multiple prevention strategies have been implemented with various degrees of success. Harm reduction efforts such as opioid substitution therapy (OST) and needle and syringe programs (NSPs) have shown to be effective at reducing HIV in IDUs9,262-264. Needle exchange was one of the first methods used in the public health arsenal to control the epidemic. NSPs not only provide sterile needles and equipment; they provide an avenue for HIV, STI, substance abuse and mental health care and treatment to a marginalized risk group. Since the mid- 1980's NSPs have emerged around the world with great success, most notably in Canada, Western Europe, and Australia260. However coverage has been stymied by controversy, government imposed regulations, and lack of available resources. Out of the 151 countries where IDU is prevalent, only 82 countries have implemented NSPs and OST is provided in 71; however coverage is variable across programs and regions260. LMIC countries have been unable to meet the WHO distribution guidelines of 200 syringes per IDU per year265. In the US, NSP support is wrought by politics and regulation. In 2009, the ban on using US federal funds for NSPs was lifted by the Executive Branch of government only to be reinstated by the Congress in the 2012 federal budget266,267.
The peril of extensive nosocomial HIV transmission has been demonstrated in major outbreaks in Russia, Libya, Romania, and other countries [42, 43]. Medical injections were implicated in each of these outbreaks. The importance attributed to unsafe medical injections in the transmission of HIV in sub-Saharan Africa has been minimized by the enormous attentions given to sexually transmitted HIV [44]. The perception that unsafe medical injections are rare in sub-Saharan Africa rests on the assurance in health workers' training and supervision, and compliance with existing safety guidelines. In 2008, studies in South Africa highlighted cases of HIV transmission in children two to nine years' old receiving immunizations in public health facilities; interviews with health care workers reported reusing syringes [45]. Working under rationing pressures and without an accurate estimate for the HIV transmission risk in an individual injection may predispose health workers to view single-use protocols for injection safety as unacceptably wasteful [46]. Programs to improve provider practices; reduce community demand for injections; support the procurement of appropriate injection commodities to eliminate re-use of syringes and needles and improve safety are still needed in these settings.
Transfusion
In higher income countries, public concern has obliged blood collection agencies and policy makers to continue to search for more sensitive HIV screening tests, despite a dramatic decrease in the transmission of HIV infection through blood transfusions268-271. The availability of HIV-1 p24 antigenic testing and state-of-the art genomic amplification techniques, while expensive, allow for the identification of the vast majority of window-phase donations270,272. The impact of other less expensive strategies on HIV transmission risk reduction, such as donor deferral and the non-use of donations from higher risk sub-populations has been highlighted in low income countries273-277. Unfortunately, these cost-effective strategies are not being applied consistently273,274,278-280. While progress towards improving safe and adequate supplies of blood is being made 278, continued government commitment is critical for ensuring quality, safety, and adequacy of the blood supply, particularly in lower income nations where challenges in capacity, logistics, and infrastructure are common.
Physical barriers to virus-cell contact
Consistent and correct condom use is estimated to provide an 80% reduction in HIV seroconversion281. Male condoms are inexpensive, widely-accessible, have few side effects, and (among many populations) are a culturally acceptable HIV prevention intervention. The number of condoms used worldwide is increasing, possibly due to increased social marketing campaigns to increase social acceptability in casual and committed relationships282. While condoms reduce the risk of HIV transmission, evidence suggests they are used inconsistently282-284. Negotiating condom use can be difficult for women as the decision to use a condom often rests with her partner.
Female condoms were designed to provide women with more control over their sexual safety, but uptake has been low285,286. Women needed considerable training and motivation to use the first generation products successfully287-290. New generation products are better designed and may be more appealing; studies are in progress291. Among couples where both partners actively participate in the decision-making process, condoms may be eschewed for other reasons: cost, feel, availability, desire for pregnancy, the belief that they are unnecessary in a ‘serious’ relationship, or religious beliefs may sway partners to have unprotected sex292-294.
Conclusions
The finding of an efficacious intervention in one venue does not guarantee success in a different cultural context. A prime example is the control of the HIV epidemic among IDUs in Australia vs. the continuing spread in Russia; lack of political support for universal NSP and a ban on OST in Russia fuel their IDU-related HIV epidemic260,295,296. A second example is the success in the 1990s with HIV prevention in Uganda, contrasted to that seen in its neighboring nations297-300. Standardized approaches for adapting interventions to new contexts have been developed, including RE-AIM301,302 and ADAPT-ITT94,303, but adaption of behavioral interventions is time-consuming and fraught with potential challenges. The tailoring a given epidemic response to local drivers of transmission is needed for both effectiveness and efficiency. Combination prevention approaches are most promising, but they require substantial success in achieving coverage metrics beyond those achieved in most global programs304-306. The good news is that the myriad of biomedical intervention strategies now demonstrated to be effective in reducing HIV transmission can be combined to make major inroads into the global pandemic307,308. Even as we research new approaches, the scientific community shares an urgent obligation to communicate current opportunities to policymakers, funders, and communities to motivate HIV control and prevention309.
Acknowledgments
Financial Support: None reported.
Footnotes
Conflicts of Interest: None reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vermund SH. The role of prevention research in HIV vaccine trials. AIDS research and human retroviruses. 1994;10(2):S303–305. [PubMed] [Google Scholar]
- 2.Sista ND, Abdool Karim Q, Hinson K, Donnell D, Eshleman SH, Vermund SH. Experience in international clinical research: the HIV Prevention Trials Network. Clinical investigation. 2011 Dec;1(12):1609–1618. doi: 10.4155/cli.11.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Millett GA, Crowley JS, Koh H, et al. A way forward: the National HIV/AIDS Strategy and reducing HIV incidence in the United States. Journal of acquired immune deficiency syndromes. 2010 Dec;55(2):S144–147. doi: 10.1097/QAI.0b013e3181fbcb04. [DOI] [PubMed] [Google Scholar]
- 4.Shilts R. And the band played on: politics, people, and the AIDS epidemic. New York, N.Y., U.S.A.: Penguin Books; 1988. [Google Scholar]
- 5.Dorell CG, Sutton MY, Oster AM, et al. Missed opportunities for HIV testing in health care settings among young African American men who have sex with men: implications for the HIV epidemic. AIDS patient care and STDs. 2011 Nov;25(11):657–664. doi: 10.1089/apc.2011.0203. [DOI] [PubMed] [Google Scholar]
- 6.Tragard A, Shrestha IB. System-wide effects of Global Fund investments in Nepal. Health policy and planning. 2010 Nov;25(1):i58–62. doi: 10.1093/heapol/czq061. [DOI] [PubMed] [Google Scholar]
- 7.Bassett IV, Walensky RP. Integrating HIV screening into routine health care in resource-limited settings. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010 May 15;50(3):S77–84. doi: 10.1086/651477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lurie P, Drucker E. An opportunity lost: HIV infections associated with lack of a national needle-exchange programme in the USA. Lancet. 1997 Mar 1;349(9052):604–608. doi: 10.1016/S0140-6736(96)05439-6. [DOI] [PubMed] [Google Scholar]
- 9.Drucker E, Lurie P, Wodak A, Alcabes P. Measuring harm reduction: the effects of needle and syringe exchange programs and methadone maintenance on the ecology of HIV. Aids. 1998;12(A):S217–230. [PubMed] [Google Scholar]
- 10.Perumal R, Padayatchi N, Stiefvater E. The whole is greater than the sum of the parts: recognising missed opportunities for an optimal response to the rapidly maturing TB-HIV co-epidemic in South Africa. BMC public health. 2009;9:243. doi: 10.1186/1471-2458-9-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makwiza I, Nyirenda L, Bongololo G, Banda T, Chimzizi R, Theobald S. Who has access to counseling and testing and anti-retroviral therapy in Malawi - an equity analysis. International journal for equity in health. 2009;8:13. doi: 10.1186/1475-9276-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girardi E, Sabin CA, Monforte AD. Late diagnosis of HIV infection: epidemiological features, consequences and strategies to encourage earlier testing. Journal of acquired immune deficiency syndromes. 2007 Sep;46(1):S3–8. doi: 10.1097/01.qai.0000286597.57066.2b. [DOI] [PubMed] [Google Scholar]
- 13.Walensky RP, Wood R, Fofana MO, et al. The clinical impact and cost-effectiveness of routine, voluntary HIV screening in South Africa. Journal of acquired immune deficiency syndromes. 2011 Jan;56(1):26–35. doi: 10.1097/QAI.0b013e3181fb8f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermund SH, Hodder SL, Justman JE, et al. Addressing research priorities for prevention of HIV infection in the United States. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010 May 15;50(3):S149–155. doi: 10.1086/651485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussein M, Jira C, Girma B. Assessment of Effective Coverage of HIV Prevention of Pregnant Mother to Child Transimission Services in Jimma Zone, South West Ethiopia. Ethiopian journal of health sciences. 2011 Aug;21(Suppl 1):1–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Boyer S, Koulla-Shiro S, Abe C, Spire B, Moatti JP. Implementing operational research to scale-up access to antiretroviral therapy for HIV infection: lessons learned from the Cameroonian experience. Current opinion in HIV and AIDS. 2011 Jul;6(4):239–244. doi: 10.1097/COH.0b013e3283478757. [DOI] [PubMed] [Google Scholar]
