Abstract
This paper reviews the scientific evidence for the safety of carbon monoxide (CO) and nitric oxide (NO) inhalation to measure pulmonary diffusing capacity (DLCO and DLNO) in pregnant women and their fetuses. In eight earlier studies, 650 pregnant women had DLCO measurements performed at various times during pregnancy, with a minimum of two to four tests per session. Both pregnant subjects that were healthy and those with medical complications were tested. No study reported adverse maternal, fetal, or neonatal outcomes from the CO inhalation in association with measuring DLCO. Eleven pregnant women, chiefly with pulmonary hypertension, and 1105 pre-term neonates, mostly with respiratory failure, were administered various dosages of NO (5–80 ppm for 4 weeks continuously in pregnant women, and 1–20 ppm for 15 min to 3 weeks for the neonates). NO treatment was found to be an effective therapy for pregnant women with pulmonary hypertension. In neonates with respiratory failure and pulmonary hypertension, NO therapy improved oxygenation and survival and has been associated with only minor, transient adverse effects. In conclusion, maternal carboxyhemoglobin ([HbCO]) levels can safely increase to 5% per testing session when the dose-exposure limit is 0.3% CO inhalation for ≤3 min, and for NO, 80 ppm for ≤ 3 min. The risk of late fetal or neonatal death from increased HbCO from diffusion testing is considerably less than the risk of death from all causes reported by the Centers for Disease Control, and is therefore considered “minimal risk”.
Keywords: Carbon monoxide, Nitric oxide, Guidelines, Recommendations, Pregnancy
1. Introduction
The 2005 guidelines for standardizing pulmonary function tests have been jointly updated, approved, and published by the American Thoracic Society (ATS) and the European Respiratory Society (ERS) (Macintyre et al., 2005; Miller et al., 2005a,b; Pellegrino et al., 2005; Wanger et al., 2005). These guidelines reflect the current knowledge in the field, and establish measures of safe clinical practice in quantifying pulmonary function. According to the ATS/ERS guidelines, only a few circumstances exist in which lung function testing is contraindicated (Miller et al., 2005b). For lung function testing that includes measurements of pulmonary diffusing capacity, there are only four contraindications: (1) chest or abdominal pain, (2) oral or facial pain, (3) stress incontinence, (4) and dementia. Pregnancy is not considered a contraindication for the measurement of pulmonary diffusing capacity (Miller et al., 2005b).
Despite the ATS/ERS guidelines, the question of whether measurement of pulmonary diffusing capacity in pregnant women is “safe” and “warranted” for themselves and their fetuses has not been studied adequately. Concern with performing diffusing capacity measurements during pregnancy stems from carbon monoxide (CO) exposure, resulting in increases in carboxyhemoglobin (HbCO) in the blood of the mother and fetus with each test. Accumulation of HbCO in excess can diminish the oxygen-carrying capacity of maternal and fetal blood to unsafe levels.
Nonetheless, determination of pulmonary diffusing capacity can be an excellent prognostic indicator for maternal and fetal outcomes, fitness, and mortality. Since impaired lung function is associated with an increase and recurrence of cardiovascular disease (Coburn et al., 1963), measuring pulmonary function in a pregnant woman may be a valuable prognostic indicator of adverse maternal (e.g. preeclampsia, gestational diabetes, cesarean section) and fetal outcomes (e.g. large for gestational age, small for gestational age infants). In fact, a forced expiratory volume in 1 s that is <80% of predicted in women who are pregnant is related to higher incidence of pre-term births (<37 weeks gestation), a higher prevalence of gestational hypertension, and a higher risk for low birth weight babies (<2500 g) (Getahun et al., 2006, 2007; Schatz et al., 2006). Most recently, a low maternal hemoglobin concentration (<10 g/dl) has been related to a higher risk for stillbirths, pre-term births, and small for gestational age babies (Gonzales et al., 2009). Because hemoglobin concentration is a determinant of pulmonary diffusing capacity, measurement of pulmonary diffusion may also relate to maternal and fetal outcomes, a possibility that has not yet been tested. Pulmonary diffusing capacity for carbon monoxide (DLCO) and nitric oxide (DLNO) at rest is also related to aerobic capacity (Zavorsky et al., 2009), a strong independent predictor of death in women (Gulati et al., 2003) and men (Myers et al., 2002). Thus, a measurement of pulmonary diffusion could be a prognostic marker for mortality in pregnancy. Low pulmonary diffusion in a pregnant woman may prompt a physician to recommend regular aerobic exercise to improve her fitness. Finally, DLCO and DLNO are sensitive indicators of the morphological changes assessed with computed tomography to detect emphysema and cystic fibrosis (Dressel et al., 2009; van der Lee et al., 2009).
What is the clinical significance of measuring alveolar membrane diffusing capacity (DM) in the pregnant female “diseased” lung? Well, the global measurement of DLCO only provides a global indication of whether gas exchange is normal or not. It does not specify where the abnormal physiology lies, whether the issue is low (or high) hemoglobin concentration, or low (or high) pulmonary capillary blood volume, or whether the problem lies solely within the alveolar-capillary membrane. A measurement of both DLCO and DLNO together allows partitioning to obtain pulmonary capillary blood volume (Vc) and DM (DM is essentially DLNO) so that there is a more precise determination of the location of the pathophysiology. Pregnant women who have a high DM/Vc ratio (which is equal to the DLNO/DLCO ratio) should be evaluated for high pulmonary artery pressure compatible with pulmonary hypertension (Bonay et al., 2004; van der Lee et al., 2006). Therefore, a disproportionate reduction in DM relative to Vc would decrease the DM/Vc ratio (and thus decrease the DLNO/DLCO ratio), which is related to a wide spectrum of pulmonary vascular diseases (Oppenheimer et al., 2006) and could apply to pregnant women. Diabetes, which can cause pulmonary microangiopathy, lowers DLNO/DLCO compared to controls (Chance et al., 2008). Thus, a DLNO and DLCO measurement could be a prognostic indicator for gestational diabetes.
Therefore, future direction of the measurements of DLCO and DLNO in pregnancy has promise as a screening tool for predicting aerobic capacity (as a determinant of mortality), and to help identify pulmonary hypertension, pulmonary vascular diseases, and gestational diabetes (including type II and possibly type I diabetes). With the goals of advancing knowledge of alveolar gas exchange during pregnancy and ensuring safe testing of lung function, the guidelines proposed herein should help to facilitate the use of pulmonary diffusing capacity measurement in the pregnant woman.
2. Carbon monoxide
Pulmonary diffusing capacity for carbon monoxide is a standard function test that measures alveolar-capillary diffusion. Because the measurement of oxygen transfer through the lung is technically difficult, and may be limited by blood flow and pulmonary tissue O2 consumption, carbon monoxide (CO) has been used as an indirect index of oxygen transfer, due to its high affinity to bonding with hemoglobin (Forster, 1957).
The standard DLCO protocol is to use the single-breath method. Following a few normal breaths, a subject first exhales to residual volume, and then inhales a standard concentration of gases (0.3% CO, 10% Helium, 21% O2, balance N2) to total lung capacity. As total lung capacity and vital capacity are minimally affected regardless of the stage of pregnancy (Alaily and Carrol, 1978; Baldwin et al., 1977; McAuliffe et al., 2002; Milne, 1979) and regardless of obesity (Eng et al., 1975), inhalation to total lung capacity is possible during all three trimesters. After a 10-s breath-hold, the patient (subject) exhales in a smooth, unforced manner, without hesitation or interruption all the way to residual volume. The first 0.75–1.0 L of expired air is discarded, and only the second liter of air (which reflects alveolar gas) is analyzed. The remainder of the expired air is discarded as well. DLCO is calculated as the total CO uptake over time divided by the alveolar partial pressure of CO (Macintyre et al., 2005).
DLCO can also be measured by rebreathing a standard concentration of gases (0.3% CO, 10% Helium, 35% O2, balance N2), with a bag volume that ranges from tidal volume (Snyder et al., 2006) to 60% of vital capacity (or 500 ml to 3.5 L) (Takahashi et al., 1995). Gases are inhaled from a closed circuit anesthesia bag, and rebreathed for 15 s at a frequency of about 30 breaths/min. The calculation of DLCO using the rebreathing technique is essentially the same as that with breath-holding. This method for DLCO is less commonly used, as it requires more expensive equipment, such as a respiratory mass spectrometer, and more complex calculations. However, because with rebreathing the CO gas is distributed in the lung more evenly, an advantage of this method, as opposed to the single-breath, is its relative insensitivity to unequal distribution of ventilation (Jansons et al., 1998; Roberts et al., 1990) and diffusion (Jansons et al., 1998; Kreukniet, 1970). Furthermore, rebreathing is preferred when subjects cannot hold their breath for long periods or, if for some reason, vital capacity is too small.
The primary safety concern with inhaling CO is the increase in fetal and maternal carboxyhemoglobin concentration ([HbCO]) resulting in diminished blood oxygen-carrying capacity. Carbon monoxide is a naturally occurring gas that is produced endogenously by catabolism of hemoglobin and other heme pigments, chiefly in the liver and spleen. Endogenous CO production is approximately 0.001–0.007 ml/min (Coburn et al., 1963, 1965). In a non-smoking individual this results in a normal carboxy-hemoglobin level of about 0.7–1.1% (Coburn et al., 1965), which increases in pregnancy (Delivoria-Papadopoulos et al., 1974). In non-smoking pregnant mothers, maternal [HbCO] is reported to be 1.1 ± 0.2% (Longo, 1976), but the range is wide, at 0.4–2.6% (Longo, 1977). Fetal [HbCO] is about 1.8 ± 0.3% (Longo, 1976), with a range of 0.4–2.8% (Longo, 1977).
