Abstract
Neurofibromatosis type 2 protein (NF2) is an underappreciated tumor suppressor involved in a broad range of nervous system tumors. Inactivation of the NF2 gene leads to neurofibromatosis type 2, which is characterized by multiple benign nervous system tumors and mutations in the gene have been demonstrated in many other tumor types as well. All tumors, regardless of location or grade, lack a fundamental control over cell cycle progression. Historically, NF2 is an unconventional tumor suppressor protein in that it does not directly influence the cell cycle. NF2 links receptors at the plasma membrane to their cytoplasmic kinases to facilitate contact inhibition. However, NF2 can also interact with an array of cytoplasmic and nuclear proteins that affect cell cycle progression. Further, through some of these pathways, NF2 may reverse the functional inhibition of conventional tumor suppressor pathways. Here we review mechanisms utilized by NF2 to regain control of the cell cycle.
Keywords: NF2, merlin, tumor suppressor, cell cycle
Introduction
Disruption or inactivation of the neurofibromatosis type 2 (NF2) gene, located on human chromosome 22, leads to the autosomal dominant cancer disorder, neurofibromatosis type 21,2. Patients with NF2 have a predisposition toward the development of a multitude of CNS tumors, most notably bilateral schwannomas, meningiomas, and ependymomas. Though these tumors are typically benign, they often convey a loss of hearing and balance, paralysis, and can lead to early death3. While the incidence of NF2 disease is relatively rare, the NF2 protein, also referred to as merlin or schwannomin, has been shown to play a pivotal role in a number of other cancers. For instance, loss or inactivation of the NF2 gene has also been described in highly aggressive mesothelioma tumors, spontaneous meningiomas and schwannomas, as well as gliomas and breast cancers4-9.
The NF2 gene product is a member of the ERM (ezrin, radixin, moesin) family of cell adhesion proteins10 and as such, NF2 binds either directly or indirectly to the actin cytoskeleton and is essential for the formation of stable adherens junctions11-14. As a cytoplasmic scaffolding protein, NF2 provides a crucial link between the extracellular environment and cell signaling pathways. Specifically, NF2 prevents the effects of aberrant mitogenic signaling on oncogenic transformation, making NF2 the only member of the ERM family to exhibit tumor suppressor activity15. At the plasma membrane, NF2 binds to receptor tyrosine kinases and integrins, such as CD44 and β1 integrin, respectively15,16 which confers responsiveness to cell confluence and growth factor availability to help maintain contact inhibition15,17,18. Consequently, loss of NF2 leads to a loss of contact inhibition and a gain of anchorage independence, further facilitating invasion and mobility of malignantly transformed cells19,20. For a detailed review of these aspects of NF2 function, please see references 19, 20, 37.
Typically, NF2 is concentrated in actin-rich areas of the cell such as fillipodia, lamellipodia, membrane ruffles, and cell to cell boundaries. Due to its predominant localization to the plasma membrane, it would not appear that NF2 significantly impacts the cell cycle, in contrast to most canonical tumor suppressors. However, several studies have demonstrated the presence of a nuclear fraction of NF2, which would facilitate this protein's ability to impact the cell cycle21-27 though the presence of nuclear NF2 could merely be indicative of its sequestration during times of rapid growth20. While NF2 has not been shown to elicit a direct effect on the cell cycle, several studies have demonstrated its influence on cell cycle progression. For example, reintroduction of NF2 induces cell cycle arrest in numerous cells types including, rat and human schwannomas, primary endothelial cells, mesotheliomas, and patient-derived meningiomas12, 27, 28-33. These studies have led to speculation that NF2 may indeed regulate progression of the cell cycle across a range of cell types. Below, we review this evidence and its implications in cell cycle regulation and tumor progression.
Nuclear NF2: In the right place, at the right time
Traditionally, NF2 has been primarily thought of as a cytoplasmic scaffold used to transduce signals from the extracellular milieu into the intracellular cues necessary for the cell to form and maintain its boundaries. While this is still considered to be a critical task for NF2, its nuclear localization hints at broader tumor suppressive capabilities. To date, NF2 has been detected in the nucleus of mouse and human glial tumors, osteosarcomas, mesotheliomas, and immortalized schwann cells, to name a few25-27, Beltrami, unpublished observation (as summarized in Table 1). While the NF2 gene lacks a canonical nuclear localization sequence, which may make the nuclear localization of NF2 difficult to detect23, it has been established that NF2 utilizes the CRM1/exportin pathway to exit the nucleus, thereby allowing the use of the CRM1-specific inhibitor, leptomycin B to to trap NF2 in the nucleus in cell culture models22,23.
Table 1.
Evidence for nuclear localization of NF2
| Regulation of nuclear translocation | Reference | Detection method* | Cell types examined | Species |
|---|---|---|---|---|
| Phosphorylation of Ser 518 | Lü et al. (2008) | IF and IHC | neurons | human |
| Thaxton et al. (2007) | IF | schwannoma | human | |
| Li et al. (2010) | IF | mesothelioma | human | |
| squamous carcinoma | ||||
| Schwann (immortalized) | ||||
| mammary epithelial | ||||
| umbilical vein endothelial | ||||
| schwannoma | mouse | |||
| G1 phase of cell cycle | Muranen et al. (2005) | NT, IF, NF, WB | glioma | human |
| IF, NF, WB | osteosarcoma | |||
| NF, WB | embryonal kidney | |||
| Unknown | Kressel and Schmucker (2002) | NT and IF | embryonic fibroblasts | mouse |
| Shollar et al. (2004) | IF, IHC, NF, WB | malignant peripheral nerve sheath | mouse | |
| Beltrami (unpublished) | IHC | glioblastoma | human | |
IF: immunofluorescence; IHC: immunohistochemistry; NF: nuclear fractionation; NT: nuclear trapping; WB: western blotting
The nuclear translocation of NF2 appears to be mediated by both its phosphorylation status and the phase of the cell cycle. NF2 is thought to exists in two conformations, known as the open and closed conformations, which are based on the phosphorylation status of serine residue number 518 (Ser518). Phosphorylation of Ser518, by either p21-activated kinases (PAKs), Rac GTPase, or protein kinase A (PKA), allows NF2 to adopt an open conformation which is considered to be inactive, as the open conformation is unable to inhibit growth in vitro34-36. However, tumor suppressive functions have ascribed to the phosphorylated form of this protein27. With respect to its nuclear localization pattern, NF2 is retained in the cytoplasm when phosphorylated at Ser518, while the unphosphorylated protein can better translocate into the nucleus26, 37-39. Phosphorylated, cytoplasmic NF2 loses its ability to control the cell cycle in schwannoma and mesothelioma cells, suggesting that nuclear translocation of NF2 is critical for its affect on the cell cycle37,38.
The subcellular distribution of NF2 is also affected by the phase of the cell cycle21, 26. The localization of NF2 during the cell cycle has been analyzed in cells synchronized using chemical agents and withdrawal of growth factors, i.e. nocodazole and serum starvation, respectively. In the late G1 phase, NF2 exits the nucleus and is predominantly localized to the cortical membrane during S and G2 phases26. NF2 is then detected in the perinuclear region during the G2/M phase transition, while during mitosis, NF2 associates with the mitotic spindle and remains in the nucleus through early G1 phase. However, NF2's nuclear localization may also be dependent on the activity of its binding partners, including PAK and Rac, both of which phosphorylate NF2, and are themselves regulated by the cell cycle.
Since NF2 is typically detected outside of the nucleus, there is a much clearer understanding of its roles and binding partners in the cytoplasm. As a scaffolding protein, NF2 has been shown to bind not only transmembrane proteins at the cell surface, but also several critical downstream effectors, each of which has distinct influences on the cell cycle.
