Abstract
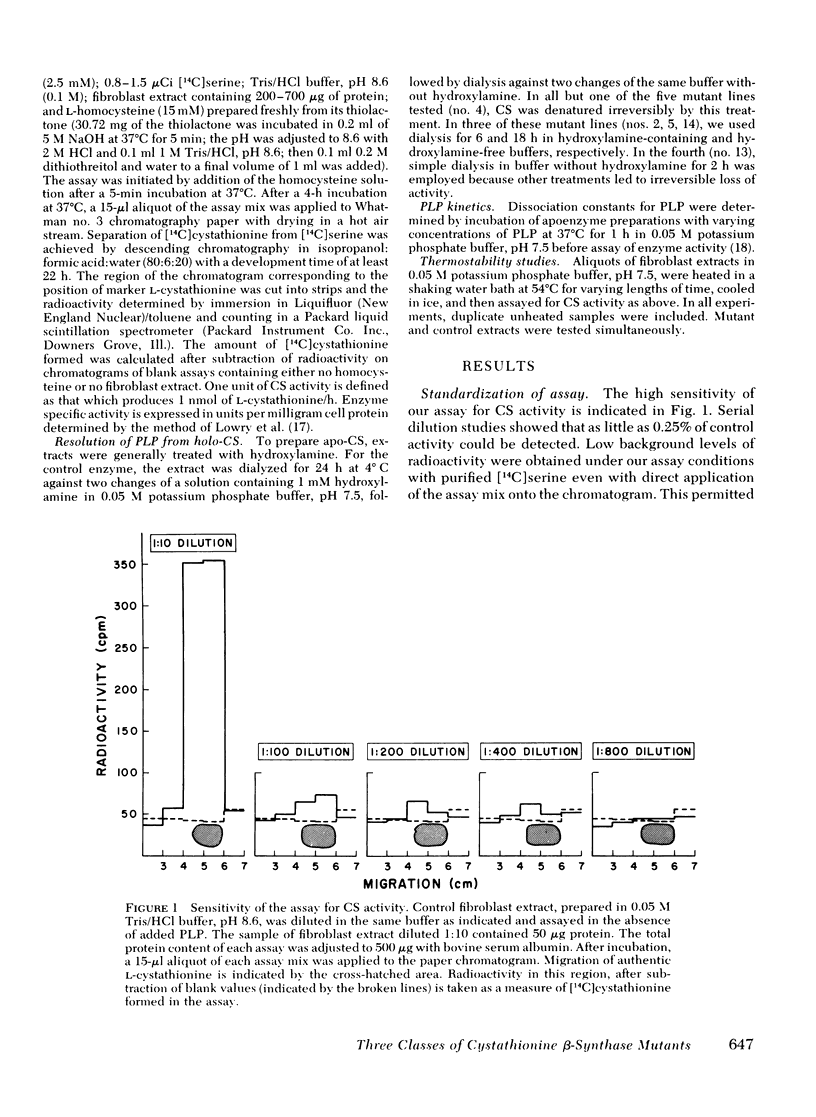
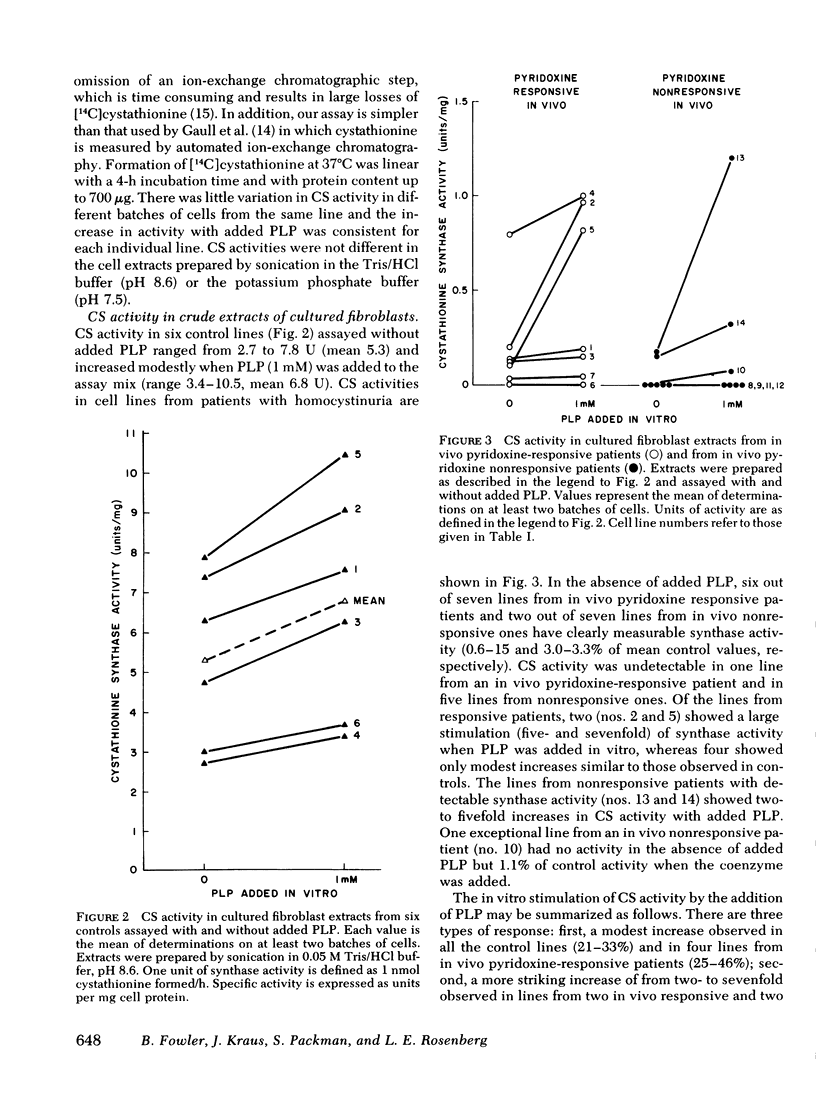
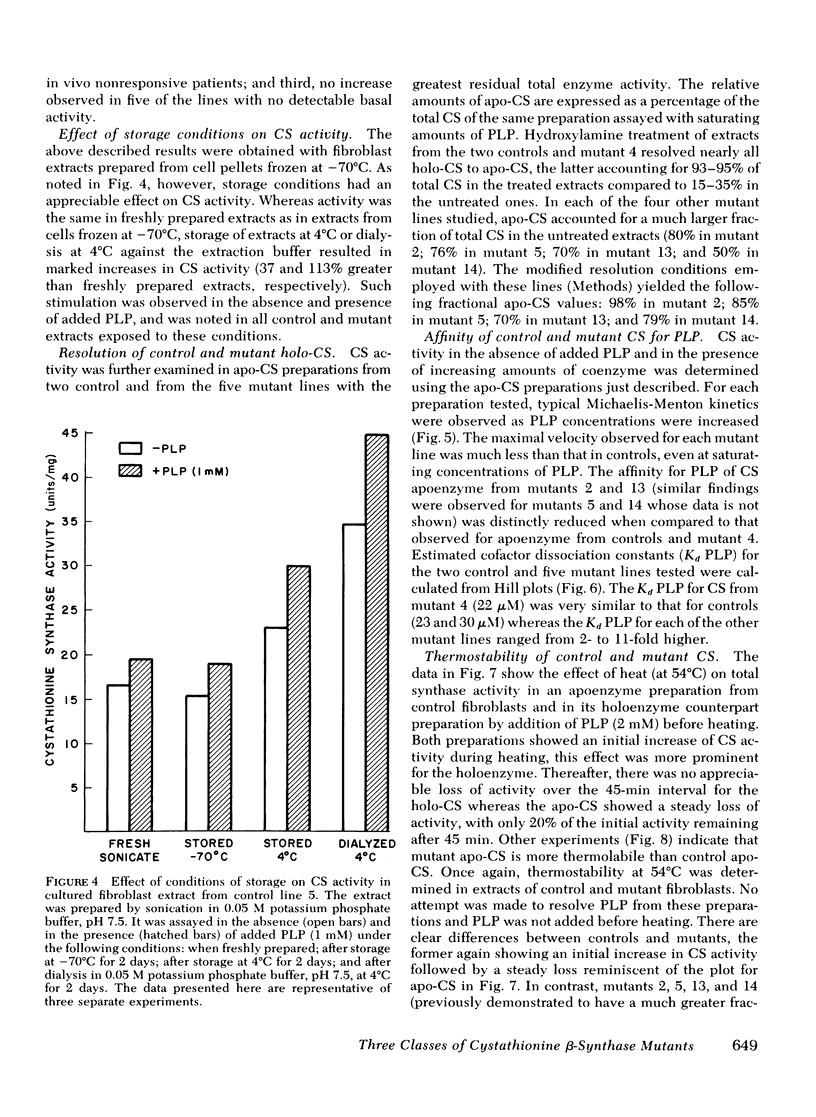
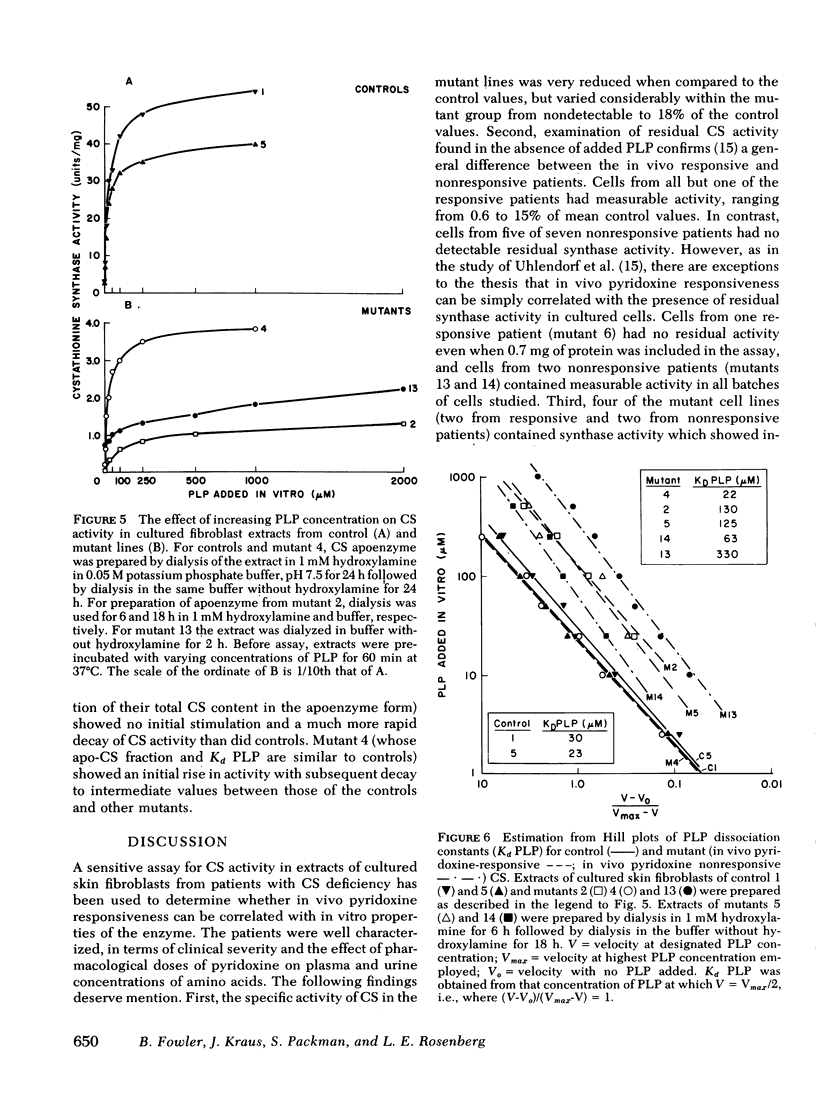
We have compared in vivo pyridoxine responsiveness with in vitro cystathionine β-synthase activity in extracts of confluent fibroblasts from 14 synthase-deficient patients. Enzyme activity was measured with and without addition of its cofactor, pyridoxal-5′-phosphate, using a radioisotopic assay which detects as little as 0.25% of control activity. Six of seven lines from responsive patients had measurable activity without the added cofactor (0.6-15% of mean control). Two of these lines showed a five- and sevenfold stimulation of cystathionine β-synthase activity with added pyridoxal-5′-phosphate; in the other four, the cofactor addition increased activity only modestly, as in controls. Two of seven lines from nonresponsive patients had measurable activity (each 3% of mean control) which increased two- and fivefold with the added cofactor. Cystathionine β-synthase activity was undetectable in one line from a responsive patient and in five lines from nonresponsive ones. To characterize control and mutant synthase further, dissociation constants for pyridoxal-5′-phosphate were estimated and thermostability (54°C) was studied in two control and five mutant lines. In one mutant, both parameters were normal; in the others, the affinity for the cofactor was reduced 3-to 11-fold and thermostability was much impaired. We conclude that at least