Tests of pulmonary diffusing capacity increase [HbCO] by approximately 0.7–0.8% per 10-s breath-hold maneuver (Forster et al., 1954; Frey et al., 1987). The standard mixture of gases in a medical grade gas tank for testing is 0.3% CO, 10% He, 21% O2, with a balance of N2. Therefore, whether the diffusing capacity measurement involves single breath-hold or rebreathing maneuvers, the increase in [HbCO] is about 0.7% per test. The ATS/ERS guidelines suggest that no more than five measurements of diffusing capacity tests be performed in one testing session for all subjects, including pregnant women (Macintyre et al., 2005). The limit of five diffusing capacity tests is not related to safety concerns with [HbCO] accumulation, but rather because each maneuver increases [HbCO] by about 0.7% and may reduce the DLCO measured in subsequent tests due to a back pressure of CO in lung capillaries. Five diffusing capacity tests in a session reduces DLCO by 1.5 ml/min/Torr, which equates to a reduction of about 5% from the first to the fifth determination (Zavorsky and Murias, 2006). These guidelines are based on DLCO measurement at rest, however. With exercise, additional testing may be warranted to match the various levels of oxygen consumption to DLCO. In that manner, the slope of the relation between oxygen consumption and DLCO would provide an indication of pulmonary microvascular regulation.
Table 1 presents the eight published studies in which DLCO measurements were performed in pregnant women using the standard inspiratory CO concentration of 0.3% CO per test (Boggess et al., 1995; Garcia-Rio et al., 1996; Gazioglu et al., 1970; Lalli and Raju, 1981; Lehmann, 1975; McAuliffe et al., 2003, 2002; Milne et al., 1977; Norregaard et al., 1989). These tests were performed at rest, and some of these women had lung and/or cardiopulmonary disease (Boggess et al., 1995; Gazioglu et al., 1970; Lalli and Raju, 1981). In each study, two to four diffusion tests were performed per session. DLCO decreased by 10–15% in the second and third trimester in both singleton and twin pregnancies compared to the first trimester and post-partum (Gazioglu et al., 1970; McAuliffe et al., 2002; Norregaard et al., 1989). Greater resistance to diffusion through the alveolar membrane (DM) and not a decrease in pulmonary capillary blood volume (Vc) accounted for the decrease in DLCO (Gazioglu et al., 1970). In pregnant women with emphysema, DLCO increases throughout pregnancy, with increased Vc being the likely cause. In pregnant women with pulmonary sarcoidosis, DLCO, alveolar membrane diffusing capacity for CO (DMCO), and Vc remain unaltered throughout pregnancy (Gazioglu et al., 1970). In the testing of 650 pregnant women and their fetuses no adverse events have been reported (Table 1).
Table 1.
Studies that measured DLCO in pregnant women.
| Study | Number and type of pregnant women | Number of diffusing capacity tests done per subject per session | Conclusion of the study |
|---|---|---|---|
| McAuliffe et al. (2003) | 112 (sea level) and 192 (4300m) healthy subjects from Peru | 2 tests per session. 1 session total (cross-sectional study) | DLCO measured in women living at altitude versus without altitude in the first, second, and third trimester compared to non-pregnant controls. DLCO was higher at altitude and DLCO decreased by about 15% by the third trimester. |
| McAuliffe et al. (2002) | 68 women with twin pregnancies and 140 women with singleton pregnancies (all healthy) | 2 tests per session. 1 session total (cross-sectional study) | To compare the differences in lung function between women with twin or singleton pregnancies, various lung function tests were performed. DLCO did not change between the first and the third trimester in women with either twin or singleton pregnancies. DLCO was 10% lower compared to non-pregnant women. |
| Milne et al. (1977) | 21 healthy subjects | 2 tests per session; 9 sessions total | DLCO was measured at 8 different time points during pregnancy and 3–5 months post-partum. DLCO decreased by 16% by the third trimester. [Hb] was lowest at 20–23 weeks gestation. |
| Lehmann (1975) | 23 healthy subjects; 8 of those reporting spontaneous reported dyspnea with pregnancy | About 2 tests per session. 5 sessions total | DLCO was measured at 12, 24, 32, and 36 weeks of gestation, and 12 weeks post-partum. Women with dyspnea in early pregnancy (12th week gestation) had a 10% decrease in DLCO. Non-dyspneic women did not show a decrease in DLCO by the 12th week. |
| Norregaard et al. (1989) | 39 healthy subjects; (10 in each trimester and 9 post-partum) | 2 series of repeated measurements (seated and supine). So 4 tests in total per session. 5 sessions in total | Lung function and postural changes with pregnancy (first, second, and third trimester), and about 2–4 weeks post-partum. DLCO decreased by about 15% by the third trimester, regardless if the measurement was done in the sitting or supine position. No change in DLCO between sitting and supine. |
| Gazioglu et al. (1970) | 24 subjects; 8 healthy, 8 valvular heart disease, 8 chronic pulmonary disease | At least 2 times per session. 4 sessions total | DLCO, DM, Vc were measured at 10, 24, and 36 weeks gestation, and 10 weeks post-partum. In normal subjects, DLCO and Dm equally decrease by 14% by 36 weeks gestation. No change in Vc. In those with emphysema, DLCO and Vc increased by 36 wks gestation with no change in DM. Those with pulmonary sarcoidosis had no change in DLCO, DM, or Vc. |
| Garcia-Rio et al. (1996) | 23 subjects; 11 healthy with dyspnea, 12 healthy asymptomatic | At least 2 times per session. 4 sessions total | DLCO was measured 12, 24, 36 weeks gestation, and 16 weeks post-partum. DLCO was not altered during pregnancy in either the non-dyspneic or dyspneic group. There was no difference in DLCO in either group. The increase in dyspnea in pregnant women could be due to an excessive increase in sensitivity to CO2 or hypoxia. |
| Boggess et al. (1995) | 9 subjects; interstitial and restrictive lung disease | About 2 times per session. 1 session total | 3 women had DLCOs ≤ 40% predicted; 6 women ≥44% predicted in the first trimester. All women had vital capacity’s ≤84% predicted. There was an association between DLCO and vital capacity (r2 = 0.63). Mean birth weight was (50th percentile) was not different between those with the most severe restrictive lung disease or the least severe restrictive lung disease. Restrictive lung disease can be tolerated in pregnancy. Exercise intolerance was common and patients may require early supplemental oxygen, DLCO < 50% better predicted the need for oxygen supplementation than did vital capacity < 1.5 L. |
All diffusion testing was accomplished with the single-breath DLCO technique. No study reported adverse outcomes on mothers or their babies before or after birth.
The current Occupational Safety and Health Administration (OSHA) permissible exposure limit (PEL) for carbon monoxide for all workers, including pregnant women, is 50 parts per million (ppm) (55 mg/m3) with an 8-h time-weighted average (TWA) concentration (28,800 s) (National Institute for Occupational Safety and Health, 2005). Thus, data presented in Table 1 demonstrate that CO inhalation at concentrations recommended by the ATS/ERS for DLCO testing is within acceptable limits of the U.S. government regulations and has been demonstrated to be safe in a number of published reports.
Overall pulmonary CO diffusion can be partitioned into the subcomponents of alveolar DM and pulmonary capillary blood volume. The equation for DLCO has been described by Roughton and Forster (1957):
where DMCO is the alveolar membrane diffusing capacity for carbon monoxide, ΘCO is the specific blood transfer conductance for CO, and Vc is the pulmonary capillary blood volume (Roughton and Forster, 1957). To obtain DMCO and Vc, DLCO has been traditionally measured at two different levels of alveolar PO2 (PAO2), e.g., at about 100–120 mmHg and about 600 mmHg. For each alveolar PO2 level, 1/DLCO is plotted on the y-axis and 1/ΘCO is plotted on the x-axis. A line is drawn through the two points and the y-intercept (1/DMCO) and slope (1/Vc) can be solved.
3. Nitric oxide
During the past 15 years, the measurement of diffusion capacity of the lung using the transfer gases CO and nitric oxide (NO) together has been developed to obtain DMCO and Vc in a single-breath maneuver. The advantage of adding NO is that its rate of combination with hemoglobin is many-fold faster than that for CO (Meyer and Piiper, 1989), and the specific blood transfer conductance for NO (ΘNO) is so great that the red cell resistance to NO (1/ΘNO) approaches zero (Johnson et al., 1996; Manier et al., 1993, 1991; Phansalkar et al., 2004; Tamhane et al., 2001; Zavorsky and Lands, 2005; Zavorsky et al., 2004). Therefore, DLNO equals membrane diffusion capacity for NO (DMNO) and is independent of either pulmonary capillary blood volume or hemoglobin concentration (van der Lee et al., 2005). The ratio of DLNO to DMCO is about 2.4 (Phansalkar et al., 2004; Tamhane et al., 2001); therefore, DMCO = DLNO/2.4. Because exposures of ≤60 ppm NO of a few seconds to a few minutes do not interfere with various cardiopulmonary parameters or DLCO in adults (Brett et al., 1998; Phansalkar et al., 2004; Sheel et al., 2001; Tamhane et al., 2001; Zavorsky and Murias, 2006), it is useful to use NO along with CO to assess alveolar-membrane function and Vc.
The ability to estimate DMCO and pulmonary capillary blood volume from the one-step simultaneous measurement of DLNO and DLCO has at least four advantages over the traditional two-step method. (1) With the standard method, cardiac output may vary between measurements of DLCO at different oxygen tensions, which then have to be interpolated to obtain DLCO at the two O2 tensions at the same cardiac output (Phansalkar et al., 2004). With the DLNO–DLCO method, all measurements are obtained at the same cardiac output and O2 tension, thus no interpolation is necessary. (2) With the traditional method, the CO gas distribution in the lungs may be different at two different oxygen tensions, but with the DLNO–DLCO method only one inspiration is required which results in a similar distribution of NO and CO throughout the lung. (3) With the traditional two-step method, there is systematic underestimation of Vc and an overestimation of DMCO, as the inspiration at two different oxygen tensions affects alveolar-capillary membrane diffusion (Hsia et al., 1995). The DLNO–DLCO method avoids this error and improves the accuracy of estimated DMCO and Vc. (4) The DLNO–DLCO method reduces the testing time and number of measurements by half, allowing for easier data collection and half the CO exposure. This is a significant advantage for investigation as well as patient care.
