Abstract
The wide-scale implementation of solar and other renewable sources of electricity requires improved means for energy storage. An intriguing strategy in this regard is the reduction of CO2 to CO, which generates an energy rich commodity chemical that can be coupled to liquid fuel production. In this work, we report an inexpensive Bismuth Carbon Monoxide Evolving Catalyst (Bi-CMEC) that can be formed upon cathodic polarization of an inert glassy carbon electrode in acidic solutions containing Bi3+ ions. This catalyst can be used in conjunction with ionic liquids to effect the electrocatalytic conversion of CO2 to CO with appreciable current density at overpotentials below 0.2 V. Bi-CMEC is selective for production of CO, operating with a Faradaic efficiency of approximately 95%. When taken together these correspond to a high energy efficiency for CO production, on par with that which has historically only been observed using expensive silver and gold cathodes.
Storage of solar and other sources of renewable electricity may be enabled by the catalytic production of fuels such as H2 or reduced carbon-containing compounds via the electrochemical reduction of H2O or CO2, respectively. The renewable production of reduced carbon compounds is especially attractive,1 as liquid fuels can directly address energy needs associated with transportation,2 which account for nearly 30% of US energy demand.3 An attractive strategy for the synthesis of carbon-based fuels is the marriage of a robust electrocatalyst for CO2 reduction with a photoelectrochemical (PEC) device or a conventional electrolyzer powered by a renewable source of electrical current. One such energy storing process is the 2e−/2H+ reduction of CO2 to CO (Eq. 1), which produces an energy rich commodity chemical. CO can be reacted with H2O via the water-gas shift (WGS) to generate H2, and this CO/H2 mixture (syngas) can be used to generate synthetic petroleum and liquid fuels using Fischer-Tropsch (FT) methods4–6 for direct integration into energy storage and distribution networks.7
Much effort has been devoted to the heterogeneous reduction of CO2 at metallic electrodes with the goal of driving selective formation of CO via Eq. 1. A number of cathode materials can facilitate this reaction, however only noble metals such as Ag and Au can catalyze this reaction with Faradaic Efficiencies (FEs) that are in excess of 80% at ambient pressures.8 The use of noble metal cathodes for production of CO has been hampered by the exorbitant cost of these materials, which eliminates their practical use on the scale required for alternative fuel synthesis. Indeed, the dearth of cost effective
| (1) |
systems that can efficiently and selectively drive Eq. 1 highlights the need for new electrode/catholyte pairings that can selectively promote the electrocatalytic conversion of CO2 to CO at appreciable rate (high current density) and low overpotential.
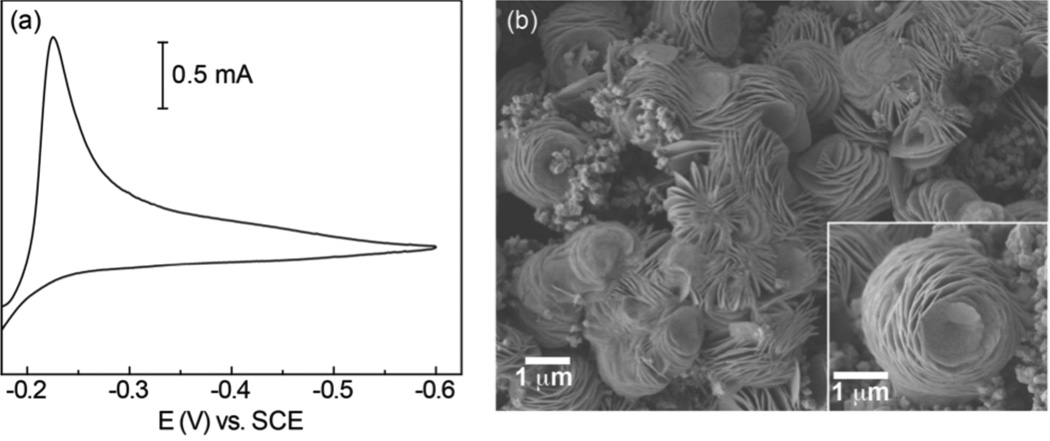
Bismuth is an attractive material for development of heterogeneous CO2 reduction catalysts, as this metal is non-toxic and has a very small environmental impact. Moreover, Bi is a byproduct of Pb, Cu and Sn refining, and has few significant commercial applications, resulting in the price of Bi being low and stable. Despite these attractive qualities, there has been only one report of electrocatalytic CO2 reduction using Bi,9 and the ability of this metal to drive CO production has not been demonstrated. As such, development of Bi-based cathodes for conversion of CO2 to CO would represent an important development for the fields of CO2 electrocatalysis and renewable energy conversion. To this end, we electrodeposited a Bi containing material onto an inert electrode substrate; reduction of an aqueous solution of 20 mM Bi(NO3)3 containing 0.5 M KBr and 1.0 M HCl using a glassy carbon electrode (GCE) produces the CV trace shown in Figure 1A, which is characterized by a broad cathodic wave. Controlled potential electrolysis (CPE) was carried out at −0.21 V versus the saturated calomel electrode (SCE; all potentials are referenced to this electrode) for quiescent acidic Bi3+ solutions until ~3 C/cm2 had been passed, leading to electrodeposition of a grey, non-lustrous material on the GCE surface. Glassy carbon was used as the working electrode to ensure that the base substrate supported negligible background activity for CO2 reduction.