Cell surface signaling molecules linking NF2 to the cell cycle
B1 integrin and CD44
NF2 has been linked to many receptor types at the plasma membrane to propagate their extracellular signals. For example, integrins have a strong influence on cell cycle progression, primarily through the induction of cyclin D1 and repression of p21 and p2740. NF2 binds to β1 integrin in Schwann cells, perhaps allowing for the signal transduction cascade triggering differentiation and/or preventing oncogenic transformation16. All ERM family proteins, especially NF2, bind the cell surface glycoprotein, CD4410, 15, 41 which is linked to the cell cycle in that it enhances cyclin E expression, and in turn suppresses that of p21 and p2742. When bound to NF2, for example, CD44 is unable to transduce growth promoting signals in response to its ligand, hyaluronate15.
ErbB receptors
NF2 has profound effects on several members of the ErbB family of receptor tyrosine kinases (RTKs) including EGFR, ErbB2, and ErbB3. In fact, NF2 utilizes a multifaceted approach to disrupt signaling through these cell surface molecules. For example, NF2 binds to EGFR, via NHE-RF1, and retains it in an inactive state, thereby blocking signaling to its downstream effectors, including Src, Raf, ERK, or Akt. Alternatively, NF2 can inhibit the internalization and recycling of activated EGFR molecules as well as the trafficking of new EGFR subunits to the cell surface14, 43-45. Inhibition of EGFR, a potent modulator of cell cycle progression through its inhibitory effects on p27, in addition to its enhancement of cyclins A, D2, and E46, promotes G1 arrest, however it has yet to be shown whether NF2 can also promote G1 arrest via an EGFR-dependent method46,47. Inactivation of the ErbB members, ErbB2 and ErbB3, by NF2 precludes the binding of ErbB2 to its downstream effector, Src, and prevents further activation of FAK and paxillin48 while also preventing activation of protein kinase B (PKB/Akt) and the mitogen-activated protein kinases, ERK1 and ERK2 in Schwann cells14. FAK and paxillin both suppress the essential cell cycle inhibitors, p21 and p2749,50. Additionally, paxillin enhances the expression of the transcription factor c-Jun, an oncoprotein which induces cyclins A and D151,52. Consequently, NF2-deficient schwannoma cells display an overabundance of EGFR, ErbB2, and ErbB3 on the cell surface and upregulation of their downstream effectors, which also serves to drive cellular proliferation14-16.
PDGFR
Platelet derived growth factor receptor (PDGFR) molecules, another group of RTKs, also accumulate on the surface of NF2 deficient schwannoma cells14, 53. Activation of these receptors facilitates the phosphorylation of β-catenin by either Src or the Rac/PAK2/JNK cascade. Upon phosphorylation of β-catenin by Src, β-catenin dissociates from the complex and destabilizes the adherens junctions. Further, phosphorylation of β-catenin by the Rac/PAK2/JNK pathway allows for the translocation of β-catenin into the nucleus, where it acts as a positive transcription factor for c-myc and cyclin D1, thus enhancing the cell cycle54. In order to form stable adherens junctions which maintain cell-cell boundaries, NF2 complexes with β-catenin and either E or N cadherin54,55. The localization of NF2 to the plasma membrane appears to be crucial in the maintenance of contact inhibition. In its role as a scaffold, NF2 thus prevents the oncogenic effects of aberrant signaling between receptors and their kinases. Independent of receptor activation, these cytoplasmic kinases have significant ties to cell cycle, providing another level to which NF2 can antagonize cell cycle progression.
Cytoplasmic kinases as intermediates linking NF2 to the cell cycle
Rac1 and Cdc42
Earlier studies have highlighted the importance of the Rho GTPases, Rac1 and Cdc42, in facilitating cell cycle progression. Specifically, these kinases are capable of enhancing both cyclin D1 expression and the transcriptional activity of E2F proteins. All of these events ultimately promote the G1 to S phase transition of the cell cycle56,57. While NF2 does not directly bind to Rac1, it serves to prevents Rac1 activation, possibly by inhibiting key GDP-GTP exchange via Rac1-associated guanine exchange factors (GEFs) 58,59. NF2 can also function upstream of Rac1, via the tight junction protein, Angiomotin, and GTPase activating protein, Rich1, to prevent Rac1 activation60. Rac1 activation is required for its localization to the plasma membrane where it allows cells to escape contact inhibition. Expression of NF2 prevents this phenomenon, and also has been shown to obstruct the motility of Rac1 transformed cells18, 31. Interestingly, a reciprocal interaction has also been observed whereby Rac1 can phosphorylate NF2 at Ser518 thus forcing NF2 into an open conformation which detaches it from the cytoskeleton18. Rac1 is also activated in response to integrin engagement to promote the expression of cyclin D1 and progression of endothelial cells to the G1 phase of the cell cycle61. Consequently, enhanced NF2 expression can negate G1 phase progression in schwannomas, which have previously shown to express aberrant Rac1 activation59, 62, 63. Cdc42 is also capable of inactivating NF2, but it is unclear whether this is a direct consequence of Cdc42 or requires Rac1 to mediate this effect18. Unlike its ability to inhibit Rac1, NF2 is unable to block the activation of Cdc42. Nevertheless, NF2 can prevent Cdc42 interaction with its downstream effectors thus blocking mitogen-activated protein kinase (MAPK) signaling62-65. Thus, redundancy and overlap among NF2 and Rac1/Cdc42 can either promote growth or prevent proliferative signaling, depending on their phosphorylation status20.
PAKs
PAKs are commonly considered downstream effectors of Rho GTPases, since phosphorylation by both Rac1 and Cdc42 are required for their activation24, 66, 67. Several studies have shown that PAKs 1 and 2 are required in order for Cdc42 and Rac1 to phosphorylate NF224, 34. However, PAKs can also act upstream of Rac1 to facilitate inactivation of NF231. As seen with Rac1, PAKs also maintain a reciprocal relationship with NF2. By binding to PAK1, NF2 can hinder Rac1/PAK1 interaction and subsequent activation of PAK168. and PAKs 2 and 3 are also subjected to inactivation by NF2, most likely via the same mechanism13, 24, 69, 70. In turn, PAKs can also contribute to the functional inactivation of NF2, and successive loss of contact inhibition18, 24, 34, 66. Regardless of where PAKs lie within this cascade, a significant relationship clearly exists between the PAKs and NF2. Some studies suggest that NF2 deficient tumors display an “oncogene addiction” to PAKs. For instance, in rat schwannoma cells which require activated PAK1 for survival, cells infected with viral vectors inducing shRNA-mediated suppression of PAK1were capable of methylating the promoter of this foreign construct thus preventing PAK1 extinction69. Elevated expression of group 1 PAKs, including PAKs 1-3, has been seen in primary schwannomas from NF2 patients and use of a group 1 specific inhibitor peptide significantly hindered several oncogenic properties of cells lacking functional NF270,71. NF2 deficient tumors may also depend on activated PAKs for survival because of their multifaceted effects on cell cycle progression. For example, PAK1 is the intermediate linking Ras to enhanced cyclin D1 expression72 which promotes progression from the G1 to S phase of the cell cycle. In addition, late in S phase, PAK1 phosphorylates histone H3 to allow for chromatin condensation of newly synthesized DNA73 and also serves to activate Aurora A and polo-like kinase 1 (plk1), which are crucial for the G2/M transition of the cell cycle74,75. Furthermore, PAK1 translocates to the nucleus during mitosis, where it plays an active role in assuring the proper segregation of chromosomes73. Taken together, these studies demonstrate several key points at which NF2 mediated inhibition of PAKs, in particular PAK1, could halt cell cycle progression.
