Summary
The tubulin homolog FtsZ provides the cytoskeletal framework and constriction force for bacterial cell division. FtsZ has an ~50 amino acid (aa) linker between the protofilament-forming globular domain and the C-terminal (Ct) peptide that binds FtsA and ZipA, tethering FtsZ to the membrane. This Ct linker is widely divergent across bacterial species and thought to be an intrinsically disordered peptide (IDP). We confirmed that the Ct linkers from three bacterial species behaved as IDPs in vitro by circular dichroism and trypsin proteolysis. We made chimeras, swapping the Escherichia coli linker for Ct linkers from other bacteria, and even for an unrelated IDP from human α-adducin. Most substitutions allowed for normal cell division, suggesting that sequence of the IDP did not matter. With few exceptions, almost any sequence appears to work. Length, however, was important: IDPs shorter than 43 or longer than 95 aa had compromised or no function. We conclude that the Ct linker functions as a flexible tether between the globular domain of FtsZ in the protofilament, and its attachment to FtsA/ZipA at the membrane. Modeling the Ct linker as a worm-like-chain, we predict that it functions as a stiff entropic spring linking the bending protofilaments to the membrane.
Keywords: bacterial cell division, tubulin, entropic spring
Introduction
FtsZ (filamentous temperature sensitive Z) is a bacterial tubulin homologue essential for cell division in almost all bacteria and in many archaea. Individual FtsZ molecules assemble into protofilaments, which further associate to make the Z ring at the site of cytokinesis. The Z ring is probably a ribbon of 3-9 parallel protofilaments connected by loose lateral interactions (Milam et al., 2012). In addition to providing the cytoskeletal framework for the division apparatus, FtsZ appears to generate the constriction force that invaginates the membrane (Osawa et al., 2008, Osawa & Erickson, 2011). The overall process of cell division has been reviewed by Adams and Errington (2009), de Boer (2010) and Lutkenhaus et al (2012); more focused reviews of FtsZ are by Erickson et al (2010) and Mingorance et al (2010).
The structure of an FtsZ subunit is shown in cartoon form in Fig. 1. The globular domain, which has the tubulin homology fold, is shown as two sub-domains colored blue and cyan. The 67 C-terminal amino acids (aa), which we refer to as the Ct tail, comprises two parts and is shown in magenta. The extreme C-terminal 15-17 aa segment, which is highly conserved across bacterial speies, binds to FtsA. This peptide also binds to ZipA in Escherichia coli, and to several other regulatory proteins. Buske and Levin (2012) separated the peptide into N-terminal conserved and C-terminal variable segments, and found that the latter affected lateral interactions in Bacillus subtilis FtsZ. For the present paper we will not distinguish different binding sites and activities within this peptide; we will refer to the 15-17 aa Ct peptide as the “FtsA-binding peptide.” FtsA and ZipA are membrane proteins, and this binding tethers FtsZ to the membrane. Crystal structures are available showing the FtsA-binding peptide bound to ZipA (Mosyak et al., 2000) and FtsA (Szwedziak et al., 2012). This peptide is shown in Fig. 1 as a beta strand and alpha helix, which is the structure the peptide assumes in a co-crystal with ZipA (Mosyak et al., 2000).
Figure 1.
FtsZ Structure. (A) Front view of the FtsZ subunit from P. aeruginosa, PDB 1OFU (Cordell et al., 2003) (modified from (Erickson et al., 2010)). This view is equivalent to that of tubulin from the outside of a microtubule. The two sub-domains of the globular domain are colored blue (Nt) and cyan (Ct). GDP and the synergy loop aa D212 (E. coli numbering) are in red. A 10 aa segment on the N terminus and the 50 aa C-terminal linker are modeled as flexible peptides in magenta. The C-terminal FtsA-binding peptide is shown in dark purple. (B.) Model of the Ct-linker stretched to 8.5 nm, or half its contour length, which would require a force of 9 pN. In this case the molecule is rotated so that the front surface, to which the Ct linker is attached, is facing left.
The ~50 aa segment between the globular domain and the FtsA-binding peptide are referred to here as the Ct linker. Several lines of evidence suggest that the Ct linker is an unstructured, intrinsically disordered protein (IDP). (a) A number of crystal structures are available where the complete protein was crystallized, but these generally show electron density only for aa 10-316 of the E. coli FtsZ (EcFtsZ). This suggests that the N-terminal aa 1-9 and the C-terminal aa 317-383 are disordered. (b) The sequence of the Ct FtsA-binding peptide is highly conserved across bacterial species, as is that of the globular domain. In contrast, the sequence of the Ct linker shows almost no sequence conservation. (c) Sequences of FtsZ were analyzed by the PONDR program (http://www.pondr.com/,(Romero et al., 2001)) and the Ct-linker region in most species was indicated as disordered (data not shown).
In the present study we have tested in vivo whether the EcFtsZ linker could be swapped with Ct linkers from other bacterial species, and with IDPs from unrelated proteins. We have also assayed linkers in vitro for evidence of disorder.
Results
In vivo assays of linker function
To examine the role of the Ct linker, we produced a number of variants and tested them for ability to function for cell division. The complementation assay used here was originally developed by Dai and Lutkenhaus (1991). JKD7-1 cells, which are a genomic ftsZ null, are supported by the expression of wild-type (wt) FtsZ from a rescue plasmid, pKD3. pKD3 is temperature sensitive for replication, and after several generations at 42°C the wt FtsZ is depleted. The mutant FtsZ to be tested is produced by the arabinose inducible plasmid, pJSB2 (Stricker & Erickson, 2003, Redick et al., 2005). In the presence of arabinose (Induction Media) and at the restrictive temperature of 42°C, the pJSB plasmid becomes the only source of FtsZ available for cell division. The ability of the mutant FtsZ to function for cell division is demonstrated by the ability of a single plated cell to form a colony. In the present study we judged a variant form of FtsZ to complement when the number of colony forming units on the Induction plate at the restrictive temperature (cells expressing FtsZ solely from pJSB) was at least 80% that on the Repression plate at the permissive temperature (cells expressing wt FtsZ solely from pKD3). This indicates that that FtsZ protein is fully functional for cell division.
