Abstract
Objective
To evaluate the effect of HIV infection on longitudinal changes in kidney function and to identify independent predictors of kidney function changes in HIV-infected individuals.
Design
A prospective cohort.
Methods
Cystatin C was measured at baseline and at the 5-year follow-up visit of the Study of Fat Redistribution and Metabolic Change in HIV infection in 554 HIV-infected participants and 230 controls. Control participants were obtained from the Coronary Artery Risk Development in Young Adults study. Glomerular filtration rate (eGFRcys) was estimated using the formula 76.7 × cysC−1.19.
Results
Compared with controls, HIV-infected participants had a greater proportion of clinical decliners (annual decrease in eGFRcys > 3 ml/min per 1.73 m2; 18 versus 13%, P=0.002) and clinical improvers (annual increase in eGFRcys > 3 ml/min per 1.73 m2; 26 versus 6%, P< 0.0001). After multivariable adjustment, HIV infection was associated with higher odds of both clinical decline (odds ratio 2.2; 95% confidence interval 1.3, 3.9, P = 0.004) and clinical improvement (odds ratio 7.3; 95% confidence interval 3.9, 13.6, P ≤ 0.0001). Among HIV-infected participants, a decrease in HIV viral load during follow-up was independently associated with clinical improvement; conversely, higher baseline and an increase in viral load during follow-up were associated with clinical decline. No individual antiretroviral drug or drug class appeared to be substantially associated with clinical decline or improvement.
Conclusion
Compared with controls, HIV-infected persons were more likely both to have clinical decline and clinical improvement in kidney function during 5 years of follow-up. The extent of viremic control had a strong association with longitudinal changes in kidney function.
Keywords: cystatin C, glomerular filtration rate, HIV, kidney, viral load
Introduction
Kidney disease is a well recognized complication of HIV infection and may become an increasingly important contributor to mortality as participants live longer in the era of highly active antiretroviral therapy (HAART). HIV-associated nephropathy (HIVAN) was recognized as a unique disease entity early in the HIV epidemic; HIVAN was associated with advanced AIDS and was characterized by rapidly progressive renal failure that often required dialysis [1]. However, since the dissemination of HAART, the incidence of HIVAN has decreased [2,3]. Kidney disease remains a major comorbidity of HIV infection [4], but is often distinct from HIVAN [5]. Crosssectional studies have described a 4–17% prevalence of reduced kidney function [estimated glomerular filtration rate (eGFR)<60 ml/min per 1.73m2] in diverse HIV-infected populations [6–9], as well as an increased prevalence of microalbuminuria [10] and proteinuria [6]. Among patients with established chronic kidney disease (CKD), HIV infection has been associated with an increased rate of kidney function decline and progression to end-stage renal disease (ESRD) [4]. Kidney disease in HIV may be primarily caused by traditional risk factors, such as hypertension [11], and by metabolic complications of HIV infection and its treatment, such as diabetes [6]. Interestingly, one recent study reported an association between viral load suppression and improved kidney function across a broad range of estimated GFR [12].
A challenge in the study of kidney disease in the HIV population is that serum creatinine is a less effective measure of GFR in the setting of comorbid illnesses [13]. Creatinine-based estimates of GFR above 60 ml/ min per 1.73 m2 are considered too imprecise to use clinically, even in the general population. Serum cystatin C is an alternative marker of kidney function, which appears to be superior to creatinine in the general population, particularly among persons without CKD (eGFR > 60 ml/min per 1.73 m2) [14]. Additionally, changes in cystatin C levels over time have been shown to be more accurate than creatinine for detecting declining kidney function in diabetic [15] and elderly participants [16]. In order to facilitate its clinical application, equations for estimating GFR from serum cystatin C have recently been developed [17].
Elevated serum levels of cystatin C are more common in HIV populations compared with HIV-negative controls [11,18,19] and are believed to represent under-recognized kidney dysfunction. In a cross-sectional study from the baseline visit of the nationally representative, multicenter Fat Redistribution and Metabolic Change in HIV infection (FRAM) cohort [11], HIV-infected participants had significantly higher cystatin C levels compared with the HIV-negative controls. Among HIV-infected participants, hypertension, lower high-density lipoprotein concentrations, lower CD4 cell count, and hepatitis C (HCV) coinfection were strongly associated with higher cystatin C levels. The main objective of the present study was to utilize follow-up measures of cystatin C from FRAM to evaluate the predictors of change in kidney function over time. A secondary objective was to compare the rates of change in kidney function among HIV-infected and control participants.