JNK
The MAPK, c-Jun N-terminal kinase (JNK) is a seemingly under-represented, downstream effector of the Rac1/PAK cascade. JNK is activated by Rac1 directly or via PAK1; however this process can be ameliorated in the presence of NF218, 56. Earlier studies detected enhanced JNK 1 and 2 activation in NF2 deficient tumors, which was assumed to be simply an indication of Rac and PAK activity18, 30, 62. However, one could argue that JNK itself provides an important link between NF2 and the cell cycle. For example, the transcription factor c-Jun, a primary target of JNK activation, is freed to homo- or heterodimerize with a fos protein (i.e. c-fos, FosB, Fra1, or Fra2) upon phosphorylation by any of the JNK isoforms to form the activator protein 1 transcriptional complex (AP-1). In NF2 deficient cells, JNK overactivation correlates with enhanced transcriptional activity by c-Jun and AP-118, 30. Therefore, it is not surprising that JNK mediated signaling via c-Jun and AP-1 results in enhanced cyclin D1 expression in NF2 null cells as the cyclin D1 promoter is upregulated by both of these proteins 30, 76. While this link between JNK activation and cell cycle progression is well established, it is not yet known whether silencing of JNK in NF2 deficient cells is capable of triggering cell cycle arrest.
Ras
Of the oncogenic kinases described here, NF2 and Ras, a member of the small GTPase family, have the longest-standing history. In fact, the ability of NF2 to inhibit Ras-mediated oncogenic transformation of NIH3T3 cells was the first report of NF2's tumor suppressor activity77. While NF2 has not been shown to directly bind Ras, it significantly hinders its downstream signaling59 by preventing Ras upregulation of AP-1 activity and cyclin D1 expression78. NF2 also prevents Ras-mediated activation of JNK, which results in suppression of AP-1 and cyclin D1 expression70. In response to serum stimulation, Ras phosphorylates ERK, which promotes its translocation to the nucleus while nuclear ERK can then act as a transcriptional activator of genes which contain serum responsive elements (SREs) within their promoters. One of these genes, Elk1, can in turn promote the expression of cyclin D1 as well as G1 phase progression. NF2 is able to ameliorate these effects by preventing Ras-mediated ERK phosphorylation59, 79. Despite the well characterized effects of NF2 on Ras mediated oncogenicity, it has yet to be determined whether NF2 overexpression can, in fact, enable Ras-transformed cells to regain control of the cell cycle.
mTOR
Aberrant signaling mediated by the serine/threonine kinase mammalian target of rapamycin (mTOR) is a characteristic of numerous malignancies, including those arising from the inherited cancer disorders, neurofibromatosis type 1 and tuberous sclerosis complex80. mTOR is a member of two distinct complexes, mTOR complex 1 and 2 (mTORC1 and mTORC2, respectively). As a member of both complexes, activation of mTOR is influenced by growth factor availability, making it a key regulator of proliferation in the tumor microenvironment81. NF2 has differing effects on mTORC1 and mTORC2, further highlighting the complexities of mTOR mediated signaling. Upon stimulation by either growth factors or nutrients, mTORC1 assembles and promotes the phosphorylation of its two key effectors, p70 S6 kinase (S6K1) and eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4E-BP1). Activation of S6K1 is not required, but certainly aides in cell cycle progression in response to mTORC1 signaling82. However, the mechanism and necessity of S6K1 involvement in cell cycle progression is still under debate83. In parallel with S6K1 activation, phosphorylation of 4E-BP1 results in the release eIF4E protein, which consequently activates genes responsible for promoting the G1 to S phase progression, mainly via cyclin D187. NF2 has been shown to be a potent inhibitor of mTORC1 signaling in mesotheliomas, meningiomas, and schwannomas as mTORC1 activation is triggered by integrin-mediated loss of NF2 or in its absence. Consequently, many NF2 deficient cell lines display constitutive mTORC1 activation, as assessed by excessive phosphorylation of S6K1 and 4E-BP1 and expression of cyclin D1. Interestingly, only NF2 deficient cells are sensitive to growth inhibition by the classic mTORC1 inhibitor, rapamycin33, 85, 86. These studies provide compelling evidence for the use of NF2 status to determine the responsiveness of a tumor to mTOR inhibitors.
Unlike mTORC1, mTORC2 is only responsive to growth factor stimulation and is not inhibited by treatment with rapamycin . In response to IGF1, EGF, or PDGF stimulation, NF2 activates mTORC2 in Schwann and arachnoid cells, the cells of origin for schwannomas and meningiomas, respectively86. However, the mechanism behind mTORC2 activation has not yet been elucidated. To propagate mTORC2 signaling, Akt1 (also referred to as protein kinase B) is phosphorylated by this complex and, accordingly, Akt1 activation is impaired in cells in which NF2 is silenced85. Interestingly, Akt1 and S6K1 are both members of the AGC kinase family, demonstrating a certain homology shared by mTORC1 and mTORC2 substrates84. The consequences of Akt1 activation of NF2 deficient cells have not yet been determined, however, Akt1 activation is typically associated with cell cycle progression. For instance, active Akt1 negatively regulates the cdk inhibitor, p27, as well as the transcription factor, FOXO. Akt1 also targets the E3 ubiquitin ligase, HDM2 (the human homolog of MDM2), to promote the degradation of p5387. While this link between NF2 and Akt1 activation may seem counterintuitive to the role of NF2 as a cell cycle inhibitor, some speculate that the activation of mTORC2 may compensate for the inactivation of mTORC1 in these malignancies86. For instance, activation of Akt1 via mTORC2 can lift repression of mTORC1 activation81. Similar to NF2, the tumor suppressor proteins of the tuberous sclerosis complex (TSC) cancer disorder, TSC1-TSC2, inhibit mTORC1 and activate mTORC2 signaling47. This pattern of differential regulation of the mTOR complexes may indicate a novel approach toward cell cycle regulation through mTOR signaling.
In response to a multitude of extracellular clues, the delicate balance is tested between NF2 and several of its cytoplasmic binding partners. As highlighted above, NF2 combats the activation of Rac1, Cdc42, PAKs, JNK, and Ras to promote growth arrest. In turn, NF2 is inhibited by Rac1, Cdc42, and PAKs to restore cellular proliferation. NF2 also has an interesting relationship with mTORC 1 and 2. Similar to another tumor suppressor protein, TSC, NF2 activates mTORC2 while suppressing mTORC1, which appears counterintuitive to its potential role as a cell cycle inhibitor. Collectively, these cytoplasmic kinases drive cell cycle progression, providing key targets through which NF2 can restore cell cycle integrity. Alternatively, these cytoplasmic interactions could offer a second line of defense in cells containing either mutant receptors or NF2, which are therefore unable to form stable cellular junctions. As described earlier, NF2 can also shuttle from the cytoplasm to the nucleus, depending upon its phosphorylation status and stage of the cell cycle. Its role in the nucleus is only currently being recognized as part of its tumor suppressive function through its binding and downregulation of a few potent oncoproteins.
NF2 interacts with nuclear oncoproteins
CRLDCAF1
Though the post-translational modification of ubiquitination occurs in the cytoplasm, ubiquitin ligases have more recently gained appreciation for their effects on the cell cycle. One such ubiquitin ligase is CRL4DCAF1, a member of the cullin-ring family of E3 ligases. In this multi-subunit complex, the adaptor (DDB1) and scaffolding (Cullin 4) subunits interact with the substrate acceptor (DCAF1). DCAF1 was first identified as the cellular binding protein for the HIV/SIV protein, Vpr, and thus originally named Vpr binding protein (VprBP). Even though Vpr is not required for HIV/SIV replication it is a heavily conserved protein among various strains and can enhance viral pathogenesis. Vpr binds to DCAF1, at the G2 phase of the cell cycle, to cause cycle arrest, which would make cells more permissive to viral infection88. However, in the absence of Vpr proteins, DCAF1 is still capable of appreciably altering cell cycle progression89. Activation of the CRL4DCAF1 complex also permits cell cycle progression by regulating expression of ubiquitin ligases that target histones, or by recruiting chromatin remodeling enzymes89,90. Recently, DCAF1 was identified as a nuclear binding partner for NF2. Whether this triggers the proteasomal mediated degradation of NF2 is still under debate27, 91. The interaction between DCAF1 and NF2 occurs only when NF2 is in its closed conformation and prevents the ubiquitin ligase function of the CRL4DCAF1 complex. As a consequence, NF2 prohibits schwannoma cells from progressing through G1 and into S phase. Interestingly, this same effect was not seen in Schwann cells indicating that DCAF1 is essential for rapid cell cycling in transformed, but not normal cells27. While this study has provided a greater appreciation for NF2's role in the nucleus, it is still unclear which cell cycle regulators NF2 specifically alters through the inhibition of the CRL4DCAF1 complex.