Like CO, NO is produced by the body and can be measured in exhaled breath. The amount of exhaled NO per breath is about 2–22 parts per billion in women (Olivieri et al., 2006). As with CO, safety is a concern when using NO in pregnant women. Nitric oxide reacts at a nearly diffusion-limited rate with oxyhemoglobin to produce methemoglobin, and reacts with deoxyhemoglobin to produce iron nitrosyl hemoglobin (HbNO). Because neither methemoglobin nor HbNO can bind oxygen, accumulation of either species can become a safety concern if it occurs to an extent that it impairs significantly the oxygen-carrying capacity of the blood. Small accumulations of methemoglobin and HbNO are of minimal safety concern, because methemoglobin (Fe3+) is reduced to ferrous hemoglobin (Fe2+) by endogenous methemoglobin reductase enzymes, with a methemoglobin half-life of 2–4 h (Blood and Power, 2007), and HbNO is eliminated with a half-life of 15–45 min (Takahashi et al., 1998). Due to the rapid reaction of NO with pulmonary capillary hemoglobin, no free NO reaches the fetus. In fact, the half-life of NO in blood is only milliseconds (Eich et al., 1996) and any free NO diffusing into pulmonary capillary blood is metabolized before reaching the systemic circulation.
In the presence of oxygen, NO converts to nitrogen dioxide (NO2), a potent oxidant that can result in pulmonary edema and lung injury. However, with the addition of 60 ppm NO in the presence of 35% oxygen in an anesthesia bag for 30 s, only about 1 ppm of NO2 will be produced (Fine, 1972; Sokol et al., 1999). Therefore, NO2 production is negligible provided care is taken to prevent the mixture of NO with air until immediately prior to inhalation.
4. Evaluating the risk of CO and NO inhalation
In several research studies, [HbCO] has been increased experimentally, providing information regarding the toxicity of inhaled CO. One study increased [HbCO] in ten healthy subjects to 15% over a period of 30 min to test a new monitoring device (Barker et al., 2006). No adverse outcomes were reported. Despite a reduction in aerobic capacity (V̇O2 peak), which decreased by 6–9% when [HbCO] was 4–7% (Ekblom and Huot, 1972; Horvath et al., 1975; Raven et al., 1974), and decreased by 15–24% when [HbCO] was 15–20% (Vogel and Gleser, 1972; Vogel et al., 1972), no adverse outcomes were reported, and [HbCO] returned to normal within 24 h.
A reduction in arterial oxyhemoglobin concentration [HbO2] (as from CO exposure) with reduction of arterial oxygen content induces systemic compensatory mechanisms. These include an increase in the fraction of oxygen extracted by the tissues, and increased blood flow, so that oxygen consumption is maintained (Vogel and Gleser, 1972). During moderate exercise, cardiac output increases (Pirnay et al., 1971; Vogel and Gleser, 1972; Vogel et al., 1972), while pH, arterial PCO2 and PO2, the alveolar-to-arterial PO2 difference and the respiratory exchange ratio are not affected, even when [HbCO] equals 18–20% (Brody and Coburn, 1970; Vogel and Gleser, 1972). Thus, appropriate compensatory mechanisms are in place when [HbCO] is increased, as high as up to levels of 20% [HbCO].
When arterial PO2 is normal (95–100 Torr), as in breathing room air, the increase in [HbCO] changes the shape of the oxyhemoglobin dissociation curve similarly as if there were only a reduction in hemoglobin concentration (Brody and Coburn, 1969). For example, a [HbCO] of 10% reduces the arterial oxygen content by the same amount as a reduction in hemoglobin concentration by 1.5 g/dl (Brody and Coburn, 1969). Therefore, a female with a hemoglobin concentration of 10.5 g/dl and 98% arterial oxyhemoglobin saturation [HbO2] would have the same arterial oxygen content as a female with a hemoglobin concentration of 12 g/dl and an arterial [HbO2] of 88% (Brody and Coburn, 1969).
The use of inhaled NO gas has been routine in the treatment of pulmonary hypertension in both newborn and adult patients for more than 10 years, providing useful information in evaluating the safety of NO for diffusion testing in pregnant women. As detailed in Table 2, a number of reports have described the use of inhaled NO in pregnant women suffering from pulmonary hypertension (Bonnin et al., 2005; Decoene et al., 2001; Goodwin et al., 1999; Lam et al., 2001; Lust et al., 1999; McMillan et al., 2002; Robinson et al., 1999). In these reports, NO was administered at delivery rates of 5–80 ppm for minutes to hours, with no reported elevations of maternal methemoglobin levels. In term and pre-term infants, inhaled NO has been given at concentrations as high as 20 ppm and for up to a week at a time (Table 3). The administration of 1–20 ppm NO to infants for days at a time was well-tolerated with the only noticeable effect being increased concentrations of methemoglobin. Even so, the methemoglobin concentrations were not high enough to warrant discontinuation of inhaled NO treatment. The production of methemoglobin is a result of the direct reaction between NO and oxyhemoglobin in the lungs. Because NO in the blood is metabolized too rapidly for it to travel from the maternal lung to the fetal circulation, there should be no concern that inhaling NO will result in fetal methemoglobinemia. Large multi-center studies with thousands of enrolled patients have demonstrated no adverse effects, including methemoglobinemia, of inhaled NO administered for days or even weeks at 20 ppm (Kinsella and Abman, 2007). In fact, studies demonstrate that inhaled NO in pre-term infants decreases the risk of cognitive impairment and abnormal neurodevelopmental outcomes (i.e. cerebral palsy, bilateral blindness, bilateral hearing loss, or development delay) 2 years later by about half, compared to a placebo group (Mestan et al., 2005). Other studies have revealed no adverse NO attributable neurodevelopmental outcomes by 2 years of age (Hintz et al., 2007; Konduri et al., 2007) in NO-exposed infants.
Table 2.
Studies and case reports of inhaled NO use in pregnant women.
| Study | Number and type of pregnant women | Concentration and length of time NO was inhaled | Outcome |
|---|---|---|---|
| Robinson et al. (1999) (case report) | 1 subject; 28 weeks gestation to delivery at 32 weeks and post-partum, with h/o HIV, pulmonary hypertension, peripartum cardiomyopathy | 5–20 ppm, 4 weeks continuous inhaled NO with episodic monitoring of methemoglobin levels. | Inhaled NO reduced pulmonary artery pressure and right ventricular pressure, prolonged continuous inhaled NO therapy may be an effective therapy in the management of pulmonary hypertension during pregnancy. |
| Lust et al. (1999) (case report) | 1 subject; delivery and post-partum, Eisenmenger’s syndrome | Per nasal cannula during delivery, after delivery 10 ppm, ICU NO was delivered via transtracheal catheter. 10 h. | Continuous inhaled NO reduced initial pulmonary arterial pressure and improved oxygenation. Inhaled NO may be used to improve oxygenation and antithrombotic effects of NO may limit the increase in pulmonary arterial pressure expected with increased cardiac output throughout labor among pts with pulmonary vascular disease. |
| Goodwin et al. (1999) (case report) | 1 subject; 36 weeks gestation with Eisenmenger’s syndrome | 20 ppm during labor. 80 ppm decreased to 60 ppm by 3rd day post-partum. Continuous during second stage of labor (45 min) and post-partum 3rd day, discontinued after 48 h. | Inhaled NO can be used to correct the hypoxemia of Eisenmenger’s syndrome. Administration of NO led to improved oxygenation and lowered pulmonary arterial pressures. Baby survived, woman died. |
| Decoene et al. (2001) (case report) | 1 subject, unexpected pulmonary hypertension that had an emergency C-section | 5 ppm 24 h continuous during labor, delivery and post-partum. | Administration of inhaled NO enabled optimal control of pulmonary hypertension. Use of inhaled NO can improve the management of urgent C-section in women with unexpected pulmonary hypertension. |
| Lam et al. (2001) (case report) | 1 subject, primigravida with primary pulmonary hypertension | 20 ppm, decreased to 10 ppm 8.5 h after delivery, NO delivered through an endotracheal tube (93 h total). | NO can be used to successfully treat primary pulmonary hypertension in pregnancy. |
| Bonnin et al. (2005) | 15 subjects with severe pulmonary hypertension, 3 were administered NO | 50 ppm. | In the 3 out of 15 subjects who were administered NO, 2 babies survived after delivery. The baby that died was delivered at 21 weeks gestation. The babies that survived at 32–34 weeks gestation. Maternal mortality was found to be 36% with pulmonary arterial hypertension. Pregnancy should be discouraged in patients with severe pulmonary hypertension. |
| McMillan et al. (2002) | 3 with pulmonary hypertension secondary to systemic lupus erythematosis (SLE) and anti-phospholipid syndrome | 40 ppm during C-section (1st case). Second case, the amount of NO was not specified. NO inhalation did not occur in third case. | 1st case patient died post-C-section. 2nd case patient died of severe heart failure after C-section. 3rd case more mild Pulmonary hypertension that was diagnosed earlier in pregnancy and had multidisciplinary management of pregnancy which is necessary for pregnant women with pulmonary hypertension. |
Table 3.