Methods
Study design
This prospective study includes 554 HIV-infected and 230 control participants followed over 5 years in the FRAM study. FRAM was designed to examine the association of HIV infection and its treatments with metabolic abnormalities and body composition. The methods have been previously described in detail [20,21]. HIV-infected participants were recruited between June 2000 and September 2002 from 16 geographically diverse sites in the United States. The demographics are similar to HIV-infected participants in the United States. [20] Follow-up data were obtained in FRAM2 from October 2004 through August 2007, approximately 5 years on average after the baseline visit. Exclusion criteria for FRAM included age less than 18 years; pregnancy; planned pregnancy within 3 months of enrollment; and contraindication to MRI or dual energy X-ray absorptiometry (DEXA) scans. Control participants in FRAM were obtained from two sites (Oakland, California, USA and Birmingham, Alabama, USA) of the Coronary Artery Risk Development in Young Adults (CARDIA) study [21] that were part of an ancillary study, the Visceral Fat and Metabolic Rate in Young Adults study (VIM). These controls have a BMI distribution similar to the CARDIA parent study and include both Whites and African–Americans. The protocol was approved by institutional review boards at all sites.
Of 1183 HIV-infected and 297 HIV-uninfected controls recruited for the baseline visit, 581 HIV-infected and 241 controls were seen at the 5-year follow-up visit. This study included all participants with measurements of cystatin C at both baseline and follow-up (n = 554 HIV-infected and 230 controls). For all comparisons with the control group, the HIV participants were restricted to those without evidence of opportunistic infection, as determined by self-report at either the baseline or follow-up visit, and between the ages 33–45 years at baseline (n = 337) to match the age range of the controls.
Kidney function
Cystatin C was measured on previously frozen sera that were stored at −70°C, by a BNII nephelometer (Siemens, Inc., Deerfield, Illinois, USA) that used a particle-enhanced immunonephelometric assay (N Latex Cystatin C) [22]. The coefficient of variation for between-run imprecision was less than 2% for cystatin C [22]. Additional characteristics of the assay have been reported previously [23]. GFR was estimated from serum cystatin C levels according to the recently published CKD Epidemiology Collaboration equation [eGFR=76.7× cysC−1.19], which was derived from a pooled analysis of 3418 individuals with CKD from four studies in which GFR was directly measured using iothalamate or EDTA clearance [17]. Cystatin C-based estimates of GFR (eGFRcys) that were greater than 120 ml/min per 1.73m2 were capped at this level, as higher estimates are unlikely to be accurate or precise. Annual change in eGFRcys from baseline to the follow-up visit was calculated from the difference in the two measurements and the elapsed time between them and expressed in ml/min per 1.73 m2 per year. ‘Clinical decline’ was defined a priori as an annual loss of GFR more than 3 ml/min per 1.73m2 per year, a level shown in prior studies to correspond to elevated mortality risk [24]. We also defined ‘clinical improvement’ as a positive change in GFR more than 3 ml/min per 1.73m2 per year. CKD was defined as an eGFR level of less than 60 ml/min per 1.73m2 . Incident CKD was defined as progression from an eGFR level of more than 60 ml/min per 1.73m2 at baseline to an eGFR level of less than 60 ml/min per 1.73m2 at the follow-up visit.
Predictors of change in kidney function
Demographic characteristics included self-reported age at baseline, sex, and race. Additional candidate predictors at baseline included self-reported history of coronary artery disease (CAD), diabetes (medication use or fasting glucose ≥ 126 mg/dl), smoking status (current, past, never; pack years), heroin use, waist circumference, systolic blood pressure, diastolic blood pressure, serum direct low-density lipoprotein (D-LDL), high-density lipoprotein (HDL), C-reactive protein (CRP), uric acid, fibrinogen, serum albumin (continuous variable, >4.0); urine albumin excretion (spot albumin/creatinine ratio) and detectable proteinuria by dipstick; total and regional subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT), obtained by MRI, methods described previously [20]; lean body mass by MRI; and medication use [antihypertensives, angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), and statins]. Additional candidate predictors evaluated only among HIV-infected participants included HIV RNA level (baseline and change from baseline during follow-up), CD4 cell count (baseline and change during follow-up), and baseline measurements or reports of: nadir CD4 cell count, self-reported duration of HIV, HAARTuse, peripheral lipoatrophy (<10th percentile of leg SAT in controls [25]), AIDS (CD4 cell count <200 or AIDS-defining opportunistic infection), and active hepatitis C infection (determined by detectable HCV RNA). In addition, use of each antiretroviral drug and class was evaluated both by ever use of the drug or class and by total duration of use through the follow-up visit.
Statistical analysis
The distributions of annual change in eGFRcys for HIV-infected and control participants were initially displayed as histograms, with reference lines at −3 and +3 to allow visual comparisons of the proportions with clinical decline, no change, and clinical improvement. The distributions were compared using the two-sample Kolmogorov–Smirnov test. Characteristics of HIV-infected participants and controls were compared and tested for statistical significance using the Mann–Whitney U test for continuous variables and Fisher’s exact test for categorical variables.