JCV T-antigen
Interest in the relationship between NF2 and JC virus (JCV) T-antigen resulted from previous studies showing an association between these two proteins in a mouse model of peripheral nerve sheath tumors25. JCV is a human polyomavirus, and the causative agent of the rare but fatal demyelinating disease, progressive multifocal leukoencephalopathy, PML92. In addition to its role in PML pathogenesis, JCV has exhibited oncogenic potential in cell culture and experimental animal models and has been detected in human medulloblastomas, astrocytomas, ependymomas, meningiomas, and numerous other mixed lineage gliomas93. Similarly, the simian homolog to JCV, SV40, has also been linked to human malignancies such as mesothelioma, meningiomas, and various grade of gliomas94,95. Due to the great variability of the detection of these viruses in human tumors, the causal role of JCV and SV40 in human malignancies is still heavily debated93. Taken together, the seemingly reciprocal relationship of NF2 and JCV in these overlapping tumor types has led our group to investigate whether NF2 can combat the oncogenic effects of polyoma viruses. To promote JC viral replication, T-antigen sequesters key cell cycle moderators, including p53 and Rb. JCV T-antigen binds to wild type p53, exclusively96 and, in fact, was first identified as a consequence of its binding to SV40 T-antigen97. Further, the ability of JCV T-antigen to bind Rb is necessary for its transformative behavior98. JCV T-antigen also enhances oncogenic signaling through the Wnt pathway by stabilizing key members, such as β-catenin and c-myc99,100 where T-antigen expressing cells contain higher levels of transcriptionally active β-catenin, as well as c-myc and cyclin D1101.
JCV T-antigen transgenic mice develop a broad range of neuronal and glial origin tumors, in particular, malignant peripheral nerve sheath tumors, similar to those seen in neurofibromatosis type 1 patients. Within these tumors an interaction between NF2 and T-antigen was detected in the nucleus of a subset of tumor cells25 and is capable of promoting the downregulation of T-antigen in human glial and neuronal cells (Beltrami, unpublished observation). While the downregulation of T-antigen expression by NF2 was able to ameliorate JCV promoter activation, the potential impact of NF2 on p53 and Rb, or Wnt signaling pathways has not yet been established. More recently NF2 is gaining appreciation for its role in the nucleus of tumor cells. While there is an incomplete understanding of its function there, a review of nuclear NF2 literature has revealed an underlying theme of interactions between NF2 and proteins known to promote viral pathogenesis. Similar to most canonical oncogenes, viral proteins and there cellular targets (T-antigen, and CRLDCAF1, respectively), have evolved to force cell cycle progression of infected cells, in order to obtain the necessary machinery for further viral replication. Through the antagonism of such viral associated proteins, NF2 can play a pivotal role allowing both viral infected and transformed, uninfected cells to regain control of the cell cycle.
Convergence of NF2 with classical tumor suppressor pathways
p53
The most commonly mutated or lost tumor suppressor protein in cancer, p53, has extensive ties to the cell cycle102. In brief, p53 is a potent inducer of G1 phase arrest, via its transcriptional activation of anti-proliferative genes, including p21, Noxa, Puma, and Perp103. While a direct interaction among NF2 and p53 has not been reported, previous literature has suggested that potential tumor suppressive synergy may exist between these two proteins. In fact, the NF2 and p53 alleles map to the same locus in the mouse genome, and loss of both genes leads to the development of highly metastatic tumors including osteosarcomas, malignant peripheral nerve sheath tumors, choroid plexus carcinomas, and mesotheliomas104,105. In addition, human meningiomas displaying NF2 loss of heterozygosity (LOH) and p53 mutations are typically of a higher grade than those with only NF2 LOH106. Further, p53 expression can overcome the oncogenic consequences of loss of NF2 in neural crest tumors, indicating that p53-specific therapies could be beneficial in NF2 deficient tumors107. These results are not surprising since NF2 has been shown to contribute to the stabilization of p53 through the downregulation of Mdm2 which also results in the enhancement of p21 promoter activity and sensitization of cells to p53-induced apoptosis108. The possibility remains, however, that NF2 also interacts with the p53 downstream effector, p21, which provides a potential link between the influence of both NF2 and p53 on cell cycle progression.
Rb
Retinoblastoma (Rb), the first described tumor suppressor protein, is a central negative regulator of cell cycle progression109. Rb is considered to function as a “pocket-protein”, whereby it binds to members of the E2F family of transcription factors, thus preventing the E2Fs from acting on downstream target genes which promote the cell cycle from G1 to S phases, including cyclins E and A, thymidine kinase, and DNA polymerase-α. When Rb is activated via phosphorylation by cyclin-dependent kinases, E2F is released, freed to heterodimerize with a binding partner (DP), leading to transcripitonal activation. In addition to its role in sequestering E2F, Rb can also recruit histone deacetylase 1 (HDAC1) to condense chromatin and hinder transcription of a given target promoter98. Though an interaction among NF2 and Rb has not been reported, several NF2 binding partners can negatively impact the function of Rb. For example, activation of EGFR, Rac1, or Cdc42 initiates a cascade resulting in the phosphorylation of Rb46,56,57. Cytoplasmic NF2, has been demonstrated to prevent Ras-mediated phosphorylation of Rb and transcriptional activation of E2F78 while nuclear binding partners of NF2, including DCAF1 and T-antigen, can force S phase entry by phosphorylation or sequestration of Rb, respectively89,98. This mechanism of sequestering Rb can also be seen with human papillomavirus E7, and adenovirus E1A proteins, further solidifying a central role for Rb inactivation as an essential step in viral oncogenesis98.
Conclusions
Traditionally, NF2 is typically viewed as a scaffolding protein primarily located at the plasma membrane, where it prevents excessive signaling via several cell surface receptors and their cytoplasmic kinases. However, NF2 inactivation is seen in an array of tumors, all of which share a dysregulation of the cell cycle. Here, we provided several lines of evidence suggesting that NF2 could prevent cell cycle progression set forth by these receptors, and serve as an unconventional tumor suppressor protein with many potential mechanisms by which it may halt the cell cycle. A fraction of NF2 has also been shown to interact with many key signal transduction pathway members in the cytoplasm as well as oncoproteins in the nucleus. The outcome of these multi-layered pathways is the inactivation of conventional tumor suppressors, such as p53 and Rb, consequently leading to cell cycle progression. In considering all of these interactions, it is likely that cell cycle control is the resulting downstream consequence of NF2 function. As such, NF2 based therapeutics may provide a dual approach whereby its traditional role as suppressor of invasion and metastasis can be targeted, as well as its proposed action as a cell cycle regulator.