In a previous study, Osawa and Erickson (2006) further investigated FtsZ mutants that were non-complementing in the simple assay, i.e., those that produced no colonies when 1000 cells were plated. When 1,000,000 cells were plated, some FtsZ mutants generated 100-500 colonies. These were attributed to suppressor mutations that arose in the E. coli genome. In that previous study we confirmed that each suppressor was a genomic mutation by curing the strain of the pJSB2 plasmid, and re-introducing the mutant FtsZ on the original plasmid. In the present study we did not repeat this replacement exercise for the three presumed suppressor strains. The frequency of occurrence was the same as for the previously verified genomic suppressors, and it was not a high priority to verify it further. That FtsZ mutant is therefore functional for cell division in the suppressor strain. In the present study we first tested all FtsZ linker substitutions for simple complementation. Those that did not complement were tested for the generation of suppressor strains. We consider FtsZ mutants non-functional if they did not complement and did not generate suppressor strains, and partially functional if they could function in a suppressor strain.
We constructed 35 different Ct-linker constructs from the original pJSB-EcFtsZ plasmid, and tested them for complementation. Results are summarized in Table 1. All new constructs contain a glycine-arginine at the start of the Ct linker (corresponding to the inserted NotI restriction site) and a leucine-glutamate at the end of the Ct linker (corresponding to the inserted XhoI restriction site). The addition of these four residues to the Ct linker had no effect on cell structure (short rods) and doubling time (data not shown). For the purposes of this paper we refer to this as Ec (Table 1, Fig. 3, Fig. 4).
Table 1.
Complementation results for all linker constructs tested.
| Ct-Linker Peptide |
Length (aa) | Ct-Linker Charge |
Complementation |
|---|---|---|---|
| Ec | 54 | 1.1 | ++ |
| Bs | 51 | 3.2 | ++ |
| Av | 54 | 3.1 | (+) |
| Cc | 179 | −11 | − |
| Rm | 257 | 1.4 | − |
| CcB | 54 | −7 | ++ |
| RmA | 54 | 2.1 | ++ |
| RmB | 54 | −3.7 | ++ |
| RmC | 54 | 0.9 | ++ |
| RmD | 54 | −0.9 | ++ |
| RmE | 54 | 2.1 | ++ |
| PQ | 154 | −8.3 | − |
| PQa | 54 | −4.7 | − |
| PQb | 54 | −0.7 | ++ |
| PQc | 54 | −2.9 | ++ |
| ΔLink | 6 | 0 | − |
| Ec-1KE | 50 | −0.9 | ++ |
| Ec-2KE | 50 | −2.9 | ++ |
| Ec-3KE | 50 | −4.9 | ++ |
| Add14 | 14 | −3 | − |
| Add24 | 24 | −1 | − |
| Add43 | 43 | 0.1 | ++ |
| Add54 | 54 | 0.1 | ++ |
| Add64blk | 64 | 2.1 | − |
| Add66 | 66 | −3.9 | ++ |
| Add70blk | 70 | 3.1 | − |
| Add76 | 76 | −1.9 | ++ |
| Add95 | 95 | −0.8 | ++ |
| Add106 | 106 | −0.8 | (+) |
| Add116blk | 116 | 1.2 | − |
| Add126blk | 126 | 2.2 | − |
| Add168blk | 168 | 0.3 | − |
| FNIII7-8 | 188 | −12.6 | − |
| FNIII7 | 94 | −8.9 | − |
| FNIII10 | 94 | −0.1 | (+) |
The first column lists all C-terminal linker constructs tested. The length reported in column two includes the GR and LE residues from the NotI and XhoI restriction sites. Complementation is reported as ++ for constructs that fully complement the ftsZ null; (+) for constructs that can complement after generation of a second site suppressor mutation; − for constructs that can neither complement nor generate suppressor strains. Abbreviations in column one refer to linkers: Ec – E. coli; Bs – B. subtilis; Av – A. vinlandii; Cc – C. crescentus; Rm R. meliloti; CcB, RmA – short segments B and A from Cc and Rm; PQ – the “charged plus PQ” IDP form ZipA; ΔLink – linker deleted; EC-1(2/3)KE – Ec linker with 1 (two or three) lys aa’s mutated to glu; Add – segments from the IDP of human adducin (see Fig. 2 for complete description of these linkers); blk – indicates that the Add peptide contained the “black sequence (Fig. 2) as its first 11 aa’s; FNIII – indicates sequences from fibronectin type III domains.
Figure 3.
Growth and morphology for cells using the indicated linkers. Growth curves show turbidity at times following the switch to Induction Media (0.2% arabinose) and 42°C. Images shown are of cells fixed 222 minutes after the switch to Induction Media.
Figure 4.
Cell morphology under dominant negative conditions: E. coli cells expressing both wt FtsZ from the pKD3 rescue plasmid (at 30°C) and mutant FtsZ from pJSB (0.2% arabinose). Each mutant contained the wt EcFtsZ up to aa 316, and the wt FtsA-binding peptide, and contained the linker indicated in the title on each frame (see Table 1 for a complete designation of the linker abbreviations). Images shown are of cells fixed 190 minutes after induction of mutant FtsZ by addition of 0.2% arabinose.
Ct-linkers from foreign FtsZ can function in E. coli
Initially, we tested the Ct linkers from other bacterial species for function in E. coli FtsZ (Table 1). The Bs construct, where the 47 aa Ct linker from B. subtilis replaced the E. coli linker, fully complemented the ftsZ null. The Av construct, containing the 50 aa Ct linker from Azotobacter vinelandii, was partially functional for cell division, but required the generation of a suppressor strain. However, two other foreign Ct-linker constructs, Cc from Caulobacter crescentus (175 aa) and Rm from Rhizobium meliloti (253 aa), did not produce any colonies in the complementation assay, and they did not generate suppressor strains. Since these Ct linkers were much longer than the native E. coli Ct linker, we tested a 50 aa segment from the Cc linker, which is the same length as the Ec Ct linker. This shorter linker, termed CcB, was fully functional for cell division. Five other Ct-linker constructs were made by dividing the 253 aa Rm Ct-linker into 50 aa segments (constructs RmA, RmB, RmC, RmD, and RmE). All five of these chimeric proteins were fully functional for cell division. The sequence of the linkers from the different bacteria, and the other IDP constructs tested, showed only about 15-20% identity to that of E. coli, which is essentially random with respect to sequence. For example, the 54 aa Ec linker showed 17, 13, 23, and 17 % identity to 54 aa linkers Bs, RmA, PQb, and Add54; the last two are completely unrelated to FtsZ, and therefore clearly random. These initial results suggested that length was the major determining factor for Ct-linker function.
