Abstract
Estradiol has potent favorable effects on brain function and behavior in animals while in human trials, the results are inconsistent. A number of potential mediating variables influencing response to estradiol have been proposed to account for this variability, one of which includes stress. We conducted a placebo-controlled study to examine joint and independent effects of estradiol and elevated levels of the stress hormone cortisol on cognition and biomarkers of aging and neurodegenerative disease. Thirty-nine healthy postmenopausal women (56-84 yrs) received 0.10 mg/d of transdermal 17β-estradiol (E2) or placebo for eight weeks. During the last four days of the trial, subjects also received 90 mg/d (30 mg TID) of oral hydrocortisone (CORT) to induce stress-level elevations in cortisol, or a matched placebo. The four groups thus included Placebo (placebo patch/placebo pill), CORT-alone (placebo patch/hydrocortisone), E2-alone (estradiol patch/placebo pill), and E2+CORT (estradiol patch/hydrocortisone). Eight weeks of E2 increased plasma estradiol by 167%, and four days of CORT increased plasma cortisol by 119%. Overall, E2 had favorable effects on verbal memory (p=0.03), working memory (p=0.02), and selective attention (p=0.04), and the magnitude of these effects was attenuated for E2+CORT. E2-alone and E2+CORT had opposing effects on plasma levels of the amyloid-β (Aβ) biomarker (Aβ40/42 ratio, p<0.05), with the more favorable response observed for E2-alone. CORT-induced increases in insulin-like growth factor-1 were blunted by E2 co-administration. Our findings indicate that cognitive and physiological responses to estradiol are adversely affected by elevated stress hormone levels of cortisol in healthy postmenopausal women.
Keywords: estrogen, stress, HPA, cognition, executive function, memory, Aβ, amyloid, IGF, postmenopausal
1. Introduction
Estrogen effects on brain structure and function have been studied extensively, and the results of in vitro and animal studies provide unequivocal support for its potent and favorable influence on neuroprotection, neurotransmission, synaptic remodeling, and neurotrophic activity including insulin-like growth factor-1 (Garcia-Segura, et al., 2010; McEwen, 2001). In light of these effects, it is no surprise that a number of animal and clinical studies report estrogen-related benefits for cognition as well (Asthana, et al., 2001; Asthana, et al., 1999; Daniel, et al., 2006; Joffe, et al., 2006; Keenan, et al., 2001; Leuner, et al., 2004; Maki, et al., 2001; Sherwin, 2006; Voytko, et al., 2009), though not without controversy (Alhola, et al., 2006; Barrett-Connor and Kritz-Silverstein, 1993; Binder, et al., 2001; Polo-Kantola, et al., 1998). One hallmark trial showing no cognitive benefit of conjugated equine estrogens administered alone or in combination with medroxyprogesterone acetate, the Women’s Health Initiative Memory Study (WHIMS) (Espeland, et al., 2004), has since had widespread consequences for standard of care regarding postmenopausal estrogen use in the United States. The WHIMS has prompted other discussions and subsequent investigations to examine a number of potential moderator variables that might account for their findings and better predict cognitive response including type of estrogen administered, form of delivery, duration and timing of exposure relative to menopause, co-administration of progesterone, extant cardiovascular or neurodegenerative disease, and lifestyle (Asthana, et al., 2009; Loucks and Berga, 2009; Sherwin, 2009).
Stress may serve as another potent moderator of response to estradiol. In an eloquent series of experiments, Shors and colleagues (Hodes and Shors, 2005; Shors, et al., 2001; Shors, et al., 1999; Shors, et al., 2004; Shors, et al., 1998; Wood and Shors, 1998; Wood, et al., 2001) demonstrated a sexually dimorphic stress response linked to estrogen. In rats, an acute stress challenge enhanced performance on an associative memory task for males but impaired performance for females. When females were estrogen-depleted (ovariectomy) or treated with an estrogen antagonist, stress-related impairments in learning were no longer evident. In the brain, although dendritic spine density of hippocampal neurons is greatest when estradiol levels are highest for naturally cycling females (Shors, et al., 2001), spine density decreases when these animals are exposed to an acute stressor (intermittent tail shocks). In contrast, but consistent with the behavioral data, spine density increased for the males in response to the same stress challenge. These findings indicate that in male rats, acute stress has favorable effects on behavior and brain morphology whereas for estradiol-exposed females, the same stressor has deleterious consequences. This innovative body of work supplements the extant literature (Dayas, et al., 2000; Gupta, et al., 2001; Viau and Meaney, 2004; Weiser and Handa, 2009) and provides compelling evidence to suggest that behavioral and biological responsivity to estrogen may be modulated by stress.
Numerous studies suggest that the targeted brain regions for estrogen- and stress-mediated effects on cognition overlap. Estrogen has selective benefits for cognitive abilities supported by the frontal lobes and hippocampus such as executive function and episodic memory (Craig, et al., 2010; Dumas, et al., 2010; Joffe, et al., 2006; Maki and Sundermann, 2009; Wegesin and Stern, 2007) while excess stress can have modulatory effects for the same abilities and brain regions (Bowman, et al., 2002; Gianaros, et al., 2007; Liston, et al., 2009; Pham, et al., 2003; Sapolsky, 2000). Stress effects are mediated by mineralocorticoid and glucocorticoid receptors abundantly distributed in the hippocampus and prefrontal cortex (Diorio, et al., 1993; McEwen, 2007) and stress-related consequences for brain structure and function depend on variables such as dose, duration of exposure, task demand, age, and gender (McEwen, 1998). Although chronic stress typically impairs episodic memory (Lupien, et al., 2005; Schwabe and Wolf, 2010), acute stress can be harmful or beneficial for executive control abilities depending on the specific demands of the task. Increasing stress can enhance performance on tasks of working memory that benefit from increased arousal, yet have a detrimental effect when there are additional attentional demands such as efficient filtering of irrelevant information or set shifting (Lewis, et al., 2008; Liston, et al., 2009).
In humans, several studies point to sexually dimorphic physiological and behavioral response to stress, including differences at the level of the hypothalamus and adrenal responsiveness to adrenocorticotropin hormone (ACTH) (Heuser, et al., 1994; Laughlin and Barrett-Connor, 2000). Despite conflicting reports in the literature about sex differences in stress response (for review, see Kajantie and Phillips, 2006), a frequent finding is that older women have increased hypothalamic-pituitary-adrenal (HPA) axis reactivity to stress that includes higher levels of circulating cortisol, the primary hormone released from the adrenal cortex in response to stress (Otte, et al., 2005). Depression, a condition that can be associated with hypersecretion of stress hormones (Banki, et al., 1987) or increased HPA reactivity to stress (Burke, et al., 2005), is sexually dimorphic with significantly higher prevalence rates in women (Kessler, et al., 1993) and a more pronounced stress-induced physiological response in depressed women vs. depressed men (Chopra, et al., 2009; Young and Ribeiro, 2006). Estrogen therapy can have antidepressant effects in depressed postmenopausal women (Birkhauser, 2002; Klaiber, et al., 1996) and can reduce plasma cortisol levels and blunt HPA reactivity to a stress challenge in healthy nondepressed postmenopausal women (Kudielka, et al., 1999; Pluchino, et al., 2005; Puder, et al., 2001). The MacArthur studies of successful aging report that elevated cortisol levels may also have a sexually dimorphic association with cognition in older adults. In this community-based longitudinal study, greater urinary cortisol excretion was associated with poorer baseline memory performance in women but not men, and change in cortisol excretion over a 2.5 year follow-up period was negatively correlated with memory performance, but again, only for the women (Seeman, et al., 1997).
Women are more likely than men to be diagnosed with Alzheimer’s disease (AD) implicating a role of sex hormones in this disparity (Birge, 1997). Increased Amyloid-β (Aβ) in brain is one well-established neuropathological trademark of the disease, which is presumed to reflect overproduction or impaired degradation and clearance. In cultured neurons, estradiol has numerous salutary effects on Aβ regulation and toxicity (Atwood, et al., 2005; Li, et al., 2000; Marin, et al., 2003) and in animal models, estradiol reverses the negative effects of estrogen depletion on brain Aβ burden (Petanceska, et al., 2000). These favorable Aβ-reducing effects of estradiol contrast with the increase in Aβ burden that occurs following exposure to acute stress (Green, et al., 2006; Kang, et al., 2007) with subsequent deleterious consequences for cognition (Catania, et al., 2009). These opposing effects of estradiol and stress on Aβ burden in animals likely have important consequences for AD-related neuroprotection when both hormones are elevated together. The role of peripheral Aβ in normal and pathological aging is unclear, and not without controversy (Okereke, et al., 2009), but higher plasma Aβ42, lower Aβ40, and thus lower Aβ40/42 ratios have been associated with increased risk of cognitive decline and neurodegenerative disease (Buerger, et al., 2009; Mayeux, et al., 2003).