- 17.Srikantiah P, Ghidinelli M, Bachani D, et al. Scale-up of national antiretroviral therapy programs: progress and challenges in the Asia Pacific region. Aids. 2010 Sep;24(3):S62–71. doi: 10.1097/01.aids.0000390091.45435.ea. [DOI] [PubMed] [Google Scholar]
- 18.Nakanjako D, Colebunders R, Coutinho AG, Kamya MR. Strategies to optimize HIV treatment outcomes in resource-limited settings. AIDS reviews. 2009 Oct-Dec;11(4):179–189. [PubMed] [Google Scholar]
- 19.Bowen A, Palasanthiran P, Sohn AH. Global challenges in the development and delivery of paediatric antiretrovirals. Drug discovery today. 2008 Jun;13(11-12):530–535. doi: 10.1016/j.drudis.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyers T, Moultrie H, Naidoo K, Cotton M, Eley B, Sherman G. Challenges to pediatric HIV care and treatment in South Africa. The Journal of infectious diseases. 2007 Dec 1;196(3):S474–481. doi: 10.1086/521116. [DOI] [PubMed] [Google Scholar]
- 21.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010 Jun 12;375(9731):2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lingappa JR, Hughes JP, Wang RS, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PloS one. 2010;5(9):e12598. doi: 10.1371/journal.pone.0012598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallett TB, Baeten JM, Heffron R, et al. Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: a modelling study. PLoS medicine. 2011 Nov;8(11):e1001123. doi: 10.1371/journal.pmed.1001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castilla J, Del Romero J, Hernando V, Marincovich B, Garcia S, Rodriguez C. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. Journal of acquired immune deficiency syndromes. 2005 Sep 1;40(1):96–101. doi: 10.1097/01.qai.0000157389.78374.45. [DOI] [PubMed] [Google Scholar]
- 25.Pedraza MA, del Romero J, Roldan F, et al. Heterosexual transmission of HIV-1 is associated with high plasma viral load levels and a positive viral isolation in the infected partner. ournal of acquired immune deficiency syndromes. 1999 Jun 1;21(2):120–125. [PubMed] [Google Scholar]
- 26.Fideli US, Allen SA, Musonda R, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS research and human retroviruses. 2001 Jul 1;17(10):901–910. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. Aids. 2008 Oct 18;22(16):2179–2185. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds SJ, Makumbi F, Nakigozi G, et al. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. Aids. 2011 Feb 20;25(4):473–477. doi: 10.1097/QAD.0b013e3283437c2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tovanabutra S, Robison V, Wongtrakul J, et al. Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand. Journal of acquired immune deficiency syndromes. 2002 Mar 1;29(3):275–283. doi: 10.1097/00126334-200203010-00008. [DOI] [PubMed] [Google Scholar]
- 30.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. The New England journal of medicine. 2000 Mar 30;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 31.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eshleman SH, Hudelson SE, Redd AD, et al. Analysis of genetic linkage of HIV from couples enrolled in the HIV Prevention Trials Network 052 trial. The Journal of infectious diseases. 2011 Dec 15;204(12):1918–1926. doi: 10.1093/infdis/jir651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen MS, McCauley M, Gamble TR. HIV treatment as prevention and HPTN 052. Current opinion in HIV and AIDS. 2012 Mar;7(2):99–105. doi: 10.1097/COH.0b013e32834f5cf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Organization WH. Guidance on couples HIV testing and counselling including antiretroviral therapy for treatment and prevention in serodiscordant couples: Recommendations for a public health approach. Geneva Switzerland: World Health Organization; Apr, 2012. 2012. 978 92 4 150197 2. [PubMed] [Google Scholar]
- 35.Smith K, Powers KA, Kashuba AD, Cohen MS. HIV-1 treatment as prevention: the good, the bad, and the challenges. Current opinion in HIV and AIDS. 2011 Jul;6(4):315–325. doi: 10.1097/COH.0b013e32834788e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PloS one. 2010;5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charlebois ED, Das M, Porco TC, Havlir DV. The effect of expanded antiretroviral treatment strategies on the HIV epidemic among men who have sex with men in San Francisco. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 Apr 15;52(8):1046–1049. doi: 10.1093/cid/cir085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hogg RS, Moore DM, Michelow WD, Montaner JS. Reduction of HIV incidence in men who have sex with men. The Lancet infectious diseases. 2010 Oct;10(10):655–656. doi: 10.1016/S1473-3099(10)70200-0. [DOI] [PubMed] [Google Scholar]
- 39.Wood E, Milloy MJ, Montaner JS. HIV treatment as prevention among injection drug users. Current opinion in HIV and AIDS. 2012 Mar;7(2):151–156. doi: 10.1097/COH.0b013e32834f9927. [DOI] [PubMed] [Google Scholar]
- 40.McDaid LM, Hart GJ. Sexual risk behaviour for transmission of HIV in men who have sex with men: recent findings and potential interventions. Current opinion in HIV and AIDS. 2010 Jul;5(4):311–315. doi: 10.1097/COH.0b013e32833a0b86. [DOI] [PubMed] [Google Scholar]
- 41.Hart GJ, Elford J. Sexual risk behaviour of men who have sex with men: emerging patterns and new challenges. Current opinion in infectious diseases. 2010 Feb;23(1):39–44. doi: 10.1097/QCO.0b013e328334feb1. [DOI] [PubMed] [Google Scholar]
- 42.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 Mar 15;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Centers for Disease C, Prevention. Vital signs: HIV prevention through care and treatment--United States. MMWR Morbidity and mortality weekly report. 2011 Dec 2;60(47):1618–1623. [PubMed] [Google Scholar]
- 44.Blair JM, McNaghten AD, Frazier EL, Skarbinski J, Huang P, Heffelfinger JD. Clinical and behavioral characteristics of adults receiving medical care for HIV infection --- Medical Monitoring Project, United States, 2007. Morbidity and mortality weekly report Surveillance summaries. 2011 Sep 2;60(11):1–20. [PubMed] [Google Scholar]
- 45.Centers for Disease C, Prevention. Results of the Expanded HIV Testing Initiative--25 jurisdictions, United States, 2007-2010. MMWR Morbidity and mortality weekly report. 2011 Jun 24;60(24):805–810. [PubMed] [Google Scholar]
- 46.Burns DN, Dieffenbach CW, Vermund SH. Rethinking prevention of HIV type 1 infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010 Sep 15;51(6):725–731. doi: 10.1086/655889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilfert CM, Sripipatana T, Spensley A, Kieffer MP, Bitarakwate E. Prevention of vertical transmission of HIV in resource-limited countries. Advances in experimental medicine and biology. 2011;697:41–57. doi: 10.1007/978-1-4419-7185-2_4. [DOI] [PubMed] [Google Scholar]
- 48.Mepham SO, Bland RM, Newell ML. Prevention of mother-to-child transmission of HIV in resource-rich and -poor settings. BJOG : an international journal of obstetrics and gynaecology. 2011 Jan;118(2):202–218. doi: 10.1111/j.1471-0528.2010.02733.x. [DOI] [PubMed] [Google Scholar]
- 49.Read JS. Prevention of mother-to-child transmission of HIV: antiretroviral strategies. Clinics in perinatology. 2010;37(4):765–776. viii. doi: 10.1016/j.clp.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Kourtis AP, Bulterys M. Mother-to-child transmission of HIV: pathogenesis, mechanisms and pathways. Clinics in perinatology. 2010 Dec;37(4):721–737. vii. doi: 10.1016/j.clp.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Coutsoudis A, Kwaan L, Thomson M. Prevention of vertical transmission of HIV-1 in resource-limited settings. Expert review of anti-infective therapy. 2010 Oct;8(10):1163–1175. doi: 10.1586/eri.10.94. [DOI] [PubMed] [Google Scholar]
- 52.Mofenson LM. Prevention in neglected subpopulations: prevention of mother-to-child transmission of HIV infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010 May 15;50(3):S130–148. doi: 10.1086/651484. [DOI] [PubMed] [Google Scholar]
- 53.Fowler MG, Gable AR, Lampe MA, Etima M, Owor M. Perinatal HIV and its prevention: progress toward an HIV-free generation. Clinics in perinatology. 2010 Dec;37(4):699–719. vii. doi: 10.1016/j.clp.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Mofenson LM. Antiretroviral drugs to prevent breastfeeding HIV transmission. Antiviral therapy. 2010;15(4):537–553. doi: 10.3851/IMP1574. [DOI] [PubMed] [Google Scholar]
- 55.McIntyre J. Use of antiretrovirals during pregnancy and breastfeeding in low-income and middle-income countries. Current opinion in HIV and AIDS. 2010 Jan;5(1):48–53. doi: 10.1097/COH.0b013e328333b8ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stringer EM, Sinkala M, Stringer JS, et al. Prevention of mother-to-child transmission of HIV in Africa: successes and challenges in scaling-up a nevirapine-based program in Lusaka, Zambia. Aids. 2003 Jun 13;17(9):1377–1382. doi: 10.1097/01.aids.0000060395.18106.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stringer JS, Sinkala M, Maclean CC, et al. Effectiveness of a city-wide program to prevent mother-to-child HIV transmission in Lusaka, Zambia. Aids. 2005 Aug 12;19(12):1309–1315. doi: 10.1097/01.aids.0000180102.88511.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Megazzini KM, Sinkala M, Vermund SH, et al. A cluster-randomized trial of enhanced labor ward-based PMTCT services to increase nevirapine coverage in Lusaka, Zambia. Aids. 2010 Jan 28;24(3):447–455. doi: 10.1097/QAD.0b013e328334b285. [DOI] [PubMed] [Google Scholar]