Functional Ct-linker peptides have a length, but not a charge requirement
To further test for potential sequence requirements, we turned to peptides from the bacterial division protein ZipA, which has a 150 aa “charged plus PQ domain” that forms a separate link between FtsZ and the membrane. This domain, called PQ in Table 1, has been considered to be an IDP (Hale & De Boer, 1997, Ohashi et al., 2002). The full length 150 aa PQ domain did not function as the Ct linker, which is consistent with the non-functionality of the longer Cc and Rm linkers. We then divided the full length PQ evenly into three 50 aa peptides, PQa, PQb, PQc, and tested those for function. PQa was non-functional, but PQb and PQc were both fully functional for cell division, consistent with lack of a sequence requirement for the linker.
We also examined the role of the total charge of the Ct linker on FtsZ function, since the native Ec Ct linker and the Bs constructs have a total charge of +1 and +3 respectively. Point mutants changing one, two or three lysines to glutamate in the E. coli Ct linker were created from the pJSB2-EcFtsZ plasmid (without the addition of the NotI and XhoI cloning sites) to create an increasing negative charge in the Ct linker. These mutants, Ec-1KE, Ec-2KE, and Ec-3KE, were all completely functional for cell division. The CcB construct, which carries a −7 charge, was functional. Overall these data suggest that the charge of the Ct linker is not important for FtsZ function.
Unstructured peptides from human α-adducin can function as the E. coli Ct-linker
To test further the lack of a sequence requirement, and to explore the length range permissible, we turned to an IDP from α-adducin, a mammalian protein completely unrelated to bacterial cell division. The initial adducin IDP was 66 aa long, so Ct linkers of different length were either truncated versions of that peptide, or were made by piecing together repeated portions of that IDP at the inserted XhoI restriction site (Fig. 2). The adducin Ct linkers were made to vary in length from 10 aa to 160 aa. We found that only adducin inserts between 43 aa and 95 aa were fully functional (Table 1). Any adducin construct outside of that length range was not able to complement. Linkers of 6, 14 and 24 aa’s did not complement and did not generate suppressor strains. However, the Add106 construct was able to generate suppressor strains, suggesting that the Add106 Ct linker is partially functional, but requires a suppressor mutation somewhere in the E. coli genome in order to function for cell division. Adducin constructs between 43 and 95 aa appeared to be fully functional.
Figure 2.
Adducin sequence and constructs tested as linkers. The adducin constructs tested are indicated as bars proportional to their length. Coloring indicates which segments of the adducin peptide are included (and repeated) in each construct. The sequence of each peptide is noted with the numbering from human α-adducin. The inserted NotI and XhoI restriction sites are included as GR or LE aa insertions, respectively.
Within the acceptable 43-95 aa length range of adducin peptides, the Add64blk and Add70blk constructs did not complement, and did not generate suppressor strains. The sequence of these constructs showed that they had a unique 11 aa sequence (QQREKTRWLNS), dubbed the “black sequence”. The presence of this peptide is noted in the constructs by “blk” (Fig. 2). Replacing this black sequence from the Add64blk, with another 13 aa sequence, gave Add66, which was fully functional. Removing the black sequence from Add116blk gave Add106, which was able to function after generating a suppressor strain.
Complementing Ct-linkers show normal cell growth and morphology
Cell proliferation was assessed via optical density at 600 nm, and cell morphology was examined via DIC light microscopy. Any Ct linker that showed complementation of the ftsZ-null phenotype on the plate-based complementation assay, also gave normal cell growth (doubling time) and morphology (short rods) in liquid culture (Fig. 3). For those Ct linkers that did not complement in the plate based assay, growth in the liquid culture was slow and plateaued at a low optical density. This was the case for adducin constructs with the black sequence and for ΔLink (Fig. 3) as well as for the other non-complementing constructs (not shown). The cell morphology showed filamentation for Add116blk and ΔLink; Add64blk showed cell lysis and a bulging phenotype. Add106 and Av Ct linkers functioned for cell division only after generation of genomic suppressor mutations. These suppressor strains grew at a rate (doubling time) comparable to that of the wt cells, and cells showed a normal, short rod morphology.
Ct-linker constructs that did not complement were tested for dominant-negative effects on FtsZ function, and thus cell morphology, by expressing the mutant Ct-linker constructs in the presence of wt FtsZ from the pKD3 rescue plasmid (Fig. 4). At the 0.2% arabinose used here, the protein expressed from pJSB2 is about 3-5 times the level of the wt FtsZ from pKD3 (Redick et al., 2005). This level of over-expression of wt FtsZ had little effect on cell morphology, but all non-complementing linker constructs showed dominant-negative effects. The Add64blk linker produced cells that tended to bulge and lyse rather than filament. The other non-complementing constructs caused moderate filamentation.
Rigid protein domains are generally non-functional as the Ct-linker
Since the flexible linker is predicted to have an end-to-end length of 5-8 nm (see Discussion) we tested whether a rigid protein of similar length could function as the linker. FNIII7-8 is a pair of FNIII domains with a length of 7 nm (Leahy et al., 1996). It did not complement and did not generate suppressors, and so is apparently non-functional. The shorter Ct-linker composed of just the FNIII7 domain was also non-functional. However, the FNIII10 domain, which has the same 3.5 nm end-to-end distance as the non-functional FNIII7 domain was able to produce slower-growing colonies. While the FNIII10 Ct-linker was below our criterion of 80% colony formation, and grew slowly, it was at least partially functional for division.
Non-complementing Ct-linkers assemble structures like Z-rings in vivo
To address the question of whether nonfunctional Ct-linkers inhibited cell division by preventing Z-ring assembly or Z-ring constriction, we used immunofluorescence microscopy to visualize FtsZ structures in vivo. Cells were grown in 0.2% arabinose at the restrictive temperature to express the mutant Ct-linker FtsZ proteins and eliminate the wt FtsZ. Z rings could be seen in cells expressing the Ec, Add106, and Add66 Ct linkers (Fig.5), as would be expected from the normal cell growth and morphology seen with these constructs (Fig. 3). Cells expressing the non-complementing Cc, Rm, ΔLink, Add14, Add24, and Add168blk were filamentous, indicating a loss of FtsZ function. However, visualization of FtsZ showed assembly of the protein into rings or ring-like structures in all of these cell samples (Fig. 5). Many of the FtsZ patches were diffuse and might be caused by nucleoid exclusion, but most cells also showed some sharp structures (white arrows) characteristic of Z rings. This suggests that the loss of FtsZ function with these Ct-linkers is not due to a defect in FtsZ polymerization or anchoring the Z ring to the membrane. It also appears that longer Ct-linkers, such at Cc, Rm, and Add168blk resulted in less clearly defined FtsZ structures in images. This could indicate that such long linkers allow for protofilaments to spread within the Z ring, resulting in a wider and fuzzier structure. The Add64blk Ct-linker, which caused cells to bulge and lyse, showed FtsZ diffuse throughout the misshapen cells with no clear FtsZ structure. This Add64blk Ct-linker is the only construct tested that appears to inhibit Z-ring assembly in vivo.