The results of animal and human studies described above implicate an interactive effect of stress- and estrogen-related neuroendocrine activities, and set the stage for the idea that cognitive and biomarker response to estrogen therapy for postmenopausal women may be modulated by elevated levels of cortisol. To test this hypothesis, we independently manipulated plasma levels of estradiol and cortisol in healthy postmenopausal women, and assessed the separate and joint effects of these hormones on cognition, mood, and the ratio of Aβ40 to Aβ42 (Aβ40/42) concentration in plasma. Treatment-related change in IGF-1 (total, unbound, IGF-binding protein-3) was also examined in light of reports to suggest that estradiol and IGF-I interact to promote neuroprotection (Azcoitia, et al., 1999; Garcia-Segura, et al., 2010) and antagonize damaging effects of adrenal steroids (Darnaudery, et al., 2006). In previous studies, we reported estradiol-related declines in circulating IGF-1 that predicted improved verbal recall in postmenopausal women with AD (Asthana, et al., 2001; Asthana, et al., 1999).
2. Methods
2.1. Study design
Using a randomized double-blind, parallel-group design, healthy postmenopausal women received either 0.10 mg/d of transdermal 17β-estradiol or a placebo skin patch (Climara®, Berlex Laboratories) for eight weeks. Skin patches were changed weekly. During the last four days of the trial, subjects were randomized to also receive 90 mg/d (30 mg TID) of oral hydrocortisone (Hydrocortone®, Merck) or a matched placebo. The resulting four treatment groups included Placebo (placebo patch/placebo pill), CORT-alone (placebo patch/hydrocortisone), E2-alone (estradiol patch/placebo pill), E2+CORT (estradiol patch/hydrocortisone). At the end of the 8-week treatment period, a 10-day progesterone challenge (200 mg/d of oral micronized progesterone, Prometrium®, Abbott Laboratories) was administered to women with an intact uterus who received the active skin patch. At baseline and week 8, cognitive tests were administered, mood was assessed, and blood was collected for assay. To control for time of day effects, all study visits took place between 9 and 11 am.
2.2. Subjects
The study was approved by the University of Washington Institutional Review Board and the Research and Development Committee of the Veterans Affairs Puget Sound Health Care System. Forty-four postmenopausal women with normal cognitive status (determined through neuropsychological testing at screening) provided written informed consent. Three women dropped out of the study prior to the baseline assessment, and thus 41 women were randomized (Placebo, n=11; E2-alone, n=10; CORT-alone, n=10; E2+CORT, n=10). Exclusion criteria included history of reproductive tissue cancer or thrombotic events, conditions affecting glucocorticoid production or clearance, significant medical illness or neurological disease that could affect cognition, and chronic or severe psychiatric illness. Current use of antipsychotic, anti-depressant, anti-convulsant, anti-coagulant, anxiolytic or sedative medication was exclusionary. When applicable, hormone therapy was discontinued two months prior to enrollment.
2.3. Cognitive and mood assessment
The cognitive battery included tests of memory, selective attention, working memory, and word fluency: tests with documented sensitivity to age, elevated stress, and estrogen (Asthana, et al., 2001; Asthana, et al., 1999; Balota, et al., 2010; Bryan and Luszcz, 2001; Meinzer, et al., 2009). Parallel test versions were administered in counterbalanced order at baseline and week 8. Subjects completed a mock testing session prior to the baseline visit to familiarize them with test procedures. Mood was assessed using the Profile of Mood States (POMS), a well-validated self-report inventory (Miller, et al., 2002).
2.3.1. Memory
Short-term memory was assessed with Story Recall, List Learning, and Delayed Match-to-Sample. For Story Recall subjects heard a brief narrative containing 44 informational bits, and were asked to recall as much as possible both immediately and after a 30-min delay. Credit was awarded for verbatim recall and accurate paraphrases. For List Learning, subjects heard a list of 12 words and were asked to recall as many items as possible across three learning trials, and then again after a 20-min delay. Delayed recall scores were subjected to analysis. For Delayed Match-To-Sample, 10 abstract geometric designs were presented in series on a touch screen monitor for 10 s and subjects were instructed to remember each design. Following a 30-min delay, 10 design triplets were displayed one set at a time, and subjects touched the single design per set that was previously studied.
2.3.2. Executive function
Executive function tests of selective attention (Stroop Color-Word Interference test), visual working memory (Self-ordered Pointing Test, SOPT), and semantic access (letter and category Word Fluency) were administered. For the Stroop, adapted for computer administration (Spieler, et al., 1996), color names were displayed on a computer screen, one at a time, in concordant or discordant font colors (e.g., the word “red” displayed in red or green font). Subjects were instructed either to read the word or to name the color as quickly as possible, and voice onset latency (VOL) and content were recorded. In each of four blocks of trials, 25% of the stimuli were concordant for content and color (word “red” printed in red font), but otherwise blocks differed only by task instruction (‘read word’ or ‘name color’). To minimize the influence of working memory on response selection and inhibition, each trial was preceded by a task instruction reminder. This adaptation to the task has been used previously to examine executive control processes in older adults (Baker, et al., 2010a; Baker, et al., 2010b). Mean VOL and total errors committed during ‘name color’ interference trials were analyzed. For the SOPT (Petrides and Milner, 1982), a multi-design array was presented on a touchscreen computer, and subjects were instructed to touch each design only once. Following each touch, the designs were randomly rearranged within the array. Subjects completed three trial blocks with a single 10-item display and number of errors was recorded. Word Fluency was measured by the total number of words generated across four 60 s trials. For this test, subjects listed words beginning with a specified letter of the alphabet in the first two trials, and exemplars of specified semantic categories (e.g., animals) for the remaining two trials.
2.4. Blood assays
Estradiol levels were measured using a direct estrogen double-antibody radioimmunoassay (RIA), with a detection limit of 10 pg/mL (DSL-4400, Beckman Coulter, Webster TX). At a mean measured value of 67 pg/mL, the intra-assay coefficient of variation (c.v.) was 5% and the inter-assay c.v. was 8%. This direct method of measurement was used to confirm correct treatment group assignment and typically results in higher levels compared to other methods that include an extraction step. Cross-reactivity was 100% for 17β-estradiol, 3.4% for estrone, 0.75% for estriol, and less than 0.5% for all other naturally occurring steroids tested. Reference values for this assay are 53–258 pg/ml (median = 115 pg/ml) for women in the follicular phase of the natural menstrual cycle, and 15–66 pg/ml (median = 39 pg/ml) for men. Reference values for postmenopausal women, provided for a similar assay kit (DSL-4800, Beckman Coulter, Webster TX), range from 6–103 pg/ml.
Cortisol was measured using RIA of unextracted plasma (MP Biomedicals, Orangeburg NY). Samples were diluted 1:100 with phosphate buffer and heated for 20 min at 80 C to denature binding globulins, and detection limit was 10 ng/mL (0.01 ng/mL per assay tube) and intra-assay c.v. was 5.6%. Cortisol-binding globulin (CBG) was measured by RIA in a 1:25 dilution of plasma (BioSource Europe S.A., Nivelles, Belgium), and detection limit was 6.25 μg/mL (0.25 μg/mL per assay tube) and intra-assay c.v. was 2.0%.
Aβ40 levels were measured by sandwich ELISA using a kit with 6E10-coated plates and a biotinylated anti-Aβ40 detection antibody (Signet Laboratories, Dedham MA). Aβ42 levels were measured using 6E10 as a capture antibody (1.5 μg/well; Signet Laboratories, Dedham MA) and biotinylated anti-Aβ42 for detection as previously described (Mehta, et al., 2000). Detergents were added to the plasma at final concentrations of 0.05% Tween-20 (both assays) and 1% sodium dodecyl sulfate (Aβ40 assay only) to improve detection of Aβ from plasma. Horseradish peroxidase–avidin was used for Aβ detection, and ELISAs were developed with tetramethylbenzidene as a peroxidase substrate. Initial reaction rate was determined by absorbance measured every 15 seconds at 650 nm, and the limit of detection for both assays was 10 pg/mL.