Figure 1.
(a) Cyclic voltammogram in 1.0 M HCl and 0.5 M KBr containing 20 mM Bi3+. (b) SEM image recorded for a Bi-modified GCE.
The morphology of the deposited material was examined by scanning electron microscopy (SEM). As shown in Figure 1B, the electrode is coated by an array of striated clusters, interspersed within a film of smaller crystallites. Magnification of the micrometer sized clusters shows that the basic morphology of this material is reminiscent of a flower or rosebud. Energy-dispersive X-ray (EDX) analysis was performed on 40 × 40 µm2 regions of several independently prepared samples of the electrodeposited material (Figure S1). The surface of the material was also analyzed by X-ray photoelectron spectroscopy (XPS). All elements detected by EDX are also accounted for by XPS (Figure S2), which identifies Bi, Cl and Br as the principal elemental components in a ratio of roughly 7:1:1 with trace amounts of O and K also present. High-resolution XPS spectra for the bismuth region reveal Bi 4f7/2 signals at 156.5 and 159.3 eV, which are in the range typical of Bi0 and Bi3+ (Figure S2B) and integrate to a ratio of ~2:1. When taken together, the EDX and XPS analyses indicate that reduction of acidic solutions of Bi3+ containing KBr leads to deposition of a microcrystalline material containing metallic Bi0 and Bi3+ ions that has incorporated a significant amount of chloride and bromide along with traces of oxygen and potassium. The presence of oxygen and halide atoms in the electrodeposited material balances the charge of the Bi3+ ions.
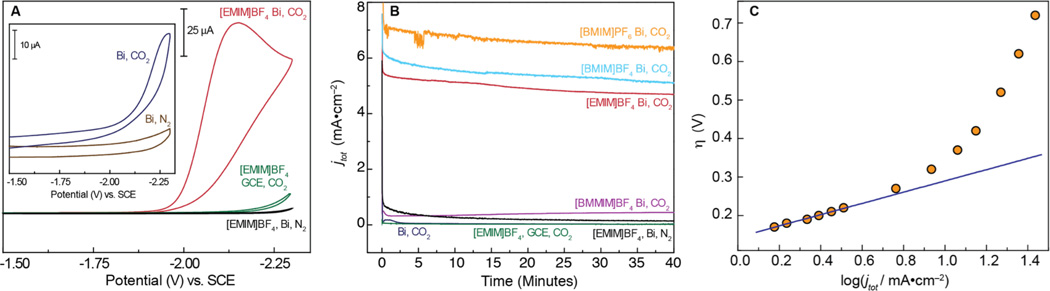
The ability of the Bi-modified electrode to electrochemically activate CO2 was assessed in MeCN, which supports a large electrochemical window and is commonly employed for CO2 electrocatalysis. As shown in the inset of Figure 2A, scanning to negative potentials in CO2 saturated solutions of MeCN containing 0.1 M TBAPF6 shows a small current enhancement as compared to the corresponding experiment under N2. 1,3-Dialkyl substituted imidazolum based ionic liquids (ILs) can strongly interact with CO2 and have found application for carbon sequestration.10,11 Moreover, the ability of such ILs to bind reduced CO2 intermediates at Ag electrodes and mediate electrochemical generation of CO at low overpotentials has also been demonstrated.12 With these properties in mind, the IL 1-ethyl-3-methylimidazolium tetrafluoroborate ([EMIM]BF4) was added to the CO2 saturated MeCN solution, resulting in a dramatic change in the resultant I/V curves. In particular, the onset of a large, irreversible cathodic wave at −1.85 V is indicative of an electrocatalytic process (Figure 2A, red trace). This current response cannot simply be attributed to reduction of [EMIM] at the electrode surface, as polarization curves recorded in the absence of CO2 did not show a reduction wave (Figure 2A, black trace), suggesting that the observed cathodic feature corresponds directly to CO2 reduction.