Among HIV-infected and control participants, multi-variable linear and logistic regression models were used to examine the association of HIV infection with annual change in eGFRcys and incident CKD, respectively. Multinomial logistic regression analysis was used to examine simultaneously the associations of HIV infection with clinical decline and clinical improvement compared with ‘no change,’ the reference group. Models were built using stepwise regression with P value of 0.05 or less for entry and retention. Sex, age, and race/ethnicity were included in every model. Interactions of age, sex, and race with HIV status were assessed for each outcome and included if they reached statistical significance. Multiple imputation utilizing the Markov chain Monte Carlo method for arbitrary missing data was used to impute missing covariates prior to covariate selection [26]. We also adjusted estimates using an inverse probability of censoring weight (IPCW) approach [27] by modeling the probability of missing follow-up data. We then used the inverse of this probability as a weight in the logistic regression analysis for those with known follow-up data to address the impact of informative censoring (i.e., lack of a follow-up visit) as a sensitivity analysis.
We applied a staged modeling approach for each outcome, fitting models with HIV infection alone; demographic adjustment, and demographic and kidney disease risk factors. In this way we were able to evaluate if the HIV effect was attenuated after correction first with demographics, or by the subsequent addition of kidney disease risk factors.
Among the HIV-infected participants only, multivariable linear and logistic regression models were used in a similar fashion to evaluate risk factors among the HIV-infected participants, with the addition of a model adding HIV-related factors.
All analyses were conducted using the SAS system, version 9.1 (SAS Institute, Inc., Cary North Carolina, USA).
Results
Baseline characteristics of 337 HIV-infected and 230 control participants are displayed in Table 1. The characteristics of all 554 HIV-infected participants were similar to the 337 age-restricted, opportunistic infectionexcluded HIV-infected participants. HIV-infected participants were more likely to be male, to be current smokers, to have CAD, diabetes, and microalbuminuria, and to use antihypertensives. Both D-LDL and HDL were lower in HIV-infected participants and CRP levels were higher.
Table 1.
Baseline characteristics of HIV-infected and controls.
| Age restricted |
||||
|---|---|---|---|---|
| HIV+ (n=337) | Controls (n=230) | P value | All HIV+ (n=554) | |
| Age at baseline (y) | 41 (37, 45) | 41 (37, 43) | 0.028 | 43 (37, 48) |
| Sex | ||||
| Female participants | 107 (32%) | 105 (46%) | 0.001 | 167 (30%) |
| Male participants | 229 (68%) | 125 (54%) | NA | 385 (70%) |
| Race | ||||
| African–American | 143 (42%) | 101 (44%) | 0.48 | 236 (43%) |
| White | 160 (48%) | 129 (56%) | NA | 266 (48%) |
| Other | 34 (10%) | 0 | NA | 52 (9%) |
| History of CAD | 18 (5%) | 0 | <0.0001 | 34 (6%) |
| Diabetic participants | 22 (7%) | 7 (3%) | 0.080 | 45 (8%) |
| Smoking status | ||||
| Current | 138 (43%) | 36 (16%) | <0.0001 | 206 (39%) |
| Past | 66 (21%) | 30 (14%) | NA | 123 (23%) |
| Never | 117 (36%) | 155 (70%) | NA | 200 (38%) |
| HCV RNA+ | 80 (24%) | 3 (1%) | <0.0001 | 122 (22%) |
| Systolic BP (mmHg) | 114 (106, 123) | 116 (108, 126) | 0.028 | 116 (107, 124) |
| Diastolic BP (mmHg) | 79 (71, 85) | 78 (71, 84) | 0.90 | 78 (71, 85) |
| Antihypertensive use | 55 (16%) | 10 (4%) | <0.0001 | 114 (21%) |
| D-LDL (mg/dl) | 110 (79, 141) | 119 (98, 145) | 0.0003 | 109 (79, 137) |
| HDL (mg/dl) | 41 (34, 53) | 52 (43, 61) | <0.0001 | 41 (34, 52) |
| CRP (mg/l) | 1.6 (0.7, 3.5) | 1.1 (0.6, 2.8) | 0.022 | 1.6 (0.7, 3.8) |
| Serum albumin (g/dl) | 4.2 (4.0, 4.5) | 4.2 (4.0, 4.4) | 0.22 | 4.2 (4.0, 4.5) |
| Urine ACR (mg/g) | 5.0 (3.0, 11.0) | 4.0 (3.0, 5.0) | <0.0001 | 5.0 (3.5, 12.0) |
| Baseline eGFRcys | 87 (72, 103) | 108 (97, 120) | <0.0001 | 86 (72, 103) |
| Baseline CKD (eGFRcys <60) | 26 (7.7%) | 2 (0.9%) | <0.0001 | 47 (8.5%) |
| HIV RNA (1000/ml) | 0.4 (0.4, 6.1) | NA | NA | 0.4 (0.4, 6.1) |
| Detectable HIV RNA | 152 (45%) | NA | NA | 251 (45%) |
| Current CD4 cells | 392 (231, 539) | NA | NA | 385 (232, 560) |
| Nadir CD4 cells | 107 (20, 231) | NA | NA | 127 (30, 252) |
| HIV duration (y) | 8.4 (5.5, 12.2) | NA | NA | 7.9 (5.2, 11.8) |
| HAART use | 294 (88%) | NA | NA | 480 (88%) |
The table displays median (IQR). ACR, albumin/creatinine ratio; BP, blood pressure; CAD, coronary artery disease; CKD, chronic kidney disease; CRP, C-reactive protein; D-LDL, direct low-density lipoprotein; eGFR, estimated glomerular filtration rate; HAART, highly active antiretroviral therapy; HCV, hepatitis C virus; HDL, high-density lipoprotein; IQR, interquartile range; NA, data not applicable for control participants; y, years.
