Abstract
Components of the complement system are known to play an important role in the cytolytic process and in chemotaxis of leukocytes. Cobra venom factor specifically cleaves C3 activity via activation of the alternative (properdin) complement pathway. It does not act directly on C3. If C3 is involved in tissue necrosis after ischemic injury, cobra venom factor might reduce tissue damage after acute coronary occlusion. Accordingly, in 14 control dogs occlusion of the left anterior descending artery was carried out for 24 h. Epicardial electrograms were recorded 15 min after occlusion, and 24 h later transmural specimens for creatine phosphokinase activity (CPK) and for histological analysis were obtained from the same sites. In another 14 experimental dogs, 20 U/kg cobra venom factor was given intravenously 30 min after occlusion. Serum complement levels fell within 2-4 h to <20% of normal. In the control dogs, the relationship between ST-segment elevation and CPK activity 24 h later was: log CPK = −0.06 ST + 1.48 (n = 111 specimens, 14 dogs, r = 0.77). In the experimental dogs, log CPK = −0.024 ST + 1.46 (n = 111 specimens, 14 dogs, r = 0.60), showing significantly different slopes (P < 0.001), i.e., less CPK depression for any level of ST-segment elevation. Histologically, 69 of 71 sites (97%) with ST-segment elevation exceeding 2 mV in the control dogs showed signs of necrosis 24 h later, whereas in the experimental group only 43 of 79 sites (54%) with abnormal ST-segment elevations showed signs of necrosis (P < 0.0005). At the same time, it was shown that the administration of cobra venom factor did not alter cardiac performance, collateral blood flow to the ischemic myocardium or the clotting system, but infiltration of polymorphonuclear leukocytes into the myocardium was decreased. It is concluded that cobra venom factor, by reducing the amount of C3 and C5 substrate available for chemotactic factor generation, or other as yet undefined mechanisms, protects the ischemic myocardium from undergoing necrosis, as judged by histology and local CPK activity. Hence, a new approach to limiting the extent of myocardial infarcts after experimental coronary occlusion, based upon inhibition of complement-dependent inflammatory processes, is demonstrated.
Full text
PDF


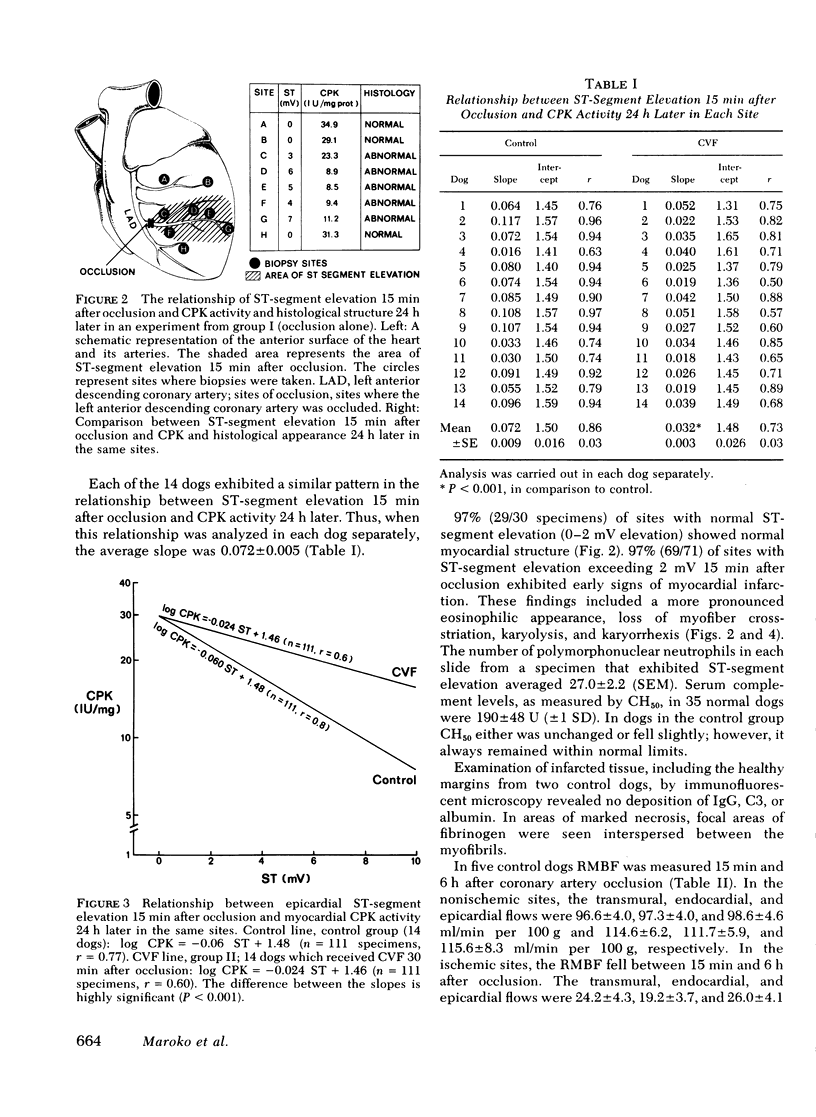





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A., Balavitch D. Cobra venom factor: evidence for its being altered cobra C3 (the third component of complement). Science. 1976 Mar 26;191(4233):1275–1276. doi: 10.1126/science.56780. [DOI] [PubMed] [Google Scholar]