Original studies of inhaled NO use in pre-term infants.
| Study | Number and gestational age of infants | Concentration and length of time NO was inhaled | Outcome |
|---|---|---|---|
| Chock et al. (2009) | Infants with a gestational age of 27 weeks with a history of premature rupture of membranes 6 infants received NO, 6 infants received placebo | 5–10 ppm NO, duration of 30 min to 14 days. | Arterial PO2 increased by 40 mmHg in the NO group versus a decrease of 11 mmHg in the placebo group. The mortality rate and incidence of bronchopulmonary dysplasia were not different between the two groups. |
| Hintz et al. (2007) | Infants < 34 weeks of age with respiratory failure. 198 subjects received NO and 200 received placebo | 5–10 ppm NO for 76 + 73 h to 14 days. | Inhaled NO did not reduce mortality or improve neurodevelopment outcomes by 18–22 months of age. However, inhaled NO did not worsen neurodevelopmental outcomes either. |
| Delsing et al. (2007) | 4 sets of twins with twin-to-twin transfusion syndrome who had severe persistent pulmonary hypertension of the newborn | 20 ppm for 2 days given to 4 sets of twins (8 neonates). | All twin-to-twin transfusion syndrome infants with severe persistent pulmonary hypertension reacted promptly to inhaled NO. |
| Mestan et al. (2005) | 70 infants at 27 weeks gestational age were given inhaled NO. 80 infants were given placebo | 10 ppm day 1. 5 ppm for the next 6 days. | This was a follow-up study to examine neurodevelopmental outcomes of children at 2 years of age who were given inhaled NO at birth. Patients treated with inhaled NO had approximately half the risk of abnormal neurodevelopment compared to placebo. There was a 47% decrease in the risk of cognitive impairment compared to placebo group. |
| Konduri et al. (2004) | Neonates born at ≥34 weeks gestation with hypoxic respiratory failure. 150 pre-term infants given NO and 149 control subjects | 5 ppm of NO which was increased to 20 ppm for 57 ±48 h. | Inhaled NO improves oxygenation but does not reduce the incidence of the use of extracorporeal membrane oxygenation or mortality when initiated at an oxygen index of 15–25 compared with > 25 in term and near-term neonates with respiratory failure. |
| Konduri et al. (2007) | 299 neonates born at ≥34 weeks gestation were randomized to receive NO or placebo. A total 150 neonates were given NO. 266 survived to age 18–24 months | 5 ppm of NO which was increased to 20 ppm for 57 ±48 h. | This was the follow-up study to the one published in 2004 (Konduri et al., 2004). Early inhaled NO therapy for hypoxic respiratory failure in term and near term infants is not associated with an increase in neurodevelopmental impairment or hearing loss at 18–14 months postnatal. |
| Schreiber et al. (2003) | 207 newborns, aged 34 weeks or less who were mechanically ventilated were randomized into NO or placebo group. A total of 105 neonates received NO | Initial dose 10 ppm for 1 day, then 5 ppm on days 2–6 (7 days total). | Significant reduction in death or bronchopulmonary dysplasia at 36 weeks of age with inhaled NO compared to controls. The use of nitric oxide in premature infants with respiratory distress syndrome decreases the incidence of chronic disease and death. |
| Kinsella and Abman (2007) | 80 pre-term neonates, with gestational age 34 weeks or less, 7 days age or less, and severe hypoxemia who were on mechanical ventilation | 5 ppm or placebo, 7 days then weaned. (40 neonates actually received NO). | Even though oxygenation improved 1 h after inhaled NO, there was only a trend toward decrease in bronchopulmonary dysplasia and no differences in rates of severe intracranial hemorrhage. |
| Kinsella et al. (2006) | 793 newborns ≤34 weeks gestation were randomly assigned to receive NO or placebo. A total of 395 neonates received NO | Randomly assigned to receive 5 ppm NO or placebo for 21 days. | No significant difference in the incidence of death or bronchopulmonary dysplasia between patients receiving NO or not. For infants with a birth weight of 1000–1250 g, inhaled NO reduced the incidence of bronchopulmonary dyplasia and overall brain injury. Inhaled NO did not increase incidence of pulmonary hemorrhage or other adverse events. |
| Clark et al. (2000) | 248 neonates with pulmonary hypertension enrolled ≥34 weeks gestation. Babies were 4 days old. 126 randomly assigned to NO group, 122 assigned to control group. | 20 ppm for 24 h, then 5 ppm for 96 h. | 30-day mortality rate was the same in both groups (8%). Chronic lung disease developed less often in neonates treated with NO. Inhaled NO reduced the extent to which extracorporeal membrane oxygenation is needed in neonates with hypoxemic respiratory failure and pulmonary hypertension. |
| Van Meurs et al. (1997) | 11 neonates, aged ≤34 weeks gestation with respiratory failure 4 h or more after birth. | Four concentrations of inhaled NO were used: 1, 5, 10, 20 ppm, and placebo for 15 min. | No significant elevations of methemoglobin were found with NO inhalation (0.6% before inhalation to 0.8% after 15 min of inhalation). NO inhalation improved the arterial PO2 to alveolar PO2 ratio in those with respiratory failure. |
To adequately assess the risks of CO and NO inhalation, it is useful to draw comparisons with exposure to these gases as a result of smoking tobacco. Each cigarette produces about 4% CO by volume (40,000 ppm), resulting in an average alveolar CO concentration of about 450 ppm (Osborne et al., 1956). The NO concentration in cigarette smoke is as much as 500 ppm (Eiserich et al., 1994; Pryor and Stone, 1993). Therefore, cigarette smoke contains many-fold higher concentrations of CO and NO than employed in a diffusing capacity test. Each cigarette smoked may increase the [HbCO] by about 1% (Russell et al., 1973a,b). One pack per day smokers have an average [HbCO] of 5–6% (Hampson and Scott, 2006; Light et al., 2007; Reddy et al., 2007; Russell et al., 1973a), and for some 10% of smokers [HbCO] are ≥7.5% (Radford and Drizd, 1982). For those that regularly smoke about 20 cigarettes during the course of a day, mean [HbCO] levels are 8.2%, with some subjects as high as 14.2% and a day-to-day variation of 0.94% (Smith et al., 1998). Therefore, smokers have high levels of [HbCO] that persist day-to-day.
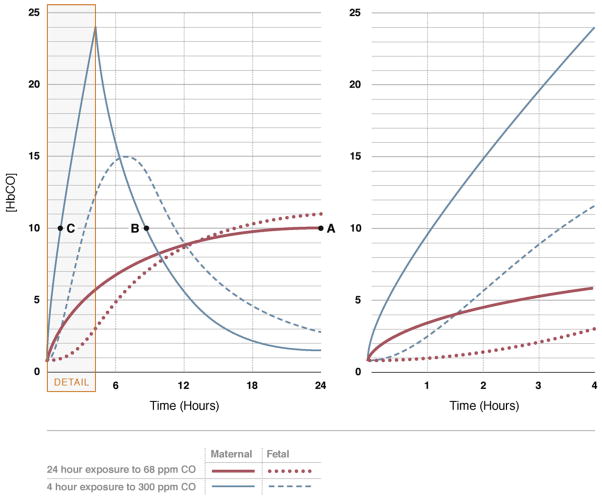
Maternal HbCO levels are 4–5% in pregnant mothers who smoke regularly (Cole et al., 1972; Davies et al., 1979) and [HbCO] is linearly related to the number of cigarettes smoked per day (Cole et al., 1972). It is not possible, however to estimate fetal [HbCO] on the basis of a single maternal blood sample without knowledge of exposure pattern. At steady state, fetal [HbCO] is higher than maternal due to a greater affinity of fetal hemoglobin for the CO and because average fetal oxygen saturations are less than maternal (Longo, 1977). The elimination half-life of CO from the fetal circulation is longer than from maternal circulation (Hill et al., 1977; Longo and Hill, 1977). When maternal and fetal [HbCO] levels are equilibrated, the mean fetal to maternal ratio for HbCO is 1.84 giving the relation: [Fetal [HbCO]% = 1.27 × maternal [HbCO]% + 0.76] (Cole et al., 1972). Fig. 1 shows the predicted maternal and fetal HbCO levels equivalent to smoking 1–1.5 packs of cigarettes per day, followed by an 8-h sleep period. Note that peak fetal HbCO levels are higher than maternal HbCO, and that, fetal HbCO lags substantially behind maternal HbCO levels until 12 h into the day (Longo, 1977). Mathematical models predict a 4-h exposure to 300 ppm CO will produce peak maternal HbCO levels of about 25% after 4 h, while fetal [HbCO] will be only 12% (Longo, 1977) (Fig. 2). Thereafter maternal [HbCO] is predicted to fall, while fetal [HbCO] peaks at 15% (Longo, 1977) (Fig. 2). From this background it is concluded that a diffusing capacity test that lasts 10–15 s per test (with exposure to 3000 ppm CO) would not appreciably increase fetal [HbCO] levels, and maternal levels of [HbCO] would increase by about 0.7% per test (Forster et al., 1954; Frey et al., 1987). Ten tests (100–150 s of total CO exposure) would increase maternal [HbCO] to about 7%. Even with more prolonged maternal exposures lasting up to 2 min fetal [HbCO] is anticipated to increase only slightly from pre-exposure levels reaching a peak about 2 h after testing. It is concluded that routine diffusing capacity testing at rest and during exercise would not increase fetal [HbCO] appreciably or to a level that would adversely affect fetal physiology.
Fig. 1.
The predicted maternal and fetal [HbCO] when a mother breaths 50 ppm CO for 16 h, followed by an 8 h period in which no CO was inspired. The level of CO exposure is equivalent to smoking about 1–1.5 packs of cigarettes per day, followed by an 8 h sleep period. Figure modified by Longo (1977) based on the mathematical model by Hill et al. (1977).
Fig. 2.
Changes in maternal and fetal [HbCO] during and after a 24 h exposure to 68 ppm CO and a 4 h exposure to 300 ppm CO. If the maternal value of 10% HbCO were obtained at equilibrium (point A), the fetal [HbCO] would be equal to about 11%. If the sample was taken at point B during the washout phase, the fetal [HbCO] would be about 14%. However, if the blood sample was taken during the uptake phase (point C), the fetal [HbCO] would be 2%. Not only is it impossible to predict fetal [HbCO] on the basis of a single maternal blood sample, but the length of time necessary to reduce the fetal [HbCO] depends on whether the concentration has already peaked or is still rising. In the case of lung diffusing testing, which could require up to about 3 min of 3000 ppm CO inhalation, the maternal [HbCO] may increase to 5%, but the fetal HbCO would hardly increase. This is typified by the enhanced “Detail” on the right panel in figure. Figures reproduced from Longo (1977).
Given the NO and CO content in cigarettes, it may be useful to extrapolate available data regarding the effects of smoking on pregnancy outcomes to evaluate the risks of inhaling NO and CO gas for lung diffusing capacity testing in pregnancy. Pregnant smokers have a late fetal plus neonatal death rate that is higher by 10 per 1000 births (to a 4.2% absolute death rate) compared to pregnant non-smokers (3.2% absolute death rate) (Butler et al., 1972). Such smoking throughout pregnancy may be compared to a testing session in which maternal [HbCO] increases to 5% and persists for about 4 h, or 0.12% of the last 20 weeks of pregnancy (4/3360 ×100 = 0.12% per testing session). Assuming a linear time dependency for adverse effects, this would increase the death rate by about 0.0012%. The Centers for Disease Control (CDC) death rate for accidental deaths is 0.04% and the death rate of all causes (accidents, homicides, suicides, diseases, cancer, infection, etc.) is about 0.8% (Kung et al., 2008). Thus, by this comparison, the total CDC death rate is 667 times more than the late fetal and neonatal death rate per testing session in which pregnant mothers [HbCO] increase to 5%. This justifies an IRB classification of the risk associated with DLCO testing in pregnant women and the resultant effects on their fetuses as “minimal risk.”