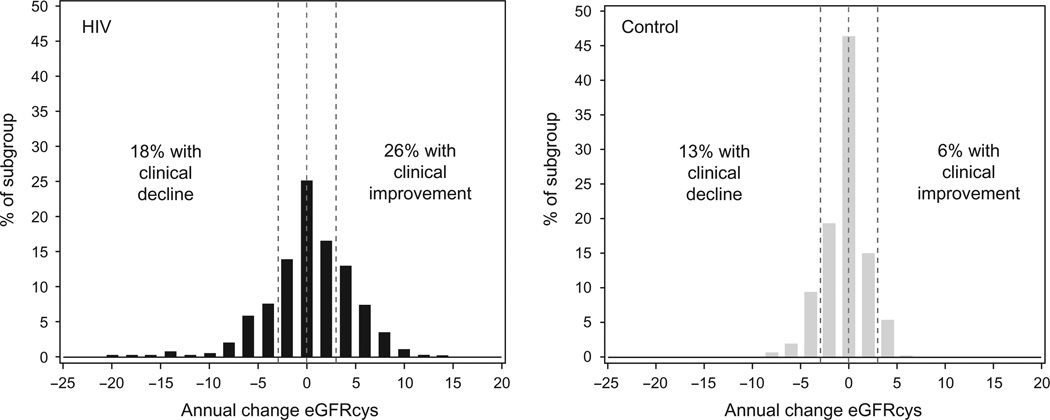
In both unadjusted and adjusted models, HIV-infected participants unexpectedly showed a slight annual improvement on average in eGFRcys (0.34 ± 4.45 ml/ min per 1.73m2 ), which was significantly different from controls who had a mean decrease in kidney function (−0.45 ± 2.25ml/min per 1.73m2 ; P=0.012). However, there was a much broader distribution (Fig. 1) within the HIV-infected cohort [standard deviation (SD) = 4.5] than in controls (SD = 2.25; P < 0.0001 for the difference in distributions). The HIV-infected cohort had both a larger proportion of clinical decliners (18 versus 13%, P= 0.002) and clinical improvers (26 versus 6%, P ≤ 0.0001) compared with controls. After adjustment for demographics and risk factors (Table 2), HIV infection was associated with twice the odds of clinical decline and seven times the odds of clinical improvement. HIV-infected participants without CKD at baseline were far more likely to develop CKD during follow-up compared with controls [8.2 vs. 0.9%, adjusted odds ratio (OR) 6.5, P= 0.014, Table 2]. We performed a sensitivity analysis of the models comparing HIV-infected and control participants in which we did not cap eGFRcys levels at 120 ml/min per 1.73 m2 (97 HIV-infected and 94 control participants were capped at baseline or follow-up or both), and results were similar. Results were also similar using IPCW in sensitivity analyses.
Fig. 1.
Distribution of annual change in cystatin C-based estimates of glomerular filtration rate (eGFRcys).
Table 2.
Odds of clinical improvement or decline and incident chronic kidney disease by HIV status.
| OR (95% CI, P value) |
||||
|---|---|---|---|---|
| HIV+ (n=337) | Controls (n=230) | Unadjusted | Adjusted | |
| Clinical improvementa | 87 (26%) | 14 (6.1%) | 6.2 (3.4, 11.3, P <0.0001) | 7.3 (3.9, 13.6, P < 0.0001) |
| Clinical declinea | 62 (18%) | 29 (13%) | 2.1 (1.3, 3.5, P=0.002) | 2.2 (1.3, 3.9, P=0.004) |
| Incident CKD at year 5b | 26 (8.2%) | 2 (0.9%) | 10.3 (2.4, 43.9, P =0.002) | 6.5 (1.5, 28.7, P = 0.014) |
CI, confidence interval; CKD, chronic kidney disease; OR, odds ratio.
Analysis using multinomial logistic regression with no change as the referent group. Clinical decline defined as annual eGFR loss −3 ml/min per 1.73 m2 or more per year; clinical improvement defined as annual eGFR improvement +3 ml/min per 1.73 m2 or more per year.
Participants without CKD at baseline (eGFRcys> 60 ml/min per 1.73 m2; n = 311 HIV infected, 228 controls).
Among the HIV-infected participants, we simultaneously evaluated predictors of clinical decline and clinical improvement using a multinomial logistic regression model with the unchanged group as the referent (Table 3). Factors associated with clinical decline included the traditional risk factors of older age and elevated serum glucose, as well as lower serum albumin levels. Of the HIV-related factors, higher baseline HIV RNA level and an increase in HIV RNA during follow-up were associated with clinical decline in kidney function. Consistent with these findings, a decrease in HIV viral load during follow-up was the only statistically significant predictor of clinical improvement in the adjusted analysis; lower baseline glucose and antihypertensive use had marginal associations with clinical improvement. Prevalence of detectable viral load at follow-up was higher in the decliners (42%) than in the unchanged (31%) and lowest in the improvers (15%, P < 0.0001).