Figure 5.
Immunofluorescence microscopy of FtsZ structures in vivo. E. coli cells expressing mutant Ct-linker constructs as the sole source of FtsZ were grown in Induction Media at 42°C for 4 hours. Cells were fixed and FtsZ was fluorescently labelled and visualized. White arrows indicate sharp structures resembling true Z-ring assembly. No FtsZ cells were grown at 42°C in Repression Media to deplete all FtsZ.
In vitro structural assays
We used circular dichroism (CD) to check for secondary structure of the full length C-terminal tails of both E. coli and C. crescentus FtsZ. For these measurements the peptides were obtained by expressing E. coli FtsZ with a TEV protease site between the globular domain and the full C-terminal tail (linker plus FtsA-binding peptide) of either E. coli or C. crescentus. The CD spectra of both Ct tails are similar to each other and to those of other intrinsically disordered peptides, such as the adducin tail (Hughes & Bennett, 1995), prothymosin alpha (Gast et al., 1995), and p21 (Kriwacki et al., 1996), suggesting the lack of any prominent secondary structure (Fig. 6a). Both linkers were estimated to have < 5% α-helix based on [θ]222 = − 40 × 103 deg cm2 dmol−1 for 100% α-helix. Consistent with the disorder, there was no unfolding transition indicated by [θ]222 as a function of temperature (Fig. 6b).
Figure 6.
Circular dichroism. (A) CD spectra for Ct tails of C. crescentus (3.4 μM, solid diamonds) and E. coli (7.2 μM open squares). (B) [θ]222 as a function of temperature.
We used trypsin proteolysis to further test for structure of the Ct tails. Trypsin cleaves peptides on the carboxyl side of basic amino acids, except when followed by proline, but cleavage can be blocked if the site is in a folded structure. Under limited trypsin digestion conditions, tandem mass spectrometry (MS/MS) analysis identified peptides corresponding to 6 of the 7 cleavable sites in E. coli (Fig. 7a) and 8 of the 10 in B. subtilis (Fig. 7b). This digestion was on full-length wt FtsZ or the full-length chimera with the Bs linker. Analysis by SDS-PAGE showed that, under these limited digestion conditions, more than 70% of the FtsZ ran at its native size, with several weak bands running slightly faster, indicating very limited digestion. The peptides identified by MS/MS are therefore only a fraction of the total protein. As a control, isolated E. coli Ct tail was also digested and it produced an identical digestion pattern as full-length E. coli FtsZ. Overall these results suggest that these two peptides are unstructured.
Figure 7.
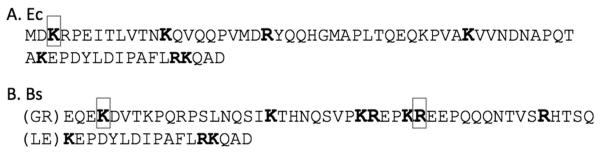
Limited trypsin digestion. Identification of cleavage sites found in the full Ct tail, as determined by MS/MS. All potential trypsin cleavage sites within E. coli (A) and B. subtilis (B) Ct tails are shown in bold. The boxed regions indicate sites where trypsin cleavage was blocked. Note that the B. subtilis sequence includes the GR and LE aa from the restriction sites, and the FtsA-binding peptide of E. coli.
Most Ct linkers have no effect on protofilament assembly in vitro. The black sequence causes protofilament pairing
We next tested whether certain linkers might interfere with protofilament assembly. Fig. 8 shows negative stain electron micrographs of wt E. coli FtsZ and three heterologous linker constructs. The functional Bs linker assembled single protofilaments identical to those of wild type E. coli, athough with some tendency to bundle or pair. The non-functional Cc linker produced normal single-stranded protofilaments, consistent with the interpretation that its lack of function is due to the exceptional length of the linker, not a defect in protofilament assembly. In contrast, the Add64blk linker assembled thicker filaments, which in places could be seen separating into pairs of protofilaments. Importantly, no single protofilaments were seen in these specimens, suggesting that the pairing was dominant.
Figure 8.
Negative stain EM images of EcFtsZ protofilaments with various C-terminal linkers. Each protein contained the E. coli globular domain (aa 1-316), the E. coli FtsA-binding peptide (aa 367-383) and the indicated linker: Ec: E. coli; Cc: C. crescentus; Bs: B. subtilis; and Add64blk: adducin64black. Scale bar: 100 nm.
Discussion
The Ct linker of FtsZ has long been thought to be an IDP and to function as a flexible tether connecting the FtsZ protofilaments to the cell membrane. In the most extreme interpretation, any IDP of appropriate length would be able to substitute for the Ct linker in EcFtsZ. In the present study we have confirmed that almost any IDP between 43 and 95 aa can function as the linker in EcFtsZ. Of 20 IDPs tested in this length range, only PQa (a 54 aa IDP from ZipA) and two adducin peptides containing the black sequence failed to function (and the Add106 and Av sequences required a genomic suppressor mutation to function).
The mechanical properties of an IDP are often fit by a model of a worm-like chain (wlc), which has only two parameters, contour length L and persistence length P. L is fixed at 0.34 nm per aa (Yang et al., 2000), giving 17 nm for 50 aa. P varies somewhat for different protein sequences. For the 72 aa Ct tail of EcFtsZ, P = 0.55 nm gave a good fit to FRET data (Ohashi et al., 2007). This is slightly higher than the 0.4-0.5 nm found for unfolded proteins stretched by AFM (Oberhauser et al., 1998, Dietz & Rief, 2004). The average end-to-end distance for this 73 aa Ct tail, in the absence of any force, was measured by FRET to be 5.2 nm (Ohashi et al., 2007), giving the value P = 0.55 nm. The formula of Rivetti et al (1996) predicts that the 50 aa linker should have an average end-to-end length of 4.4 nm.
The constriction force will cause the wlc to behave as an entropic spring, and the end-to-end distance will increase with force. An approximate formula is used to fit the distance vs force for a wlc (Bustamante et al., 1994). To stretch the Ct linker to half its contour length (8.5 nm end-to- end) requires a force of 9.3 pN. This is nearly twice the ~5 pN force generated by single motor molecules. The entropic spring of the Ct linker is therefore relatively stiff, and unlikely to be highly extended by the (still unknown) constriction force of the Z ring.