5. Conclusions
Based on the evidence provided in this review, we submit that CO and NO inhalation for testing of pulmonary diffusing capacity is a safe procedure that may be justified by improved medical scare. Given that the elimination half-life of [HbCO] in maternal blood is about 3.8–4 h (Lawther and Commins, 1970; Selvakumar et al., 1993), or 74 min when given 100% oxygen (Weaver et al., 2000), the following recommendations are provided in Table 4. We suggest different acceptable concentrations for pregnant and non-pregnant women. When CO exposure in a pregnant woman is ≤ 3 min at a concentration of 0.3% (3000 ppm), fetal [HbCO] will not rise appreciably post-testing. A maximum of 3 testing sessions per trimester is recommended during pregnancy when DLCO is measured (9 sessions in total throughout pregnancy, with a minimum of 48 h between each session). Only if DLNO is measured, then a maximum of 5 testing sessions per trimester is recommended during pregnancy (15 sessions in total, with a minimum of 48 h between each session). If testing pregnant smokers whose maternal [HbCO] is already at about 5%, then CO exposure that is ≤1 min at a concentration of 0.3% (3000 ppm) can be allowed. Even though each pulmonary diffusion test of 10–15 s in length is separated by 4 min of rest breathing room air, the exposure time within each testing session is suggested to be cumulative over a typical 30–45 min session. Therefore, five 15 s DLCO tests (rebreathing), or four 10 s single-breath DLCO tests equates to 50–75 s of total CO exposure time resulting in an increase of [HbCO] by 3–4%, since each DLCO test increases [HbCO] by 0.6–0.8% per test.
Table 4.
Recommendations for CO and NO inhalation at rest and during exercise in pregnant women and non-pregnant individuals per testing session.
| Condition | Recommended CO inhalation exposure time and dosage resulting in a recommended acceptable [HbCO] concentration | Recommended NO inhalation exposure time and dosage |
|---|---|---|
| 1. Men and non-pregnant women who are asymptomatic | ||
| Rest (AHA Class A1–3) | [HbCO] = 10% when 0.3% CO is inhaled for ≤6 min after which breathing room air ensuesa | 80 ppm over 6 min |
| Exercise (AHA Class A1–3) | [HbCO] = 5% when 0.3% CO is inhaled for ≤3 min after which breathing room air ensues | 80 ppm over 3 min |
| 2. Men and non-pregnant women with cardiovascular disease | ||
| Rest (AHA Class B–D) | [HbCO] = 5% when 0.3% CO is inhaled for ≤3 min after which breathing room air ensues | 80 ppm over 3 min |
| Exercise (AHA class B and C) | [HbCO] = 5% when 0.3% CO is inhaled for ≤3 min after which breathing room air ensues | 80 ppm over 3 min |
| 3. Asymptomatic pregnant womenb | ||
| Rest (AHA Class A1–3) | [HbCO] = 5% when 0.3% CO is inhaled for ≤3 min after which breathing room air ensuesc | 80 ppm over 3 min |
| Exercise (AHA Class A1–3) | [HbCO] = 5% when 0.3% CO is inhaled for ≤3 min after which breathing room air ensuesc | 80 ppm over 3 min |
| 4. Pregnant women with cardiovascular diseaseb | ||
| Rest (AHA class B–D)* | [HbCO] = 4% when 0.3% CO is inhaled for ≤3 min after which breathing room air ensuesc | 80 ppm over 3 min |
| Exercise (AHA Class B and C) | [HbCO] = 4% when 0.3% CO is inhaled for ≤3 min after which breathing room air ensuesc | 80 ppm over 3 min |
The American Heart Association (AHA) Risk Stratification Criteria (Fletcher et al., 2001; Fuster et al., 1996) Class A. Apparently Healthy
- Children, adolescents, men < 45 years of age, and women < 55 years of age who have no symptoms or known presence of heart disease or major cardiovascular disease risk (CVD) factors.
- Men ≥45 years of age or women ≥55 years who have no symptoms or known presence of heart disease with less than two major CVD risk factors.
- Men ≥45 years of age or women ≥55 years who have no symptoms or known presence of heart disease with more than two major CVD risk factors.
Class B. Presence of known, stable cardiovascular disease with low risk for complications. Includes individuals with any of the following diagnoses:
- Coronary artery disease who condition is stable and who have the clinical characteristics described below.
- Valvular heart disease, excluding severe valvular stenosis or regurgitation.
- Congenital heart disease.
- Cardiomyopathy, ejection fraction ≤30%.
- Exercise test abnormalities that do not meet the criteria for Class C.
Clinical characteristics
- New York Heart Association Class 1 or 2.
- Exercise capacity ≤6 METS.
- No evidence of congestive heart failure.
- No evidence of myocardial ischemia or angina at rest or on the exercise test at or below 6 METS.
- Appropriate rise in systolic blood pressure during exercise.
- Absence of sustained or non-sustained ventricular tachycardia at rest or with exercise.
Class C. Those at moderate to high risk for cardiac complications during exercise and/or unable to self-regulate activity or understand recommended activity level. Includes individuals with any of the following diagnoses:
- Cardiovascular disease with the clinical characteristics outlined below.
- Valvular heart disease, excluding severe valvular stenosis or regurgitation.
- Congenital heart disease.
- Cardiomyopathy, ejection fraction ≤30%.
- Complex ventricular arrhythmias not well-controlled.
Clinical characteristics
- New York Heart Association Class 3 or 4.
- Exercise capacity < 6 METS.
- Angina of ischemia ST depression at workload < 6 METS.
- Fall in systolic blood pressure below resting during exercise.
- Non-sustained ventricular tachycardia at rest or with exercise.
- Previous episode of primary cardiac arrest.
- A medical problem that the physician may be life threatening.
Class D. Unstable cardiovascular disease with activity restriction. Includes individuals with:
- Unstable angina.
- Severe and symptomatic valvular stenosis or regurgitation.
- Congenital heart disease.
- Heart failure that is not compensated.
- Uncontrolled arrhythmias.
- Other medical condition that could be aggravated by exercise. No physical exercise is recommended.
CVD risk factors are:
- Age: Men ≥45 years of age; women ≥55 years of age.
- Family history: Myocardial infarction, coronary revascularization, or sudden death before age 55 years in father or other first degree male relative, or before 65 years of age in mother or other female first degree relative.
- Cigarette smoking: Current smoker or those who have quit within the past 6 months.
- Sedentary lifestyle: Not participating in a least 30 min of moderate intensity (40–60% of oxygen uptake reserve) on at least 3 days per week for at least 3 months.
- Obesity: Body mass index ≥30 kg/m2 or waist girth > 102 cm (40 in.) for men and >88 cm (35 in.) for women.
- Hypertension: Systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, confirmed by measurements on at least two separate occasions, or on anti-hypertensive medication.
- Dylipidemia: Low density lipoprotein cholesterol ≥130 mg/dl (3.37 mmol/l) or high density lipoprotein cholesterol < 40 mg/dl (1.04 mmol/l) or on lipid lowering medication. If total serum cholesterol is all that is available use ≥200 mg/dl (5.18 mmol/l).
- Prediabetes: Impaired fasting glucose ≥100 mg/dl (5.50 mmol/l) but <126 mg/dl (6.93 mmol/l) or impaired glucose tolerance from a 2 h oral glucose tolerance test ≥140 mg/dl (7.70 mmol/l) confirmed by measurements on at least two separate occasions.
CVD risk factors are obtained from the American College of Sports Medicine Handbook (ACSM, 2009).
If neurophysiological skills are being tested in a research study (i.e. visuomotor coordination, visuospacial functioning, short and long term semantic memory) (Amitai et al., 1998), HbCO levels are recommended not to exceed 5%.
Inhalation of 100% oxygen to reduce the half-life of CO to 74 min (Weaver et al., 2000) is not recommended because of the potential toxic effects of oxygen on the mother and fetus. Tests of diffusing capacity (with 0.3% CO inhalation) increase HbCO by about 0.6–0.8% per 10 s breath-hold maneuver (Forster et al., 1954; Frey et al., 1987) or 15 s rebreathing maneuver. Therefore, one can use this as a template for estimating the [HbCO] when it is not measured (i.e. one diffusion test increases HbCO by 0.6–0.8%). Four rebreathing diffusion tests of 15 s each or 4 single-breath diffusion tests of 10 s each should be separated by 4 min between each test. The total CO exposure time in this example is therefore 40–60 s and is estimated to increase HbCO by 2.4–3.2% (0.6% × 4 tests, or 0.8% ×4 tests).
A maximum of 3 testing sessions per trimester is recommended during pregnancy in which maternal HbCO can increase up to 5% per session when 0.3% CO exposure is ≤ 3 min. Therefore, a maximum of 9 sessions in total throughout pregnancy are recommended, and sessions should be separated by 48 h. If only DLNO is measured during pregnancy, then a maximum of 5 testing sessions per trimester is thought permissible when ≤80 ppm NO is inhaled for ≤3 min per session (15 sessions in total throughout pregnancy). If testing pregnant smokers, in which maternal levels are already 5%, and a measurement of diffusing capacity is needed, then CO exposure that is ≤1 min at a concentration of 0.3% (3000 ppm) can be allowed.