Table 3.
Characteristics independently associated with clinical decline or improvement among HIV-infected participants.
| Adjusted odds ratios (n=554) |
||||||
|---|---|---|---|---|---|---|
| Decline vs. unchangeda |
Improvement vs. unchangeda |
|||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Demographic factors | ||||||
| Female vs. male participants | 1.05 | (0.63, 1.75) | 0.85 | 0.92 | (0.55, 1.52) | 0.73 |
| African–American vs.White | 1.26 | (0.77, 2.05) | 0.36 | 0.97 | (0.60, 1.57) | 0.91 |
| Other vs. White | 1.10 | (0.47, 2.58) | 0.83 | 1.25 | (0.61, 2.57) | 0.54 |
| Age (per decade) | 1.34 | (1.02, 1.77) | 0.036 | 0.85 | (0.65, 1.13) | 0.26 |
| Kidney disease risk factors | ||||||
| Antihypertensive use | 0.58 | (0.32, 1.06) | 0.074 | 1.66 | (0.97,2.83) | 0.065 |
| Baseline glucose: 100–125 vs. <100 mg/dl | 2.50 | (1.10, 5.71) | 0.030 | 0.92 | (0.33, 2.55) | 0.87 |
| Baseline glucose: >125 vs. <100 mg/dl | 2.38 | (1.04, 5.43) | 0.040 | 0.16 | (0.02, 1.27) | 0.081 |
| Baseline albumin (g/dl) | 0.56 | (0.31, 0.99) | 0.045 | 1.08 | (0.62, 1.87) | 0.79 |
| HIV-related factors | ||||||
| Baseline HIV RNA (log 10) | 1.35 | (1.00, 1.83) | 0.048 | 0.77 | (0.52, 1.12) | 0.16 |
| Change in HIV RNA (log 10) | 1.38 | (1.08, 1.77) | 0.011 | 0.53 | (0.37, 0.75) | 0.0003 |
Analysis using multinomial logistic regression with no change as the referent group. Clinical decline defined as annual eGFR loss −3 ml/min per 1.73 m2 or more per year; clinical improvement defined as annual eGFR improvement +3 ml/min per 1.73 m2 or more per year.
Similar results were obtained in a multivariable linear regression model of annual eGFRcys change among all HIV-infected participants (Table 4). Older age, higher baseline glucose, and nonuse of antihypertensives were all strongly associated with declining kidney function. We compared separately the effects of ACE inhibitor/ARB use and ‘other’ antihypertensives on annual change in eGFRcysC. Although the results were similar with overlapping confidence intervals, the effect of ACE/ ARB [+1.01 ml/min per 1.73m2, 95% confidence interval (CI) −0.38, 2.41] was if anything weaker than ‘other’ antihypertensive use (+1.93 ml/min per 1.73 m2 , 95% CI 0.73, 3.14). Hypolipidemic medication use was associated with worsening kidney function. Although directionally similar, the point estimate was modestly stronger for fibrates (−1.80ml/min per 1.73 m2 per year, 95% CI −3.23 to −0.37) than statins (−0.98 ml/min per 1.73 m per year, 95% CI −2.17 to +0.20). Unexpectedly, higher HDL levels at baseline were associated with worsening kidney function, and higher baseline LDL levels were associated with improving kidney function. However, in an exploratory analysis that replaced baseline lipid values with lipid changes during follow-up, we found that an increase in HDL level during follow-up was associated with improving kidney function (+0.53 ml/ min per 1.73m per 10mg/dl, P=0.0005), whereas an increase in LDL level during follow-up was associated with declining kidney function (−0.14ml/min per 1.73m per 10mg/dl, P=0.007). African–American race (P=0.31), baseline proteinuria (P=0.33), HCV infection (P=0.36), and heroin use (P=0.77) did not have statistically significant associations with longitudinal changes in kidney function.
Table 4.