Acid-ethanol extraction of IGF-I was performed before quantification using RIA. The intra-assay c.v. was 4.7% for total IGF-I, 9.4% for free IGF-I and 10.2% for IGF binding protein-3 (IGFBP-3), and the inter-assay c.v. was 11.6% for total IGF-I, 15.8% for unbound IGF-I and 15.2% for IGFBP-3. To permit the assessment of elevated circulating cortisol controlling for cortisol-induced increases in insulin, plasma insulin levels were quantified using RIA as previously described (Craft, et al., 1999). All samples were run in duplicate in a single assay.
2.5. Statistical analysis
The general analytic strategy was first to residualize week 8 data from baseline using multiple regression and correlation (MRC) procedures to create scores that reflect change due to treatment, controlling for baseline differences. For compliance measures (plasma estradiol, cortisol), separate two-way (E2-treatment, CORT-treatment) univariate analyses of variance (ANOVA) were performed. The cognitive data were subjected to similarly structured multivariate analyses of variance (MANOVAs) by cognitive domain on the specified outcome measures of delayed recall and executive function. Mood was assessed using a two-way ANOVA. The analytic model used to examine the primary biomarker outcomes of Aβ40/42 ratio and total IGF-1 was the same as that used for the cognitive outcomes (2 by 2 MANOVA). Potential treatment effects on plasma levels of free (unbound) IGF-1, IGFBP-3, and insulin were examined in exploratory analyses using ANOVA. For all analyses, age, education, and body mass index (BMI) were statistically considered as covariates. When a MANOVA proved significant at the 0.05 level, group differences for each outcome measure were further examined using ANOVA and t-tests. To assess associations between treatment-related changes in biomarkers and cognitive outcomes, MRC analyses were conducted on residualized week 8 values. Missing values (Aβ data unavailable for n=1) were handled using case-wise deletion.
3. Results
3.1. Subjects and compliance
Thirty-nine subjects completed the study as two women failed to complete the 8-week assessment (n=1 Placebo with CVA, n=1 E2-alone with breakthrough bleeding). The four groups were comparable with respect to age, education, body mass index (BMI), history of hormone therapy, and hysterectomy status at study outset (p>0.47, Table 1). Plasma hormone concentrations at baseline and week 8 are provided in Table 2. Estradiol levels were elevated for women who received the active patch (main effect of E2-tx, F1,35=72.7, p=0.00001), achieving concentrations similar to those observed during the follicular phase of the menstrual cycle in younger women. Levels rose by 144% for E2-alone and by 191% for E2+CORT: a group-wise difference that did not reach statistical significance (p=0.23). Endogenous estradiol levels decreased from baseline by 20% for the CORT-alone group. Four days of hydrocortisone administration elevated plasma cortisol levels for both CORT-treated groups (main effect of CORT-tx, F1,35=20.5, p=0.0001), rising by 127% for CORT-alone and 110% for E2+CORT: a group-wise difference that was not statistically significant (p=0.37). The cortisol levels achieved in our study fell within the physiologic range for older adults (Schoorlemmer, et al., 2009). CBG concentration dropped for CORT-treated subjects (main effect of CORT-tx, F1,35=4.7, p=0.04) and for E2-treated subjects (main effect of E2-tx, F1,35=8.4, p=0.007), but not disproportionately so for women who received the combined regimen of E2+CORT (no E2-tx by CORT-tx interaction, p=0.85).
Table 1.
Subject characteristics by treatment group at study entry
| Placebo | CORT - alone | E2 - alone | E2 + CORT | |
|---|---|---|---|---|
| Subjects, no. | 10 | 10 | 10 | 9 |
| Requiring washout, no. | 4 | 6 | 3 | 2 |
| Hysterectomy, no. | 7 | 5 | 4 | 4 |
|
| ||||
| Age, yrs | 70.2 (8.2) | 71.7 (8.0) | 74.1 (8.9) | 71.2 (4.5) |
| Education, yrs | 13.8 (2.7) | 14.5 (2.7) | 15.5 (1.9) | 14.2 (2.6) |
| Body mass index, m2/kg | 27.7 (3.5) | 28.6 (7.5) | 27.0 (3.4) | 28.7 (6.0) |
Means (SD) are provided unless otherwise indicated. Abbreviations: Subjects = no. of completers per treatment group; Requiring washout = no. of subjects per treatment group requiring a 2-month washout prior to study entry; Hysterectomy = no. of hysterectomized subjects per treatment group at the time of study entry; Age = age in years at time of study entry; Education = number of years of formal education completed; Body mass index = body mass calculation based on height (m2) and weight (kg). There were no baseline differences between treatment groups for any of the variables listed (p>0.47).
Table 2.
Hormone levels by treatment group at baseline and week 8
| Hormone | Wk | Placebo | CORT-alone | E2-alone | E2+CORT | Reference |
|---|---|---|---|---|---|---|
| Estradiol, pg/mL1 | 0 | 46.9 (10.2) | 49.3 (11.6) | 51.5 (4.9) | 53.6 (10.3) | 6 – 103 |
| 8 | 44.2 (9.1) | *39.2 (11.1) | *125.7 (52.2) | *156.2 (32.3) | ||
|
| ||||||
| Total cortisol, μM2 | 0 | 0.26 (0.06) | 0.22 (0.06) | 0.27 (0.11) | 0.20 (0.05) | 0 – .70 |
| 8 | 0.25 (0.08) | *0.50 (0.22) | 0.22 (0.04) | *0.42 (0.18) | ||
|
| ||||||
| CBG, μM1,2 | 0 | 0.88 (0.17) | 0.91 (0.14) | 0.98 (0.10) | 0.90 (0.22) | 0.77 – 2.96 |
| 8 | 0.89 (0.14) | †0.85 (0.15) | *0.89 (0.15) | *0.75 (0.23) | ||
|
| ||||||
| Free IGF-1, ng/mL2 | 0 | 0.73 (0.25) | 0.85 (0.49) | 0.75 (0.22) | 0.87 (0.27 | — |
| 8 | 0.69 (0.25) | *1.33 (0.62) | 0.71 (0.18) | *1.13 (0.33) | ||
|
| ||||||
| IGFBP-3, μg/mL2 | 0 | 4.9 (1.6) | 5.3 (1.3) | 5.4 (1.5) | 5.1 (1.9) | 2.5 – 6.6 |
| 8 | 5.4 (1.5) | †4.6 (1.0) | 5.0 (1.5) | 3.9 (0.9) | ||
|
| ||||||
| Total IGF-1, ng/L3 | 0 | 89.3 (43.3) | 121.4 (24.6) | 135.0 (92.3) | 109.0 (33.8) | 30 – 228 |
| 8 | 95.0 (44.1) | *178.8 (36.3) | 110.9 (51.2) | 117.1 (32.6) | ||
|
| ||||||
| Insulin, μU/mL3 | 0 | 11.7 (4.4) | 17.3 (10.6) | 12.3 (2.8) | 12.9 (2.8) | 1 – 58 |
| 8 | 11.7 (4.3) | *21.2 (9.2) | 13.0 (2.7) | 14.1 (3.3) | ||
Means (SD) at week 0 (baseline) and week 8. Abbreviations: Wk = week; CBG = cortisol-binding globulin; IGF = insulin-like growth factor; IGFBP-3 = insulin-like growth factor binding protein – 3. Reference ranges for E2 (for postmenopausal women), total cortisol, and CBG were provided by the manufacturer of the assay kits, older adult ranges for IGFBP-3 and total IGF-1 were based on levels reported in a large cohort study (Friedrich, et al., 2008), and range for insulin was based on levels observed in a large nondiabetic adult population (Yeni-Komshian, et al., 2000). Reliable reference values for free (unbound) IGF-1 were not available. At baseline, there were no differences between treatment groups (ANOVA, p>0.17). After treatment, change in estradiol levels did not differ across E2-treated groups (p=0.23), and change in cortisol levels did not differ across CORT-tx groups (p=0.37). Relative to Placebo, fasting plasma concentrations of total IGF-I and insulin increased for CORT-alone (p<0.001) but not E2+CORT (p>0.30), and total IGF-1 trended down for E2-alone relative to E2+CORT (p=0.09).