The repeatability for DLNO (within session variability) is 17 ml/min/mmHg in non-pregnant women and men (Zavorsky and Murias, 2006). The reproducibility of DLNO (week to week variability) from these same subjects is 20 ml/min/mmHg (Murias and Zavorsky, 2007). Thus, we not expect the variability of DLNO to be different during pregnancy since DLNO is minimally affected by hemoglobin changes (van der Lee et al., 2005). Furthermore, as changes in hemoglobin concentration only vary by 1 g/dl throughout pregnancy or over a 28-day menstrual cycle (McAuliffe et al., 2002; Vellar, 1974), the small 10–15% reduction in DLCO in the third trimester of pregnancy has little to do with maternal hemoglobin variability. The repeatability and reproducibility of DLCO is 3 and 5 ml/min/mmHg, respectively in a non-pregnant state (Murias and Zavorsky, 2007; Zavorsky and Murias, 2006) and is not expected to vary during pregnancy.
Footnotes
Conflicts of interest
No author has any actual or potential conflicts of interest pertaining to this manuscript.
References
- ACSM. ACSM’s Guidelines for Exercise Testing and Prescription. 8. Lippincott Williams & Wilkins; Baltimore: 2009. [Google Scholar]
- Alaily AB, Carrol KB. Pulmonary ventilation in pregnancy. Br J Obstet Gynaecol. 1978;85:518–524. doi: 10.1111/j.1471-0528.1978.tb15626.x. [DOI] [PubMed] [Google Scholar]
- Amitai Y, Zlotogorski Z, Golan-Katzav V, Wexler A, Gross D. Neuropsychological impairment from acute low-level exposure to carbon monoxide. Arch Neurol. 1998;55:845–848. doi: 10.1001/archneur.55.6.845. [DOI] [PubMed] [Google Scholar]
- Baldwin GR, Moorthi DS, Whelton JA, MacDonnell KF. New lung functions and pregnancy. Am J Obstet Gynecol. 1977;127:235–239. doi: 10.1016/0002-9378(77)90460-4. [DOI] [PubMed] [Google Scholar]
- Barker SJ, Curry J, Redford D, Morgan S. Measurement of carboxyhemoglobin and methemoglobin by pulse oximetry: a human volunteer study. Anesthesiology. 2006;105:892–897. doi: 10.1097/00000542-200611000-00008. [DOI] [PubMed] [Google Scholar]
- Blood AB, Power GG. In vitro and in vivo kinetic handling of nitrite in blood: effects of varying hemoglobin oxygen saturation. Am J Physiol Heart Circ Physiol. 2007;293:H1508–H1517. doi: 10.1152/ajpheart.01259.2006. [DOI] [PubMed] [Google Scholar]
- Boggess KA, Easterling TR, Raghu G. Management and outcome of pregnant women with interstitial and restrictive lung disease. Am J Obstet Gynecol. 1995;173:1007–1014. doi: 10.1016/0002-9378(95)91318-1. [DOI] [PubMed] [Google Scholar]
- Bonay M, Bancal C, de Zuttere D, Arnoult F, Saumon G, Camus F. Normal pulmonary capillary blood volume in patients with chronic infiltrative lung disease and high pulmonary artery pressure. Chest. 2004;126:1460–1466. doi: 10.1378/chest.126.5.1460. [DOI] [PubMed] [Google Scholar]
- Bonnin M, Mercier FJ, Sitbon O, Roger-Christoph S, Jais X, Humbert M, Audibert F, Frydman R, Simonneau G, Benhamou D. Severe pulmonary hypertension during pregnancy: mode of delivery and anesthetic management of 15 consecutive cases. Anesthesiology. 2005;102:1133–1137. doi: 10.1097/00000542-200506000-00012. discussion 1135A–1136A. [DOI] [PubMed] [Google Scholar]
- Brett SJ, Chambers J, Bush A, Rosenthal M, Evans TW. Pulmonary response of normal human subjects to inhaled vasodilator substances. Clin Sci (Lond) 1998;95:621–627. doi: 10.1042/cs0950621. [DOI] [PubMed] [Google Scholar]
- Brody JS, Coburn RF. Carbon monoxide-induced arterial hypoxemia. Science. 1969;164:1297–1298. doi: 10.1126/science.164.3885.1297. [DOI] [PubMed] [Google Scholar]
- Brody JS, Coburn RF. Effects of elevated carboxyhemoglobin on gas exchange in the lung. Ann N Y Acad Sci. 1970;174:255–260. doi: 10.1111/j.1749-6632.1970.tb49793.x. [DOI] [PubMed] [Google Scholar]
- Butler NR, Goldstein H, Ross EM. Cigarette smoking in pregnancy: its influence on birth weight and perinatal mortality. Br Med J. 1972;2:127–130. doi: 10.1136/bmj.2.5806.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance WW, Rhee C, Yilmaz C, Dane DM, Pruneda ML, Raskin P, Hsia CC. Diminished alveolar microvascular reserves in type-2 diabetes mellitus reflect systemic microangiopathy. Diabetes Care. 2008;31:1596–1601. doi: 10.2337/dc07-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chock VY, Van Meurs KP, Hintz SR, Ehrenkranz RA, Lemons JA, Kendrick DE, Stevenson DK. Inhaled nitric oxide for preterm premature rupture of membranes, oligohydramnios, and pulmonary hypoplasia. Am J Perinatol. 2009;26:317–322. doi: 10.1055/s-0028-1104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342:469–474. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- Coburn RF, Blakemore WS, Forster RE. Endogenous carbon monoxide production in man. J Clin Invest. 1963;42:1172–1178. doi: 10.1172/JCI104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn RF, Forster RE, Kane PB. Considerations of the physiological variables that determine the blood carboxyhemoglobin concentration in man. J Clin Invest. 1965;44:1899–1910. doi: 10.1172/JCI105296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PV, Hawkins LH, Roberts D. Smoking during pregnancy and its effects on the fetus. J Obstet Gynaecol Br Commonw. 1972;79:782–787. doi: 10.1111/j.1471-0528.1972.tb12920.x. [DOI] [PubMed] [Google Scholar]
- Davies JM, Latto IP, Jones JG, Veale A, Wardrop CA. Effects of stopping smoking for 48 hours on oxygen availability from the blood: a study on pregnant women. Br Med J. 1979;2:355–356. doi: 10.1136/bmj.2.6186.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoene C, Bourzoufi K, Moreau D, Narducci F, Crepin F, Krivosic-Horber R. Use of inhaled nitric oxide for emergency Cesarean section in a woman with unexpected primary pulmonary hypertension. Can J Anaesth. 2001;48:584–587. doi: 10.1007/BF03016836. [DOI] [PubMed] [Google Scholar]
- Delivoria-Papadopoulos M, Coburn RF, Forster RE. Cyclic variation of rate of carbon monoxide production in normal women. J Appl Physiol. 1974;36:49–51. doi: 10.1152/jappl.1974.36.1.49. [DOI] [PubMed] [Google Scholar]
- Delsing B, Lopriore E, Blom N, Te Pas AB, Vandenbussche FP, Walther FJ. Risk of persistent pulmonary hypertension of the neonate in twin-to-twin transfusion syndrome. Neonatology. 2007;92:134–138. doi: 10.1159/000101433. [DOI] [PubMed] [Google Scholar]
- Dressel H, Filser L, Fischer R, Marten K, Muller-Lisse U, de la Motte D, Nowak D, Huber RM, Jorres RA. Lung diffusing capacity for nitric oxide and carbon monoxide in relation to morphological changes as assessed by computed tomography in patients with cystic fibrosis. BMC Pulm Med. 2009;9:30. doi: 10.1186/1471-2466-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Jr, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- Eiserich JP, Vossen V, O’Neill CA, Halliwell B, Cross CE, van der Vliet A. Molecular mechanisms of damage by excess nitrogen oxides: nitration of tyrosine by gas-phase cigarette smoke. FEBS Lett. 1994;353:53–56. doi: 10.1016/0014-5793(94)01011-0. [DOI] [PubMed] [Google Scholar]
- Ekblom B, Huot R. Response to submaximal and maximal exercise at different levels of carboxyhemoglobin. Acta Physiol Scand. 1972;86:474–482. doi: 10.1111/j.1748-1716.1972.tb05350.x. [DOI] [PubMed] [Google Scholar]
- Eng M, Butler J, Bonica JJ. Respiratory function in pregnant obese women. Am J Obstet Gynecol. 1975;123:241–245. doi: 10.1016/0002-9378(75)90192-1. [DOI] [PubMed] [Google Scholar]
- Fine DH. Critical evaluation of Saltzman technique for NOx analysis in the 0–100 ppm range. Environ Sci Technol. 1972;6:348–350. [Google Scholar]
- Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, Froelicher VF, Leon AS, Pina IL, Rodney R, Simons-Morton DA, Williams MA, Bazzarre T. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694–1740. doi: 10.1161/hc3901.095960. [DOI] [PubMed] [Google Scholar]
- Forster RE. Exchange of gases between alveolar air and pulmonary capillary blood: pulmonary diffusing capacity. Physiol Rev. 1957;37:391–452. doi: 10.1152/physrev.1957.37.4.391. [DOI] [PubMed] [Google Scholar]
- Forster RE, Fowler WS, Bates DV, Van Lingen B. The absorption of carbon monoxide by the lungs during breath-holding. J Clin Invest. 1954;33:1135–1145. doi: 10.1172/JCI102987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey TM, Crapo RO, Jensen RL, Elliott CG. Diurnal variation of the diffusing capacity of the lung: is it real? Am Rev Respir Dis. 1987;136:1381–1384. doi: 10.1164/ajrccm/136.6.1381. [DOI] [PubMed] [Google Scholar]
- Fuster V, Gotto AM, Libby P, Loscalzo J, McGill HC. 27th Bethesda Conference: matching the intensity of risk factor management with the hazard for coronary disease events. Task Force 1 Pathogenesis of coronary disease: the biologic role of risk factors. J Am Coll Cardiol. 1996;27:964–976. doi: 10.1016/0735-1097(96)00014-9. [DOI] [PubMed] [Google Scholar]
- Garcia-Rio F, Pino JM, Gomez L, Alvarez-Sala R, Villasante C, Villamor J. Regulation of breathing and perception of dyspnea in healthy pregnant women. Chest. 1996;110:446–453. doi: 10.1378/chest.110.2.446. [DOI] [PubMed] [Google Scholar]