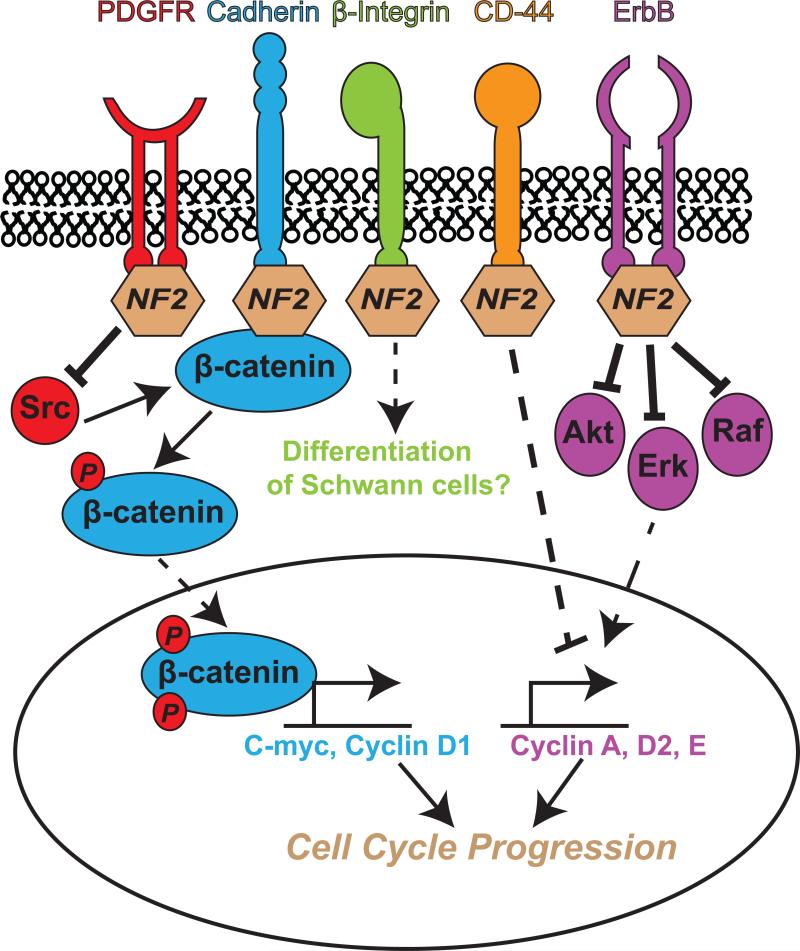
Figure 1. Interaction of NF2 with cell surface receptors can alter cell cycle progression through several signaling pathways.
NF2 is predominantly localized to the plasma membrane where it interacts with various cell surface receptors, each with an established effect on the cell cycle. Through its interactions with PDGFR and Src or cadherins, NF2 prevents the phosphorylation of β-catenin. In doing so, β-catenin cannot translocate to the nucleus and act as a positive transcription factor for cell cycle enhancers, c-myc and cyclin D1. In a less understood interaction, NF2 binds to β-integrins to promote the differentiation of Schwann cells, which may result in the halting of the cell cycle. Similar to all ERM proteins, NF2 interacts with CD-44, perhaps to act as a negative regulator of cyclins A, D2, and E. NF2 also networks with ErbB receptors 1, 2, and 3, and acts as an inhibitor of Akt, ERK, and Raf. This interruption of ErbB signaling may disrupt the effects of these receptors on cell cycle progression.
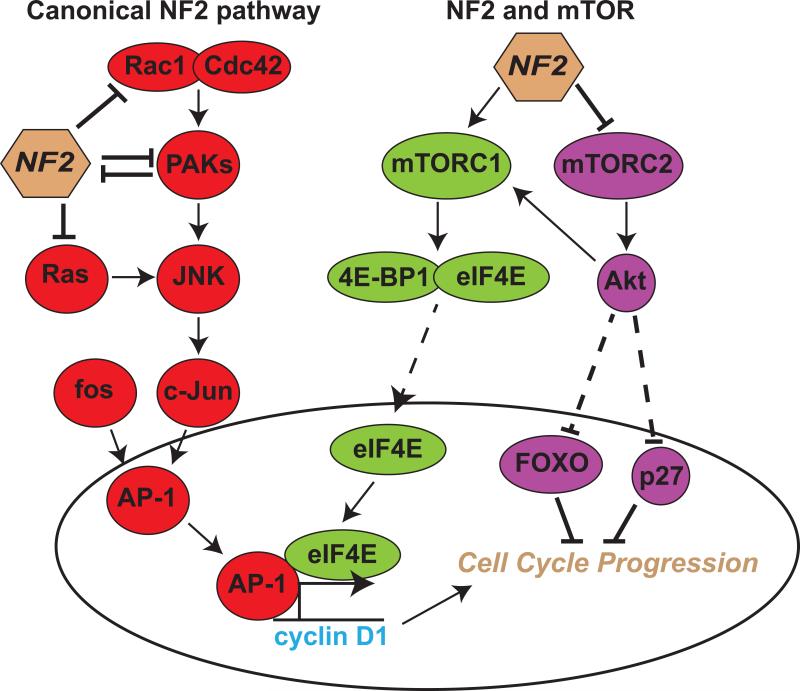
Figure 2. The canonical NF2 pathway and an alternative pathway via mTOR for affecting the cell cycle.
In the cytoplasm, NF2 is known to disrupt the downstream signaling of Rac1 and Cdc42 at several points. In addition to directly inhibiting the Rac1/Cdc42 complex, NF2 can also inhibit activation of PAKs, and Ras. In either exchange, NF2 prevents the activation of JNK and c-Jun, and the assembly of the AP-1 transcription complex. A reciprocal interaction also occurs in this pathway whereby PAKs can inhibit the actions of NF2. Interestingly, NF2 has differential effects on mTORC1 and mTORC2. By inhibiting mTORC2, NF2 prevents the activation of Akt and its repression of FOXO and p27, known inhibitors of cell cycle progression. However, NF2 enhances the activation of mTORC1 and subsequent dissociation of transcription factors, 4E-BP1 and eIF4E. When freed, eIF4E, along with AP-1, can amplify the transcriptional activation of cyclin D1, demonstrating the overlapping nature of these separate pathways.
References
- 1.Rouleau GA, Wertelecki W, Haines JL, Hobbs WJ, Trofatter JA, Seizinger BR, et al. Genetic linkage of bilateral acoustic neurofibromatosis to a DNA marker on chromosome 22. Nature. 1987;329:246–8. doi: 10.1038/329246a0. [DOI] [PubMed] [Google Scholar]
- 2.Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;75:826. doi: 10.1016/0092-8674(93)90501-g. [DOI] [PubMed] [Google Scholar]
- 3.Martuza RL, Eldridge R. Neurofibromatosis 2 (bilateral acoustic neurofibromatosis). N Engl J Med. 1988;318:684–8. doi: 10.1056/NEJM198803173181106. [DOI] [PubMed] [Google Scholar]
- 4.Morrow KA, Das S, Metge BJ, Ye K, Mulekar MS, Tucker JA, et al. Loss of tumor suppressor Merlin in advanced breast cancer is due to post-translational regulation. J Biol Chem. 2011;286:40376–85. doi: 10.1074/jbc.M111.250035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruttledge MH, Sarrazin J, Rangaratnam S, Phelan CM, Twist E, Merel P, et al. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat Genet. 1994;6:180–4. doi: 10.1038/ng0294-180. [DOI] [PubMed] [Google Scholar]
- 6.Kimura Y, Koga H, Araki N, Mugita N, Fujita N, Takeshima H, et al. The involvement of calpain-dependent proteolysis of the tumor suppressor NF2 (merlin) in schwannomas and meningiomas. Nat Med. 1998;4:915–22. doi: 10.1038/nm0898-915. [DOI] [PubMed] [Google Scholar]
- 7.Evans DG, Huson SM, Donnai D, Neary W, Blair V, Newton V, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84:603–18. [PubMed] [Google Scholar]
- 8.Hitotsumatsu T, Iwaki T, Kitamoto T, Mizoguchi M, Suzuki SO, Hamada Y, et al. Expression of neurofibromatosis 2 protein in human brain tumors: an immunohistochemical study. Acta Neuropathol. 1997;93:225–32. doi: 10.1007/s004010050608. [DOI] [PubMed] [Google Scholar]
- 9.Lau YK, Murray LB, Houshmandi SS, Xu Y, Gutmann DH, Yu Q. Merlin is a potent inhibitor of glioma growth. Cancer Res. 2008;68:5733–42. doi: 10.1158/0008-5472.CAN-08-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsukita S, Yonemura S. ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol. 1997;9:70–5. doi: 10.1016/s0955-0674(97)80154-8. [DOI] [PubMed] [Google Scholar]
- 11.Curto M, McClatchey AI. Nf2/Merlin: a coordinator of receptor signalling and intercellular contact. Br J Cancer. 2008;98:256–62. doi: 10.1038/sj.bjc.6604002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiederhold T, Lee MF, James M, Neujahr R, Smith N, Murthy A, et al. Magicin, a novel cytoskeletal protein associates with the NF2 tumor suppressor merlin and Grb2. Oncogene. 2004;23:8815–25. doi: 10.1038/sj.onc.1208110. [DOI] [PubMed] [Google Scholar]
- 13.Xiao GH, Gallagher R, Shetler J, Skele K, Altomare DA, Pestell RG, et al. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol Cell Biol. 2005;25:2384–94. doi: 10.1128/MCB.25.6.2384-2394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lallemand D, Manent J, Couvelard A, Watilliaux A, Siena M, Chareyre F, et al. Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene. 2009;28:854–65. doi: 10.1038/onc.2008.427. [DOI] [PubMed] [Google Scholar]
- 15.Morrison H, Sherman LS, Legg J, Banine F, Isacke C, Haipek CA, et al. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15:968–80. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obremski VJ, Hall AM, Fernandez-Valle C. Merlin, the neurofibromatosis type 2 gene product, and beta1 integrin associate in isolated and differentiating Schwann cells. J Neurobiol. 1998;37:487–501. [PubMed] [Google Scholar]
- 17.Grönholm M, Teesalu T, Tyynelä J, Piltti K, Böhling T, Wartiovaara K, et al. Characterization of the NF2 protein merlin and the ERM protein ezrin in human, rat, and mouse central nervous system. Mol Cell Neurosci. 2005;28:683–93. doi: 10.1016/j.mcn.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell. 2001;1:63–72. doi: 10.1016/s1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 19.McClatchey AI. Merlin and ERM proteins: unappreciated roles in cancer development? Nat Rev Cancer. 2003;3:877–83. doi: 10.1038/nrc1213. [DOI] [PubMed] [Google Scholar]
- 20.McClatchey AI, Giovannini M. Membrane organization and tumorigenesis--the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19:2265–77. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- 21.Shaw RJ, McClatchey AI, Jacks T. Localization and functional domains of the neurofibromatosis type II tumor suppressor, merlin. Cell Growth Differ. 1998;9:287–96. [PubMed] [Google Scholar]