- 59.Mandala J, Torpey K, Kasonde P, et al. Prevention of mother-to-child transmission of HIV in Zambia: implementing efficacious ARV regimens in primary health centers. BMC public health. 2009;9:314. doi: 10.1186/1471-2458-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torpey K, Kabaso M, Kasonde P, et al. Increasing the uptake of prevention of mother-to-child transmission of HIV services in a resource-limited setting. BMC health services research. 2010;10:29. doi: 10.1186/1472-6963-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Lettow M, Bedell R, Landes M, et al. Uptake and outcomes of a prevention-of mother-to-child transmission (PMTCT) program in Zomba district, Malawi. BMC public health. 2011;11:426. doi: 10.1186/1471-2458-11-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oladokun RE, Awolude O, Brown BJ, et al. Service uptake and performance of the prevention of mother-to-child transmission (PMTCT) programme in Ibadan, Nigeria. African journal of medicine and medical sciences. 2010 Jun;39(2):81–87. [PubMed] [Google Scholar]
- 63.Karamagi CA, Tumwine JK, Tylleskar T, Heggenhougen K. Antenatal HIV testing in rural eastern Uganda in 2003: incomplete rollout of the prevention of mother-to-child transmission of HIV programme? BMC international health and human rights. 2006;6:6. doi: 10.1186/1472-698X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bulterys M, Ellington S, Kourtis AP. HIV-1 and breastfeeding: biology of transmission and advances in prevention. Clinics in perinatology. 2010 Dec;37(4):807–824. ix–x. doi: 10.1016/j.clp.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 65.Peltzer K, Jones D, Weiss SM, Shikwane E. Promoting male involvement to improve PMTCT uptake and reduce antenatal HIV infection: a cluster randomized controlled trial protocol. BMC public health. 2011;11:778. doi: 10.1186/1471-2458-11-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bond V, Chase E, Aggleton P. Stigma, HIV/AIDS and prevention of mother-to-child transmission in Zambia. Evaluation and Program Planning. 2002;25:347–356. [Google Scholar]
- 67.Sprague C, Chersich MF, Black V. Health system weaknesses constrain access to PMTCT and maternal HIV services in South Africa: a qualitative enquiry. AIDS research and therapy. 2011;8:10. doi: 10.1186/1742-6405-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doherty TM, McCoy D, Donohue S. Health system constraints to optimal coverage of the prevention of mother-to-child HIV transmission programme in South Africa: lessons from the implementation of the national pilot programme. African health sciences. 2005 Sep;5(3):213–218. doi: 10.5555/afhs.2005.5.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ciampa PJ, Burlison JR, Blevins M, et al. Improving retention in the early infant diagnosis of HIV program in rural Mozambique by better service integration. Journal of acquired immune deficiency syndromes. 2011 Sep 1;58(1):115–119. doi: 10.1097/QAI.0b013e31822149bf. [DOI] [PubMed] [Google Scholar]
- 70.Ciampa PJ, Tique JA, Juma N, et al. Addressing Poor Retention of Infants Exposed to HIV: A Quality Improvement Study in Rural Mozambique. Journal of acquired immune deficiency syndromes. 2012 Jun 1;60(2):e46–52. doi: 10.1097/QAI.0b013e31824c0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bratt JH, Torpey K, Kabaso M, Gondwe Y. Costs of HIV/AIDS outpatient services delivered through Zambian public health facilities. Tropical medicine & international health: TM & IH. 2011 Jan;16(1):110–118. doi: 10.1111/j.1365-3156.2010.02640.x. [DOI] [PubMed] [Google Scholar]
- 72.Ekouevi DK, Stringer E, Coetzee D, et al. Health facility characteristics and their relationship to coverage of PMTCT of HIV services across four African countries: the PEARL study. PloS one. 2012;7(1):e29823. doi: 10.1371/journal.pone.0029823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stringer EM, Sinkala M, Kumwenda R, et al. Personal risk perception, HIV knowledge and risk avoidance behavior, and their relationships to actual HIV serostatus in an urban African obstetric population. Journal of acquired immune deficiency syndromes. 2004 Jan 1;35(1):60–66. doi: 10.1097/00126334-200401010-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bajunirwe F, Muzoora M. Barriers to the implementation of programs for the prevention of mother-to-child transmission of HIV: a cross-sectional survey in rural and urban Uganda. AIDS research and therapy. 2005 Oct 28;2:10. doi: 10.1186/1742-6405-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nkuoh GN, Meyer DJ, Tih PM, Nkfusai J. Barriers to men's participation in antenatal and prevention of mother-to-child HIV transmission care in Cameroon, Africa. Journal of midwifery & women's health. 2010 Jul-Aug;55(4):363–369. doi: 10.1016/j.jmwh.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 76.Doherty T, Chopra M, Nkonki L, Jackson D, Greiner T. Effect of the HIV epidemic on infant feeding in South Africa: “When they see me coming with the tins they laugh at me”. Bulletin of the World Health Organization. 2006 Feb;84(2):90–96. doi: 10.2471/blt.04.019448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reithinger R, Megazzini K, Durako SJ, Harris DR, Vermund SH. Monitoring and evaluation of programmes to prevent mother to child transmission of HIV in Africa. Bmj. 2007 Jun 2;334(7604):1143–1146. doi: 10.1136/bmj.39211.527488.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stringer EM, Ekouevi DK, Coetzee D, et al. Coverage of nevirapine-based services to prevent mother-to-child HIV transmission in 4 African countries. JAMA : the journal of the American Medical Association. 2010 Jul 21;304(3):293–302. doi: 10.1001/jama.2010.990. [DOI] [PubMed] [Google Scholar]
- 79.Coetzee D, Hilderbrand K, Boulle A, Draper B, Abdullah F, Goemaere E. Effectiveness of the first district-wide programme for the prevention of mother-to-child transmission of HIV in South Africa. Bulletin of the World Health Organization. 2005 Jul;83(7):489–494. [PMC free article] [PubMed] [Google Scholar]
- 80.Colvin M, Chopra M, Doherty T, et al. Operational effectiveness of single-dose nevirapine in preventing mother-to-child transmission of HIV. Bulletin of the World Health Organization. 2007 Jun;85(6):466–473. doi: 10.2471/BLT.06.033639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stringer EM, Chi BH, Chintu N, et al. Monitoring effectiveness of programmes to prevent mother-to-child HIV transmission in lower-income countries. Bulletin of the World Health Organization. 2008 Jan;86(1):57–62. doi: 10.2471/BLT.07.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Experts/Researchers UUWECH. Medicine VUSo. Vanderbilt University School of Medicine; 2009. Consultative meeting on: Evaluating the impact of prevention of mother-to-child transmission of HIV (PMTCT) services in low- and middle-income countries in averting new HIV infections in children and improving child survival; pp. 1–42. [Google Scholar]
- 83.Peltzer K, Sikwane E, Majaja M. Factors associated with short-course antiretroviral prophylaxis (dual therapy) adherence for PMTCT in Nkangala district, South Africa. Acta paediatrica. 2011 Sep;100(9):1253–1257. doi: 10.1111/j.1651-2227.2011.02253.x. [DOI] [PubMed] [Google Scholar]
- 84.Cook RE, Ciampa PJ, Sidat M, et al. Predictors of successful early infant diagnosis of HIV in a rural district hospital in Zambezia, Mozambique. Journal of acquired immune deficiency syndromes. 2011 Apr;56(4):e104–109. doi: 10.1097/QAI.0b013e318207a535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perez F, Mukotekwa T, Miller A, et al. Implementing a rural programme of prevention of mother-to-child transmission of HIV in Zimbabwe: first 18 months of experience. Tropical medicine & international health: TM & IH. 2004 Jul;9(7):774–783. doi: 10.1111/j.1365-3156.2004.01264.x. [DOI] [PubMed] [Google Scholar]
- 86.Manzi M, Zachariah R, Teck R, et al. High acceptability of voluntary counselling and HIV-testing but unacceptable loss to follow up in a prevention of mother-to-child HIV transmission programme in rural Malawi: scaling-up requires a different way of acting. Tropical medicine & international health: TM & IH. 2005 Dec;10(12):1242–1250. doi: 10.1111/j.1365-3156.2005.01526.x. [DOI] [PubMed] [Google Scholar]
- 87.Nyandiko WM, Otieno-Nyunya B, Musick B, et al. Outcomes of HIV-exposed children in western Kenya: efficacy of prevention of mother to child transmission in a resource-constrained setting. Journal of acquired immune deficiency syndromes. 2010 May 1;54(1):42–50. doi: 10.1097/QAI.0b013e3181d8ad51. [DOI] [PubMed] [Google Scholar]
- 88.Jones SA, Sherman GG, Varga CA. Exploring socio-economic conditions and poor follow-up rates of HIV-exposed infants in Johannesburg, South Africa. AIDS care. 2005 May;17(4):466–470. doi: 10.1080/09540120412331319723. [DOI] [PubMed] [Google Scholar]
- 89.Braun M, Kabue MM, McCollum ED, et al. Inadequate coordination of maternal and infant HIV services detrimentally affects early infant diagnosis outcomes in Lilongwe, Malawi. Journal of acquired immune deficiency syndromes. 2011 Apr 15;56(5):e122–128. doi: 10.1097/QAI.0b013e31820a7f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Youngleson MS, Nkurunziza P, Jennings K, Arendse J, Mate KS, Barker P. Improving a mother to child HIV transmission programme through health system redesign: quality improvement, protocol adjustment and resource addition. PloS one. 2010;5(11):e13891. doi: 10.1371/journal.pone.0013891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leatherman S, Ferris TG, Berwick D, Omaswa F, Crisp N. The role of quality improvement in strengthening health systems in developing countries. International journal for quality in health care : journal of the International Society for Quality in Health Care/ISQua. 2010 Aug;22(4):237–243. doi: 10.1093/intqhc/mzq028. [DOI] [PubMed] [Google Scholar]
- 92.Langley G, N K, Nolan T . The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. San Francisco, CA: Jossey-Bass; 1996. [Google Scholar]
- 93.Doherty T, Chopra M, Nsibande D, Mngoma D. Improving the coverage of the PMTCT programme through a participatory quality improvement intervention in South Africa. BMC public health. 2009;9:406. doi: 10.1186/1471-2458-9-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wingood GM, DiClemente RJ. The ADAPT-ITT model: a novel method of adapting evidence-based HIV Interventions. Journal of acquired immune deficiency syndromes. 2008 Mar 1;47(1):S40–46. doi: 10.1097/QAI.0b013e3181605df1. [DOI] [PubMed] [Google Scholar]