- Gazioglu K, Kaltreider NL, Rosen M, Yu PN. Pulmonary function during pregnancy in normal women and in patients with cardiopulmonary disease. Thorax. 1970;25:445–450. doi: 10.1136/thx.25.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getahun D, Ananth CV, Peltier MR, Smulian JC, Vintzileos AM. Acute and chronic respiratory diseases in pregnancy: associations with placental abruption. Am J Obstet Gynecol. 2006;195:1180–1184. doi: 10.1016/j.ajog.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Getahun D, Ananth CV, Oyelese Y, Peltier MR, Smulian JC, Vintzileos AM. Acute and chronic respiratory diseases in pregnancy: associations with spontaneous premature rupture of membranes. J Matern Fetal Neonatal Med. 2007;20:669–675. doi: 10.1080/14767050701516063. [DOI] [PubMed] [Google Scholar]
- Gonzales GF, Steenland K, Tapia V. Maternal hemoglobin level and fetal outcome at low and high altitudes. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1477–R1485. doi: 10.1152/ajpregu.00275.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin TM, Gherman RB, Hameed A, Elkayam U. Favorable response of Eisenmenger syndrome to inhaled nitric oxide during pregnancy. Am J Obstet Gynecol. 1999;180:64–67. doi: 10.1016/s0002-9378(99)70151-1. [DOI] [PubMed] [Google Scholar]
- Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, Al-Hani AJ, Black HR. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003;108:1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- Hampson NB, Scott KL. Use of a noninvasive pulse CO-oximeter to measure blood carboxyhemoglobin levels in bingo players. Respir Care. 2006;51:758–760. [PubMed] [Google Scholar]
- Hill EP, Hill JR, Power GG, Longo LD. Carbon monoxide exchanges between the human fetus and mother: a mathematical model. Am J Physiol. 1977;232:H311–H323. doi: 10.1152/ajpheart.1977.232.3.H311. [DOI] [PubMed] [Google Scholar]
- Hintz SR, Van Meurs KP, Perritt R, Poole WK, Das A, Stevenson DK, Ehrenkranz RA, Lemons JA, Vohr BR, Heyne R, Childers DO, Peralta-Carcelen M, Dusick A, Johnson YR, Morris B, Dillard R, Vaucher Y, Steichen J, Adams-Chapman I, Konduri G, Myers GJ, de Ungria M, Tyson JE, Higgins RD. Neurodevelopmental outcomes of premature infants with severe respiratory failure enrolled in a randomized controlled trial of inhaled nitric oxide. J Pediatr. 2007;151:16–22. 22 e11–e13. doi: 10.1016/j.jpeds.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath SM, Raven PB, Dahms TE, Gray DJ. Maximal aerobic capacity at different levels of carboxyhemoglobin. J Appl Physiol. 1975;38:300–303. doi: 10.1152/jappl.1975.38.2.300. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Chuong CJ, Johnson RL., Jr Critique of conceptual basis of diffusing capacity estimates: a finite element analysis. J Appl Physiol. 1995;79:1039–1047. doi: 10.1152/jappl.1995.79.3.1039. [DOI] [PubMed] [Google Scholar]
- Jansons H, Fokkens JK, van der Tweel I, Kreukniet J. Re-breathing vs single-breath TLCO in patients with unequal ventilation and diffusion. Respir Med. 1998;92:18–24. doi: 10.1016/s0954-6111(98)90026-9. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Jr, Heigenhauser GJF, Hsia CCW, Jones NL, Wagner PD. Determinants of gas exchange and acid-balance during exercise. In: Rowell LB, Shepard JT, editors. Handbook of Physiology. Section 12: Exercise: Regulation and Integration of Multiple Systems. Oxford University Press; New York: 1996. pp. 515–584. [Google Scholar]
- Kinsella JP, Abman SH. Inhaled nitric oxide in the premature newborn. J Pediatr. 2007;151:10–15. doi: 10.1016/j.jpeds.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS, George TN, Southgate WM, Carriedo H, Couser RJ, Mammel MC, Hall DC, Pappagallo M, Sardesai S, Strain JD, Baier M, Abman SH. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;355:354–364. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- Konduri GG, Solimano A, Sokol GM, Singer J, Ehrenkranz RA, Singhal N, Wright LL, Van Meurs K, Stork E, Kirpalani H, Peliowski A. A randomized trial of early versus standard inhaled nitric oxide therapy in term and near-term newborn infants with hypoxic respiratory failure. Pediatrics. 2004;113:559–564. doi: 10.1542/peds.113.3.559. [DOI] [PubMed] [Google Scholar]
- Konduri GG, Vohr B, Robertson C, Sokol GM, Solimano A, Singer J, Ehrenkranz RA, Singhal N, Wright LL, Van Meurs K, Stork E, Kirpalani H, Peliowski A, Johnson Y. Early inhaled nitric oxide therapy for term and near-term newborn infants with hypoxic respiratory failure: neurodevelopmental follow-up. J Pediatr. 2007;150:235–240. 240 e231. doi: 10.1016/j.jpeds.2006.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreukniet J. Relation between rebreathing CO-diffusing capacity of the lung and unequal ventilation. Scand J Respir Dis. 1970;51:49–54. [PubMed] [Google Scholar]
- Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56:1–120. [PubMed] [Google Scholar]
- Lalli CM, Raju L. Pregnancy and chronic obstructive pulmonary disease. Chest. 1981;80:759–761. doi: 10.1378/chest.80.6.759. [DOI] [PubMed] [Google Scholar]
- Lam GK, Stafford RE, Thorp J, Moise KJ, Jr, Cairns BA. Inhaled nitric oxide for primary pulmonary hypertension in pregnancy. Obstet Gynecol. 2001;98:895–898. doi: 10.1016/s0029-7844(01)01549-6. [DOI] [PubMed] [Google Scholar]
- Lawther PJ, Commins BT. Cigarette smoking and exposure to carbon monoxide. Ann N Y Acad Sci. 1970;174:135–147. doi: 10.1111/j.1749-6632.1970.tb49780.x. [DOI] [PubMed] [Google Scholar]
- Lehmann V. Dyspnea in pregnancy. J Perinat Med. 1975;3:154–160. doi: 10.1515/jpme.1975.3.3.154. [DOI] [PubMed] [Google Scholar]
- Light A, Grass C, Pursely D, Krause J. Carboxyhemoglobin levels in smokers vs nonsmokers in a smoking environment. Respir Care; Presented at the 52nd Annual Meeting of American Association for Respiratory Care (AARC); December 2006; Las Vegas. 2007. http://www.rcjournal.com/abstracts/2007/?id=aarc07_111. [Google Scholar]
- Longo LD. Carbon monoxide: effects on oxygenation of the fetus in utero. Science. 1976;194:523–525. doi: 10.1126/science.973133. [DOI] [PubMed] [Google Scholar]
- Longo LD. The biological effects of carbon monoxide on the pregnant woman, fetus, and newborn infant. Am J Obstet Gynecol. 1977;129:69–103. doi: 10.1016/0002-9378(77)90824-9. [DOI] [PubMed] [Google Scholar]
- Longo LD, Hill EP. Carbon monoxide uptake and elimination in fetal and maternal sheep. Am J Physiol. 1977;232:H324–H330. doi: 10.1152/ajpheart.1977.232.3.H324. [DOI] [PubMed] [Google Scholar]
- Lust KM, Boots RJ, Dooris M, Wilson J. Management of labor in Eisenmenger syndrome with inhaled nitric oxide. Am J Obstet Gynecol. 1999;181:419–423. doi: 10.1016/s0002-9378(99)70572-7. [DOI] [PubMed] [Google Scholar]
- Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- Manier G, Moinard J, Stoicheff H. Pulmonary diffusing capacity after maximal exercise. J Appl Physiol. 1993;75:2580–2585. doi: 10.1152/jappl.1993.75.6.2580. [DOI] [PubMed] [Google Scholar]
- Manier G, Moinard J, Techoueyres P, Varene N, Guenard H. Pulmonary diffusion limitation after prolonged strenuous exercise. Respir Physiol. 1991;83:143–153. doi: 10.1016/0034-5687(91)90024-d. [DOI] [PubMed] [Google Scholar]
- McAuliffe F, Kametas N, Rafferty GF, Greenough A, Nicolaides K. Pulmonary diffusing capacity in pregnancy at sea level and at high altitude. Respir Physiol Neurobiol. 2003;134:85–92. doi: 10.1016/s1569-9048(02)00212-4. [DOI] [PubMed] [Google Scholar]
- McAuliffe F, Kametas N, Costello J, Rafferty GF, Greenough A, Nicolaides K. Respiratory function in singleton and twin pregnancy. BJOG. 2002;109:765–769. doi: 10.1111/j.1471-0528.2002.01515.x. [DOI] [PubMed] [Google Scholar]
- McMillan E, Martin WL, Waugh J, Rushton I, Lewis M, Clutton-Brock T, Townend JN, Kilby MD, Gordon C. Management of pregnancy in women with pulmonary hypertension secondary to SLE and anti-phospholipid syndrome. Lupus. 2002;11:392–398. doi: 10.1191/0961203302lu216xx. [DOI] [PubMed] [Google Scholar]
- Mestan KK, Marks JD, Hecox K, Huo D, Schreiber MD. Neurodevelopmental outcomes of premature infants treated with inhaled nitric oxide. N Engl J Med. 2005;353:23–32. doi: 10.1056/NEJMoa043514. [DOI] [PubMed] [Google Scholar]
- Meyer M, Piiper J. Nitric oxide (NO), a new test gas for study of alveolar-capillary diffusion. Eur Respir J. 1989;2:494–496. [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005a;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. General considerations for lung function testing. Eur Respir J. 2005b;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- Milne JA. The respiratory response to pregnancy. Postgrad Med J. 1979;55:318–324. doi: 10.1136/pgmj.55.643.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JA, Mills RJ, Coutts JR, Macnaughton MC, Moran F, Pack AI. The effect of human pregnancy on the pulmonary transfer factor for carbon monoxide as measured by the single-breath method. Clin Sci Mol Med. 1977;53:271–276. doi: 10.1042/cs0530271. [DOI] [PubMed] [Google Scholar]