Figure 2.
(a) CV traces recorded for Bi-modified and bare GCEs in MeCN containing 20 mM [EMIM]BF4; Inset: Bi-modified GCE in MeCN without IL. (b) Total current density (jtot) profiles for Bi-CMEC and GCE at −1.95 V in MeCN. (c) Total current density (jtot) Tafel plot for Bi-CMEC with 20 mM [BMIM]PF6 in MeCN. All electrochemistry was performed using 0.1 M TBAPF6 as electrolyte.
In order to establish that the electrocatalytic response shown in Figure 2A corresponded to conversion of CO2 to a reduced carbon product, CPE experiments were performed for a CO2 saturated solution of MeCN containing 20 mM [EMIM]BF4 using a Bi-modified GCE. After initiating the CPE at −1.95 V, the reaction headspace was periodically analyzed by gas chromatography (GC). This analysis showed CO to be the sole gaseous product formed during the electrolysis experiment. After 60 min, the CPE was discontinued and the amount of CO in the headspace was quantified; the measured CO levels corresponded to a FE of nearly 95% for the 2e−/2H+ conversion of CO2 to CO, with an average partial current density of jCO = 3.77 ± 0.7 mA/cm2 (Figure 2B, Table 1). Repetition of this experiment under N2 leads to negligible current density (Figure 2B, black trace) and no CO production, indicating that the CO formed under an atmosphere of CO2 is not simply a product of IL or solvent decomposition. Similarly, repeating this experiment under CO2 but in the absence of [EMIM] results in a nearly 40-fold decrease in current density (Figure 2B, light blue trace) and a substantial reduction in FE for CO production (Table 1). Taken together, these control experiments demonstrate that the IL is integral to the observed electrocatalysis, which is distinguished by high current densities for the selective production of CO from CO2.
Table 1.
Faradaic efficiencies (FE) and current densities for electrocatalytic reduction of CO2 to CO at an applied potential of −1.95 V vs. SCE in MeCN.
| Electrode | Ionic Liquid | FECO | jco (mA/cm2) |
|---|---|---|---|
| GCE | [EMIM]BF4 | Trace | <0.03a |
| Bi-CMEC | [EMIM]BF4 | 93 ± 7 % | 3.77 ± 0.7 |
| Bi-CMEC | None | 48 ± 13% | 0.11 ± 0.1 |
| Bi-CMEC | [BMIM]BF4 | 95 ± 6% | 5.51 ± 1.2 |
| Bi-CMEC | [BMIM]PF6 | 90 ± 9% | 4.82 ± 0.7 |
| Bi-CMEC | [BMMIM]BF4 | 76 ± 6% | 0.67 ± 0.5 |
Total current density
Additional experiments demonstrate that the observed electrocatalysis cannot simply be attributed to homogeneous CO2 reduction mediated by the IL. If the observed electrocatalysis were homogeneous in nature, the identity of the working electrode should have minimal impact on the observed chemistry. Unlike those obtained using a Bi-modified electrode, CV traces recorded for 20 mM [EMIM]BF4 in MeCN under CO2 with a glassy carbon working electrode showed virtually no current enhancement upon scanning to negative potentials (Figure 2A, green trace). Similarly, CPE of the CO2 saturated solution of MeCN containing [EMIM] at −1.95 V using a GCE resulted in negligible charge being passed over the course of a 60 min experiment, and did not produce CO (Figure 2B, Table 1). Accordingly, the Bi-modified electrode is intimately involved in the electrocatalytic conversion of CO2 to CO, and represents the first Bismuth-Carbon Monoxide Evolving Catalyst (Bi-CMEC).