ANOVA, main effect of E2-tx;
ANOVA, main effect of CORT-tx;
ANOVA, E2-tx by CORT-tx interaction
p<0.05;
p<0.10: change from week 0 to week 8
3.2. Cognition and mood
Eight weeks of E2 improved delayed recall (MANOVA, main effect of E2-tx, F3,30=3.0, p=0.04). Subsequent univariate analyses indicated that Story Recall was the primary contributor to this finding: retention or “savings” of encoded information improved for E2-treated women (main effect of E2-tx, F1,35=5.2, p=0.03, Fig. 1A). Post-hoc ANOVAs were conducted to examine the relative contribution of each E2-treated group; when E2+CORT was omitted from the analysis the results were robust (F1,28=5.0, p=0.03, Cohen’s f=0.42), whereas when E2-alone was omitted the effect was attenuated (F1,27=2.1, p=0.16, Cohen’s f=0.28). In addition, the results of pairwise comparisons using t-tests indicated that memory performance improved over baseline for E2-alone (p=0.05), but not for E2+CORT (p=0.48). Ceiling effects on List learning and floor effects on Delayed Match-to-Sample likely reduced their contribution to the omnibus analysis.
Figure 1.
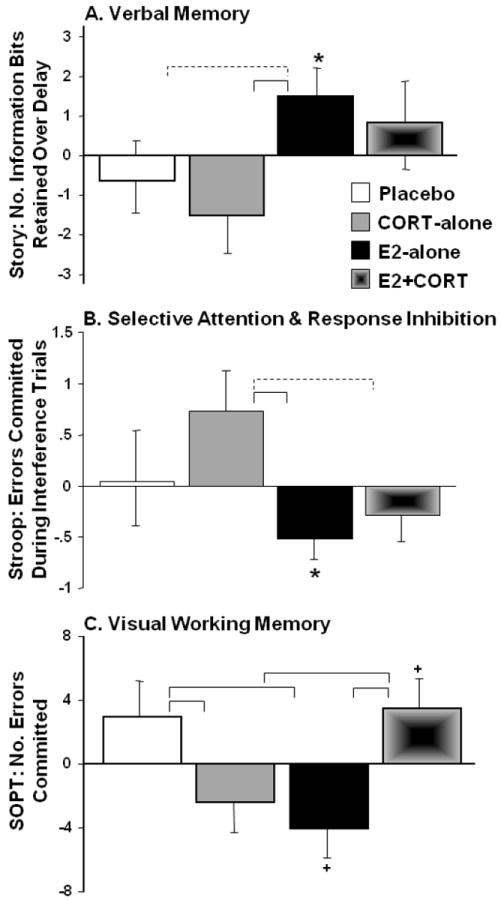
Mean (SE) change at week 8 vs. baseline, expressed as residual values, in cognitive performance across the four treatment arms for (A) Verbal memory (Story Recall, N=39), (B) Selective attention and response inhibition (Stroop, N=39) and (C) Visual working memory (Self-Ordered Pointing Test, SOPT, N=35; data loss for 1 sub/group). Brackets indicate group differences in treatment response (solid, p<0.05; dashed, p<0.10, 2-tailed) and symbols indicate change from baseline (*p<0.05; +p<0.10, 2-tailed).
For executive function, the MANOVA indicated that E2+CORT had a different effect than either E2- or CORT-alone (E2-tx by CORT-tx interaction, F5,29=5.5, p=0.004). Selective attention as measured by Stroop performance (errors on interference trials) improved with E2 (main effect of E2-tx, F1,35=4.6, p=0.04, Fig. 1B). The results of post-hoc ANOVAs to examine the relative contribution of the two E2-treated groups indicated that the effect was larger for E2-alone (F1,28=3.4, p=0.07, Cohen’s f=0.35) than for E2+CORT (F1,27=1.7, p=0.20, Cohen’s f=0.25). In addition, the number of Stroop errors reliably decreased from baseline to week 8 for E2-alone (p=0.03) but not E2+CORT (p=0.26). Visual working memory (SOPT errors) differed across treatment groups (E2-tx by CORT-tx interaction, F1,31=13.3, p=0.001, Fig. 1C), and the results of pairwise comparisons vs. Placebo indicated improved performance for E2-alone (t16=2.6, p=0.02) and for CORT-alone (t16=2.4, p=0.03), but not for E2+CORT (t15=-0.06, p=0.95). Word Fluency (letter and category combined) was not affected by E2 or CORT administration. Although the overall analysis of mood as measured by total score on the POMS failed to indicate a treatment effect, when one outlier was removed (wk 8 score > 3 SD of the group mean), the results indicated similar improvements in mood for both E2-treated groups (main effect of E2-tx, F1,34=6.9, p=0.01; when E2-alone omitted: p=0.06; when E2+CORT omitted: p=0.06). The results for Story Recall, Stroop, and SOPT remained unchanged when adjusted for treatment-related changes in mood. For analyses of cognitive outcomes, age, education, and BMI were non-contributory.
3.3. Biomarkers in plasma
E2+CORT had different effects on plasma concentrations of Aβ40/42, and total IGF-1 than either E2- or CORT-alone (MANOVA, E2-tx by CORT-tx interaction, F2,32=5.8, p=0.007). The subsequent univariate analysis of Aβ40/42 indicated that this ratio differed for the two E2-treated groups (E2-tx by CORT-tx interaction, F1,33=4.28, p=0.05, Fig. 2), tending to move in a more favorable upward direction at week 8 relative to baseline for E2-alone (p=0.09) while dropping for E2+CORT (p=0.03). Separate analyses by Aβ species indicated that while Aβ42 levels tended to increase over baseline with CORT administration (p=0.08), Aβ40 levels increased for E2-alone (p=0.0001) and decreased for E2+CORT (p=0.02). As a consequence, treatment effects on Aβ40/42 were most pronounced for E2+CORT (lower Aβ40/higher Aβ42).
Figure 2.
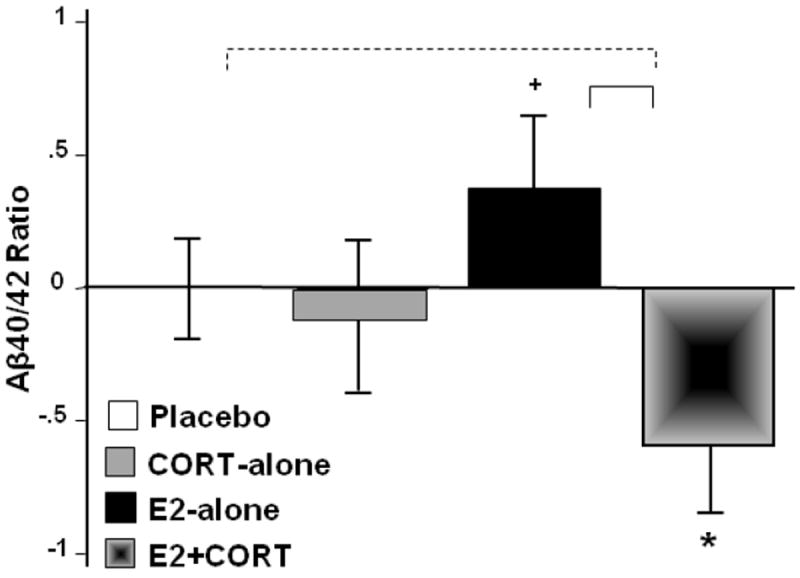
Mean (SE) change at week 8 relative to baseline, expressed as residual values, in fasting plasma concentrations of Aβ40 (pg/mL) to Aβ42 (pg/mL) ratio (N=37; missing data for 1 sub/CORT-tx group) across the four treatment arms. Brackets indicate group differences in treatment response (solid, p<0.05; dashed, p<0.10, 2-tailed) and symbols indicate change from baseline (*p<0.05; +p<0.10, 2-tailed).