- 22.Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci U S A. 1999;96:9112–7. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kressel M, Schmucker B. Nucleocytoplasmic transfer of the NF2 tumor suppressor protein merlin is regulated by exon 2 and a CRM1-dependent nuclear export signal in exon 15. Hum Mol Genet. 2002;11:2269–78. doi: 10.1093/hmg/11.19.2269. [DOI] [PubMed] [Google Scholar]
- 24.Kissil JL, Johnson KC, Eckman MS, Jacks T. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem. 2002;277:10394–9. doi: 10.1074/jbc.M200083200. [DOI] [PubMed] [Google Scholar]
- 25.Shollar D, Del Valle L, Khalili K, Otte J, Gordon J. JCV T-antigen interacts with the neurofibromatosis type 2 gene product in a transgenic mouse model of malignant peripheral nerve sheath tumors. Oncogene. 2004;23:5459–67. doi: 10.1038/sj.onc.1207728. [DOI] [PubMed] [Google Scholar]
- 26.Muranen T, Grönholm M, Renkema GH, Carpén O. Cell cycle-dependent nucleocytoplasmic shuttling of the neurofibromatosis 2 tumour suppressor merlin. Oncogene. 2005;24:1150–8. doi: 10.1038/sj.onc.1208283. [DOI] [PubMed] [Google Scholar]
- 27.Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140:477–90. doi: 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda K, Saeki Y, Gonzalez-Agosti C, Ramesh V, Chiocca EA. Inhibition of NF2-negative and NF2-positive primary human meningioma cell proliferation by overexpression of merlin due to vector-mediated gene transfer. J Neurosurg. 1999;91:85–92. doi: 10.3171/jns.1999.91.1.0085. [DOI] [PubMed] [Google Scholar]
- 29.Schulze KM, Hanemann CO, Müller HW, Hanenberg H. Transduction of wild-type merlin into human schwannoma cells decreases schwannoma cell growth and induces apoptosis. Hum Mol Genet. 2002;11:69–76. doi: 10.1093/hmg/11.1.69. [DOI] [PubMed] [Google Scholar]
- 30.Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 2003;17:1090–100. doi: 10.1101/gad.1054603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada T, Lopez-Lago M, Giancotti FG. Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J Cell Biol. 2005;171:361–71. doi: 10.1083/jcb.200503165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poulikakos PI, Xiao GH, Gallagher R, Jablonski S, Jhanwar SC, Testa JR. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006;25:5960–8. doi: 10.1038/sj.onc.1209587. [DOI] [PubMed] [Google Scholar]
- 33.López-Lago MA, Okada T, Murillo MM, Socci N, Giancotti FG. Loss of the tumor suppressor gene NF2, encoding merlin, constitutively activates integrin-dependent mTORC1 signaling. Mol Cell Biol. 2009;29:4235–49. doi: 10.1128/MCB.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao GH, Beeser A, Chernoff J, Testa JR. p21-activated kinase links Rac/Cdc42 signaling to merlin. J Biol Chem. 2002;277:883–6. doi: 10.1074/jbc.C100553200. [DOI] [PubMed] [Google Scholar]
- 35.Tang X, Jang SW, Wang X, Liu Z, Bahr SM, Sun SY, et al. Akt phosphorylation regulates the tumour-suppressor merlin through ubiquitination and degradation. Nat Cell Biol. 2007;9:1199–207. doi: 10.1038/ncb1641. [DOI] [PubMed] [Google Scholar]
- 36.Laulajainen M, Muranen T, Carpén O, Grönholm M. Protein kinase A-mediated phosphorylation of the NF2 tumor suppressor protein merlin at serine 10 affects the actin cytoskeleton. Oncogene. 2008;27:3233–43. doi: 10.1038/sj.onc.1210988. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Cooper J, Karajannis MA, Giancotti FG. Merlin: a tumour suppressor with functions at the cell cortex and in the nucleus. EMBO Rep. 2012;13:204–15. doi: 10.1038/embor.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lü J, Zou J, Wu H, Cai L. Compensative shuttling of merlin to phosphorylation on serine 518 in vestibular schwannoma. Laryngoscope. 2008;118:169–74. doi: 10.1097/MLG.0b013e3181566594. [DOI] [PubMed] [Google Scholar]
- 39.Thaxton C, Lopera J, Bott M, Baldwin ME, Kalidas P, Fernandez-Valle C. Phosphorylation of the NF2 tumor suppressor in Schwann cells is mediated by Cdc42-Pak and requires paxillin binding. Mol Cell Neurosci. 2007;34:231–42. doi: 10.1016/j.mcn.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Walker JL, Assoian RK. Integrin-dependent signal transduction regulating cyclin D1 expression and G1 phase cell cycle progression. Cancer Metastasis Rev. 2005;24:383–93. doi: 10.1007/s10555-005-5130-7. [DOI] [PubMed] [Google Scholar]
- 41.Sainio M, Zhao F, Heiska L, Turunen O, den Bakker M, Zwarthoff E, et al. Neurofibromatosis 2 tumor suppressor protein colocalizes with ezrin and CD44 and associates with actin-containing cytoskeleton. J Cell Sci. 1997;110(Pt 18):2249–60. doi: 10.1242/jcs.110.18.2249. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Bi G, Wen P, Yang W, Ren X, Tang T, et al. Down-regulation of CD44 contributes to the differentiation of HL-60 cells induced by ATRA or HMBA. Cell Mol Immunol. 2007;4:59–63. [PubMed] [Google Scholar]
- 43.Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cole BK, Curto M, Chan AW, McClatchey AI. Localization to the cortical cytoskeleton is necessary for Nf2/merlin-dependent epidermal growth factor receptor silencing. Mol Cell Biol. 2008;28:1274–84. doi: 10.1128/MCB.01139-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scoles DR, Yong WH, Qin Y, Wawrowsky K, Pulst SM. Schwannomin inhibits tumorigenesis through direct interaction with the eukaryotic initiation factor subunit c (eIF3c). Hum Mol Genet. 2006;15:1059–70. doi: 10.1093/hmg/ddl021. [DOI] [PubMed] [Google Scholar]
- 46.Peng D, Fan Z, Lu Y, DeBlasio T, Scher H, Mendelsohn J. Anti-epidermal growth factor receptor monoclonal antibody 225 up-regulates p27KIP1 and induces G1 arrest in prostatic cancer cell line DU145. Cancer Res. 1996;56:3666–9. [PubMed] [Google Scholar]
- 47.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104–15. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Houshmandi SS, Emnett RJ, Giovannini M, Gutmann DH. The neurofibromatosis 2 protein, merlin, regulates glial cell growth in an ErbB2- and Src-dependent manner. Mol Cell Biol. 2009;29:1472–86. doi: 10.1128/MCB.01392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bryant P, Zheng Q, Pumiglia K. Focal adhesion kinase controls cellular levels of p27/Kip1 and p21/Cip1 through Skp2-dependent and -independent mechanisms. Mol Cell Biol. 2006;26:4201–13. doi: 10.1128/MCB.01612-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding Q, Grammer JR, Nelson MA, Guan JL, Stewart JE, Gladson CL. p27Kip1 and cyclin D1 are necessary for focal adhesion kinase regulation of cell cycle progression in glioblastoma cells propagated in vitro and in vivo in the scid mouse brain. J Biol Chem. 2005;280:6802–15. doi: 10.1074/jbc.M409180200. [DOI] [PubMed] [Google Scholar]