three general classes of cystathionine β-synthase mutants exist: those with no residual activity; those with reduced activity and normal affinity for pyridoxal-5′ phosphate; and those with reduced activity and a reduced affinity for the cofactor. Pyridoxine responsiveness in vivo cannot be correlated simply with the presence or absence of residual synthase activity in vitro or with stimulation of in vitro enzyme activity by cofactor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber G. W., Spaeth G. L. The successful treatment of homocystinuria with pyridoxine. J Pediatr. 1969 Sep;75(3):463–478. doi: 10.1016/s0022-3476(69)80274-x. [DOI] [PubMed] [Google Scholar]
- Bond J. S. A comparison of the proteolytic susceptibility of several rat liver enzymes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):333–339. doi: 10.1016/0006-291x(71)90757-1. [DOI] [PubMed] [Google Scholar]
- CARSON N. A., NEILL D. W. Metabolic abnormalities detected in a survey of mentally backward individuals in Northern Ireland. Arch Dis Child. 1962 Oct;37:505–513. doi: 10.1136/adc.37.195.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatagner F., Gicquel Y., Portemer C., Tixier M. Inactivation of cystathionase and of cysteine sulfinic acid decarboxylase by proteolytic enzymes: effect of pyridoxal phosphate. Experientia. 1970 Jun 15;26(6):602–604. doi: 10.1007/BF01898711. [DOI] [PubMed] [Google Scholar]