The cortisol-induced rise in plasma concentration of total IGF-I was blocked when estradiol levels were elevated (E2-tx by CORT-tx interaction, F1,35=6.2, p=0.02, Table 2). IGF-I levels differed for the two CORT-treated groups (p=0.0004), increasing for CORT-alone (vs. Placebo, p=0.0001) but not E2+CORT (vs. Placebo, p=0.31). In addition, total IGF-1 levels tended to be lower for E2-alone relative to E2+CORT (p=0.09). Consistent with our findings from a previous study (Asthana, et al., 2001; Asthana, et al., 1999), treatment-related changes in estradiol and IGF-I levels for the group as a whole were negatively correlated (r=-0.40, p=0.01), and lower IGF-I levels tended to predict better verbal memory performance (r=-0.28, p=0.08). For CORT-treated subjects, free (unbound) IGF-I levels increased (main effect of CORT-tx, F1,35=23.9, p=0.00001) and IGFBP-3 levels decreased (main effect of CORT-tx, F1,35=6.5, p=0.02) (Table 2).
Exploratory analyses of treatment effects on fasting plasma insulin indicated that the cortisol-induced rise was blocked by E2 (E2-tx by CORT-tx interaction, F1,33=4.1, p=0.05). BMI was included as a covariate in this analysis given that it tended to vary with insulin levels at baseline (r=0.30, p=0.07). As expected, change in plasma concentrations of cortisol and insulin were positively correlated for all subjects (r=0.64, p=0.00001). Although the pattern of results (mean values) observed for insulin and IGF-I were similar (Table 2), these measures were correlated only for subjects who did not receive E2 (r=0.52, p=0.02). When change in insulin was included as a covariate in the analyses of cognitive outcomes, none of the E2-related effects were altered. That is, cortisol-induced increases in insulin did not account for the effects observed in the E2+CORT group. When the analysis of SOPT was adjusted for change in insulin, favorable treatment effects for the CORT-alone group were no longer apparent (p=0.32) suggesting that benefits for this group on task performance were likely attributable to increased insulin rather than increased cortisol per se.
4. Discussion
We examined the independent and combined effects of E2 and CORT administration on cognition, Aβ, and IGF-1 in healthy postmenopausal women. Eight weeks of unopposed transdermal E2 increased verbal retention on Story Recall and improved performance on the Stroop, and these effects were more pronounced for women who received E2-alone. E2-alone also improved working memory performance on the SOPT; this benefit was blocked for women who received E2+CORT. E2 administered with and without CORT had opposing effects on the Aβ biomarker in plasma (Aβ40/42) with the more favorable response observed with E2-alone. Although four days of CORT increased fasting levels of IGF-1, these effects were attenuated by E2. Together, these findings implicate an interactive effect of estradiol and cortisol on cognitive and biological processes, and suggest that the favorable cognitive effects of estradiol therapy may be modulated by elevated levels of plasma cortisol.
In our study, we observed E2-related improvements in verbal episodic memory, visual working memory, and selective attention for older postmenopausal women, consistent with reports of others (Dumas, et al., 2010; Keenan, et al., 2001; Maki and Resnick, 2000; Smith, et al., 2006). We are the first to show however that when plasma concentration of cortisol is exogenously elevated modeling high levels of physiological stress in these women, the estrogen-related benefit is altered. This detrimental effect of E2+CORT relative to E2-alone was predicted in light of the animal work by Shors and colleagues described above showing that favorable effects of estradiol on trace conditioning, a prefrontal cortex-dependent task (Gilmartin and Helmstetter, 2010), could be reduced or abolished by an acute stress challenge.
Our results are consistent with a recent study in which cognition was impaired when a stress challenge was paired with estradiol (Newhouse, et al., 2010). In this randomized trial, nondemented postmenopausal women pretreated with three months of oral estradiol (comparable in dose to that administered in our study) or placebo received a monoamine depletion challenge, the Trier Social Stress Test (TSST), and cognitive testing on three separate days. Following the TSST, estradiol-treated women performed worse on tests of simple attention, psychomotor speed, and select tasks of episodic verbal memory. Cognitive outcomes were largely unaffected by monoamine depletion. Mood disturbance, measured using the POMS at three time points within a 7-hour period, increased for estradiol-treated subjects after the monoamine depletion and TSST. In our study, mood improved with E2-treatment, with or without elevated cortisol. However, our participants completed the POMS at baseline and at the end of the 8-week trial, four days after cortisol levels were abruptly elevated.
One interpretation of the Newhouse et al. data is that estradiol blunted stress-induced enhancements on tasks that benefit from increased arousal, including those of simple attention and psychomotor speed. Although Newhouse et al. did not measure cortisol until at least 90 minutes post-TSST when levels typically return to baseline under comparable conditions (Kudielka, et al., 1999), other studies that examined the time course of stress-related changes in blood chemistry demonstrated clear estradiol-blunting effects on stress hormones and catecholamines (Ceresini, et al., 2000; Komesaroff, et al., 1999; Kudielka, et al., 1999). Any further comparisons between our and the Newhouse et al. study are limited by important methodological differences relating to a longer-lasting and exogenous manipulation of cortisol, and the inclusion of an E2-alone group in our study that allowed us to examine E2 effects with and without a stress challenge. Nonetheless, together these results provide additional support for an interactive effect of estradiol and physiological stress on cognitive function in postmenopausal women.
Cortisol attenuated the favorable estrogenic effects on verbal episodic memory (retention) and selective attention, and neutralized estradiol-related improvements in visual working memory. These results suggest that cortisol may also blunt or block the actions of estradiol in the brain. The pattern of results for visual working memory differed slightly from that observed for the other cognitive tasks such that estradiol-related improvement was reversed altogether when cortisol levels were elevated for the E2+CORT group. For the CORT-alone group, improvement on this task was attributable to increased insulin rather than to direct effects of elevated circulating cortisol. Nonetheless, our findings are consistent with the animal work of Wood and Shors (Wood and Shors, 1998) who demonstrated that associative learning, supported by brain regions that also contribute to working memory (Kronforst-Collins and Disterhoft, 1998), benefits from stress in the absence of estradiol or from estradiol in the absence of stress but under conditions of estradiol plus stress, performance deteriorates. Elevated stress hormone-related enhancements in working memory, a task that benefits from increased arousal (Lewis, et al., 2008), have also been reported by others (Cornelisse, et al., 2010; Weerda, et al., 2010; Yuen, et al., 2010). Estradiol increases HPA axis responsiveness to stress (Gupta, et al., 2001; Viau and Meaney, 2004; Weiser and Handa, 2009) and alters monoaminergic activity in prefrontal brain circuits affecting arousal and cognitive function (Jacome, et al., 2010; Luine, et al., 1998). Thus, we speculate that the combined action of estradiol plus cortisol may have additive or synergistic effects on the chemical neuromodulatory inputs to prefrontal circuits affecting arousal with deleterious consequences for working memory as predicted by the inverted U-shaped dose-response function.
The role of peripheral Aβ in normal and pathological aging is controversial, (Okereke, et al., 2009), but higher plasma Aβ42, lower Aβ40, and thus lower Aβ40/42 ratios have been associated with increased risk of cognitive decline and neurodegenerative disease (Buerger, et al., 2009; Mayeux, et al., 2003). E2-alone increased the Aβ40/42 ratio in plasma but this effect was attenuated or reversed for E2+CORT. Although any interpretations must be considered speculative, the E2-related rise in Aβ40/42 ratio is presumably favorable in this trial given salutary effects of E2-alone on cognition and the results of our earlier work showing a similar effect of a cognition-enhancing intervention on this ratio. In this randomized controlled trial targeting age- and disease-related disturbances in brain energy metabolism in healthy older adults and individuals with mild cognitive impairment, 21 days of intranasal insulin raised the Aβ40/42 ratio in plasma, improved cognition, and suppressed cortisol levels (Reger, et al., 2008). If the predictive value of the Aβ40/42 ratio as a marker of cognitive decline in nondemented older adult populations continues to gain support over time, then this interactive effect of estradiol and cortisol on the Aβ biomarker, if replicated in larger trials, may have important cognitive implications for E2-treated women with abnormally high levels of circulating stress hormones.
Circulating levels of IGF-I were elevated for CORT-alone but not for E2+CORT. Consistent with the results from our earlier studies (Asthana, et al., 2001; Asthana, et al., 1999), E2-alone tended to have a suppressive effect on IGF-I. Although the cortisol-related rise in IGF-I that we observed has also been reported by others in response to exogenous glucocorticoid administration (Borges, et al., 1999) and in conditions of chronic hypercortisolemia such as Cushing’s syndrome (Bang, et al., 1993) or depression (Weber-Hamann, et al., 2009), the modulating effect of estradiol on the IGF-1 response to cortisol has not been previously described. This interactive effect of estradiol and cortisol may account, at least in part, for discrepant reports in the literature regarding estradiol effects on IGF-I although the impact of other variables such as dose and route of delivery also need to be weighed (Davis, et al., 2008).