Two rigid linkers, FNIII7 and FNIII7-8 from fibronectin, failed to function. These have end-to-end distances of 3.5 and 7 nm, bracketing the spacing predicted for the IDP linker. However, another FNIII domain, FNIII10, gave slow-growing colonies, which confirms that it is at least partially functional. We note that FNIII10 was the weakest domain mechanically when fibronectin was stretched by AFM (Oberhauser et al., 2002). The AFM required a force of 100 pN to unravel FNIII10, but this was at a high pulling speed. It is possible that under the much weaker, but slow and steady pulling by the Z ring, the FNIII10 domain unravels into an IDP. This will need to be tested by new approaches.
In vitro assembly monitored by negative stain electron microscopy showed that one non-functional linker, Cc, assembled one-stranded protofilaments that were identical to those of wt FtsZ. The Bs linker, which was functional, also produced mostly one-stranded protofilaments. However it also gave some protofilaments in closely spaced pairs. These were presumably paired in solution with close lateral contacts between the pairs. The small separation of the protofilaments likely occurred after contact with the UV-treated carbon film. We note that full-length BsFtsZ has a pronounced tendency to bundle, mediated largely by the six “C-terminal variable” aa’s (Buske & Levin, 2012). Our present results suggest that the Bs linker may contribute to a bundling tendency, but this is relatively weak and does not interfere with function. The adducin black sequence, in contrast, caused a much stronger pairing of protofilaments. No single protofilaments were found, and the separation of the bundles into protofilament pairs was seen less often. These paired protofilaments likely retain the ability to bind FtsA and ZipA, which may distort the membrane and cause the observed cell lysis.
We can now estimate the distance of the protofilament from the membrane. The FtsA-binding peptide, which can also bind ZipA, is at the end of the Ct linker. A recent crystal structure shows Thermotoga maritima FtsA bound to the FtsA-binding peptide of FtsZ (Szwedziak et al., 2012). From the PDB file 4A2A we measured a distance of 3.8 nm between the first aa of the FtsZ peptide and V390, the last structured aa of FtsA. There is a 13 aa peptide, presumably an IDP, between the globular domain of FtsA and the amphipathic helix. This has a contour length of 4.4 nm, and if stretched to half the coutour length would add 2.2 nm. This would place the C-terminal side of the FtsZ globular domain about 11-14 nm from the inner surface of the membrane (5-8 nm for the FtsZ linker, plus ~6 nm for FtsA). A modest conundrum is posed by ZipA, since its FtsZ-binding site is on the end of a 150 aa IDP, which would have an average relaxed end-to-end distance of 7.7 nm (Ohashi et al., 2007). This is slightly longer than the 6 nm length of the FtsA link, suggesting that ZipA tethers may bear little or no tension. Thus the attachment of FtsZ to the membrane may include a mixture of stiff and soft entropic springs mediated by FtsA and ZipA.
One might expect that, if the linker is really functioning as an IDP, one could insert a small globular domain like YFP without affecting function. However, in a previous study we found that YFP(Venus) inserted at aa 326 or 346 did not complement and did not generate suppressor strains (Osawa & Erickson, 2005). Inserts of 20 aa (LSLIHIWRARADVYKRGxyz, structure unknown) at these two positions were also non-functional. A YFP insert at aa 331 did function, apparently by generating suppressor strains. In the globular domain of YFP, the N and C termini are about 2 nm apart, which would add the equivalent of a few aa to the length of the linker. In addition, the first five and last eleven aa of YFP are unstructured, and would add to the length (Ohashi et al., 2007). The total increase in length of the linker from the inserted YFP would be about 20 aa. However, since the 95 aa adducin IDP was functional, there must be something other than the length that causes a problem with the YFP inserts. The structure and function of the FtsZ linker still has some mysteries.
Experimental Procedures
Cloning
All variant forms of FtsZ presented in this paper were constructed in E. coli FtsZ in a pJSB2 plasmid, which is derived from pBAD and has an arabinose-inducible promoter (Stricker & Erickson, 2003, Redick et al., 2005). Novel restriction sites were inserted into the FtsZ sequence at the start and end of the Ct linker (NotI before aa317 and XhoI after aa366) to produce the EcFtsZ construct presented here. Alternative linker sequences to splice into FtsZ were amplified via PCR to contain a 5′ GCGGCCGC (NotI, where the GC indicates nucleotides already present in the FtsZ sequence, thus requiring the insertion of only 6 nt) and a CTCGAG 3 (XhoI)’. This results in aa pairs GR and LE inserted before and after the linker. The B. subtilis linker sequence (EQEK…RHTSQ) was amplified from the pBS58 plasmid (Wang & Lutkenhaus, 1993). The R. melliloti linker sequence (RTAA…QMRS) was amplified from pJC06 (provided by W. Margolin, (Margolin et al., 1991)). The A. vinelandii linker sequence (TRAD…INPQ) was taken from pET11b-AzbFtsZ (Lu et al., 1998). The C. crescentus linker sequence (GASI…DDAE) was amplified from a pET15b plasmid codon optimized for E. coli, provided Dr. M. Schumacher, Duke). The ZipA PQ domain sequences were taken from the E. coli ZipA PQ domain previously characterized as unstructured (Ohashi et al., 2002). The various adducin Ct-linker sequences were put together from a 66 aa peptide of human α-adducin (residues 440-505) previously characterized as unstructured (Ohashi et al., 2007). Sequences were repeated according to the color code in Figure 2. Each is labelled as Add#, where the # indicates the length of the adducin peptide. Amplified PCR products were restricted with NotI and XhoI and ligated into the EcFtsZ construct at the complementary cut sites. Resultant plasmids were verified by Sanger sequencing across the ligation sites. The ΔLink FtsZ construct was made by PCR amplification of the Ec FtsZ plasmid with primers 5′gggctcgagaaagcgccggattatctggatatccccagc3′ and 5′ggagcggccgccgatacctgtcgcaacaacggttacgcg3′, followed by the blunt end ligation of the PCR product to produce an FtsZ construct in which the Ct linker was replaced with a serine-glycine dipeptide. Charge mutants of the EcFtsZ linker were made by the directed mutagenesis of lysines (K319, K329, K356) to glutamate. Ec-1KE refers broadly to the K319E, K329E or K356E mutations. Ec-2KE refers to both K319E/K329E and K329E/K356E. Ec-3KE refers to the K319E/K329E/K356E triple mutant.