- 95.Baek C, Rutenberg N. Implementing programs for the prevention of mother-to-child HIV transmission in resource-constrained settings: Horizons studies, 1999-2007. Public health reports. 2010 Mar-Apr;125(2):293–304. doi: 10.1177/003335491012500220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horwood C, Haskins L, Vermaak K, Phakathi S, Subbaye R, Doherty T. Prevention of mother to child transmission of HIV (PMTCT) programme in KwaZulu-Natal, South Africa: an evaluation of PMTCT implementation and integration into routine maternal, child and women's health services. Tropical medicine & international health : TM & IH. 2010 Jun 17; doi: 10.1111/j.1365-3156.2010.02576.x. [DOI] [PubMed] [Google Scholar]
- 97.Futterman D, Shea J, Besser M, et al. Mamekhaya: a pilot study combining a cognitive-behavioral intervention and mentor mothers with PMTCT services in South Africa. AIDS care. 2010 Sep;22(9):1093–1100. doi: 10.1080/09540121003600352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tshibumbu DD. Prevention of mother to child transmission of HIV (PMTCT) programme in KwaZulu-Natal, South Africa: an evaluation of PMTCT implementation and integration into routine maternal, child and women's health services. South Africa: Master of Public Health, University of South Africa; 2006. [DOI] [PubMed] [Google Scholar]
- 99.Kasenga F, Byass P, Emmelin M, Hurtig AK. The implications of policy changes on the uptake of a PMTCT programme in rural Malawi: first three years of experience. Global health action. 2009:2. doi: 10.3402/gha.v2i0.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Friend DR, Doncel GF. Combining prevention of HIV-1, other sexually transmitted infections and unintended pregnancies: Development of dual-protection technologies. Antiviral research. 2010 Dec;88(1):S47–54. doi: 10.1016/j.antiviral.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 101.Reynolds HW, Janowitz B, Wilcher R, Cates W. Contraception to prevent HIV-positive births: current contribution and potential cost savings in PEPFAR countries. Sexually transmitted infections. 2008 Oct;84(2):ii49–53. doi: 10.1136/sti.2008.030049. [DOI] [PubMed] [Google Scholar]
- 102.Reynolds HW, Janowitz B, Homan R, Johnson L. The value of contraception to prevent perinatal HIV transmission. Sexually transmitted diseases. 2006 Jun;33(6):350–356. doi: 10.1097/01.olq.0000194602.01058.e1. [DOI] [PubMed] [Google Scholar]
- 103.Wilcher R, Petruney T, Reynolds HW, Cates W. From effectiveness to impact: contraception as an HIV prevention intervention. Sexually transmitted infections. 2008 Oct;84(2):ii54–60. doi: 10.1136/sti.2008.030098. [DOI] [PubMed] [Google Scholar]
- 104.Hladik W, Stover J, Esiru G, Harper M, Tappero J. The contribution of family planning towards the prevention of vertical HIV transmission in Uganda. PloS one. 2009;4(11):e7691. doi: 10.1371/journal.pone.0007691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.King R, Estey J, Allen S, et al. A family planning intervention to reduce vertical transmission of HIV in Rwanda. Aids. 1995 Jul;9(1):S45–51. [PubMed] [Google Scholar]
- 106.Wanyenze RK, Tumwesigye NM, Kindyomunda R, et al. Uptake of family planning methods and unplanned pregnancies among HIV-infected individuals: a cross-sectional survey among clients at HIV clinics in Uganda. Journal of the International AIDS Society. 2011;14:35. doi: 10.1186/1758-2652-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stuart GS. Fourteen million women with limited options: HIV/AIDS and highly effective reversible contraception in sub-Saharan Africa. Contraception. 2009 Nov;80(5):412–416. doi: 10.1016/j.contraception.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 108.Johnson KB, Akwara P, Rutstein SO, Bernstein S. Fertility preferences and the need for contraception among women living with HIV: the basis for a joint action agenda. Aids. 2009 Nov;23(1):S7–S17. doi: 10.1097/01.aids.0000363773.83753.27. [DOI] [PubMed] [Google Scholar]
- 109.Mark KE, Meinzen-Derr J, Stephenson R, et al. Contraception among HIV concordant and discordant couples in Zambia: a randomized controlled trial. Journal of women's health. 2007 Oct;16(8):1200–1210. doi: 10.1089/jwh.2006.0238. [DOI] [PubMed] [Google Scholar]
- 110.Massad LS, Evans CT, Wilson TE, et al. Contraceptive use among U.S. women with HIV. Journal of women's health. 2007 Jun;16(5):657–666. doi: 10.1089/jwh.2006.0204. [DOI] [PubMed] [Google Scholar]
- 111.Rutenberg N, Baek C. Field experiences integrating family planning into programs to prevent mother-to-child transmission of HIV. Studies in family planning. 2005 Sep;36(3):235–245. doi: 10.1111/j.1728-4465.2005.00064.x. [DOI] [PubMed] [Google Scholar]
- 112.Hoffman IF, Martinson FE, Powers KA, et al. The year-long effect of HIV-positive test results on pregnancy intentions, contraceptive use, and pregnancy incidence among Malawian women. Journal of acquired immune deficiency syndromes. 2008 Apr 1;47(4):477–483. doi: 10.1097/QAI.0b013e318165dc52. [DOI] [PubMed] [Google Scholar]
- 113.Adair T. Unmet need for contraception among HIV-positive women in Lesotho and implications for mother-to-child transmission. Journal of biosocial science. 2009 Mar;41(2):269–278. doi: 10.1017/S0021932008003076. [DOI] [PubMed] [Google Scholar]
- 114.Kaida A, Laher F, Strathdee SA, et al. Contraceptive use and method preference among women in Soweto, South Africa: the influence of expanding access to HIV care and treatment services. PloS one. 2010;5(11):e13868. doi: 10.1371/journal.pone.0013868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Todd CS, Stibich MA, Laher F, et al. Influence of culture on contraceptive utilization among HIV-positive women in Brazil, Kenya, and South Africa. AIDS and behavior. 2011 Feb;15(2):454–468. doi: 10.1007/s10461-010-9848-z. [DOI] [PubMed] [Google Scholar]
- 116.Stanwood NL, Cohn SE, Heiser JR, Pugliese M. Contraception and fertility plans in a cohort of HIV-positive women in care. Contraception. 2007 Apr;75(4):294–298. doi: 10.1016/j.contraception.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Delvaux T, Nostlinger C. Reproductive choice for women and men living with HIV: contraception, abortion and fertility. Reproductive health matters. 2007 May;15(29 Suppl):46–66. doi: 10.1016/S0968-8080(07)29031-7. [DOI] [PubMed] [Google Scholar]
- 118.Allen S, Stephenson R, Weiss H, et al. Pregnancy, hormonal contraceptive use, and HIV-related death in Rwanda. Journal of women's health. 2007 Sep;16(7):1017–1027. doi: 10.1089/jwh.2006.0151. [DOI] [PubMed] [Google Scholar]
- 119.Kongnyuy EJ, Soskolne V, Adler B. Hormonal contraception, sexual behaviour and HIV prevalence among women in Cameroon. BMC women's health. 2008;8:19. doi: 10.1186/1472-6874-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mitchell HS, Stephens E. Contraception choice for HIV positive women. Sexually transmitted infections. 2004 Jun;80(3):167–173. doi: 10.1136/sti.2003.008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weinberg A, Forster-Harwood J, McFarland EJ, et al. Resistance to antiretrovirals in HIV-infected pregnant women. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2009 May;45(1):39–42. doi: 10.1016/j.jcv.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 122.Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. The Lancet infectious diseases. 2012 Jan;12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bank UUWW. Hormal contraception and HIV: Technical statement. Vol. 2012. World Health Organization: WHO; Feb 16, 2012. [PubMed] [Google Scholar]
- 124.Stringer EM, Kaseba C, Levy J, et al. A randomized trial of the intrauterine contraceptive device vs hormonal contraception in women who are infected with the human immunodeficiency virus. American journal of obstetrics and gynecology. 2007 Aug;197(2):144 e141–148. doi: 10.1016/j.ajog.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Curtis KM, Nanda K, Kapp N. Safety of hormonal and intrauterine methods of contraception for women with HIV/AIDS: a systematic review. Aids. 2009 Nov;23(1):S55–67. doi: 10.1097/01.aids.0000363778.58203.b6. [DOI] [PubMed] [Google Scholar]
- 126.Heikinheimo O, Lahteenmaki P. Contraception and HIV infection in women. Human reproduction update. 2009 Mar-Apr;15(2):165–176. doi: 10.1093/humupd/dmn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sinei SK, Morrison CS, Sekadde-Kigondu C, Allen M, Kokonya D. Complications of use of intrauterine devices among HIV-1-infected women. Lancet. 1998 Apr 25;351(9111):1238–1241. doi: 10.1016/S0140-6736(97)10319-1. [DOI] [PubMed] [Google Scholar]
- 128.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010 Dec 30;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baeten J, Donnell D, Ndase P, et al. Abstract #29: ARV PrEP for HIV-1 Prevention among Heterosexual Men and Women; Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; March 5-8, 2012; Seattle, WA, USA. [Google Scholar]
- 130.Thigpen MC, Kebaabetswe PM, Smith DK, et al. Abstract # WELBC01: Daily oral antiretroviral use for the prevention of HIV infection in heterosexually active young adults in Botswana: results from teh TDF2 study; Paper presented at: 6th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; July 17-20, 2011; Rome, Italy. [Google Scholar]
- 131.Grohskopf L, Gvetadze R, Pathak S, et al. Abstract # FRLBC102: Preliminary analysis of biomedical data from the phase II clinical safety trial of tenofovir disoproxil fumarate (TDF) for HIV-1 pre-exposure prophylaxis (PrEP) among U S men who have sex with men (MSM); Paper presented at: XVIII International AIDS Conference; July 18-23, 2010; Vienna, Austria. [Google Scholar]
- 132.The Review Team for NDA21-752/S-30. Memorandum: Background Package for NDA 21-752/Supplement 30. 2012:1–47. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM303213.pdf.
- 133.Van Damme L, Corneli A, Ahmed K, et al. Abstract # 32LB: The FEM-PrEP Trial of Emtricitabine/Tenofovir Disoproxil Fumarate (Truvada) among African Women; Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; March 5-8, 2012; Seattle, WA, USA. [Google Scholar]
- 134.NIAID/NIH. NIH Discontinues Tenofovir Vaginal Gel in ‘VOICE’ HIV Prevention Study: Product Safe but No More Effective than Placebo. [Accessed June 3, 2012];NIAID Media Availability. 2011 http://www.niaid.nih.gov/news/newsreleases/2011/Pages/VOICEdiscontinued.aspx.
- 135.NIAID/NIH. NIH Modifies ‘VOICE’ HIV Prevention Study in Women: Oral Tenofovir Discontinued in Clinical Trial. [Accessed June 3, 2012];NIAID Media Availability. 2011 http://www.niaid.nih.gov/news/newsreleases/2011/Pages/VOICEmodified.aspx.