The significance of elevated or suppressed peripheral levels of IGF-I in blood for cognition remains unclear. In our study, women who improved the most in verbal memory tended to have the greatest treatment-related drop in IGF-I, providing replicable confirmation of our previous report (Asthana, et al., 1999). The results of cross-sectional studies suggest that age-related declines in IGF-I and cognition are linked (Aleman and Torres-Aleman, 2009), however chronic elevations in IGF-I that characterize clinically depressed populations or adults with excessive growth hormone production can also be associated with deleterious consequences for brain function (Deuschle, et al., 1997; Leon-Carrion, et al., 2010). Although the data from our study are limited in their ability to resolve this controversy, they do attest to an interactive effect of estradiol and cortisol on circulating levels of IGF-I such that high levels of estradiol and cortisol, when paired, have different effects than high levels of either hormone alone.
Our study had several limitations, including a small sample size per treatment arm and relatively short duration of exposure to elevated estradiol and cortisol. In addition, although administering hydrocortisone for four days successfully increased circulating cortisol to high physiological levels (Schoorlemmer, et al., 2009) and produced similar cognitive impairments to those reported with in other studies of exogenous or endogenous manipulation of cortisol levels (Wolkowitz, et al., 1990), endogenous alterations of cortisol accompanying increased stress are associated with other hormonal changes (e.g., ACTH) that may have independent effects on the central nervous system (McEwen, 2008). One goal of this study was to model the Shors et al. animal work that examined the interactive effects of estradiol and acute stress. As a consequence, exposure duration to these hormones was relatively brief and may not adequately characterize interactive effects under conditions of chronic stress (Bowman, et al., 2002). Finally, given that synthetic glucocorticoids differ with respect to their pharmacokinetic profiles and that time-of-day impacts not only direction but also magnitude of cortisol-related effects on cognition (deKloet, et al., 1999; Lupien, et al., 2002), the generalizability of our results may be limited if a different glucocorticoid is administered or when assessments occur later in the day as endogenous levels begin to fall.
In summary, our findings indicate that the effects of estradiol administration on cognition, Aβ, and IGF-1 are modified when circulating levels of cortisol are elevated, and that stress effects on IGF-1 are blocked with estradiol administration. That is, elevated cortisol may have deleterious effects for E2-treated postmenopausal women, but E2 administration may offset the effects of high stress. One mechanistic account of estrogenic activity in the brain, the ‘healthy cell bias of estrogen action’ hypothesis, predicts a favorable response when the target of estrogen’s action is healthy, but deleterious consequences when viability of this target is compromised (Brinton, 2008). Consistent with this idea, stress-induced injury may create a window of vulnerability for negative estrogenic effects on affected targets with a net result of reducing, blocking, or even reversing favorable estrogenic actions. In the wake of compelling evidence from animal studies demonstrating striking benefits of estradiol on brain structure and function, it may be prudent to further examine the interactions of cortisol, stress, and other moderator variables before conceding defeat with respect to the cognition-enhancing efficacy of estradiol for postmenopausal women.
Acknowledgments
This work was supported by the Office of Research and Development Medical Research Service, the Geriatric Research, Education and Clinical Center of the Department of Veterans Affairs, and the National Alliance for Research on Schizophrenia and Depression (NARSAD). Berlex Laboratories provided the transdermal estradiol patches and matching placebo. We are grateful to the members of our laboratory who contributed many hours to this project including Karen Enstrom, RN, Karen Hyde, RN, Darla Chapman, RN, Donna Davis, RN, Jaime Tidwell, Dana Belongia, Tracia Clark, Amy Morgan, Michelle Keeling, and Margaret Pagoria. We would also like to thank Elizabeth Colasurdo and Kathy Haugk for their assistance with the assays.
Footnotes
Disclosure statement
There are no conflicts of interest for any of the authors. The principal investigator, Dr. Baker, had full access to all of the data and takes responsibility for the integrity and accuracy of all analyses that were conducted without input from the funding agencies.
References
- Aleman A, Torres-Aleman I. Circulating insulin-like growth factor I and cognitive function: neuromodulation throughout the lifespan. Prog Neurobiol. 2009;89(3):256–65. doi: 10.1016/j.pneurobio.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Alhola P, Polo-Kantola P, Erkkola R, Portin R. Estrogen therapy and cognition: a 6-year single-blind follow-up study in postmenopausal women. Neurology. 2006;67(4):706–9. doi: 10.1212/01.wnl.0000230135.10179.86. [DOI] [PubMed] [Google Scholar]
- Asthana S, Baker LD, Craft S, Stanczyk FZ, Veith RC, Raskind MA, Plymate SR. High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology. 2001;57(4):605–12. doi: 10.1212/wnl.57.4.605. [DOI] [PubMed] [Google Scholar]
- Asthana S, Brinton RD, Henderson VW, McEwen BS, Morrison JH, Schmidt PJ. Frontiers proposal. National Institute on Aging “bench to bedside: estrogen as a case study”. Age (Dordr) 2009;31(3):199–210. doi: 10.1007/s11357-009-9087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asthana S, Craft S, Baker LD, Raskind MA, Birnbaum RS, Lofgreen CP, Veith RC, Plymate SR. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology. 1999;24(6):657–77. doi: 10.1016/s0306-4530(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Meethal SV, Liu T, Wilson AC, Gallego M, Smith MA, Bowen RL. Dysregulation of the hypothalamic-pituitary-gonadal axis with menopause and andropause promotes neurodegenerative senescence. Journal of neuropathology and experimental neurology. 2005;64(2):93–103. doi: 10.1093/jnen/64.2.93. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Garcia-Segura LM. Neuroprotective effects of estradiol in the adult rat hippocampus: interaction with insulin-like growth factor-I signalling. J Neurosci Res. 1999;58:815–22. doi: 10.1002/(sici)1097-4547(19991215)58:6<815::aid-jnr8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Cholerton BA, Plymate SR, Fishel MA, Watson GS, Duncan GE, Mehta PD, Craft S. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J Alzheimers Dis. 2010a;22(2):569–79. doi: 10.3233/JAD-2010-100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch Neurol. 2010b;67(1):1–9. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Tse CS, Hutchison KA, Spieler DH, Duchek JM, Morris JC. Predicting conversion to dementia of the Alzheimer’s type in a healthy control sample: the power of errors in Stroop color naming. Psychol Aging. 2010;25(1):208–18. doi: 10.1037/a0017474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang P, Degerblad M, Thoren M, Schwander J, Blum W, Hall K. Insulin-like growth factor (IGF) I and II and IGF binding protein (IGFBP) 1, 2 and 3 in serum from patients with Cushing’s syndrome. Acta Endocrinol (Copenh) 1993;128(5):397–404. [PubMed] [Google Scholar]
- Banki CM, Bissette G, Arato M, O’Connor L, Nemeroff CB. CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry. 1987;144(7):873–7. doi: 10.1176/ajp.144.7.873. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Kritz-Silverstein D. Estrogen replacement therapy and cognitive function in older women. JAMA. 1993;269(20):2637–41. [PubMed] [Google Scholar]
- Binder EF, Schechtman KB, Birge SJ, Williams DB, Kohrt WM. Effects of hormone replacement therapy on cognitive performance in elderly women. Maturitas. 2001;38(2):137–46. doi: 10.1016/s0378-5122(00)00214-0. [DOI] [PubMed] [Google Scholar]
- Birge S. The role of estrogen in the treatment of Alzheimer’s disease. Neurology. 1997;48(Suppl 7):S36–S41. doi: 10.1212/wnl.48.5_suppl_7.36s. [DOI] [PubMed] [Google Scholar]
- Birkhauser M. Depression, menopause and estrogens: is there a correlation? Maturitas. 2002;41(Suppl 1):S3–8. doi: 10.1016/s0378-5122(02)00009-9. [DOI] [PubMed] [Google Scholar]