- Ballow M., Cochrane C. G. Two anticomplementary factors in cobra venom: hemolysis of guinea pig erythrocytes by one of them. J Immunol. 1969 Nov;103(5):944–952. [PubMed] [Google Scholar]
- Borer J. S., Redwood D. R., Levitt B., Cagin N., Bianchi C., Vallin H., Epstein S. E. Reduction in myocardial ischemia with nitroglycerin or nitroglycerin plus phenylephrine administered during acute myocardial infarction. N Engl J Med. 1975 Nov 13;293(20):1008–1012. doi: 10.1056/NEJM197511132932002. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Come P. C., Flaherty J. T., Baird M. G., Rouleau J. R., Weisfeldt M. L., Greene H. L., Becker L., Pitt B. Reversal by phenylephrine of the beneficial effects of intravenous nitroglycerin in patients with acute myocardial infarction. N Engl J Med. 1975 Nov 13;293(20):1003–1007. doi: 10.1056/NEJM197511132932001. [DOI] [PubMed] [Google Scholar]
- Domenech R. J., Hoffman J. I., Noble M. I., Saunders K. B., Henson J. R., Subijanto S. Total and regional coronary blood flow measured by radioactive microspheres in conscious and anesthetized dogs. Circ Res. 1969 Nov;25(5):581–596. doi: 10.1161/01.res.25.5.581. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Ruddy S., Knostman J. D., Carpenter C. B., Austen K. F. The functional significance of complement. Adv Nephrol Necker Hosp. 1974;4:15–35. [PubMed] [Google Scholar]
- Flaherty J. T., Reid P. R., Kelly D. T., Taylor D. R., Weisfeldt M. L., Pitt B. Intravenous nitroglycerin in acute myocardial infarction. Circulation. 1975 Jan;51(1):132–139. doi: 10.1161/01.cir.51.1.132. [DOI] [PubMed] [Google Scholar]
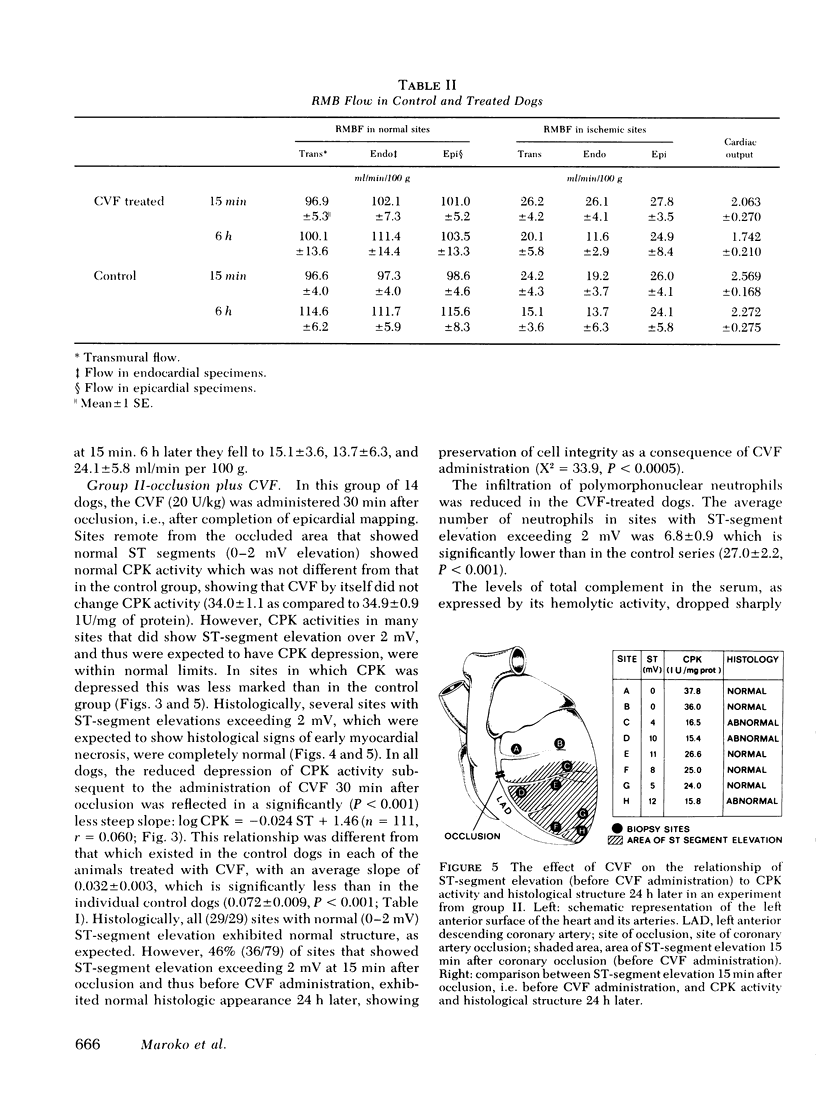
- Ginks W. R., Sybers H. D., Maroko P. R., Covell J. W., Sobel B. E., Ross J., Jr Coronary artery reperfusion. II. Reduction of myocardial infarct size at 1 week after the coronary occlusion. J Clin Invest. 1972 Oct;51(10):2717–2723. doi: 10.1172/JCI107091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- Harnarayan C., Bennett M. A., Pentecost B. L., Brewer D. B. Quantitative study of infarcted myocardium in cardiogenic shock. Br Heart J. 1970 Nov;32(6):728–732. doi: 10.1136/hrt.32.6.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. H., Ward P. A. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med. 1971 Apr 1;133(4):885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld J. W., Jr, Borer J. S., Goldstein R. E., Barrett M. J., Epstein S. E. Reduction in severity and extent of myocardial infarction when nitroglycerin and methoxamine are administered during coronary occlusion. Circulation. 1974 Feb;49(2):291–297. doi: 10.1161/01.cir.49.2.291. [DOI] [PubMed] [Google Scholar]