- 51.Tatsumi Y, Cho YY, He Z, Mizuno H, Seok Choi H, Bode AM, et al. Involvement of the paxillin pathway in JB6 Cl41 cell transformation. Cancer Res. 2006;66:5968–74. doi: 10.1158/0008-5472.CAN-05-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaulian E. AP-1--The Jun proteins: Oncogenes or tumor suppressors in disguise? Cell Signal. 2010;22:894–9. doi: 10.1016/j.cellsig.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 53.Ammoun S, Cunliffe CH, Allen JC, Chiriboga L, Giancotti FG, Zagzag D, et al. ErbB/HER receptor activation and preclinical efficacy of lapatinib in vestibular schwannoma. Neuro Oncol. 2010;12:834–43. doi: 10.1093/neuonc/noq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou L, Ercolano E, Ammoun S, Schmid MC, Barczyk MA, Hanemann CO. Merlin-deficient human tumors show loss of contact inhibition and activation of Wnt/β-catenin signaling linked to the PDGFR/Src and Rac/PAK pathways. Neoplasia. 2011;13:1101–12. doi: 10.1593/neo.111060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rangwala R, Banine F, Borg JP, Sherman LS. Erbin regulates mitogen-activated protein (MAP) kinase activation and MAP kinase-dependent interactions between Merlin and adherens junction protein complexes in Schwann cells. J Biol Chem. 2005;280:11790–7. doi: 10.1074/jbc.M414154200. [DOI] [PubMed] [Google Scholar]
- 56.Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–2. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 57.Gjoerup O, Lukas J, Bartek J, Willumsen BM. Rac and Cdc42 are potent stimulators of E2F-dependent transcription capable of promoting retinoblastoma susceptibility gene product hyperphosphorylation. J Biol Chem. 1998;273:18812–8. doi: 10.1074/jbc.273.30.18812. [DOI] [PubMed] [Google Scholar]
- 58.Ryu CH, Kim SW, Lee KH, Lee JY, Kim H, Lee WK, et al. The merlin tumor suppressor interacts with Ral guanine nucleotide dissociation stimulator and inhibits its activity. Oncogene. 2005;24:5355–64. doi: 10.1038/sj.onc.1208633. [DOI] [PubMed] [Google Scholar]
- 59.Morrison H, Sperka T, Manent J, Giovannini M, Ponta H, Herrlich P. Merlin/neurofibromatosis type 2 suppresses growth by inhibiting the activation of Ras and Rac. Cancer Res. 2007;67:520–7. doi: 10.1158/0008-5472.CAN-06-1608. [DOI] [PubMed] [Google Scholar]
- 60.Yi C, Troutman S, Fera D, Stemmer-Rachamimov A, Avila JL, Christian N, et al. A tight junction-associated Merlin-angiomotin complex mediates Merlin's regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell. 2011;19:527–40. doi: 10.1016/j.ccr.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mettouchi A, Klein S, Guo W, Lopez-Lago M, Lemichez E, Westwick JK, et al. Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol Cell. 2001;8:115–27. doi: 10.1016/s1097-2765(01)00285-4. [DOI] [PubMed] [Google Scholar]
- 62.Kaempchen K, Mielke K, Utermark T, Langmesser S, Hanemann CO. Upregulation of the Rac1/JNK signaling pathway in primary human schwannoma cells. Hum Mol Genet. 2003;12:1211–21. doi: 10.1093/hmg/ddg146. [DOI] [PubMed] [Google Scholar]
- 63.Flaiz C, Kaempchen K, Matthies C, Hanemann CO. Actin-rich protrusions and nonlocalized GTPase activation in Merlin-deficient schwannomas. J Neuropathol Exp Neurol. 2007;66:608–16. doi: 10.1097/nen.0b013e318093e555. [DOI] [PubMed] [Google Scholar]
- 64.Chadee DN, Xu D, Hung G, Andalibi A, Lim DJ, Luo Z, et al. Mixed-lineage kinase 3 regulates B-Raf through maintenance of the B-Raf/Raf-1 complex and inhibition by the NF2 tumor suppressor protein. Proc Natl Acad Sci U S A. 2006;103:4463–8. doi: 10.1073/pnas.0510651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhan Y, Modi N, Stewart AM, Hieronimus RI, Liu J, Gutmann DH, et al. Regulation of mixed lineage kinase 3 is required for Neurofibromatosis-2-mediated growth suppression in human cancer. Oncogene. 2011;30:781–9. doi: 10.1038/onc.2010.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rong R, Surace EI, Haipek CA, Gutmann DH, Ye K. Serine 518 phosphorylation modulates merlin intramolecular association and binding to critical effectors important for NF2 growth suppression. Oncogene. 2004;23:8447–54. doi: 10.1038/sj.onc.1207794. [DOI] [PubMed] [Google Scholar]
- 67.Szczepanowska J. Involvement of Rac/Cdc42/PAK pathway in cytoskeletal rearrangements. Acta Biochim Pol. 2009;56:225–34. [PubMed] [Google Scholar]
- 68.Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell. 2003;12:841–9. doi: 10.1016/s1097-2765(03)00382-4. [DOI] [PubMed] [Google Scholar]
- 69.Yi C, Wilker EW, Yaffe MB, Stemmer-Rachamimov A, Kissil JL. Validation of the p21-activated kinases as targets for inhibition in neurofibromatosis type 2. Cancer Res. 2008;68:7932–7. doi: 10.1158/0008-5472.CAN-08-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirokawa Y, Tikoo A, Huynh J, Utermark T, Hanemann CO, Giovannini M, et al. A clue to the therapy of neurofibromatosis type 2: NF2/merlin is a PAK1 inhibitor. Cancer J. 2004;10:20–6. doi: 10.1097/00130404-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 71.Chow HY, Stepanova D, Koch J, Chernoff J. p21-Activated kinases are required for transformation in a cell-based model of neurofibromatosis type 2. PLoS One. 2010;5:e13791. doi: 10.1371/journal.pone.0013791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nheu T, He H, Hirokawa Y, Walker F, Wood J, Maruta H. PAK is essential for RAS-induced upregulation of cyclin D1 during the G1 to S transition. Cell Cycle. 2004;3:71–4. [PubMed] [Google Scholar]
- 73.Li F, Adam L, Vadlamudi RK, Zhou H, Sen S, Chernoff J, et al. p21-activated kinase 1 interacts with and phosphorylates histone H3 in breast cancer cells. EMBO Rep. 2002;3:767–73. doi: 10.1093/embo-reports/kvf157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell. 2005;20:237–49. doi: 10.1016/j.molcel.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 75.Maroto B, Ye MB, von Lohneysen K, Schnelzer A, Knaus UG. P21-activated kinase is required for mitotic progression and regulates Plk1. Oncogene. 2008;27:4900–8. doi: 10.1038/onc.2008.131. [DOI] [PubMed] [Google Scholar]
- 76.Guo ZY, Hao XH, Tan FF, Pei X, Shang LM, Jiang XL, et al. The elements of human cyclin D1 promoter and regulation involved. Clin Epigenetics. 2011;2:63–76. doi: 10.1007/s13148-010-0018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tikoo A, Varga M, Ramesh V, Gusella J, Maruta H. An anti-Ras function of neurofibromatosis type 2 gene product (NF2/Merlin). J Biol Chem. 1994;269:23387–90. [PubMed] [Google Scholar]