- 136.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 Sep 3;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Quinones-Mateu ME, Vanham G. HIV microbicides: where are we now? Current HIV research. 2012 Jan 1;10(1):1–2. doi: 10.2174/157016212799304724. [DOI] [PubMed] [Google Scholar]
- 138.McEnery R. Oral tenofovir arm of VOICE trial discontinued early. IAVI report : newsletter on international AIDS vaccine research. 2011 Sep-Oct;15(5):21. [PubMed] [Google Scholar]
- 139.Morris GC, Lacey CJ. Microbicides and HIV prevention: lessons from the past, looking to the future. Current opinion in infectious diseases. 2010 Feb;23(1):57–63. doi: 10.1097/QCO.0b013e328334de6d. [DOI] [PubMed] [Google Scholar]
- 140.Nuttall J. Microbicides in the prevention of HIV infection: current status and future directions. Drugs. 2010 Jul 9;70(10):1231–1243. doi: 10.2165/10898650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 141.Kelly CG, Shattock RJ. Specific microbicides in the prevention of HIV infection. Journal of internal medicine. 2011 Dec;270(6):509–519. doi: 10.1111/j.1365-2796.2011.02454.x. [DOI] [PubMed] [Google Scholar]
- 142.Belec L, Jenabian MA, Charpentier C, Saidi H. Combinatorial prevention of HIV transmission in women: the case for a vaginal microbicide. Future microbiology. 2011 Jul;6(7):731–737. doi: 10.2217/fmb.11.64. [DOI] [PubMed] [Google Scholar]
- 143.Pirrone V, Thakkar N, Jacobson JM, Wigdahl B, Krebs FC. Combinatorial approaches to the prevention and treatment of HIV-1 infection. Antimicrobial agents and chemotherapy. 2011 May;55(5):1831–1842. doi: 10.1128/AAC.00976-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hladik F, Doncel GF. Preventing mucosal HIV transmission with topical microbicides: challenges and opportunities. Antiviral research. 2010 Dec;88(1):S3–9. doi: 10.1016/j.antiviral.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Underhill K, Operario D, Mimiaga MJ, Skeer MR, Mayer KH. Implementation science of pre-exposure prophylaxis: preparing for public use. Current HIV/AIDS reports. 2010 Nov;7(4):210–219. doi: 10.1007/s11904-010-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Myers GM, Mayer KH. Oral preexposure anti-HIV prophylaxis for high-risk U.S. populations: current considerations in light of new findings. AIDS patient care and STDs. 2011 Feb;25(2):63–71. doi: 10.1089/apc.2010.0222. [DOI] [PubMed] [Google Scholar]
- 147.Romanelli F, Murphy B. Systemic preexposure prophylaxis for human immunodeficiency virus infection. Pharmacotherapy. 2010 Oct;30(10):1021–1030. doi: 10.1592/phco.30.10.1021. [DOI] [PubMed] [Google Scholar]
- 148.van de Vijver DA, Boucher CA. The risk of HIV drug resistance following implementation of pre-exposure prophylaxis. Current opinion in infectious diseases. 2010 Dec;23(6):621–627. doi: 10.1097/QCO.0b013e32833ff1e6. [DOI] [PubMed] [Google Scholar]
- 149.Panlilio AL, Cardo DM, Grohskopf LA, Heneine W, Ross CS Service USPH. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2005 Sep 30;54(RR-9):1–17. [PubMed] [Google Scholar]
- 150.Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. The New England journal of medicine. 1997 Nov 20;337(21):1485–1490. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- 151.Chin RL. Postexposure prophylaxis for HIV. Emergency medicine clinics of North America. 2010 May;28(2):421–429. doi: 10.1016/j.emc.2010.01.013. Table of Contents. [DOI] [PubMed] [Google Scholar]
- 152.Landovitz RJ, Currier JS. Clinical practice. Postexposure prophylaxis for HIV infection. The New England journal of medicine. 2009 Oct 29;361(18):1768–1775. doi: 10.1056/NEJMcp0904189. [DOI] [PubMed] [Google Scholar]
- 153.Bryant J, Baxter L, Hird S. Non-occupational postexposure prophylaxis for HIV: a systematic review. Health technology assessment. 2009 Feb;13(14):iii, ix–x. 1–60. doi: 10.3310/hta13140. [DOI] [PubMed] [Google Scholar]
- 154.Tolle MA, Schwarzwald HL. Postexposure prophylaxis against human immunodeficiency virus. American family physician. 2010 Jul 15;82(2):161–166. [PubMed] [Google Scholar]
- 155.Almeda J, Casabona J, Allepuz A, et al. Recommendations for non-occupational postexposure HIV prophylaxis. Spanish Working Group on Non-Occupational Postexposure HIV Prophylaxis of the Catalonian Center for Epidemiological Studies on AIDS and the AIDS Study Group. Enfermedades infecciosas y microbiologia clinica. 2002 Oct;20(8):391–400. doi: 10.1016/s0213-005x(02)72826-7. [DOI] [PubMed] [Google Scholar]
- 156.Myles JE, Hirozawa A, Katz MH, Kimmerling R, Bamberger JD. Postexposure prophylaxis for HIV after sexual assault. JAMA : the journal of the American Medical Association. 2000 Sep 27;284(12):1516–1518. doi: 10.1001/jama.284.12.1516-a. [DOI] [PubMed] [Google Scholar]
- 157.Nielsen-Saines K, Watts H, Veloso VG, et al. Abstract # 124LB: Phase III Randomized Trial of the Safety and Efficacy of 3 Neonatal ARV Regimens for Prevention of Intrapartum HIV-1 Transmission: NICHD HPTN 040/PACTG 1043; Paper presented at: 18th Conference on Retroviruses and Opportunistic Infections; February 27-March 2, 2011; Boston, MA, USA. [Google Scholar]
- 158.Mirochnick M, Nielsen-Saines K, Pilotto JH, et al. Nelfinavir and Lamivudine pharmacokinetics during the first two weeks of life. The Pediatric infectious disease journal. 2011 Sep;30(9):769–772. doi: 10.1097/INF.0b013e3182242950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mirochnick M, Nielsen-Saines K, Pilotto JH, et al. Nevirapine concentrations in newborns receiving an extended prophylactic regimen. Journal of acquired immune deficiency syndromes. 2008 Mar 1;47(3):334–337. [PubMed] [Google Scholar]
- 160.NIAID/NIH. 18th Conference on Retroviruses and Opportunistic Infections: Day Four: Selected Highlights of NIH-supported Research Preventing Mother-to-Child Transmission, HIV Transmission Factors among Key Topics Presented. [Accessed June 3, 2012];NIAID Media Availability. 2011 http://www.niaid.nih.gov/news/newsreleases/2011/Pages/CROIwed11.aspx.
- 161.Bongaarts J, Reining P, Way P, Conant F. The relationship between male circumcision and HIV infection in African populations. Aids. 1989 Jun;3(6):373–377. doi: 10.1097/00002030-198906000-00006. [DOI] [PubMed] [Google Scholar]
- 162.Wamai RG, Morris BJ, Bailis SA, et al. Male circumcision for HIV prevention: current evidence and implementation in sub-Saharan Africa. Journal of the International AIDS Society. 2011;14:49. doi: 10.1186/1758-2652-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Weiss HA, Quigley MA, Hayes RJ. Male circumcision and risk of HIV infection in sub-Saharan Africa: a systematic review and meta-analysis. Aids. 2000 Oct 20;14(15):2361–2370. doi: 10.1097/00002030-200010200-00018. [DOI] [PubMed] [Google Scholar]
- 164.Siegfried N, Muller M, Volmink J, et al. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane database of systematic reviews. 2003;(3):CD003362. doi: 10.1002/14651858.CD003362. [DOI] [PubMed] [Google Scholar]
- 165.Weiss HA, Thomas SL, Munabi SK, Hayes RJ. Male circumcision and risk of syphilis, chancroid, and genital herpes: a systematic review and meta-analysis. Sexually transmitted infections. 2006 Apr;82(2):101–109. doi: 10.1136/sti.2005.017442. discussion 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007 Feb 24;369(9562):657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 167.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007 Feb 24;369(9562):643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 168.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS medicine. 2005 Nov;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.White RG, Glynn JR, Orroth KK, et al. Male circumcision for HIV prevention in sub-Saharan Africa: who, what and when? Aids. 2008 Sep 12;22(14):1841–1850. doi: 10.1097/QAD.0b013e32830e0137. [DOI] [PubMed] [Google Scholar]
- 170.Auvert B, Buve A, Lagarde E, et al. Male circumcision and HIV infection in four cities in sub-Saharan Africa. Aids. 2001 Aug;15(4):S31–40. doi: 10.1097/00002030-200108004-00004. [DOI] [PubMed] [Google Scholar]
- 171.Shaffer DN, Bautista CT, Sateren WB, et al. The protective effect of circumcision on HIV incidence in rural low-risk men circumcised predominantly by traditional circumcisers in Kenya: two-year follow-up of the Kericho HIV Cohort Study. Journal of acquired immune deficiency syndromes. 2007 Aug 1;45(4):371–379. doi: 10.1097/QAI.0b013e318095a3da. [DOI] [PubMed] [Google Scholar]
- 172.Drain PK, Halperin DT, Hughes JP, Klausner JD, Bailey RC. Male circumcision, religion, and infectious diseases: an ecologic analysis of 118 developing countries. BMC infectious diseases. 2006;6:172. doi: 10.1186/1471-2334-6-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Westercamp N, Bailey RC. Acceptability of male circumcision for prevention of HIV/AIDS in sub-Saharan Africa: a review. AIDS and behavior. 2007 May;11(3):341–355. doi: 10.1007/s10461-006-9169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Edgil D, Stankard P, Forsythe S, et al. Voluntary medical male circumcision: logistics, commodities, and waste management requirements for scale-up of services. PLoS medicine. 2011 Nov;8(11):e1001128. doi: 10.1371/journal.pmed.1001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Templeton DJ. Male circumcision to reduce sexual transmission of HIV. Current opinion in HIV and AIDS. 2010 Jul;5(4):344–349. doi: 10.1097/COH.0b013e32833a46d3. [DOI] [PubMed] [Google Scholar]
- 176.Weiss HA, Dickson KE, Agot K, Hankins CA. Male circumcision for HIV prevention: current research and programmatic issues. Aids. 2010 Oct;24(4):S61–69. doi: 10.1097/01.aids.0000390708.66136.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Muula AS, Prozesky HW, Mataya RH, Ikechebelu JI. Prevalence of complications of male circumcision in Anglophone Africa: a systematic review. BMC urology. 2007;7:4. doi: 10.1186/1471-2490-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lagarde E, Dirk T, Puren A, Reathe RT, Bertran A. Acceptability of male circumcision as a tool for preventing HIV infection in a highly infected community in South Africa. Aids. 2003 Jan 3;17(1):89–95. doi: 10.1097/00002030-200301030-00012. [DOI] [PubMed] [Google Scholar]
- 179.Wang AL, Duke W, Schmid GP. Print media reporting of male circumcision for preventing HIV infection in sub-Saharan Africa. Bulletin of the World Health Organization. 2009 Aug;87(8):595–603. doi: 10.2471/BLT.09.066704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Boily MC, Desai K, Masse B, Gumel A. Incremental role of male circumcision on a generalised HIV epidemic through its protective effect against other sexually transmitted infections: from efficacy to effectiveness to population-level impact. Sexually transmitted infections. 2008 Oct;84(2):ii28–34. doi: 10.1136/sti.2008.030346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Begley EB, Jafa K, Voetsch AC, Heffelfinger JD, Borkowf CB, Sullivan PS. Willingness of men who have sex with men (MSM) in the United States to be circumcised as adults to reduce the risk of HIV infection. PloS one. 2008;3(7):e2731. doi: 10.1371/journal.pone.0002731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Grund JM, Hennink MM. A qualitative study of sexual behavior change and risk compensation following adult male circumcision in urban Swaziland. AIDS care. 2012;24(2):245–251. doi: 10.1080/09540121.2011.596516. [DOI] [PubMed] [Google Scholar]