Multivariable linear model of annual eGFRcys change in HIV-infected participantsa
| Adjusted effect (ml/min per 1.73 m2 per year), n =554 |
|||
|---|---|---|---|
| Effect | 95% CI | P value | |
| Demographic factors | |||
| Female vs. male participants | 0.25 | (−0.65, 1.15) | 0.59 |
| African–American vs. White | −0.45 | (−1.32, 0.42) | 0.31 |
| Other vs. White | 0.19 | (−1.17,1.56) | 0.78 |
| Age (per decade) | −0.60 | (−1.09, −0.11) | 0.016 |
| Kidney disease risk factors | |||
| Baseline glucose >125 vs. <100 mg/dl | −4.46 | (−6.19, −2.74) | <0.0001 |
| Baseline glucose: 100–125 vs. <100 mg/dl | −1.42 | (−3.08, 0.23) | 0.092 |
| Antihypertensive use | 1.78 | (0.79, 2.78) | 0.0004 |
| Hypolipidemic use | −1.38 | (−2.43, −0.32) | 0.011 |
| Baseline HDL (/10 mg/dl) | −0.39 | (−0.65, −0.13) | 0.003 |
| Baseline D-LDL (/10 mg/dl) | 0.14 | (0.05, 0.24) | 0.002 |
| HIV-related factors | |||
| Baseline HIV RNA (log 10) | −1.07 | (−1.63, −0.51) | 0.0002 |
| Change in HIV RNA (log 10) | −1.34 | (−1.86, −0.83) | <0.0001 |
| Change in CD4 cells (/100) | 0.18 | (−0.00, 0.37) | 0.056 |
| Saquinavir duration (per year of use through follow-up visit) | 0.38 | (0.04, 0.72) | 0.028 |
Multivariable linear regression analysis.
Of the HIV-specific variables, higher baseline HIV RNA level and increases in HIV RNA during follow-up were strongly and significantly associated with declining kidney function (Table 4). An increase in CD4 cell count during follow-up was associated with improvement. Use of saquinavir was associated with modest but statistically significant improvement. No other antiretroviral drug or drug class had a statistically significant association with longitudinal changes in kidney function. Tenofovir use was rare at the baseline visit; however, by the time of the follow-up visit, a majority of participants (59%) had been exposed to tenofovir (average duration of use was 2 years).
Discussion
In this nationally representative sample of HIV-infected persons, we found that HIV-infected participants were more likely than HIV-negative controls to have clinically significant decline in kidney function and to progress to CKD prospectively after 5 years of follow-up. However, we also found that 26% of HIV-infected persons manifested clinically significant improvements in kidney function. Changes in HIV viral load were strongly associated with both improvements and declines in kidney function that were observed in HIV-infected participants. Our results suggest that HIV viral replication is a primary pathogenic factor in the development of kidney disease in HIV-infected persons and a potential therapeutic target for HIV-related kidney disease.
The importance of viral load in the development and progression of HIV-related kidney disease is supported by laboratory studies and clinical trials. Direct viral infection of renal tubular cells and podocytes has been associated with the development of HIVAN in transgenic mouse models and human renal biopsies [28]. It follows therefore that treatment of active viral replication with HAART may slow the progression of kidney dysfunction. Several cross-sectional studies and one retrospective cohort study have suggested an association of HAARTuse with higher eGFR and decreased incidence of HIVAN [2,3,19,29].
Our study provides evidence that kidney function may improve in HIV-infected persons with suppression of viral load. This is in stark contrast to most other forms of CKD, such as the nephropathies of diabetes and hypertension, in which progression of GFR loss may be slowed, but rarely reversed. In a recent analysis of data from the AIDS Clinical Trials Group (ACTG) Longitudinal Linked Randomized Trials (ALLRT) study [12], full virologic suppression was associated with an average increase in eGFRcr of 9.2 ml/min per 1.73 m2 over 160 weeks (approximately 3 ml/min per 1.73m2 per year) in participants with CD4 cell counts less than 200 cells/µl with evidence of kidney damage at baseline. Renal benefits of antiretroviral therapy (ART) were also observed in the Development of Anti-Retroviral Therapy in Africa (DART) [30] and the Strategies for Management of Antiretroviral Therapy (SMART) [31] trials. In SMART, continuous viral suppression was associated with both a reduced ESRD incidence [31] and an improvement in cystatin C levels compared with interrupted therapy [32].
Our study is also in agreement with previous findings that HIV infection is a risk factor for kidney function decline and incident CKD [4]. Just as a decrease from baseline in viral load was associated with clinical improvement, an increase from baseline in viral load was associated with clinical decline. The higher incidence of CKD may in part be explained by lower mean eGFRcys at the baseline visit [11].
Chronic inflammation, hypertension, and metabolic abnormalities such as insulin resistance and dyslipidemia also appear to be important risk factors for worsening kidney function in our study. Markers of inflammation are known predictors of kidney function decline [33]; in this study, lower serum albumin (a negative acute phase response protein) was significantly associated with clinical decline but CRP was not. Higher fasting glucose was one of the strongest predictors of clinical decline. A decrease in HDL and an increase in D-LDL from baseline to follow-up were also associated with worsening kidney function. However, we found that hypolipidemic medications, particularly fibrates, were associated with modest declines in kidney function, a finding that should be investigated in future studies. In a prior cross-sectional analysis of the baseline visit of FRAM [11], hypertension was associated with worse kidney function. In this follow-up study, baseline blood pressure did not appear to be associated with longitudinal changes in kidney function. Antihypertensive use, however, was protective of kidney function, though there was no added benefit of ACE inhibitor or ARB use over ‘other’ antihypertensives. Together, these findings reinforce the importance of blood pressure as a modifiable risk factor in this population.