- Efremova L. L., Florent'ev V. L., Goryachenkova E. V. Production of apoenzymes of serine sulfhydrase and cystathionine-beta-synthase and their interaction with pyridoxal-5'-phosphate and its analogs. Mol Biol. 1974 Jul;8(1):123–131. [PubMed] [Google Scholar]
- GERRITSEN T., VAUGHN J. G., WAISMAN H. A. The identification of homocystine in the urine. Biochem Biophys Res Commun. 1962 Dec 19;9:493–496. doi: 10.1016/0006-291x(62)90114-6. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Campbell B. K., Gartler S. M. Cystathionine synthase activity in human lymphocytes: induction by phytohemagglutinin. J Clin Invest. 1972 Apr;51(4):1034–1037. doi: 10.1172/JCI106863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holten D., Wicks W. D., Kenney F. T. Studies on the role of vitamin B6 derivatives in regulating tyrosine alpha-ketoglutarate transaminase activity in vitro and in vivo. J Biol Chem. 1967 Mar 10;242(5):1053–1059. [PubMed] [Google Scholar]
- Hunter J. E., Harper A. E. Stability of some pyridoxal phosphate-dependent enzymes in vitamin B-6 deficient rats. J Nutr. 1976 May;106(5):653–664. doi: 10.1093/jn/106.5.653. [DOI] [PubMed] [Google Scholar]
- Kashiwamata S., Greenberg D. M. Studies on cystathionine synthase of rat liver. Properties of the highly purified enzyme. Biochim Biophys Acta. 1970 Sep 16;212(3):488–500. doi: 10.1016/0005-2744(70)90255-x. [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Rosenberg L. E. On the mechanism of pyridoxine responsive homocystinuria. II. Properties of normal and mutant cystathionine beta-synthase from cultured fibroblasts. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4821–4825. doi: 10.1073/pnas.71.12.