- 183.Weiss HA, Plummer ML, Changalucha J, et al. Circumcision among adolescent boys in rural northwestern Tanzania. Tropical medicine & international health : TM & IH. 2008 Aug;13(8):1054–1061. doi: 10.1111/j.1365-3156.2008.02107.x. [DOI] [PubMed] [Google Scholar]
- 184.Mwandi Z, Murphy A, Reed J, et al. Voluntary medical male circumcision: translating research into the rapid expansion of services in Kenya, 2008-2011. PLoS medicine. 2011 Nov;8(11):e1001130. doi: 10.1371/journal.pmed.1001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lissouba P, Taljaard D, Rech D, et al. Adult male circumcision as an intervention against HIV: an operational study of uptake in a South African community (ANRS 12126) BMC infectious diseases. 2011;11:253. doi: 10.1186/1471-2334-11-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tobian AA, Grabowski MK, Kigozi G, et al. High-risk human papillomavirus prevalence is associated with HIV infection among heterosexual men in Rakai, Uganda. Sexually transmitted infections. 2012 May 24; doi: 10.1136/sextrans-2012-050524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Albero G, Castellsague X, Giuliano AR, Bosch FX. Male circumcision and genital human papillomavirus: a systematic review and meta-analysis. Sexually transmitted diseases. 2012 Feb;39(2):104–113. doi: 10.1097/OLQ.0b013e3182387abd. [DOI] [PubMed] [Google Scholar]
- 188.Marrazzo JM, Cates W. Interventions to prevent sexually transmitted infections, including HIV infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 Dec;53(3):S64–78. doi: 10.1093/cid/cir695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vanbuskirk K, Winer RL, Hughes JP, et al. Circumcision and acquisition of human papillomavirus infection in young men. Sexually transmitted diseases. 2011 Nov;38(11):1074–1081. doi: 10.1097/OLQ.0b013e31822e60cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Larke N, Thomas SL, Dos Santos Silva I, Weiss HA. Male circumcision and human papillomavirus infection in men: a systematic review and meta-analysis. The Journal of infectious diseases. 2011 Nov;204(9):1375–1390. doi: 10.1093/infdis/jir523. [DOI] [PubMed] [Google Scholar]
- 191.Backes DM, Bleeker MC, Meijer CJ, et al. Male circumcision is associated with a lower prevalence of human papillomavirus-associated penile lesions among Kenyan men. International journal of cancer Journal international du cancer. 2012 Apr 15;130(8):1888–1897. doi: 10.1002/ijc.26196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tobian AA, Kong X, Wawer MJ, et al. Circumcision of HIV-infected men and transmission of human papillomavirus to female partners: analyses of data from a randomised trial in Rakai, Uganda. The Lancet infectious diseases. 2011 Aug;11(8):604–612. doi: 10.1016/S1473-3099(11)70038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wawer MJ, Tobian AA, Kigozi G, et al. Effect of circumcision of HIV-negative men on transmission of human papillomavirus to HIV-negative women: a randomised trial in Rakai, Uganda. Lancet. 2011 Jan 15;377(9761):209–218. doi: 10.1016/S0140-6736(10)61967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mabey D, Ndowa F, Latif A. What have we learned from sexually transmitted infection research in sub-Saharan Africa? Sexually transmitted infections. 2010 Dec;86(7):488–492. doi: 10.1136/sti.2009.041632. [DOI] [PubMed] [Google Scholar]
- 195.Jozkowski K, Rosenberger JG, Schick V, Herbenick D, Novak DS, Reece M. Relations between circumcision status, sexually transmitted infection history, and HIV serostatus among a national sample of men who have sex with men in the United States. AIDS patient care and STDs. 2010 Aug;24(8):465–470. doi: 10.1089/apc.2010.0082. [DOI] [PubMed] [Google Scholar]
- 196.Smith JS, Moses S, Hudgens MG, et al. Increased risk of HIV acquisition among Kenyan men with human papillomavirus infection. The Journal of infectious diseases. 2010 Jun 1;201(11):1677–1685. doi: 10.1086/652408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Smith DK, Taylor A, Kilmarx PH, et al. Male circumcision in the United States for the prevention of HIV infection and other adverse health outcomes: report from a CDC consultation. Public health reports. 2010 Jan-Feb;125(1):72–82. doi: 10.1177/00333549101250S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gray RH, Serwadda D, Kong X, et al. Male circumcision decreases acquisition and increases clearance of high-risk human papillomavirus in HIV-negative men: a randomized trial in Rakai, Uganda. The Journal of infectious diseases. 2010 May 15;201(10):1455–1462. doi: 10.1086/652184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Serwadda D, Wawer MJ, Makumbi F, et al. Circumcision of HIV-infected men: effects on high-risk human papillomavirus infections in a randomized trial in Rakai, Uganda. The Journal of infectious diseases. 2010 May 15;201(10):1463–1469. doi: 10.1086/652185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wawer MJ, Makumbi F, Kigozi G, et al. Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet. 2009 Jul 18;374(9685):229–237. doi: 10.1016/S0140-6736(09)60998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bridges JF, Selck FW, Gray GE, McIntyre JA, Martinson NA. Condom avoidance and determinants of demand for male circumcision in Johannesburg, South Africa. Health policy and planning. 2011 Jul;26(4):298–306. doi: 10.1093/heapol/czq064. [DOI] [PubMed] [Google Scholar]
- 202.Gust DA, Kretsinger K, Pals SL, et al. Male circumcision as an HIV prevention intervention in the U.S.: Influence of health care providers and potential for risk compensation. Preventive medicine. 2011 Mar-Apr;52(3-4):270–273. doi: 10.1016/j.ypmed.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 203.Kigozi G, Gray RH, Wawer MJ, et al. The safety of adult male circumcision in HIV-infected and uninfected men in Rakai, Uganda. PLoS medicine. 2008 Jun 3;5(6):e116. doi: 10.1371/journal.pmed.0050116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mehta SD, Gray RH, Auvert B, et al. Does sex in the early period after circumcision increase HIV-seroconversion risk? Pooled analysis of adult male circumcision clinical trials. Aids. 2009 Jul 31;23(12):1557–1564. doi: 10.1097/QAD.0b013e32832afe95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kelly R, Kiwanuka N, Wawer MJ, et al. Age of male circumcision and risk of prevalent HIV infection in rural Uganda. Aids. 1999 Feb 25;13(3):399–405. doi: 10.1097/00002030-199902250-00013. [DOI] [PubMed] [Google Scholar]
- 206.Baeten JM, Celum C, Coates TJ. Male circumcision and HIV risks and benefits for women. Lancet. 2009 Jul 18;374(9685):182–184. doi: 10.1016/S0140-6736(09)61311-8. [DOI] [PubMed] [Google Scholar]
- 207.Sanchez J, Sal YRVG, Hughes JP, et al. Male circumcision and risk of HIV acquisition among MSM. Aids. 2011 Feb 20;25(4):519–523. doi: 10.1097/QAD.0b013e328340fd81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Vermund SH, Qian HZ. Circumcision and HIV prevention among men who have sex with men: no final word. JAMA : the journal of the American Medical Association. 2008 Oct 8;300(14):1698–1700. doi: 10.1001/jama.300.14.1698. [DOI] [PubMed] [Google Scholar]
- 209.Schneider JA, Michaels S, Gandham SR, et al. A protective effect of circumcision among receptive male sex partners of Indian men who have sex with men. AIDS and behavior. 2012 Feb;16(2):350–359. doi: 10.1007/s10461-011-9982-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Millett GA, Flores SA, Marks G, Reed JB, Herbst JH. Circumcision status and risk of HIV and sexually transmitted infections among men who have sex with men: a meta-analysis. JAMA : the journal of the American Medical Association. 2008 Oct 8;300(14):1674–1684. doi: 10.1001/jama.300.14.1674. [DOI] [PubMed] [Google Scholar]
- 211.Buchbinder SP, Vittinghoff E, Heagerty PJ, et al. Sexual risk, nitrite inhalant use, and lack of circumcision associated with HIV seroconversion in men who have sex with men in the United States. Journal of acquired immune deficiency syndromes. 2005 May 1;39(1):82–89. doi: 10.1097/01.qai.0000134740.41585.f4. [DOI] [PubMed] [Google Scholar]
- 212.Mavhu W, Hatzold K, Laver SM, et al. Acceptability of early infant male circumcision as an HIV prevention intervention in Zimbabwe: a qualitative perspective. PloS one. 2012;7(2):e32475. doi: 10.1371/journal.pone.0032475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Waters E, Stringer E, Mugisa B, Temba S, Bowa K, Linyama D. Acceptability of neonatal male circumcision in Lusaka, Zambia. AIDS care. 2012;24(1):12–19. doi: 10.1080/09540121.2011.587508. [DOI] [PubMed] [Google Scholar]
- 214.Mugwanya KK, Whalen C, Celum C, Nakku-Joloba E, Katabira E, Baeten JM. Circumcision of male children for reduction of future risk for HIV: acceptability among HIV serodiscordant couples in Kampala, Uganda. PloS one. 2011;6(7):e22254. doi: 10.1371/journal.pone.0022254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Perez F, Aung KD, Ndoro T, Engelsmann B, Dabis F. Participation of traditional birth attendants in prevention of mother-to-child transmission of HIV services in two rural districts in Zimbabwe: a feasibility study. BMC public health. 2008;8:401. doi: 10.1186/1471-2458-8-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wambura M, Mwanga JR, Mosha JF, Mshana G, Mosha F, Changalucha J. Acceptability of medical male circumcision in the traditionally circumcising communities in Northern Tanzania. BMC public health. 2011;11:373. doi: 10.1186/1471-2458-11-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Waiswa P, Kemigisa M, Kiguli J, Naikoba S, Pariyo GW, Peterson S. Acceptability of evidence-based neonatal care practices in rural Uganda - implications for programming. BMC pregnancy and childbirth. 2008;8:21. doi: 10.1186/1471-2393-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Madhivanan P, Krupp K, Chandrasekaran V, Karat SC, Reingold AL, Klausner JD. Acceptability of male circumcision among mothers with male children in Mysore, India. Aids. 2008 May 11;22(8):983–988. doi: 10.1097/QAD.0b013e3282ffde52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mshana G, Wambura M, Mwanga J, Mosha J, Mosha F, Changalucha J. Traditional male circumcision practices among the Kurya of North-eastern Tanzania and implications for national programmes. AIDS care. 2011 Sep;23(9):1111–1116. doi: 10.1080/09540121.2011.554518. [DOI] [PubMed] [Google Scholar]
- 220.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine. 2009 Dec 3;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 221.Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine. 2012 Apr 5;366(14):1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Paris RM, Kim JH, Robb ML, Michael NL. Prime-boost immunization with poxvirus or adenovirus vectors as a strategy to develop a protective vaccine for HIV-1. Expert review of vaccines. 2010 Sep;9(9):1055–1069. doi: 10.1586/erv.10.106. [DOI] [PubMed] [Google Scholar]