Growth of JKD7-1 Cells
The JKD7-1/pKD3 E. coli strain was developed by Dai and Lutkenhaus (1991), and has been useful to test for complementation of ftsZ mutants (Redick et al., 2005). JKD7-1 is an ftsZ null due to a kanamycin casette insertion into the genomic ftsZ. Wt FtsZ for cell division is provided by the pKD3 plasmid, which is temperature sensitive for replication. The cell strain was maintained at 30°C in Repression Media: Luria-Bertani media containing 100 μg mL−1 of ampicillin (selecting for pKD3 plasmid), 100 μg mL−1 of kanamycin (selecting for the kanamycin inserted at genomic ftsZ), 34 μg mL−1 of chloramphenicol (selecting for the pJSB2 plasmid containing the variant ftsZ), and 0.2% (w/v) glucose (suppressing expression of the pJSB2-FtsZ protein).
Complementation
JKD7-1/pKD3 cells containing pJSB2-FtsZ with Ct-linker variants were grown in Repression Media at 30°C overnight. The cultures were then diluted 1:1000, and 103 cells were plated on dishes containing Induction Media: Luria-Bertani-agar media containing 100 μg mL−1 kanamycin, 34 μg mL−1 chloramphenicol and 0.2% (w/v) arabinose (to induce expression of the FtsZ protein from pJSB2). Plates were grown at 42°C to eliminate the temperature sensitive pKD3 rescue plasmid. In Induction Media at 42°C, the pJSB plasmid is the only source of FtsZ for cell division. An equal volume of cells was also plated on Repression Media plates and grown at 30°C, to assess the total number of cells plated. A serial dilution of JKD7-1/pKD3 cells were also plated on Repression Media at the permissive temperature to further asses the total number of cells plated.
Growth Curves and Cell Morphology
JKD7-1 cells with pJSB-FtsZ were grown in liquid Repression Media at 30°C with shaking overnight. To track the ability of various FtsZ proteins to function for cell division and normal cell morphology, an aliquot of these cells was transferred to liquid Induction Media at 42°C with shaking. Cell growth was assayed as the optical density at 600 nm at various timepoints folllowing the transfer to the restrictive temperature. 222 minutes after switching to Induction Media and 42°C, cell samples were fixed in cold methanol for DIC microscopy. Some mutants of FtsZ that did not complement were assessed for dominant-negative effects. Non-complementing protein was expressed in Induction Media containing 100 μg mL−1 ampicillin at 30°C, to maintain the pKD3 rescue plasmid. The expression of wt FtsZ from pKD3 should allow for cell division to occur, while testing whether the non-complementing FtsZ would interfere with the wt FtsZ. These cells were also fixed in cold methanol and observed for cell morphology 190 minutes after induction of mutant FtsZ with 0.2% arabinose.
Immunofluorescence Microscopy of FtsZ Structures
JKD7-1 cells were grown overnight in Repression Media at 30°C. The cells were then diluted 1:1,000 in Induction Media and grown at 42°C to an OD600 of ~0.5. These cells were fixed in paraformaldehyde, formaldehyde and sodium phosphate buffer, as described by Addinall et al. (1996). Fixed cells were washed three times with PBST (PBS plus 0.05% Tween 20) and resuspended in 25 mM Tris-HCl, 50 mM glucose, 10 mM EDTA, pH 8.0. Ten microliters of resuspended cells were allowed to adhere to poly-L-lysine coated slides for 10 minutes, and then permeabilized with 100 μL of 2 mg mL−1 lysozyme for 5 minutes. The slides were washed three times in PBST. Cells were blocked and stained as described by Addinall et al. (1996) using 3.1 μg mL−1 of affinity-purified rabbit anti-EcFtsZ 5435 (Lu et al., 1998) as the primary antibody and 2 μg mL−1 of goat anti-rabbit immunoglobulin G conjugated to Oregon Green-500 (Molecular Probes) as the secondary antibody. Slides were washed in PBST after staining and viewed immediately on a Zeiss Axiophot microscope with 100× magnification using a plan-Neofluar oil immersion lens (numerical aperture 1.3). Images were captured with a cooled charge-coupled device camera (Coolsnap HQ; Roper, Ottobrun, Germany) and manipulated using Adobe Photoshop.
Expression and Purification of Ct Tails
C-terminal tails, including the linker and the FtsA-binding peptide, were prepared as fusions to the globular domain of EcFtsZ. These genes were all cloned into pET-11B for expression and used for limited trypsin digestion: (a) MFE…EcFtsZg…GIG–MDKR…EcTail…KQAD (where MFE…EcFtsZg…GIGg indicates the globular domain beginning with MFE and ending in…GIG, and MDKR…EcTail…KQAD indicates the full Ct tail); (b) MFE…EcFtsZg…GIG–grEQEK…Bslinker…HTSQleKEPDYLDIPAFLRKQAD (where gr and le indicate the aa’s introduced by the cloning sites); and (c) MFE…EcFtsZg…GIG–grGASI…Cclinker…DAEleKEPDYLDIPAFLRKQAD. To study the Ct tails isolated from the globular domain we inserted a TEV protease site (ENLYFQS/G, where / indicates the cut site) after aa316 of the EcFtsZ globular domain: (d) MFE…EcFtsZg…GIG–grenlyfqgMDKR…EcTail…KQADwgdpnsssvdklaaalehhhhhh (where wgd…hhh indicates the added tryptophan, histag and additional aa); and (e) FE…EcFtsZg…GIGgrenlyfqgGASI…Cclinker…DDAEwleKEPDYLDIPAFLRKQAD. Note that all of these expressed proteins had the E. coli FtsA-binding peptide.