In the cross-sectional analysis of the baseline visit of FRAM, proteinuria, HCV coinfection, and heroin use were significantly associated with higher cystatin C levels among HIV-infected participants [11]. None was a statistically significant predictor of declining kidney function in the present study, though all had point estimates in the expected direction. African–American race has historically been viewed as a risk factor for HIVAN, but was not a statistically significant predictor of decline in this analysis. We note that confidence intervals were wide enough to leave open the possibility of important effects for these predictors.
As the variety of antiretroviral drugs has proliferated, clinicians have become aware of the risk profiles of individual drugs. Tenofovir and indinavir are the most widely cited nephrotoxic agents, though there have been case reports of renal dysfunction with many of the other drugs [34]. Notably, despite widespread use of tenofovir at the follow-up visit, it did not appear to be associated with worsening kidney function in our study. Indinavir and efavirenz were associated with higher cystatin C levels at the baseline visit of FRAM [11] but did not appear to have substantial effects on changes in this follow-up study. Current use of indinavir has fallen considerably, which may explain why it was not a statistically significant predictor of kidney function decline. Interestingly, saquinavir appeared to be associated with modest improvement in eGFRcys, though it appeared to have little association with clinically significant improvement. Although this finding should be evaluated in future studies, this association should be interpreted in the context of the large number of candidate antiretroviral predictors studied.
The inclusion of participants with higher CD4 cell counts extends the generalizability of our results compared with studies that examined participants in clinical drug trials who have more advanced disease. The large proportion of participants with a detectable viral load allowed us to examine the role of active viral replication on longitudinal changes in kidney function.
Our study was limited by the lack of a direct measure of GFR. However, this limitation is shared by all large studies of kidney disease in HIV-infected persons. In addition, the formula for eGFRcys was derived from a CKD cohort and has not been validated in an HIV-infected population. As in all observational cohort studies, there may be important unknown confounders for which either we did not adjust or we incompletely captured in our multivariate analysis. The participants who were lost to follow-up or who died may have led to downward bias in observed differences between the HIV and control groups. However, use of IPCW in sensitivity analyses found results similar to the primary analysis, suggesting that study dropout did not unduly influence the results.
In conclusion, HIV-infected participants were both more likely to have clinically significant kidney function decline and more likely to have clinically significant improvement compared with controls during a 5-year follow-up. Longitudinal kidney function changes in HIV infection appeared to be strongly mediated by changes in viral load, though fasting glucose levels and other metabolic abnormalities may also play an important role. Further studies should evaluate the prognostic implications for HIV-infected persons with dynamic changes in kidney function compared to those who have steady eGFR levels, both on kidney and cardiovascular outcomes.
Supplementary Material
Acknowledgements
FRAM is supported by grants from the National Institutes of Health (R01-DK-57508, R01-HL-74814, and R01-HL-53359). NIH support for this project is also provided through grants to General Clinical Research Centers (M01-RR00036, M01-RR00051, M01-RR00052, M01-RR00054, M01-RR00083, M01-RR00636, and M01-RR00865). M.G.S. is supported by an Established Investigator Award from the American Heart Association.
References
- 1.Humphreys MH. Human immunodeficiency virus-associated glomerulosclerosis. Kidney Int. 1995;48:311–320. doi: 10.1038/ki.1995.299. [DOI] [PubMed] [Google Scholar]
- 2.Lucas GM, Eustace JA, Sozio S, Mentari EK, Appiah KA, Moore RD. Highly active antiretroviral therapy and the incidence of HIV-1-associated nephropathy: a 12-year cohort study. AIDS. 2004;18:541–546. doi: 10.1097/00002030-200402200-00022. [DOI] [PubMed] [Google Scholar]
- 3.Szczech LA, Gupta SK, Habash R, Guasch A, Kalayjian R, Appel R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66:1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 4.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Volberding PA, O′Hare AM. The impact of HIV on chronic kidney disease outcomes. Kidney Int. 2007;72:1380–1387. doi: 10.1038/sj.ki.5002541. [DOI] [PubMed] [Google Scholar]
- 5.Wyatt CM, Morgello S, Katz-Malamed R, Wei C, Klotman ME, Klotman PE, D′Agati VD. The spectrum of kidney disease in patients with AIDS in the era of antiretroviral therapy. Kidney Int. 2009;75:428–434. doi: 10.1038/ki.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernando SK, Finkelstein FO, Moore BA, Weissman S. Prevalence of chronic kidney disease in an urban HIV infected population. Am J Med Sci. 2008;335:89–94. doi: 10.1097/MAJ.0b013e31812e6b34. [DOI] [PubMed] [Google Scholar]