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Nakagawa H. Studies on cystathionine synthetase characteristics of purified rat liver enzyme. J Biochem. 1971 Apr;69(4):711–723. doi: 10.1093/oxfordjournals.jbchem.a129520. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Longhi R. C., Fleisher L. D., Tallan H. H., Gaull G. E. Cystathionine beta-synthase deficiency: a qualitative abnormality of the deficient enzyme modified by vitamin B6 therapy. Pediatr Res. 1977 Feb;11(2):100–103. doi: 10.1203/00006450-197702000-00003. [DOI] [PubMed] [Google Scholar]
- MUDD S. H., FINKELSTEIN J. D., IRREVERRE F., LASTER L. HOMOCYSTINURIA: AN ENZYMATIC DEFECT. Science. 1964 Mar 27;143(3613):1443–1445. doi: 10.1126/science.143.3613.1443. [DOI] [PubMed] [Google Scholar]
- Mudd S. H., Edwards W. A., Loeb P. M., Brown M. S., Laster L. Homocystinuria due to cystathionine synthase deficiency: the effect of pyridoxine. J Clin Invest. 1970 Sep;49(9):1762–1773. doi: 10.1172/JCI106394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd S. H., Finkelstein J. D., Irreverre F., Laster L. Transsulfuration in mammals. Microassays and tissue distributions of three enzymes of the pathway. J Biol Chem. 1965 Nov;240(11):4382–4392. [PubMed] [Google Scholar]
- Porter P. N., Grishaver M. S., Jones O. W. Characterization of human cystathionine beta-synthase. Evidence for the identity of human L-serine dehydratase and cystathionine beta-synthase. Biochim Biophys Acta. 1974 Sep 11;364(1):128–139. doi: 10.1016/0005-2744(74)90140-5. [DOI] [PubMed] [Google Scholar]