- KENT J. F., FIFE E. H., Jr Precise standardization of reagents for complement fixation. Am J Trop Med Hyg. 1963 Jan;12:103–116. doi: 10.4269/ajtmh.1963.12.103. [DOI] [PubMed] [Google Scholar]
- Klein P. G., Wellensiek H. J. Multiple nature of the third component of guinea-pig complement. I. Separation and characterization of three factors a, b, and c, essential for haemolysis. Immunology. 1965 Jun;8(6):590–603. [PMC free article] [PubMed] [Google Scholar]
- Libby P., Maroko P. R., Bloor C. M., Sobel B. E., Braunwald E. Reduction of experimental myocardial infarct size by corticosteroid administration. J Clin Invest. 1973 Mar;52(3):599–607. doi: 10.1172/JCI107221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroko P. R., Bernstein E. F., Libby P., DeLaria G. A., Covell J. W., Ross J., Jr, Braunwald E. Effects of intraaortic balloon counterpulsation on the severity of myocardial ischemic injury following acute coronary occlusion. Counterpulsation and myocardial injury. Circulation. 1972 Jun;45(6):1150–1159. doi: 10.1161/01.cir.45.6.1150. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Braunwald E. Modification of myocardial infarction size after coronary occlusion. Ann Intern Med. 1973 Nov;79(5):720–733. doi: 10.7326/0003-4819-79-5-720. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Davidson D. M., Libby P., Hagan A. D., Braunwald E. Effects of hyaluronidase administration on myocardial ischemic injury in acute infarction. A preliminary study in 24 patients. Ann Intern Med. 1975 Apr;82(4):516–520. doi: 10.7326/0003-4819-82-4-516. [DOI] [PubMed] [Google Scholar]
- Maroko P. R. Editorial: Assessing myocardial damage in acute infarcts. N Engl J Med. 1974 Jan 17;290(3):158–159. doi: 10.1056/NEJM197401172900310. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Kjekshus J. K., Sobel B. E., Watanabe T., Covell J. W., Ross J., Jr, Braunwald E. Factors influencing infarct size following experimental coronary artery occlusions. Circulation. 1971 Jan;43(1):67–82. doi: 10.1161/01.cir.43.1.67. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Libby P., Bloor C. M., Sobel B. E., Braunwald E. Reduction by hyaluronidase of myocardial necrosis following coronary artery occlusion. Circulation. 1972 Sep;46(3):430–437. doi: 10.1161/01.cir.46.3.430. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Libby P., Covell J. W., Sobel B. E., Ross J., Jr, Braunwald E. Precordial S-T segment elevation mapping: an atraumatic method for assessing alterations in the extent of myocardial ischemic injury. The effects of pharmacologic and hemodynamic interventions. Am J Cardiol. 1972 Feb;29(2):223–230. doi: 10.1016/0002-9149(72)90633-9. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Libby P., Ginks W. R., Bloor C. M., Shell W. E., Sobel B. E., Ross J., Jr Coronary artery reperfusion. I. Early effects on local myocardial function and the extent of myocardial necrosis. J Clin Invest. 1972 Oct;51(10):2710–2716. doi: 10.1172/JCI107090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroko P. R., Libby P., Sobel B. E., Bloor C. M., Sybers H. D., Shell W. E., Covell J. W., Braunwald E. Effect of glucose-insulin-potassium infusion on myocardial infarction following experimental coronary artery occlusion. Circulation. 1972 Jun;45(6):1160–1175. doi: 10.1161/01.cir.45.6.1160. [DOI] [PubMed] [Google Scholar]
- Muller J. E., Maroko P. R., Braunwald E. Evaluation of precordial electrocardiographic mapping as a means of assessing changes in myocardial ischemic injury. Circulation. 1975 Jul;52(1):16–27. doi: 10.1161/01.cir.52.1.16. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Fjellström K. E. Isolation of the anticomplementary protein from cobra venom and its mode of action on C3. J Immunol. 1971 Dec;107(6):1666–1672. [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Polley M. J., Calcott M. A. Formation and functional significance of a molecular complex derived from the second and the fourth component of human complement. J Exp Med. 1967 Feb 1;125(2):359–380. doi: 10.1084/jem.125.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Jr A new concept of immunosuppression in hypersensitivity reactions and in transplantation immunity. Surv Ophthalmol. 1966 Aug;11(4):498–505. [PubMed] [Google Scholar]
- Page D. L., Caulfield J. B., Kastor J. A., DeSanctis R. W., Sanders C. A. Myocardial changes associated with cardiogenic shock. N Engl J Med. 1971 Jul 15;285(3):133–137. doi: 10.1056/NEJM197107152850301. [DOI] [PubMed] [Google Scholar]
- Pelides L. J., Reid D. S., Thomas M., Shillingford J. P. Inhibition by -blockade of the ST segment elevation after acute myocardial infarction in man. Cardiovasc Res. 1972 May;6(3):295–301. doi: 10.1093/cvr/6.3.295. [DOI] [PubMed] [Google Scholar]