The performance of Bi-CMEC on GCE was also assessed using more viscous ILs in MeCN. Titration of either the BF4− or PF6− salts of 1-butyl-3-methylimidazolium ([BMIM]) into acetonitrile gave rise to polarization curves similar to that observed for [EMIM]BF4 (Figure S3). Similarly, CPE of MeCN solutions containing 0.1 M TBAPF6 and 20 mM [BMIM]X (X = BF4 or PF6) at −1.95 V lead to the rapid production of CO with near quantitative FEs (Table 1). Notably, the CPEs produced only trace levels of H2 and no detectable formate, oxalate or glyoxalate, which are often observed upon electrochemical reduction of CO2 in organic solvents.13,14 This result is especially notable, as there are few materials that can electrocatalyze the conversion of CO2 to CO in organic catholyte with even modest FEs.15 Moreover, the few metals that drive this electrocatalytic process with reasonable current densities do so only upon application of very large overpotentials.16
While Bi-CMEC with [BMIM]X ILs generates CO with FEs that are comparable to that observed with [EMIM], current densities for CO production (jCO) using the [BMIM] ILs were slightly higher than for the [EMIM] experiments (Table 1, Figure 2B) and are similar to those obtained using Ag or Au cathodes.12,15,17 The Bi-CMEC system is robust under these conditions and displays steady current densities for CO production over several hours (Figure S4).
The energy efficiency of electrocatalytic CO production by Bi-CMEC can be calculated using the expression below (Eq. 2), in which E°CO2/CO represents the standard reduction potential for conversion of CO2 to CO under the CPE conditions and
| (2) |
E is the applied potential.18 Determination of the energy efficiency of the Bi-CMEC system requires an estimation of the standard potential of the CO2/CO redox couple (E°CO2/CO) and calculation of the overpotential (η) at which CPE is carried out. The position of E°CO2/CO is dependent on the proton donating ability of the electrolyte solution.19 For the present system, deprotonation of the central imidazolium carbon of the [EMIM] and [BMIM] ILs is the most likely source of protons to drive the 2e−/2H+ conversion of CO2 to CO. The pKa value for deprotonation of these 1,3-dialkylimidazolium cations is approximately 32 in MeCN.20 The low proton availability of the current system drives E°CO2/CO to more negative potential, as this standard redox couple can be estimated to be −1.78 V based on the pKa values for deprotonation of [EMIM] and [BMIM].21 By contrast, the IL 1-butyl-2,3-dimethylimidazolium BF4 ([BMMIM]BF4), which bears a methyl substituent at the imidazolium-2-position cannot be deprotonated as readily, and does not induce CO2 reduction catalysis with appreciable current density (Figure 2b, Table 1) over the same potential range (Figure S3d). These results indicate that proton transfer from the imidiazolium helps drive the observed CO2 reduction chemistry and suggests other organic acids may be able to promote a similar electrocatalysis.
Given that Bi-CMEC drives selective CO formation while operating with appreciable current density at E = −1.95 V, the overpotential for this process is only 0.165 V. This low overpotential coupled with the high FEs corresponds to an energy efficiency of over 85%. Both the low overpotential and high energy efficiency distinguish Bi-CMEC as a promising platform for electrocatalytic CO production, as both these values compare favorably to those obtained using Ag and Au based electrocatalysts, which are among the most efficient existing platforms for electrolytic generation of CO from CO2.12,15,17
The variation in partial current density of CO for Bi-CMEC on glassy carbon was measured as a function of applied overpotential in CO2 saturated MeCN containing 20 mM [BMIM]PF6. These data were obtained by performing stepped-potential electrolyses between E = −1.95 and −2.5 V, with commensurate quantification of the gaseous products by GC. The FE for CO production remains high as the applied η is increased, however the resulting Tafel plot constructed from these data (Figure 2c) begins to deviate from linearity as the applied potential exceeds −2.1 V. This curvature may be due to uncompensated iR drop caused by the surface resistivity of the GCE. The Tafel data is linear in the range of η = 0.165 – 0.275 V, with a slope of 139 mV/decade. This value, which is close to 118 mV/dec, points to a mechanistic pathway in which initial electron transfer to generate a surface adsorbed CO2•− species is rate determining. This mechanism has been invoked for CO2 reduction at many heterogeneous electrodes.22
The electrocatalytic reduction of CO2 offers a promising route to the conversion of renewable sources of electric current to carbon based fuels when coupled to the 4e−/4H+ splitting of water. A two-compartment cell for CO2 electrocatalysis allowed CO production at the Bi-CMEC modified electrode to be coupled to water oxidation. In these experiments, the anode compartment consisted of a piece of platinum-gauze in aqueous phosphate buffer (pH ~ 7.4) and the cathode compartment was comprised of the Bi-CMEC modified GCE immersed in CO2 saturated MeCN containing 0.1 M TBAPF6 and 20 mM [BMIM]PF6. The two compartments were separated by a Nafion membrane. CV analysis of this split cell arrangement shows the same intense catalytic wave for CO2 reduction at approximately −1.9 V (Figure S5, inset) that was observed using the single solvent arrangement.