- Scriver C. R., Rosenberg L. E. Amino acid metabolism and its disorders. Major Probl Clin Pediatr. 1973;10:1–478. [PubMed] [Google Scholar]
- Seashore M. R., Durant J. L., Rosenberg L. E. Studies of the mechanism of pyridoxine-responsive homocystinuria. Pediatr Res. 1972 Mar;6(3):187–196. doi: 10.1203/00006450-197203000-00007. [DOI] [PubMed] [Google Scholar]
- Snell E. E. Analogs of pyridoxal or pyridoxal phosphate: relation of structure to binding with apoenzymes and to catalytic activity. Vitam Horm. 1970;28:265–290. doi: 10.1016/s0083-6729(08)60897-3. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. Studies on the sulfhydryl groups of L-aspartate beta-decarboxylase. Biochemistry. 1968 Sep;7(9):3240–3247. doi: 10.1021/bi00849a029. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. The effects of various vitamin B6 5'-phosphate derivatives on the structure and activities of L-aspartate beta-decarboxylase. Biochemistry. 1969 Mar;8(3):1056–1065. doi: 10.1021/bi00831a037. [DOI] [PubMed] [Google Scholar]
- Uhlendorf B. W., Conerly E. B., Mudd S. H. Homocystinuria: studies in tissue culture. Pediatr Res. 1973 Jul;7(7):645–658. doi: 10.1203/00006450-197307000-00008. [DOI] [PubMed] [Google Scholar]
- Uhlendorf B. W., Mudd S. H. Cystathionine synthase in tissue culture derived from human skin: enzyme defect in homocystinuria. Science. 1968 May 31;160(3831):1007–1009. doi: 10.1126/science.160.3831.1007. [DOI] [PubMed] [Google Scholar]