- Murias JM, Zavorsky GS. Short-term variability of nitric oxide diffusing capacity and its components. Respir Physiol Neurobiol. 2007;157:316–325. doi: 10.1016/j.resp.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health, NIOSH Publication 2005-149, Carbon Monoxide. 2005. NIOSH Pocket Guide to Chemical Hazards. [Google Scholar]
- Norregaard O, Schultz P, Ostergaard A, Dahl R. Lung function and postural changes during pregnancy. Respir Med. 1989;83:467–470. doi: 10.1016/s0954-6111(89)80127-1. [DOI] [PubMed] [Google Scholar]
- Olivieri M, Talamini G, Corradi M, Perbellini L, Mutti A, Tantucci C, Malerba M. Reference values for exhaled nitric oxide (reveno) study. Respir Res. 2006;7:94. doi: 10.1186/1465-9921-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer BW, Berger KI, Hadjiangelis NP, Norman RG, Rapoport DM, Goldring RM. Membrane diffusion in diseases of the pulmonary vasculature. Respir Med. 2006;100:1247–1253. doi: 10.1016/j.rmed.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Osborne JS, Adamek S, Hobbs ME. Some components of gas phase of cigarette smoke. Anal Chem. 1956;28:211. [Google Scholar]
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, Mac-Intyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- Phansalkar AR, Hanson CM, Shakir AR, Johnson RL, Jr, Hsia CC. Nitric oxide diffusing capacity and alveolar microvascular recruitment in sarcoidosis. Am J Respir Crit Care Med. 2004;169:1034–1040. doi: 10.1164/rccm.200309-1287OC. [DOI] [PubMed] [Google Scholar]
- Pirnay F, Dujardin J, Deroanne R, Petit JM. Muscular exercise during intoxication by carbon monoxide. J Appl Physiol. 1971;31:573–575. doi: 10.1152/jappl.1971.31.4.573. [DOI] [PubMed] [Google Scholar]
- Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. discussion 27–18. [DOI] [PubMed] [Google Scholar]
- Radford EP, Drizd TA. Advance Data from Vital and Health Statistics No 76. PHS 82-1250. U.S. Department of Health and Human Services; Hyattsville, MD: 1982. Mar 17, Blood carbon monoxide levels in persons 3 to 74 years of age, United States, 1976–1980. [PubMed] [Google Scholar]
- Raven PB, Drinkwater BL, Ruhling RO, Bolduan N, Taguchi S, Gliner J, Horvath SM. Effect of carbon monoxide and peroxyacetyl nitrate on man’s maximal aerobic capacity. J Appl Physiol. 1974;36:288–293. doi: 10.1152/jappl.1974.36.3.288. [DOI] [PubMed] [Google Scholar]
- Reddy AP, Zaremba ML, Reddy SP. Noninvasive pulse co-oximetry as a tool to detect smoking status in an outpatient setting. Chest. 2007;132:490. [Google Scholar]
- Roberts CM, MacRae KD, Seed WA. Multi-breath and single breath helium dilution lung volumes as a test of airway obstruction. Eur Respir J. 1990;3:515–520. [PubMed] [Google Scholar]
- Robinson JN, Banerjee R, Landzberg MJ, Thiet MP. Inhaled nitric oxide therapy in pregnancy complicated by pulmonary hypertension. Am J Obstet Gynecol. 1999;180:1045–1046. doi: 10.1016/s0002-9378(99)70686-1. [DOI] [PubMed] [Google Scholar]
- Roughton FJW, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 1957;11:290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- Russell MA, Cole PV, Brown E. Absorption by non-smokers of carbon monoxide from room air polluted by tobacco smoke. Lancet. 1973a;1:576–579. doi: 10.1016/s0140-6736(73)90718-6. [DOI] [PubMed] [Google Scholar]
- Russell MA, Wilson C, Cole PV, Idle M, Feyerabend C. Comparison of increases in carboxyhaemoglobin after smoking “extra-mild” and “non-mild” cigarettes. Lancet. 1973b;2:687–690. doi: 10.1016/s0140-6736(73)92533-6. [DOI] [PubMed] [Google Scholar]
- Schatz M, Dombrowski MP, Wise R, Momirova V, Landon M, Mabie W, Newman RB, Rouse DJ, Lindheimer M, Miodovnik M, Caritis SN, Leveno KJ, Meis P, Wapner RJ, Paul RH, O’Sullivan MJ, Varner MW, Thurnau GR, Conway DL. Spirometry is related to perinatal outcomes in pregnant women with asthma. Am J Obstet Gynecol. 2006;194:120–126. doi: 10.1016/j.ajog.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003;349:2099–2107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- Selvakumar S, Sharan M, Singh MP. A mathematical model for the elimination of carbon monoxide in humans. J Theor Biol. 1993;162:321–336. doi: 10.1006/jtbi.1993.1090. [DOI] [PubMed] [Google Scholar]
- Sheel AW, Edwards MR, Hunte GS, McKenzie DC. Influence of inhaled nitric oxide on gas exchange during normoxic and hypoxic exercise in highly trained cyclists. J Appl Physiol. 2001;90:926–932. doi: 10.1152/jappl.2001.90.3.926. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Guy TD, Stiles MF, Morton MJ, Collie BB, Ingebrethsen BJ, Robinson JH. A repeatable method for determination of carboxyhemoglobin levels in smokers. Hum Exp Toxicol. 1998;17:29–34. doi: 10.1177/096032719801700105. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Beck KC, Hulsebus ML, Breen JF, Hoffman EA, Johnson BD. Short term hypoxic exposure at rest and during exercise reduces lung water in healthy humans. J Appl Physiol. 2006;101:1623–1632. doi: 10.1152/japplphysiol.00481.2006. [DOI] [PubMed] [Google Scholar]
- Sokol GM, Van Meurs KP, Wright LL, Rivera O, Thorn WJ, III, Chu PM, Sams RL. Nitrogen dioxide formation during inhaled nitric oxide therapy. Clin Chem. 1999;45:382–387. [PubMed] [Google Scholar]
- Takahashi H, Iwabuchi K, Kudo Y, Tomoike H, Niizeki K, Uchida K, Takahashi K. Simultaneous measurement of pulmonary diffusing capacity for CO and cardiac output by a rebreathing method in patients with pulmonary diseases. Intern Med. 1995;34:330–338. doi: 10.2169/internalmedicine.34.330. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Kobayashi H, Tanaka N, Sato T, Takizawa N, Tomita T. Nitrosyl hemoglobin in blood of normoxic and hypoxic sheep during nitric oxide inhalation. Am J Physiol. 1998;274:H349–357. doi: 10.1152/ajpheart.1998.274.1.H349. [DOI] [PubMed] [Google Scholar]
- Tamhane RM, Johnson RL, Jr, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest. 2001;120:1850–1856. doi: 10.1378/chest.120.6.1850. [DOI] [PubMed] [Google Scholar]
- van der Lee I, Zanen P, Biesma DH, van den Bosch JM. The effect of red cell transfusion on nitric oxide diffusing capacity. Respiration. 2005;72:512–516. doi: 10.1159/000087676. [DOI] [PubMed] [Google Scholar]
- van der Lee I, Zanen P, Grutters JC, Snijder RJ, van den Bosch JM. Diffusing capacity for nitric oxide and carbon monoxide in patients with diffuse parenchymal lung disease and pulmonary arterial hypertension. Chest. 2006;129:378–383. doi: 10.1378/chest.129.2.378. [DOI] [PubMed] [Google Scholar]
- van der Lee I, Gietema HA, Zanen P, van Klaveren RJ, Prokop M, Lammers JW, van den Bosch JM. Nitric oxide diffusing capacity versus spirometry in the early diagnosis of emphysema in smokers. Respir Med. 2009;103:1892–1897. doi: 10.1016/j.rmed.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Van Meurs KP, Rhine WD, Asselin JM, Durand DJ. Response of premature infants with severe respiratory failure to inhaled nitric oxide. Preemie NO Collaborative Group. Pediatr Pulmonol. 1997;24:319–323. doi: 10.1002/(sici)1099-0496(199711)24:5<319::aid-ppul3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Vellar OD. Changes in hemoglobin concentration and hematocrit during the menstrual cycle. I A cross-sectional study. Acta Obstet Gynecol Scand. 1974;53:243–246. doi: 10.3109/00016347409162164. [DOI] [PubMed] [Google Scholar]
- Vogel JA, Gleser MA. Effect of carbon monoxide on oxygen transport during exercise. J Appl Physiol. 1972;32:234–239. doi: 10.1152/jappl.1972.32.2.234. [DOI] [PubMed] [Google Scholar]
- Vogel JA, Gleser MA, Wheeler RC, Whitten BK. Carbon monoxide and physical work capacity. Arch Environ Health. 1972;24:198–203. doi: 10.1080/00039896.1972.10666069. [DOI] [PubMed] [Google Scholar]
- Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- Weaver LK, Howe S, Hopkins R, Chan KJ. Carboxyhemoglobin half-life in carbon monoxide-poisoned patients treated with 100% oxygen at atmospheric pressure. Chest. 2000;117:801–808. doi: 10.1378/chest.117.3.801. [DOI] [PubMed] [Google Scholar]
- Zavorsky GS, Lands LC. Lung diffusion capacity for nitric oxide and carbon monoxide is impaired similarly following short-term graded exercise. Nitric Oxide. 2005;12:31–38. doi: 10.1016/j.niox.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Zavorsky GS, Murias JM. A small amount of inhaled nitric oxide does not increase lung diffusing capacity. Eur Respir J. 2006;27:1251–1257. doi: 10.1183/09031936.06.00146805. [DOI] [PubMed] [Google Scholar]
- Zavorsky GS, Quiron KB, Massarelli PS, Lands LC. The relationship between single-breath diffusion capacity of the lung for nitric oxide and carbon monoxide during various exercise intensities. Chest. 2004;125:1019–1027. doi: 10.1378/chest.125.3.1019. [DOI] [PubMed] [Google Scholar]
- Zavorsky GS, Wilson B, Harris JK, Kim DJ, Carli F, Mayo NE. Pulmonary diffusion and aerobic capacity: is there a relation? Does obesity matter? Acta Physiol (Oxf) 2009 doi: 10.1111/j.1748-1716.2009.02059.x. [DOI] [PubMed] [Google Scholar]