FtsZ constructs were cloned in pET-11B expression vectors (except for the TEV-Ectail in pET-24B) and transformed in E. coli strain C41 (Miroux & Walker, 1996) for expression. Cultures were grown in LB with 100 μg mL−1 of ampicillin at 37°C to OD600 0.6-1 and induced by adding 0.5 mM IPTG. Pelleted cells were resuspended in lysis buffer (50 mM Tris pH 7.9, 1 mM EDTA, 10% glycerol), and lysed by sonication. Soluble FtsZ protein was precipitated with 30% ammonium sulfate. Full-length FtsZ was then further purified by chromatography on a source Q 10/10 column (GE Healthcare, Pittsburgh, PA) with a linear gradient of 50-500 mM KCl in lysis buffer. Peak fractions were identified by SDS-PAGE and stored at −80°C. For isolation of the Ct tail, TEV protease was added (0.022 mg mL−1) to the final pellet and incubated at 4°C overnight. C. crescentus linker was then further purified by chromatography on a source Q 10/10 column (50 mM Tris pH 7.9, 1 mM EDTA, 10 % glycerol, 50-500 mM KCl). Peak fractions were identified by SDS-PAGE. E. coli Ct tail samples were dialyzed in 20 mM Tris pH 7.4, 300 mM KCl and bound to a column of TALON Metal Affinity Resin (Clontech Laboratories, Inc). Elution from the column was achieved by adding 150 mM imidazole. The sample was then dialysed into 50 mM CAPS pH 10, 1 mM EDTA, and 10% glycerol (CAPS buffer – the high pH was used to enhance binding to the column) before chromatography on resource Q with a linear gradient of 50-500 mM KCl in CAPS buffer. All samples were further concentrated and buffer exchanged using Amicon Ultra Centrifugal Filters Ultracel- 3K or 10k (Millipore). The concentration of the EcFtsZ Ct tail was determined by OD280 from the single inserted trp. Otherwise, bicinchoninic acid assay (Pierce, Rockford, IL.) was used to measure protein concentration of full-length FtsZ, correcting for the 0.75 reduced color of EcFtsZ relative to the bovine serum albumin standard (Lu et al., 1998).
Circular Dichroism
An AVIV 202 spectropolarimeter with 1 mm cells was used to record CD spectra. Far-UV CD spectra were collected over a range of 204-260 nm. Data were collected each 1 nm and averaged over 2 acquisitions. The sample was held at 25°C. Isolated E. coli Ct tail at 7.2 μM and isolated C. crescentus Ct tail chimera at 3.4 μM were in 10 mM sodium phosphate buffer (pH 7.4), 190 mM NaF, and 2 mM KF. Wavelength scans were corrected for buffer contributions and converted to molar ellipticity. [θ]222 was monitored as a function of temperature from 4°C to 98°C with 2°C steps and 1 nm bandwidth. Samples were allowed 2 minutes to equilibrate. Data were averaged over 2 repeats.
Trypsin Digestion and Mass Spectrometry
Proteolysis was performed in HMK100 buffer (50 mM Hepes pH 7.7, 5 mM MgAc, and 100 mM KAc) with 1 μg μL−1 of E. coli FtsZ, or the chimera of B. subtilis Ct tail. Proteomics grade trypsin from porcine pancreas (Sigma) was added at a 500:1 FtsZ:trypsin ratio (2 ng μL−1 of trypsin) in a total volume of 50 μL. Trypsin digestion was at room temperature for 10 minutes. The reaction was stopped by adding 2 μL of 10% trifluoroacetic acid (TFA). Proteolyzed peptides were desalted and placed in 0.1% TFA using ZipTip C18 resin (Millipore) prior to electrospray MS. MS/MS mass analysis of the ZipTip purified peptides was performed on a 6520 Accurate-Mass Q-TOF LC/MS (Agilent Technologies). The program Spectrum Mill MS Proteomics was used to analyze tryspin cleavage sites (http://spectrummill.mit.edu/).
Electron microscopy
Several linker constructs were selected for analysis of in vitro assembly by electron microscopy. Expression proteins for these were prepared and purified as described for wild type E. coli FtsZ (as described above). 2 μM FtsZ in assembly buffer (50 mM HEPES, pH 7.5, 100 mM KCl, 5 mM MgAc) was induced to assemble by addition of GTP to 0.5 mM. After two minutes at room temperature, negatively stained specimens were prepared on UV treated carbon films as described previously (Milam et al., 2012). Uranyl acetate was applied as quickly as possible, within a few seconds after the protein was applied to the carbon film, since we noted that longer times led to continued accumulation of protofilaments, and formation of some bundles.
Acknowledgements
Supported by NIH grant R01 GM66014 to HPE and F31 GM090677 to KAJAG.
List of Abbreviations
- FtsZ
filamentous temperature senstive Z
- Ct
C-terminal
- aa
amino acid(s)
- IDP
intrinsically disordered peptide
- wt
wild-type
- CD
circular dichroism
- MS/MS
tandem mass spectrometry
- SDS-PAGE
sodium dodecyl sulfate – polyacrylamide gel electrophoresis
- OD
optical density
- IPTG
isopropyl β-D-1-thiogalactopyranoside
- EDTA
ethylenediaminetetraacetic acid
- CAPS
N-cyclohexyl-3-aminopropanesulfonic acid
- TEV
tobacco etch virus
- Hepes
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- wlc
worm-like chain
- PBST
phosphate buffered saline with 0.05% Tween 20
References
- Adams DW, Errington J. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol. 2009;7:642–653. doi: 10.1038/nrmicro2198. [DOI] [PubMed] [Google Scholar]
- Addinall SG, Bi EF, Lutkenhaus J. FtsZ ring formation in fts mutants. J Bacteriol. 1996;178:3877–3884. doi: 10.1128/jb.178.13.3877-3884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske PJ, Levin PA. Extreme C terminus of bacterial cytoskeletal protein FtsZ plays fundamental role in assembly independent of modulatory proteins. J Biol Chem. 2012;287:10945–10957. doi: 10.1074/jbc.M111.330324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante C, Marko JF, Siggia ED, Smith S. Entropic elasticity of lambda-phage DNA. Science. 1994;265:1599–1600. doi: 10.1126/science.8079175. [DOI] [PubMed] [Google Scholar]
- Cordell SC, Robinson EJ, Lowe J. Crystal structure of the SOS cell division inhibitor SulA and in complex with FtsZ. Proc Natl Acad Sci U S A. 2003;100:7889–7894. doi: 10.1073/pnas.1330742100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai K, Lutkenhaus J. ftsZ is an essential cell division gene in Escherichia coli. J Bacteriol. 1991;173:3500–3506. doi: 10.1128/jb.173.11.3500-3506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer PA. Advances in understanding E. coli cell fission. Curr Opin Microbiol. 2010;13:730–737. doi: 10.1016/j.mib.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz H, Rief M. Exploring the energy landscape of GFP by single-molecule mechanical experiments. Proc Natl Acad Sci U S A. 2004;101:16192–16197. doi: 10.1073/pnas.0404549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson HP, Anderson DE, Osawa M. FtsZ in Bacterial Cytokinesis: Cytoskeleton and Force Generator All in One. Microbiol Mol Biol Rev. 2010;74:504–528. doi: 10.1128/MMBR.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gast K, Damaschun H, Eckert K, Schulze-Forster K, Maurer HR, Muller-Frohne M, Zirwer D, Czarnecki J, Damaschun G. Prothymosin alpha: a biologically active protein with random coil conformation. Biochemistry. 1995;34:13211–13218. doi: 10.1021/bi00040a037. [DOI] [PubMed] [Google Scholar]