- Pinckard R. N., Olson M. S., Giclas P. C., Terry R., Boyer J. T., O'Rourke R. A. Consumption of classical complement components by heart subcellular membranes in vitro and in patients after acute myocardial infarction. J Clin Invest. 1975 Sep;56(3):740–750. doi: 10.1172/JCI108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwood D. R., Smith E. R., Epstein S. E. Coronary artery occlusion in the conscious dog. Effects of alterations in heart rate and arterial pressure on the degree of myocardial ischemia. Circulation. 1972 Aug;46(2):323–332. doi: 10.1161/01.cir.46.2.323. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Erdmann F., Carpenter C. B., Galvanek E. G. Experimental dysfibrinogenemia: in vivo studies with arvin. Blood. 1971 Jun;37(6):664–674. [PubMed] [Google Scholar]
- Ruddy S., Gigli I., Austen K. F. The complement system of man (second of four parts). N Engl J Med. 1972 Sep 14;287(11):545–549. doi: 10.1056/NEJM197209142871106. [DOI] [PubMed] [Google Scholar]
- Smith E. R., Redwood D. R., McCarron W. E., Epstein S. E. Coronary artery occlusion in the conscious dog. Effects of alterations in arterial pressure produced by nitroglycerin, hemorrhage, and alpha-adrenergic agonists on the degree of myocardial ischemia. Circulation. 1973 Jan;47(1):51–57. doi: 10.1161/01.cir.47.1.51. [DOI] [PubMed] [Google Scholar]
- Sommers H. M., Jennings R. B. Ventricular fibrillation and myocardial necrosis after transient ischemia. Effect of treatment with oxygen, procainamide, reserpine, and propranolol. Arch Intern Med. 1972 May;129(5):780–789. [PubMed] [Google Scholar]
- Utley J., Carlson E. L., Hoffman J. I., Martinez H. M., Buckberg G. D. Total and regional myocardial blood flow measurements with 25 micron, 15 micron, 9 micron, and filtered 1-10 micron diameter microspheres and antipyrine in dogs and sheep. Circ Res. 1974 Mar;34(3):391–405. doi: 10.1161/01.res.34.3.391. [DOI] [PubMed] [Google Scholar]
- Ward P. A. Complement-derived leukotactic factors in pathological fluids. J Exp Med. 1971 Sep 1;134(3 Pt 2):109s–113s. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YACHNIN S. THE HEMOLYSIS OF RED CELLS FROM PATIENTS WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA BY PARTIALLY PURIFIED SUB-COMPONENTS OF THE THIRD COMPLEMENT COMPONENT. J Clin Invest. 1965 Sep;44:1534–1546. doi: 10.1172/JCI105260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yipintsoi T., Dobbs W. A., Jr, Scanlon P. D., Knopp T. J., Bassingthwaighte J. B. Regional distribution of diffusible tracers and carbonized microspheres in the left ventricle of isolated dog hearts. Circ Res. 1973 Nov;33(5):573–587. doi: 10.1161/01.res.33.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]