- 78.Kim H, Lim JY, Kim YH, Park SH, Lee KH, Han H, et al. Inhibition of ras-mediated activator protein 1 activity and cell growth by merlin. Mol Cells. 2002;14:108–14. [PubMed] [Google Scholar]
- 79.Lim JY, Kim H, Kim YH, Kim SW, Huh PW, Lee KH, et al. Merlin suppresses the SRE-dependent transcription by inhibiting the activation of Ras-ERK pathway. Biochem Biophys Res Commun. 2003;302:238–45. doi: 10.1016/s0006-291x(03)00124-4. [DOI] [PubMed] [Google Scholar]
- 80.Lodish MB, Stratakis CA. Endocrine tumours in neurofibromatosis type 1, tuberous sclerosis and related syndromes. Best Pract Res Clin Endocrinol Metab. 2010;24:439–49. doi: 10.1016/j.beem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 82.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–16. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fenton TR, Gout IT. Functions and regulation of the 70kDa ribosomal S6 kinases. Int J Biochem Cell Biol. 2011;43:47–59. doi: 10.1016/j.biocel.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 84.Zhou H, Huang S. The complexes of mammalian target of rapamycin. Curr Protein Pept Sci. 2010;11:409–24. doi: 10.2174/138920310791824093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.James MF, Han S, Polizzano C, Plotkin SR, Manning BD, Stemmer-Rachamimov AO, et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29:4250–61. doi: 10.1128/MCB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.James MF, Stivison E, Beauchamp R, Han S, Li H, Wallace MR, et al. Regulation of mTOR complex 2 signaling in neurofibromatosis 2-deficient target cell types. Mol Cancer Res. 2012;10:649–59. doi: 10.1158/1541-7786.MCR-11-0425-T. [DOI] [PubMed] [Google Scholar]
- 87.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 88.Hrecka K, Gierszewska M, Srivastava S, Kozaczkiewicz L, Swanson SK, Florens L, et al. Lentiviral Vpr usurps Cul4-DDB1[VprBP] E3 ubiquitin ligase to modulate cell cycle. Proc Natl Acad Sci U S A. 2007;104:11778–83. doi: 10.1073/pnas.0702102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O'Connell BC, Harper JW. Ubiquitin proteasome system (UPS): what can chromatin do for you? Curr Opin Cell Biol. 2007;19:206–14. doi: 10.1016/j.ceb.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 90.Higa LA, Zhang H. Stealing the spotlight: CUL4-DDB1 ubiquitin ligase docks WD40-repeat proteins to destroy. Cell Div. 2007;2:5. doi: 10.1186/1747-1028-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang J, Chen J. VprBP targets Merlin to the Roc1-Cul4A-DDB1 E3 ligase complex for degradation. Oncogene. 2008;27:4056–64. doi: 10.1038/onc.2008.44. [DOI] [PubMed] [Google Scholar]
- 92.ZuRhein G, Chou SM. Particles resembling papova viruses in human cerebral demyelinating disease. Science. 1965;148:1477–9. doi: 10.1126/science.148.3676.1477. [DOI] [PubMed] [Google Scholar]
- 93.White MK, Gordon J, Reiss K, et al. Human polyomaviruses and brain tumors. Brain Res Brain Res Rev. 2005;50:69–85. doi: 10.1016/j.brainresrev.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 94.Rascoe PA, Jupiter D, Cao X, Littlejohn JE, Smythe WR. Molecular pathogenesis of malignant mesothelioma. Expert Rev Mol Med. 2012;14:e12. doi: 10.1017/erm.2012.6. [DOI] [PubMed] [Google Scholar]
- 95.Qi F, Carbone M, Yang H, Gaudino G. Simian virus 40 transformation, malignant mesothelioma and brain tumors. Expert Rev Respir Med. 2011;5:683–97. doi: 10.1586/ers.11.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krynska B, Gordon J, Otte J, Franks R, Knobler R, DeLuca A, et al. Role of cell cycle regulators in tumor formation in transgenic mice expressing the human neurotropic virus, JCV, early protein. J Cell Biochem. 1997;67:223–30. doi: 10.1002/(sici)1097-4644(19971101)67:2<223::aid-jcb7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 97.Lane DP, Crawford LV. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979;278:261–3. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- 98.Helt AM, Galloway DA. Mechanisms by which DNA tumor virus oncoproteins target the Rb family of pocket proteins. Carcinogenesis. 2003;24:159–69. doi: 10.1093/carcin/24.2.159. [DOI] [PubMed] [Google Scholar]
- 99.Gan DD, Khalili K. Interaction between JCV large T-antigen and beta-catenin. Oncogene. 2004;23:483–90. doi: 10.1038/sj.onc.1207018. [DOI] [PubMed] [Google Scholar]
- 100.Gan DD, Reiss K, Carrill T, Del Valle L, Croul S, Giordano A, et al. Involvement of Wnt signaling pathway in murine medulloblastoma induced by human neurotropic JC virus. Oncogene. 2001;20:4864–70. doi: 10.1038/sj.onc.1204670. [DOI] [PubMed] [Google Scholar]
- 101.Bhattacharyya R, Noch EK, Khalili K. A novel role of Rac1 GTPase in JCV T-antigen-mediated beta-catenin stabilization. Oncogene. 2007;26:7628–36. doi: 10.1038/sj.onc.1210576. [DOI] [PubMed] [Google Scholar]
- 102.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 103.Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, Kozak MM, et al. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell. 2011;145:571–83. doi: 10.1016/j.cell.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McClatchey AI, Saotome I, Mercer K, Crowley D, Gusella JF, Bronson RT, et al. Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly metastatic tumors. Genes Dev. 1998;12:1121–33. doi: 10.1101/gad.12.8.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Altomare DA, Vaslet CA, Skele KL, De Rienzo A, Devarajan K, Jhanwar SC, et al. A mouse model recapitulating molecular features of human mesothelioma. Cancer Res. 2005;65:8090–5. doi: 10.1158/0008-5472.CAN-05-2312. [DOI] [PubMed] [Google Scholar]
- 106.Chang Z, Guo CL, Ahronowitz I, Stemmer-Rachamimov AO, MacCollin M, Nunes FP. A role for the p53 pathway in the pathology of meningiomas with NF2 loss. J Neurooncol. 2009;91:265–70. doi: 10.1007/s11060-008-9721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Robanus-Maandag E, Giovannini M, van der Valk M, Niwa-Kawakita M, Abramowski V, Antonescu C, et al. Synergy of Nf2 and p53 mutations in development of malignant tumours of neural crest origin. Oncogene. 2004;23:6541–7. doi: 10.1038/sj.onc.1207858. [DOI] [PubMed] [Google Scholar]
- 108.Kim H, Kwak NJ, Lee JY, Choi BH, Lim Y, Ko YJ, et al. Merlin neutralizes the inhibitory effect of Mdm2 on p53. J Biol Chem. 2004;279:7812–8. doi: 10.1074/jbc.M305526200. [DOI] [PubMed] [Google Scholar]
- 109.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]