- 223.Vaccari M, Poonam P, Franchini G. Phase III HIV vaccine trial in Thailand: a step toward a protective vaccine for HIV. Expert review of vaccines. 2010 Sep;9(9):997–1005. doi: 10.1586/erv.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fairley CK, Read TR. Vaccination against sexually transmitted infections. Current opinion in infectious diseases. 2012 Feb;25(1):66–72. doi: 10.1097/QCO.0b013e32834e9aeb. [DOI] [PubMed] [Google Scholar]
- 225.Bar-On ES, Goldberg E, Fraser A, Vidal L, Hellmann S, Leibovici L. Combined DTP-HBV-HIB vaccine versus separately administered DTP-HBV and HIB vaccines for primary prevention of diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae B (HIB) Cochrane database of systematic reviews. 2009;(3):CD005530. doi: 10.1002/14651858.CD005530.pub2. [DOI] [PubMed] [Google Scholar]
- 226.Garland SM, Skinner SR, Brotherton JM. Adolescent and young adult HPV vaccination in Australia: achievements and challenges. Preventive medicine. 2011 Oct;53(1):S29–35. doi: 10.1016/j.ypmed.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 227.Humiston SG, Rosenthal SL. Challenges to vaccinating adolescents: vaccine implementation issues. The Pediatric infectious disease journal. 2005 Jun;24(6 Suppl):S134–140. doi: 10.1097/01.inf.0000166161.12087.94. [DOI] [PubMed] [Google Scholar]
- 228.Mast EE, Williams IT, Alter MJ, Margolis HS. Hepatitis B vaccination of adolescent and adult high-risk groups in the United States. Vaccine. 1998 Nov;(16 Suppl):S27–29. doi: 10.1016/s0264-410x(98)00288-6. [DOI] [PubMed] [Google Scholar]
- 229.Suh CA, Saville A, Daley MF, et al. Effectiveness and Net Cost of Reminder/Recall for Adolescent Immunizations. Pediatrics. 2012 May 7; doi: 10.1542/peds.2011-1714. [DOI] [PubMed] [Google Scholar]
- 230.Liddon N, Pulley L, Cockerham WC, Lueschen G, Vermund SH, Hook EW. Parents'/guardians' willingness to vaccinate their children against genital herpes. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2005 Sep;37(3):187–193. doi: 10.1016/j.jadohealth.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 231.Rainey JJ, Watkins M, Ryman TK, Sandhu P, Bo A, Banerjee K. Reasons related to non-vaccination and under-vaccination of children in low and middle income countries: findings from a systematic review of the published literature, 1999-2009. Vaccine. 2011 Oct 26;29(46):8215–8221. doi: 10.1016/j.vaccine.2011.08.096. [DOI] [PubMed] [Google Scholar]
- 232.Offit PA. Studying complementary and alternative therapies. JAMA : the journal of the American Medical Association. 2012 May 2;307(17):1803–1804. doi: 10.1001/jama.2012.518. [DOI] [PubMed] [Google Scholar]
- 233.Offit PA. Should childhood vaccination be mandatory? Yes. Bmj. 2012;344:e2434. doi: 10.1136/bmj.e2434. [DOI] [PubMed] [Google Scholar]
- 234.Szmuness W, Stevens CE, Zang EA, Harley EJ, Kellner A. A controlled clinical trial of the efficacy of the hepatitis B vaccine (Heptavax B): a final report. Hepatology. 1981 Sep-Oct;1(5):377–385. doi: 10.1002/hep.1840010502. [DOI] [PubMed] [Google Scholar]
- 235.Szmuness W, Stevens CE, Harley EJ, et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. The New England journal of medicine. 1980 Oct 9;303(15):833–841. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- 236.Garcia F, Leon A, Gatell JM, Plana M, Gallart T. Therapeutic vaccines against HIV infection. Human vaccines & immunotherapeutics. 2012 May 1;8(5) doi: 10.4161/hv.19555. [DOI] [PubMed] [Google Scholar]
- 237.Modjarrad K, Vermund SH. An addition to the effect of treating co-infections on HIV-1 viral load. The Lancet infectious diseases. 2011 Feb;11(2):81. doi: 10.1016/S1473-3099(11)70013-5. [DOI] [PubMed] [Google Scholar]
- 238.Modjarrad K, Vermund SH. Effect of treating co-infections on HIV-1 viral load: a systematic review. The Lancet infectious diseases. 2010 Jul;10(7):455–463. doi: 10.1016/S1473-3099(10)70093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Barnabas RV, Webb EL, Weiss HA, Wasserheit JN. The role of coinfections in HIV epidemic trajectory and positive prevention: a systematic review and meta-analysis. Aids. 2011 Aug 24;25(13):1559–1573. doi: 10.1097/QAD.0b013e3283491e3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mbabazi PS, Andan O, Fitzgerald DW, Chitsulo L, Engels D, Downs JA. Examining the relationship between urogenital schistosomiasis and HIV infection. PLoS neglected tropical diseases. 2011 Dec;5(12):e1396. doi: 10.1371/journal.pntd.0001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hayes R, Watson-Jones D, Celum C, van de Wijgert J, Wasserheit J. Treatment of sexually transmitted infections for HIV prevention: end of the road or new beginning? Aids. 2010 Oct;24(4):S15–26. doi: 10.1097/01.aids.0000390704.35642.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.White RG, Orroth KK, Glynn JR, et al. Treating curable sexually transmitted infections to prevent HIV in Africa: still an effective control strategy? Journal of acquired immune deficiency syndromes. 2008 Mar 1;47(3):346–353. doi: 10.1097/QAI.0b013e318160d56a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Steen R, Wi TE, Kamali A, Ndowa F. Control of sexually transmitted infections and prevention of HIV transmission: mending a fractured paradigm. Bulletin of the World Health Organization. 2009 Nov;87(11):858–865. doi: 10.2471/BLT.08.059212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Siddappa NB, Hemashettar G, Shanmuganathan V, et al. Schistosoma mansoni enhances host susceptibility to mucosal but not intravenous challenge by R5 Clade C SHIV. PLoS neglected tropical diseases. 2011 Aug;5(8):e1270. doi: 10.1371/journal.pntd.0001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chenine AL, Shai-Kobiler E, Steele LN, et al. Acute Schistosoma mansoni infection increases susceptibility to systemic SHIV clade C infection in rhesus macaques after mucosal virus exposure. PLoS neglected tropical diseases. 2008;2(7):e265. doi: 10.1371/journal.pntd.0000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ayash-Rashkovsky M, Chenine AL, Steele LN, et al. Coinfection with Schistosoma mansoni reactivates viremia in rhesus macaques with chronic simian-human immunodeficiency virus clade C infection. Infection and immunity. 2007 Apr;75(4):1751–1756. doi: 10.1128/IAI.01703-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chenine AL, Buckley KA, Li PL, et al. Schistosoma mansoni infection promotes SHIV clade C replication in rhesus macaques. Aids. 2005 Nov 4;19(16):1793–1797. doi: 10.1097/01.aids.0000189857.51935.0b. [DOI] [PubMed] [Google Scholar]
- 248.Mugwanya K, Baeten JM, Mugo NR, Irungu E, Ngure K, Celum C. High-dose valacyclovir HSV-2 suppression results in greater reduction in plasma HIV-1 levels compared with standard dose acyclovir among HIV-1/HSV-2 coinfected persons: a randomized, crossover trial. The Journal of infectious diseases. 2011 Dec 15;204(12):1912–1917. doi: 10.1093/infdis/jir649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. The Journal of infectious diseases. 2007 Nov 15;196(10):1500–1508. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 250.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. The New England journal of medicine. 2010 Feb 4;362(5):427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Baeten JM, Strick LB, Lucchetti A, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. The Journal of infectious diseases. 2008 Dec 15;198(12):1804–1808. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mayaud P, Legoff J, Weiss HA, et al. Impact of acyclovir on genital and plasma HIV-1 RNA, genital herpes simplex virus type 2 DNA, and ulcer healing among HIV-1-infected African women with herpes ulcers: a randomized placebo-controlled trial. The Journal of infectious diseases. 2009 Jul 15;200(2):216–226. doi: 10.1086/599991. [DOI] [PubMed] [Google Scholar]
- 253.Dunne EF, Whitehead S, Sternberg M, et al. Suppressive acyclovir therapy reduces HIV cervicovaginal shedding in HIV- and HSV-2-infected women, Chiang Rai, Thailand. Journal of acquired immune deficiency syndromes. 2008 Sep 1;49(1):77–83. doi: 10.1097/QAI.0b013e3181831832. [DOI] [PubMed] [Google Scholar]
- 254.Ouedraogo A, Nagot N, Vergne L, et al. Impact of suppressive herpes therapy on genital HIV-1 RNA among women taking antiretroviral therapy: a randomized controlled trial. Aids. 2006 Nov 28;20(18):2305–2313. doi: 10.1097/QAD.0b013e328010238d. [DOI] [PubMed] [Google Scholar]
- 255.Drake AL, Roxby AC, Ongecha-Owuor F, et al. Valacyclovir suppressive therapy reduces plasma and breast milk HIV-1 RNA levels during pregnancy and postpartum: a randomized trial. The Journal of infectious diseases. 2012 Feb;205(3):366–375. doi: 10.1093/infdis/jir766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.(UNAIDS) JUNPoHA. Global report: UNAIDS report on the global AIDS epidemic 2010. 2010. [Google Scholar]
- 257.Shurtleff D, Lawrence D. HIV and Substance Abuse: A Commentary. Current HIV research. 2012 May 10; doi: 10.2174/157016212802138760. [DOI] [PubMed] [Google Scholar]
- 258.Centers for Disease C. HIV in the United States: At A Glance. Vol. 2012 Centers for Disease Control website: Centers for Disease Control; Mar, 2012. [Google Scholar]
- 259.Organization WH. HIV/AIDS Fact Sheet. Vol. 2011 World Health Organization website: World Health Organization; Nov, 2011. [Google Scholar]
- 260.Mathers BM, Degenhardt L, Ali H, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010 Mar 20;375(9719):1014–1028. doi: 10.1016/S0140-6736(10)60232-2. [DOI] [PubMed] [Google Scholar]
- 261.Des Jarlais DC, Arasteh K, Gwadz M. Increasing HIV prevention and care for injecting drug users. Lancet. 2010 Mar 20;375(9719):961–963. doi: 10.1016/S0140-6736(10)60314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Strathdee SA, Shoptaw S, Dyer TP, Quan VM, Aramrattana A for the Substance Use Scientific Committee of the HIVPTN. Towards combination HIV prevention for injection drug users: addressing addictophobia, apathy and inattention. Current opinion in HIV and AIDS. 2012 Apr 11; doi: 10.1097/COH.0b013e32835369ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Degenhardt L, Mathers B, Vickerman P, Rhodes T, Latkin C, Hickman M. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet. 2010 Jul 24;376(9737):285–301. doi: 10.1016/S0140-6736(10)60742-8. [DOI] [PubMed] [Google Scholar]
- 264.Wodak A, Maher L. The effectiveness of harm reduction in preventing HIV among injecting drug users. New South Wales public health bulletin. 2010 Mar-Apr;21(3-4):69–73. doi: 10.1071/NB10007. [DOI] [PubMed] [Google Scholar]
- 265.WHO/UNAIDS/UNICEF. Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. World Health Organization; 2011. [Google Scholar]
- 266.Barr S. Needle-Exchange Programs Face New Federal Funding Ban. KHN: Kaiser Health News; Dec 21, Dec 21, 2011. Public Health Politics. [Google Scholar]
- 267.Staff OoNDCP. Federal Funding Ban on Needle Exchange Programs. Vol. 2012. US White House; 2012. Jan 5, www.whitehouse.gov/blog. [Google Scholar]