CPE experiments using the split electrode/electrolyte arrangement showed initial current densities of approximately 9 mA/cm2 with a FE of 52% for generation of CO (Figure S5). Together, these data correspond to a TOF of approximately 300 s−1 for generation of CO, based on the electrochemical surface area of the Bi-CMEC electrode. Permeation of the Nafion membrane by water caused a gradual decrease in CO evolution activity to jtot = 0.25 mA/cm2 with a FE of 39% for CO formation. This drop in FE for CO generation is due to enhanced production of HCO2H from CO2 in the presence of an aqueous catholyte.9 The Bi-CMEC assembly is robust under these conditions. Extended CPEs of over 12 hours showed no additional decay in current density and produced over 85,000 cumulative surface turnovers over the course of the experiment, which is significantly higher than that obtained using a Ag cathode with an [EMIM]BF4 cocatalyst.12 It is expected that improved mass transport using a flow-cell, gas diffusion electrode or other advanced cell design23,24 would enable even higher current densities (TOFs) for CO production by Bi-CMEC. Additional improvements in activity may also be attained simply by improving ohmic contact between the Bi-CMEC and underlying GCE or by using an alternative substrate entirely.
Despite its low cost, Bi has been virtually ignored as a cathode material for CO2 electrolysis. As we have shown, however, electrodeposition of a material containing Bi0 and Bi3+ produces a catalyst that can efficiently drive CO generation in the presence of an IL. The activity of Bi-CMEC rivals the best noble-metal cathodes described to date, as this Bi0/Bi3+ assembly catalyzes the conversion of CO2 to CO with high selectivity and FE. At present, the importance of the Bi3+ ions to the efficacy of Bi-CMEC is unclear. It is possible that the interface between Bi0 and Bi3+ sites serves to stabilize CO2•− intermediates at the electrode surface in a fashion similar to that proposed for metal oxides on etched electrodes.25,26 Alternatively, the exceptional activity of Bi-CMEC may be derived from in-situ reduction of the Bi0/Bi3+ material to generate metastable surfaces with enhanced catalytic properties. The use of imidazolium ILs may be especially critical in this regard,27 as recent work has demonstrated that interplay between adsorbed imidazolium cations and CO2 at bulk platinum electrodes can significantly enhance electrocatalytic CO2 reduction.28 Future efforts to uncover the atomic-level structure of Bi-CMEC will be critical to understanding the mechanism by which this system activates CO2, and more generally, may provide a rational route for the development of additional inexpensive electrode materials for CO evolution.
Supplementary Material
ACKNOWLEDGMENT
We thank Rachel C. Pupillo and Prof. Thomas P. Beebe (UD) for assistance with XPS and SEM analysis. Financial support for this work was provided through an Institutional Development Award (IDeA) from the NIGMS of the NIH under grant number P20GM103541. Additional financial support was provided, by the American Chemical Society Petroleum Research Fund, the University of Delaware and through a DuPont Young Professor Award to JR. Instrumentation in UD’s Surface Analysis Facility was purchased with funding from the NSF (CHE-9814477 and DMR-9724307).
Footnotes
ASSOCIATED CONTENT
Supporting Information. Experimental methods and electrochemistry data. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interests. The following patent application is related to the work here: 61/779666, “System and Process for Electrochemical Conversion of Carbon Dioxide to Carbon Monoxide” (JR).