- 7.Cheung CY, Wong KM, Lee MP, Liu YL, Kwok H, Chung R, et al. Prevalence of chronic kidney disease in Chinese HIV-infected patients. Nephrol Dial Transplant. 2007;22:3186–3190. doi: 10.1093/ndt/gfm350. [DOI] [PubMed] [Google Scholar]
- 8.Lucas GM, Mehta SH, Atta MG, Kirk GD, Galai N, Vlahov D, Moore RD. End-stage renal disease and chronic kidney disease in a cohort of African-American HIV-infected and at-risk HIV-seronegative participants followed between 1988 and 2004. AIDS. 2007;21:2435–2443. doi: 10.1097/QAD.0b013e32827038ad. [DOI] [PubMed] [Google Scholar]
- 9.Mocroft A, Kirk O, Gatell J, Reiss P, Gargalianos P, Zilmer K, et al. Chronic renal failure among HIV-1-infected patients. AIDS. 2007;21:1119–1127. doi: 10.1097/QAD.0b013e3280f774ee. [DOI] [PubMed] [Google Scholar]
- 10.Szczech LA, Grunfeld C, Scherzer R, Canchola JA, van der Horst C, Sidney S, et al. Microalbuminuria in HIV infection. AIDS. 2007;21:1003–1009. doi: 10.1097/QAD.0b013e3280d3587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odden MC, Scherzer R, Bacchetti P, Szczech LA, Sidney S, Grunfeld C, Shlipak MG. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: the FRAM study. Arch Intern Med. 2007;167:2213–2219. doi: 10.1001/archinte.167.20.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalayjian RC, Franceschini N, Gupta SK, Szczech LA, Mupere E, Bosch RJ, et al. Suppression of HIV-1 replication by antiretro-viral therapy improves renal function in persons with low CD4 cell counts and chronic kidney disease. AIDS. 2008;22:481–487. doi: 10.1097/QAD.0b013e3282f4706d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the modification of diet in renal disease and Cock-croft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005;16:459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 14.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 15.Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol. 2005;16:1404–1412. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shlipak MG, Katz R, Kestenbaum B, Fried LF, Newman AB, Siscovick DS, et al. Rate of kidney function decline in the elderly: a comparison using creatinine and cystatin C. Am J Nephrol. 2009 doi: 10.1159/000212381. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones CY, Jones CA, Wilson IB, Knox TA, Levey AS, Spiegelman D, et al. Cystatin C and creatinine in an HIV cohort: the nutrition for healthy living study. Am J Kidney Dis. 2008;51:914–924. doi: 10.1053/j.ajkd.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaroszewicz J, Wiercinska-Drapalo A, Lapinski TW, Prokopo-wicz D, Rogalska M, Parfieniuk A. Does HAART improve renal function? An association between serum cystatin C concentration, HIV viral load and HAART duration. Antivir Ther. 2006;11:641–645. [PubMed] [Google Scholar]
- 20.Tien PC, Benson C, Zolopa AR, Sidney S, Osmond D, Grunfeld C. The study of fat redistribution and metabolic change in HIV infection (FRAM): methods, design, and sample characteristics. Am J Epidemiol. 2006;163:860–869. doi: 10.1093/aje/kwj111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 22.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Ne-phelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 23.Shlipak MG, Wassel Fyr CL, Chertow GM, Harris TB, Kritch-evsky SB, Tylavsky FA, et al. Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2006;17:254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 24.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168:2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosmiski LA, Bacchetti P, Kotler DP, Heymsfield SB, Lewis CE, Shlipak MG, et al. Relationship of fat distribution with adipo-kines in human immunodeficiency virus infection. J Clin Endocrinol Metab. 2008;93:216–224. doi: 10.1210/jc.2007-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 27.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 28.Ross MJ, Klotman PE. Recent progress in HIV-associated nephropathy. J Am Soc Nephrol. 2002;13:2997–3004. doi: 10.1097/01.asn.0000040750.40907.99. [DOI] [PubMed] [Google Scholar]
- 29.Ifudu O, Rao TK, Tan CC, Fleischman H, Chirgwin K, Friedman EA. Zidovudine is beneficial in human immunodeficiency virus associated nephropathy. Am J Nephrol. 1995;15:217–221. doi: 10.1159/000168835. [DOI] [PubMed] [Google Scholar]
- 30.Reid A, Stohr W, Walker AS, Williams IG, Kityo C, Hughes P, et al. Severe renal dysfunction and risk factors associated with renal impairment in HIV-infected adults in Africa initiating antiretroviral therapy. Clin Infect Dis. 2008;46:1271–1281. doi: 10.1086/533468. [DOI] [PubMed] [Google Scholar]
- 31.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 32.Mocroft A, Wyatt C, Szczech L, Neuhaus J, El-Sadr W, Tracy R, et al. Interruption of antiretroviral therapy is associated with increased plasma cystatin C. AIDS. 2009;23:71–82. doi: 10.1097/QAD.0b013e32831cc129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fried L, Solomon C, Shlipak M, Seliger S, Stehman-Breen C, Bleyer AJ, et al. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol. 2004;15:3184–3191. doi: 10.1097/01.ASN.0000146422.45434.35. [DOI] [PubMed] [Google Scholar]
- 34.Berns JS, Kasbekar N. Highly active antiretroviral therapy and the kidney: an update on antiretroviral medications for nephrologists. Clin J Am Soc Nephrol. 2006;1:117–129. doi: 10.2215/CJN.00370705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.