- 268.Lackritz EM, Satten GA, Aberle-Grasse J, et al. Estimated risk of transmission of the human immunodeficiency virus by screened blood in the United States. The New England journal of medicine. 1995 Dec 28;333(26):1721–1725. doi: 10.1056/NEJM199512283332601. [DOI] [PubMed] [Google Scholar]
- 269.Dreier J, Gotting C, Wolff C, Petersen N, Kleesiek K. Recent experience with human immunodeficiency virus transmission by cellular blood products in Germany: antibody screening is not sufficient to prevent transmission. Vox sanguinis. 2002 Feb;82(2):80–83. doi: 10.1046/j.0042-9007.2001.00144.x. [DOI] [PubMed] [Google Scholar]
- 270.Velati C, Romano L, Fomiatti L, Baruffi L, Zanetti AR, Group SR. Impact of nucleic acid testing for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus on the safety of blood supply in Italy: a 6-year survey. Transfusion. 2008 Oct;48(10):2205–2213. doi: 10.1111/j.1537-2995.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 271.Weinberg PD, Hounshell J, Sherman LA, et al. Legal, financial, and public health consequences of HIV contamination of blood and blood products in the 1980s and 1990s. Annals of internal medicine. 2002 Feb 19;136(4):312–319. doi: 10.7326/0003-4819-136-4-200202190-00011. [DOI] [PubMed] [Google Scholar]
- 272.Ling AE, Robbins KE, Brown TM, et al. Failure of routine HIV-1 tests in a case involving transmission with preseroconversion blood components during the infectious window period. JAMA : the journal of the American Medical Association. 2000 Jul 12;284(2):210–214. doi: 10.1001/jama.284.2.210. [DOI] [PubMed] [Google Scholar]
- 273.Wake DJ, Cutting WA. Blood transfusion in developing countries: problems, priorities and practicalities. Tropical doctor. 1998 Jan;28(1):4–8. doi: 10.1177/004947559802800104. [DOI] [PubMed] [Google Scholar]
- 274.McFarland W, Mvere D, Shandera W, Reingold A. Epidemiology and prevention of transfusion-associated human immunodeficiency virus transmission in sub-Saharan Africa. Vox sanguinis. 1997;72(2):85–92. doi: 10.1046/j.1423-0410.1997.7220085.x. [DOI] [PubMed] [Google Scholar]
- 275.McFarland W, Kahn JG, Katzenstein DA, Mvere D, Shamu R. Deferral of blood donors with risk factors for HIV infection saves lives and money in Zimbabwe. Journal of acquired immune deficiency syndromes and human retrovirology: official publication of the International Retrovirology Association. 1995 Jun 1;9(2):183–192. [PubMed] [Google Scholar]
- 276.Cruz JR, Perez-Rosales MD, Zicker F, Schmunis GA. Safety of blood supply in the Caribbean countries: role of screening blood donors for markers of hepatitis B and C viruses. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2005 Dec;34(2):S75–80. doi: 10.1016/s1386-6532(05)80038-1. [DOI] [PubMed] [Google Scholar]
- 277.Schutz R, Savarit D, Kadjo JC, et al. Excluding blood donors at high risk of HIV infection in a west African city. Bmj. 1993 Dec 11;307(6918):1517–1519. doi: 10.1136/bmj.307.6918.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Centers for Disease C, Prevention. Progress toward strengthening national blood transfusion services--14 countries, 2008-2010. MMWR Morbidity and mortality weekly report. 2011 Nov 25;60(46):1577–1582. [PubMed] [Google Scholar]
- 279.Lackritz EM. Prevention of HIV transmission by blood transfusion in the developing world: achievements and continuing challenges. Aids. 1998;12(A):S81–86. [PubMed] [Google Scholar]
- 280.Heyns Adu P, Benjamin RJ, Swanevelder JP, et al. Prevalence of HIV-1 in blood donations following implementation of a structured blood safety policy in South Africa. JAMA : the journal of the American Medical Association. 2006 Feb 1;295(5):519–526. doi: 10.1001/jama.295.5.519. [DOI] [PubMed] [Google Scholar]
- 281.Weller S, Davis K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane database of systematic reviews. 2002;(1):CD003255. doi: 10.1002/14651858.CD003255. [DOI] [PubMed] [Google Scholar]
- 282.Tavory I, Swidler A. Condom Semiotics: Meaning and Condom use in Rural Malawi. American Sociological Review. 2009;74(2):171–189. [Google Scholar]
- 283.Lagarde E, Carael M, Glynn JR, et al. Educational level is associated with condom use within non-spousal partnerships in four cities of sub-Saharan Africa. Aids. 2001 Jul 27;15(11):1399–1408. doi: 10.1097/00002030-200107270-00009. [DOI] [PubMed] [Google Scholar]
- 284.Irungu E, Chersich MF, Sanon C, et al. Changes in sexual behaviour among HIV-infected women in west and east Africa in the first 24 months after delivery. Aids. 2012 May 15;26(8):997–1007. doi: 10.1097/QAD.0b013e3283524ca1. [DOI] [PubMed] [Google Scholar]
- 285.Agha S. Intention to use the female condom following a mass-marketing campaign in Lusaka, Zambia. American journal of public health. 2001 Feb;91(2):307–310. doi: 10.2105/ajph.91.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Pool R. Acceptability of the female condom and vaginal spermicidal products in Uganda. Sexual health exchange. 1999;(1):5–7. [PubMed] [Google Scholar]
- 287.Galvao LW, Oliveira LC, Diaz J, et al. Effectiveness of female and male condoms in preventing exposure to semen during vaginal intercourse: a randomized trial. Contraception. 2005 Feb;71(2):130–136. doi: 10.1016/j.contraception.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 288.Beksinska M, Smit J, Joanis C, Usher-Patel M, Potter W. Female condom technology: new products and regulatory issues. Contraception. 2011 Apr;83(4):316–321. doi: 10.1016/j.contraception.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 289.Macaluso M, Blackwell R, Jamieson DJ, et al. Efficacy of the male latex condom and of the female polyurethane condom as barriers to semen during intercourse: a randomized clinical trial. American journal of epidemiology. 2007 Jul 1;166(1):88–96. doi: 10.1093/aje/kwm046. [DOI] [PubMed] [Google Scholar]
- 290.Macaluso M, Lawson ML, Hortin G, et al. Efficacy of the female condom as a barrier to semen during intercourse. American journal of epidemiology. 2003 Feb 15;157(4):289–297. doi: 10.1093/aje/kwf212. [DOI] [PubMed] [Google Scholar]
- 291.Joanis C, Beksinska M, Hart C, Tweedy K, Linda J, Smit J. Three new female condoms: which do South-African women prefer? Contraception. 2011 Mar;83(3):248–254. doi: 10.1016/j.contraception.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 292.MacPhail C, Campbell C. 'I think condoms are good but, aai, I hate those things': condom use among adolescents and young people in a Southern African township. Social science & medicine. 2001 Jun;52(11):1613–1627. doi: 10.1016/s0277-9536(00)00272-0. [DOI] [PubMed] [Google Scholar]
- 293.Agha S, Karlyn A, Meekers D. The promotion of condom use in non-regular sexual partnerships in urban Mozambique. Health policy and planning. 2001 Jun;16(2):144–151. doi: 10.1093/heapol/16.2.144. [DOI] [PubMed] [Google Scholar]
- 294.Grimley DM, Hook EW, 3rd, DiClemente RJ, Lee PA. Condom use among low-income African American males attending an STD clinic. American journal of health behavior. 2004 Jan-Feb;28(1):33–42. doi: 10.5993/ajhb.28.1.4. [DOI] [PubMed] [Google Scholar]
- 295.Kwon JA, Iversen J, Maher L, Law MG, Wilson DP. The impact of needle and syringe programs on HIV and HCV transmissions in injecting drug users in Australia: a model-based analysis. Journal of acquired immune deficiency syndromes. 2009 Aug 1;51(4):462–469. doi: 10.1097/QAI.0b013e3181a2539a. [DOI] [PubMed] [Google Scholar]
- 296.Aceijas C, Hickman M, Donoghoe MC, Burrows D, Stuikyte R. Access and coverage of needle and syringe programmes (NSP) in Central and Eastern Europe and Central Asia. Addiction. 2007 Aug;102(8):1244–1250. doi: 10.1111/j.1360-0443.2007.01848.x. [DOI] [PubMed] [Google Scholar]
- 297.Stoneburner RL, Low-Beer D. Sexual partner reductions explain human immunodeficiency virus declines in Uganda: comparative analyses of HIV and behavioural data in Uganda, Kenya, Malawi, and Zambia. International journal of epidemiology. 2004 Jun;33(3):624. doi: 10.1093/ije/dyh141. [DOI] [PubMed] [Google Scholar]
- 298.Hallett TB, Aberle-Grasse J, Bello G, et al. Declines in HIV prevalence can be associated with changing sexual behaviour in Uganda, urban Kenya, Zimbabwe, and urban Haiti. Sexually transmitted infections. 2006 Apr;82(1):i1–8. doi: 10.1136/sti.2005.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Bunnell R, Opio A, Musinguzi J, et al. HIV transmission risk behavior among HIV-infected adults in Uganda: results of a nationally representative survey. Aids. 2008 Mar 12;22(5):617–624. doi: 10.1097/QAD.0b013e3282f56b53. [DOI] [PubMed] [Google Scholar]
- 300.Stoneburner RL, Low-Beer D. Population-level HIV declines and behavioral risk avoidance in Uganda. Science. 2004 Apr 30;304(5671):714–718. doi: 10.1126/science.1093166. [DOI] [PubMed] [Google Scholar]
- 301.Dzewaltowski DA, Glasgow RE, Klesges LM, Estabrooks PA, Brock E. RE-AIM: evidence-based standards and a Web resource to improve translation of research into practice. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2004 Oct;28(2):75–80. doi: 10.1207/s15324796abm2802_1. [DOI] [PubMed] [Google Scholar]
- 302.Dzewaltowski DA, Estabrooks PA, Klesges LM, Bull S, Glasgow RE. Behavior change intervention research in community settings: how generalizable are the results? Health promotion international. 2004 Jun;19(2):235–245. doi: 10.1093/heapro/dah211. [DOI] [PubMed] [Google Scholar]
- 303.Latham TP, Sales JM, Boyce LS, et al. Application of ADAPT-ITT: adapting an evidence-based HIV prevention intervention for incarcerated African American adolescent females. Health promotion practice. 2010 May;11(3 Suppl):53S–60S. doi: 10.1177/1524839910361433. [DOI] [PubMed] [Google Scholar]
- 304.Matovu JK. Preventing HIV transmission in married and cohabiting HIV-discordant couples in sub-Saharan Africa through combination prevention. Current HIV research. 2010 Sep;8(6):430–440. doi: 10.2174/157016210793499303. [DOI] [PubMed] [Google Scholar]
- 305.Granich R, Crowley S, Vitoria M, et al. Highly active antiretroviral treatment as prevention of HIV transmission: review of scientific evidence and update. Current opinion in HIV and AIDS. 2010 Jul;5(4):298–304. doi: 10.1097/COH.0b013e32833a6c32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.DeGruttola V, Smith DM, Little SJ, Miller V. Developing and evaluating comprehensive HIV infection control strategies: issues and challenges. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010 May 15;50(3):S102–107. doi: 10.1086/651480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kurth AE, Celum C, Baeten JM, Vermund SH, Wasserheit JN. Combination HIV prevention: significance, challenges, and opportunities. Current HIV/AIDS reports. 2011 Mar;8(1):62–72. doi: 10.1007/s11904-010-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Padian NS, McCoy SI, Karim SS, et al. HIV prevention transformed: the new prevention research agenda. Lancet. 2011 Jul 16;378(9787):269–278. doi: 10.1016/S0140-6736(11)60877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Katsidzira L, Hakim JG. HIV prevention in southern Africa: why we must reassess our strategies? Tropical medicine & international health : TM & IH. 2011 Sep;16(9):1120–1130. doi: 10.1111/j.1365-3156.2011.02807.x. [DOI] [PubMed] [Google Scholar]