- Borges MH, Pinto AC, DiNinno FB, Camacho-Hubner C, Grossman A, Kater CE, Lengyel AM. IGF-I levels rise and GH responses to GHRH decrease during long-term prednisone treatment in man. J Endocrinol Invest. 1999;22(1):12–7. doi: 10.1007/BF03345472. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113(2):401–10. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31(10):529–37. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J, Luszcz MA. Adult age differences in self-ordered pointing task performance: contributions from working memory, executive function and speed of information processing. J Clin Exp Neuropsychol. 2001;23(5):608–19. doi: 10.1076/jcen.23.5.608.1250. [DOI] [PubMed] [Google Scholar]
- Buerger K, Frisoni G, Uspenskaya O, Ewers M, Zetterberg H, Geroldi C, Binetti G, Johannsen P, Rossini PM, Wahlund LO, Vellas B, Blennow K, Hampel H. Validation of Alzheimer’s disease CSF and plasma biological markers: the multicentre reliability study of the pilot European Alzheimer’s Disease Neuroimaging Initiative (E-ADNI) Exp Gerontol. 2009;44(9):579–85. doi: 10.1016/j.exger.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–56. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N, Almeida OF. The amyloidogenic potential and behavioral correlates of stress. Molecular psychiatry. 2009;14(1):95–105. doi: 10.1038/sj.mp.4002101. [DOI] [PubMed] [Google Scholar]
- Ceresini G, Freddi M, Morganti S, Rebecchi I, Modena AB, Rinaldi M, Manca C, Amaducci A, Del Rio G, Valenti G. The effects of transdermal estradiol on the response to mental stress in postmenopausal women: a randomized trial. Am J Med. 2000;109(6):463–8. doi: 10.1016/s0002-9343(00)00523-4. [DOI] [PubMed] [Google Scholar]
- Chopra KK, Ravindran A, Kennedy SH, Mackenzie B, Matthews S, Anisman H, Bagby RM, Farvolden P, Levitan RD. Sex differences in hormonal responses to a social stressor in chronic major depression. Psychoneuroendocrinology. 2009;34(8):1235–41. doi: 10.1016/j.psyneuen.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Cornelisse S, van Stegeren AH, Joels M. Implications of psychosocial stress on memory formation in a typical male versus female student sample. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Petrova A, Plymate S, Raskind M, Grimwood K, Veith RC. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry. 1999;56(12):1135–40. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- Craig MC, Brammer M, Maki PM, Fletcher PC, Daly EM, Rymer J, Giampietro V, Picchioni M, Stahl D, Murphy DG. The interactive effect of acute ovarian suppression and the cholinergic system on visuospatial working memory in young women. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147(1):607–14. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Darnaudery M, Perez-Martin M, Belizaire G, Maccari S, Garcia-Segura LM. Insulin-like growth factor 1 reduces age-related disorders induced by prenatal stress in female rats. Neurobiol Aging. 2006;27(1):119–27. doi: 10.1016/j.neurobiolaging.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Davis SR, Stuckey BG, Norman RJ, Papalia MA, Drillich A, Bell RJ. Effects of the route of estrogen administration on insulinlike growth factor-I, IGF binding protein-3, and insulin resistance in healthy postmenopausal women: results from a randomized, controlled study. Menopause. 2008;15(6):1065–9. doi: 10.1097/gme.0b013e318174f16e. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Xu Y, Buller KM, Day TA. Effects of chronic oestrogen replacement on stress-induced activation of hypothalamic-pituitary-adrenal axis control pathways. J Neuroendocrinol. 2000;12(8):784–94. doi: 10.1046/j.1365-2826.2000.00527.x. [DOI] [PubMed] [Google Scholar]
- deKloet E, Oitzl M, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22(10):422–6. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Deuschle M, Blum WF, Strasburger CJ, Schweiger U, Weber B, Korner A, Standhardt H, Gotthardt U, Schmider J, Pflaum CD, Heuser I. Insulin-like growth factor-I (IGF-I) plasma concentrations are increased in depressed patients. Psychoneuroendocrinology. 1997;22(7):493–503. doi: 10.1016/s0306-4530(97)00046-2. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13(9):3839–47. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Increased memory load-related frontal activation after estradiol treatment in postmenopausal women. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2959–68. doi: 10.1001/jama.291.24.2959291/24/2959. [DOI] [PubMed] [Google Scholar]
- Friedrich N, Alte D, Volzke H, Spilcke-Liss E, Ludemann J, Lerch MM, Kohlmann T, Nauck M, Wallaschofski H. Reference ranges of serum IGF-1 and IGFBP-3 levels in a general adult population: results of the Study of Health in Pomerania (SHIP) Growth Horm IGF Res. 2008;18(3):228–37. doi: 10.1016/j.ghir.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Arevalo MA, Azcoitia I. Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: new advances. Progress in brain research. 2010;181:251–72. doi: 10.1016/S0079-6123(08)81014-X. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35(2):795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn Mem. 2010;17(6):289–96. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2006;26(35):9047–56. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats. Brain Res. 2001;888(2):356–65. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Heuser I, Gotthardt U, Schweiger U, Schmider J, Lammers C, Dettling M, Holsboer F. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: Importance of gender. Neurobiol Aging. 1994;15(2):227–31. doi: 10.1016/0197-4580(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Shors TJ. Distinctive stress effects on learning during puberty. Horm Behav. 2005;48(2):163–71. doi: 10.1016/j.yhbeh.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V. Estradiol and ERbeta agonists enhance recognition memory, and DPN, an ERbeta agonist, alters brain monoamines. Neurobiol Learn Mem. 2010 doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13(3):411–22. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151–78. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(25):10673–8. doi: 10.1073/pnas.0700148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan PA, Ezzat WH, Ginsburg K, Moore GJ. Prefrontal cortex as the site of estrogen’s effect on cognition. Psychoneuroendocrinology. 2001;26(6):577–90. doi: 10.1016/s0306-4530(01)00013-0. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2-3):85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Klaiber EL, Broverman DM, Vogel W, Peterson LG, Snyder MB. Individual differences in changes in mood and platelet monoamine oxidase (MAO) activity during hormonal replacement therapy in menopausal women. Psychoneuroendocrinology. 1996;21(7):575–92. doi: 10.1016/s0306-4530(96)00023-6. [DOI] [PubMed] [Google Scholar]
- Komesaroff P, Esler M, Sudhir K. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal. J Clin Endocrinol Metab. 1999;84(2):606–10. doi: 10.1210/jcem.84.2.5447. [DOI] [PubMed] [Google Scholar]
- Kronforst-Collins MA, Disterhoft JF. Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiol Learn Mem. 1998;69(2):147–62. doi: 10.1006/nlme.1997.3818. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, Kirschbaum C. Psychological and endocrine responses to psychosocial stress and dexamethasone/corticotropin-releasing hormone in healthy postmenopausal women and young controls: the impact of age and a two-week estradiol treatment. Neuroendocrinology. 1999;70(6):422–30. doi: 10.1159/000054504. [DOI] [PubMed] [Google Scholar]
- Laughlin G, Barrett-Connor E. Sexual dimorphism in the influence of advanced aging on adrenal hormone levels: The Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85(10):3561–8. doi: 10.1210/jcem.85.10.6861. [DOI] [PubMed] [Google Scholar]
- Leon-Carrion J, Martin-Rodriguez JF, Madrazo-Atutxa A, Soto-Moreno A, Venegas-Moreno E, Torres-Vela E, Benito-Lopez P, Galvez MA, Tinahones FJ, Leal-Cerro A. Evidence of cognitive and neurophysiological impairment in patients with untreated naive acromegaly. J Clin Endocrinol Metab. 2010;95(9):4367–79. doi: 10.1210/jc.2010-0394. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors TJ. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29(7):883–90. doi: 10.1016/j.psyneuen.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS, Nikolova A, Chang DJ, Weekes NY. Examination stress and components of working memory. Stress. 2008;11(2):108–14. doi: 10.1080/10253890701535160. [DOI] [PubMed] [Google Scholar]
- Li R, Shen Y, Yang LB, Lue LF, Finch C, Rogers J. Estrogen enhances uptake of amyloid beta-protein by microglia derived from the human cortex. Journal of neurochemistry. 2000;75(4):1447–54. doi: 10.1046/j.1471-4159.2000.0751447.x. [DOI] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009;106(3):912–7. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks TL, Berga SL. Does postmenopausal estrogen use confer neuroprotection? Semin Reprod Med. 2009;27(3):260–74. doi: 10.1055/s-0029-1216279. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34(2):149–62. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, Tu MT. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30(3):225–42. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Wilkinson CW, Briere S, Ng Ying Kin NM, Meaney MJ, Nair NP. Acute modulation of aged human memory by pharmacological manipulation of glucocorticoids. J Clin Endocrinol Metab. 2002;87(8):3798–807. doi: 10.1210/jcem.87.8.8760. [DOI] [PubMed] [Google Scholar]