REFERENCES
- 1.Olah GA, Prakash GKS, Goeppert A. J. Am. Chem. Soc. 2011;133:12881–12898. doi: 10.1021/ja202642y. [DOI] [PubMed] [Google Scholar]
- 2.Centi G, Perathoner S. Catal. Today. 2009;148:191– 205. [Google Scholar]
- 3.Annual Energy Review 2011. US Energy Information Administration; Sep, 2012. [Google Scholar]
- 4.Rofer-DePoorter CK. Chem. Rev. 2012;81:447–474. [Google Scholar]
- 5.Vennestrøm PNR, Osmundsen CM, Christensen CH, Taarning E. Angew. Chem. Int. Ed. 2011;50:10502– 10509. doi: 10.1002/anie.201102117. [DOI] [PubMed] [Google Scholar]
- 6.Takeshita T, Yamaji K. Energy Policy. 2008;36:2773– 2784. [Google Scholar]
- 7.Kintisch E. Science. 2008;320:306–308. doi: 10.1126/science.320.5874.306. [DOI] [PubMed] [Google Scholar]
- 8.Hori Y, Wakebe H, Tsukamoto T, Koga O. Electrochimica Acta. 1994;39:1833–1839. [Google Scholar]
- 9.Komatsu S, Yanagihara T, Hiraga Y, Tanaka M, Kunugi A. Denki Kagaku. 1995;63:217–224. [Google Scholar]
- 10.Cadena C, Anthony JL, Shah JK, Morrow TI, Brennecke JF, Maginn EJ. J. Am. Chem. Soc. 2004;126:5300–5308. doi: 10.1021/ja039615x. [DOI] [PubMed] [Google Scholar]
- 11.Brennecke JF, Gurkan BE. J. Phys. Chem. Lett. 2010;1:3459–3464. [Google Scholar]
- 12.Rosen BA, Salehi-Khojin A, Thorson MR, Zhu W, Whipple DT, Kenis PJA, Masel RI. Science. 2011;334:643–644. doi: 10.1126/science.1209786. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda S, Takagi T, Ito K. Bull. Chem. Soc. Jap. 1987;60:2517–2522. [Google Scholar]
- 14.Amatore C, Saveant JM. J. Am. Chem. Soc. 1981;103:5021–5023. [Google Scholar]
- 15.Hori Y. In: Modern Aspects of Electrochemistry. Vayenas CG, White RE, Gamboa-Aldeco ME, editors. Vol. 42. New York: Springer; 2002. pp. 89–189. [Google Scholar]
- 16.Ikeda S, Takagi T, Ito K. Bull. Chem. Soc. Jap. 1987;60:2517–2522. [Google Scholar]
- 17.Chen Y, Li CW, Kanan MW. J. Am. Chem. Soc. 2012;134:19969–19972. doi: 10.1021/ja309317u. [DOI] [PubMed] [Google Scholar]
- 18.Whipple DT, Kenis PJA. J. Phys. Chem. Lett. 2010;1:3451–3458. [Google Scholar]
- 19.Costentin C, Drouet S, Robert M, Saveant JM. Science. 2012;338:90–94. doi: 10.1126/science.1224581. [DOI] [PubMed] [Google Scholar]
- 20.Magill AM, Cavell KJ, Yates BF. J. Am. Chem. Soc. 2004;126:8717–8724. doi: 10.1021/ja038973x. [DOI] [PubMed] [Google Scholar]
- 21.Calculation of E°CO2/CO for the electrolysis conditions employed is presented in the Supporting Information.
- 22.Gattrell M, Gupta N, Co AJ. Electroanal. Chem. 2006;594:1–19. [Google Scholar]
- 23.Hara K, Kudo A, Sakata T. J. Electroanal. Chem. 1995;391:141–147. [Google Scholar]
- 24.Whipple DT, Finke EC, Kenis PJA. Electrochem. and Solid-State Lett. 2010;13:B109–B111. [Google Scholar]
- 25.Chen Y, Kanan MW. J. Am. Chem. Soc. 2012;134:1986–1989. doi: 10.1021/ja2108799. [DOI] [PubMed] [Google Scholar]
- 26.Li CW, Kanan MW. J. Am. Chem. Soc. 2012;134:7231–7234. doi: 10.1021/ja3010978. [DOI] [PubMed] [Google Scholar]
- 27.Schernich S, Laurin M, Lykhach Y, Steinrück H-P, Tsud N, Skála T, Prince KC, Taccardi N, Matolín V, Wasserscheid P, Libuda J. J. Phys. Chem. Lett. 2013;4:30–35. doi: 10.1021/jz301856a. [DOI] [PubMed] [Google Scholar]
- 28.Rosen BA, Haan JL, Mukherjee P, Braunschweig B, Zhu W, Salehi-Khojin A, Dlott DD, Masel RI. J. Phys. Chem. C. 2012;116:15307–15312. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.