- Hale CA, De Boer PAJ. Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E-coli. Cell. 1997;88:175–185. doi: 10.1016/s0092-8674(00)81838-3. [DOI] [PubMed] [Google Scholar]
- Hughes CA, Bennett V. Adducin: a physical model with implications for function in assembly of spectrin-actin complexes. J Biol Chem. 1995;270:18990–18996. doi: 10.1074/jbc.270.32.18990. [DOI] [PubMed] [Google Scholar]
- Kriwacki RW, Hengst L, Tennant L, Reed SI, Wright PE. Structural studies of p21Waf1/Cip1/Sdi1 in the free and Cdk2-bound state: conformational disorder mediates binding diversity. Proc Natl Acad Sci U S A. 1996;93:11504–11509. doi: 10.1073/pnas.93.21.11504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy DJ, Aukhil I, Erickson HP. 2.0 Å crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84:155–164. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- Lu C, Stricker J, Erickson HP. FtsZ from Escherichia coli, Azotobacter vinelandii, and Thermotoga maritima - quantitation, GTP hydrolysis, and assembly. Cell Motil Cytoskel. 1998;40:71–86. doi: 10.1002/(SICI)1097-0169(1998)40:1<71::AID-CM7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J, Pichoff S, Du S. Bacterial cytokinesis: From Z ring to divisome. Cytoskeleton (Hoboken) 2012;69:778–790. doi: 10.1002/cm.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin W, Corbo JC, Long SR. Cloning and characterization of a Rhizobium meliloti homolog of the Escherichia coli cell division gene ftsZ. Journal of Bacteriology. 1991;173:5822–5830. doi: 10.1128/jb.173.18.5822-5830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam SL, Osawa M, Erickson HP. Negative-Stain Electron Microscopy of Inside-Out FtsZ Rings Reconstituted on Artificial Membrane Tubules Show Ribbons of Protofilaments. Biophys J. 2012;103:59–68. doi: 10.1016/j.bpj.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingorance J, Rivas G, Velez M, Gomez-Puertas P, Vicente M. Strong FtsZ is with the force: mechanisms to constrict bacteria. Trends Microbiol. 2010;18:348–356. doi: 10.1016/j.tim.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Miroux B, Walker JE. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol. 1996;260:289–298. doi: 10.1006/jmbi.1996.0399. [DOI] [PubMed] [Google Scholar]
- Mosyak L, Zhang Y, Glasfeld E, Haney S, Stahl M, Seehra J, Somers WS. The bacterial cell-division protein ZipA and its interaction with an FtsZ fragment revealed by X-ray crystallography. EMBO J. 2000;19:3179–3191. doi: 10.1093/emboj/19.13.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser AF, Badilla-Fernandez C, Carrion-Vazquez M, Fernandez JM. The mechanical hierarchies of fibronectin observed with single-molecule AFM. J Mol Biol. 2002;319:433–447. doi: 10.1016/S0022-2836(02)00306-6. [DOI] [PubMed] [Google Scholar]
- Oberhauser AF, Marszalek PE, Erickson HP, Fernandez JM. The molecular elasticity of the extracellular matrix protein tenascin. Nature. 1998;393:181–185. doi: 10.1038/30270. [DOI] [PubMed] [Google Scholar]
- Ohashi T, Galiacy SD, Briscoe G, Erickson HP. An experimental study of GFP-based FRET, with application to intrinsically unstructured proteins. Protein Sci. 2007;16:1429–1438. doi: 10.1110/ps.072845607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T, Hale CA, De Boer PA, Erickson HP. Structural Evidence that the P/Q Domain of ZipA Is an Unstructured, Flexible Tether between the Membrane and the C-Terminal FtsZ-Binding Domain. J Bacteriol. 2002;184:4313–4315. doi: 10.1128/JB.184.15.4313-4315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Anderson DE, Erickson HP. Reconstitution of contractile FtsZ rings in liposomes. Science. 2008;320:792–794. doi: 10.1126/science.1154520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Erickson HP. Probing the domain structure of FtsZ by random truncation and insertion of GFP. Microbiology. 2005;151:4033–4043. doi: 10.1099/mic.0.28219-0. [DOI] [PubMed] [Google Scholar]
- Osawa M, Erickson HP. FtsZ from divergent foreign bacteria can function for cell division in Escherichia coli. J Bacteriol. 2006;188:7132–7140. doi: 10.1128/JB.00647-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Erickson HP. Inside-out Z rings - constriction with and without GTP hydrolysis. Molec Microbiol. 2011;81:571–579. doi: 10.1111/j.1365-2958.2011.07716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redick SD, Stricker J, Briscoe G, Erickson HP. Mutants of FtsZ Targeting the Protofilament Interface: Effects on Cell Division and GTPase Activity. J Bacteriol. 2005;187:2727–2736. doi: 10.1128/JB.187.8.2727-2736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivetti C, Guthold M, Bustamante C. Scanning force microscopy of DNA deposited onto mica: equilibration versus kinetic trapping studied by statistical polymer chain analysis. J Mol Biol. 1996;264:919–932. doi: 10.1006/jmbi.1996.0687. [DOI] [PubMed] [Google Scholar]
- Romero P, Obradovic Z, Li X, Garner EC, Brown CJ, Dunker AK. Sequence complexity of disordered protein. Proteins. 2001;42:38–48. doi: 10.1002/1097-0134(20010101)42:1<38::aid-prot50>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Stricker J, Erickson HP. In vivo characterization of Escherichia coli ftsZ mutants: effects on Z-ring structure and function. J Bacteriol. 2003;185:4796–4805. doi: 10.1128/JB.185.16.4796-4805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwedziak P, Wang Q, Freund SM, Lowe J. FtsA forms actin-like protofilaments. EMBO J. 2012;31:2249–2260. doi: 10.1038/emboj.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lutkenhaus J. The FtsZ protein of Bacillus subtilis is localized at the division site and has GTPase activity that is dependent upon FtsZ concentration. Mol.Microbiol. 1993;9:435–442. doi: 10.1111/j.1365-2958.1993.tb01705.x. [DOI] [PubMed] [Google Scholar]
- Yang G, Cecconi C, Baase WA, Vetter IR, Breyer WA, Haack JA, Matthews BW, Dahlquist FW, Bustamante C. Solid-state synthesis and mechanical unfolding of polymers of T4 lysozyme. Proc Natl Acad Sci U S A. 2000;97:139–144. doi: 10.1073/pnas.97.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]