- Maki P, Resnick S. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol of Aging. 2000;21:373–83. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Maki PM, Sundermann E. Hormone therapy and cognitive function. Hum Reprod Update. 2009;15(6):667–81. doi: 10.1093/humupd/dmp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Zonderman AB, Resnick SM. Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. Am J Psychiatry. 2001;158(2):227–33. doi: 10.1176/appi.ajp.158.2.227. [DOI] [PubMed] [Google Scholar]
- Marin R, Guerra B, Morales A, Diaz M, Alonso R. An ICI 182,780-sensitive, membrane-related estrogen receptor contributes to estrogenic neuroprotective actions against amyloid-beta toxicity. Annals of the New York Academy of Sciences. 2003;1007:108–16. doi: 10.1196/annals.1286.011. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Honig LS, Tang MX, Manly J, Stern Y, Schupf N, Mehta PD. Plasma A[beta]40 and A[beta]42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology. 2003;61(9):1185–90. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91(6):2785–801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2-3):174–85. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, Pirttila T, Mehta S, Sersen E, Aisen P, Wisniewski H. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol. 2000;57:100–5. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Wilser L, Eulitz C, Rockstroh B, Conway T, Gonzalez-Rothi L, Crosson B. Neural signatures of semantic and phonemic fluency in young and old adults. J Cogn Neurosci. 2009;21(10):2007–18. doi: 10.1162/jocn.2009.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Conney J, Rasgon N, Fairbanks L, Small G. Mood symptoms and cognitive performance in women estrogen users and nonusers and men. JAGS. 2002;50(11):1826–30. doi: 10.1046/j.1532-5415.2002.50511.x. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Dumas J, Wilkins H, Coderre E, Sites CK, Naylor M, Benkelfat C, Young SN. Estrogen treatment impairs cognitive performance after psychosocial stress and monoamine depletion in postmenopausal women. Menopause. 2010;17(4):860–73. doi: 10.1097/gme.0b013e3181e15df4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereke OI, Xia W, Selkoe DJ, Grodstein F. Ten-year change in plasma amyloid beta levels and late-life cognitive decline. Arch Neurol. 2009;66(10):1247–53. doi: 10.1001/archneurol.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30(1):80–91. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Petanceska SS, Nagy V, Frail D, Gandy S. Ovariectomy and 17beta-estradiol modulate the levels of Alzheimer’s amyloid beta peptides in brain. Neurology. 2000;54(12):2212–7. doi: 10.1212/wnl.54.12.2212. [DOI] [PubMed] [Google Scholar]
- Petrides M, Milner B. Deficits on subject-order tasks after frontal- and termpoal-lobe lesions in man. Neuropsychologia. 1982;20(3):249–62. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17(4):879–86. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Genazzani AD, Bernardi F, Casarosa E, Pieri M, Palumbo M, Picciarelli G, Gabbanini M, Luisi M, Genazzani AR. Tibolone, transdermal estradiol or oral estrogen-progestin therapies: effects on circulating allopregnanolone, cortisol and dehydroepiandrosterone levels. Gynecol Endocrinol. 2005;20(3):144–9. doi: 10.1080/09513590400021169. [DOI] [PubMed] [Google Scholar]
- Polo-Kantola P, Portin R, Polo O, Helenius H, Irjala K, Erkkola R. The effect of short-term estrogen replacement therapy on cognition: a randomized, double-blind, cross-over trial in postmenopausal women. Obstet Gynecol. 1998;91(3):459–66. doi: 10.1016/s0029-7844(97)00700-x. [DOI] [PubMed] [Google Scholar]
- Puder A, Freda P, Goland R, Wardlaw H. Estrogen modulates the hypothalamic-pituitary-adrenal and inflammatory cytokine repsonses to endotoxin in women. J Clin Endocrinol Metab. 2001;86(6):2403–8. doi: 10.1210/jcem.86.6.7528. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70(6):440–8. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–35. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Schoorlemmer RM, Peeters GM, van Schoor NM, Lips P. Relationships between cortisol level, mortality and chronic diseases in older persons. Clin Endocrinol (Oxf) 2009;71(6):779–86. doi: 10.1111/j.1365-2265.2009.03552.x. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Learning under stress impairs memory formation. Neurobiology of learning and memory. 2010;93(2):183–8. doi: 10.1016/j.nlm.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. The Journal of clinical endocrinology and metabolism. 1997;82(8):2458–65. doi: 10.1210/jcem.82.8.4173. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138(3):1021–6. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol. 2009;5(11):620–7. doi: 10.1038/nrendo.2009.193. [DOI] [PubMed] [Google Scholar]
- Shors T, Chua C, Falduto J. Sex differencesand opposite effects of stress on dendritic spine density in male versus female hippocampus. J Neurosci. 2001;21(16):6292–7. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors T, Pickett J, Wood G, Paczynski M. Acute stress persistently enhances estrogen levels in the female rat. Stress. 1999;3(2):163–71. doi: 10.3109/10253899909001120. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Falduto J, Leuner B. The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur J Neurosci. 2004;19(1):145–50. doi: 10.1046/j.1460-9568.2003.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9(3):419–23. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- Smith YR, Love T, Persad CC, Tkaczyk A, Nichols TE, Zubieta JK. Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. J Clin Endocrinol Metab. 2006;91(11):4476–81. doi: 10.1210/jc.2006-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, Faust ME. Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer’s type. J Exp Psychol Hum Percept Perform. 1996;22(2):461–79. doi: 10.1037//0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Alpha1 adrenoreceptors mediate the stimulatory effects of oestrogen on stress-related hypothalamic-pituitary-adrenal activity in the female rat. J Neuroendocrinol. 2004;16(1):72–8. doi: 10.1111/j.1365-2826.2004.01122.x. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Tinkler GP, Browne C, Tobin JR. Neuroprotective effects of estrogen therapy for cognitive and neurobiological profiles of monkey models of menopause. Am J Primatol. 2009;71(9):794–801. doi: 10.1002/ajp.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Hamann B, Blum WF, Kratzsch J, Gilles M, Heuser I, Deuschle M. Insulin-like growth factor-I (IGF-I) serum concentrations in depressed patients: relationship to saliva cortisol and changes during antidepressant treatment. Pharmacopsychiatry. 2009;42(1):23–8. doi: 10.1055/s-0028-1085442. [DOI] [PubMed] [Google Scholar]
- Weerda R, Muehlhan M, Wolf OT, Thiel CM. Effects of acute psychosocial stress on working memory related brain activity in men. Hum Brain Mapp. 2010 doi: 10.1002/hbm.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegesin DJ, Stern Y. Effects of hormone replacement therapy and aging on cognition: evidence for executive dysfunction. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14(3):301–28. doi: 10.1080/13825580600802893. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159(2):883–95. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Weingartner H, Thompson K, Breier A, Doran A, Rubinow D, Pickar D. Cognitive effects of corticosteroids. Am J Psychiatry. 1990;147(10):1297–303. doi: 10.1176/ajp.147.10.1297. [DOI] [PubMed] [Google Scholar]
- Wood G, Shors T. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA. 1998;95(7):1066–71. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci. 2001;115(1):175–87. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Yeni-Komshian H, Carantoni M, Abbasi F, Reaven GM. Relationship between several surrogate estimates of insulin resistance and quantification of insulin-mediated glucose disposal in 490 healthy nondiabetic volunteers. Diabetes Care. 2000;23(2):171–5. doi: 10.2337/diacare.23.2.171. [DOI] [PubMed] [Google Scholar]
- Young EA, Ribeiro SC. Sex differences in the ACTH response to 24H metyrapone in depression. Brain Res. 2006;1126(1):148–55. doi: 10.1016/j.brainres.2006.05.053. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]


