Abstract
The primary goal of this study was to characterize the response of chondrocyte-seeded agarose constructs to varying concentrations of several key nutrients in a chondrogenic medium, within the overall context of optimizing the key nutrients and the placement of nutrient channels for successful growth of cartilage tissue constructs large enough to be clinically relevant in the treatment of osteoarthritis (OA). To this end, chondrocyte–agarose constructs (ø4×2.34 mm, 30×106 cells/mL) were subjected to varying supplementation levels of insulin (0× to 30× relative to standard supplementation), transferrin (0× to 30×), selenous acid (0× to 10×), ascorbate (0× to 30×), and glucose (0× to 3×). The quality of resulting engineered tissue constructs was evaluated by their compressive modulus (E-Y), tensile modulus (E+Y), hydraulic permeability (k), and content of sulfated glycosaminoglycans (sGAG) and collagen (COL); DNA content was also quantified. Three control groups from two separate castings of constructs (1× concentrations of all medium constituents) were used. After 42 days of culture, values in each of these controls were, respectively, E-Y=518±78, 401±113, 236±67 kPa; E+Y=1420±430, 1140±490, 1240±280 kPa; k=2.3±0.8×10−3, 5.4±7.0×10−3, 3.3±1.3×10−3 mm4/N·s; sGAG=7.8±0.3, 6.3±0.4, 4.1±0.5%/ww; COL=1.3±0.2, 1.1±0.3, 1.4±0.4%/ww; and DNA=11.5±2.2, 12.1±0.6, 5.2±2.8 μg/disk. The presence of insulin and ascorbate was essential, but their concentrations may drop as low as 0.3× without detrimental effects on any of the measured properties; excessive supplementation of ascorbate (up to 30×) was detrimental to E-Y, and 30× insulin was detrimental to both E+Y and E-Y. The presence of glucose was similarly essential, and matrix elaboration was significantly dependent on its concentration (p<10−6), with loss of functional properties, composition, and cellularity observed at ≤0.3×; excessive glucose supplementation (up to 3×) showed no detrimental effects. In contrast, transferrin and selenous acid had no influence on matrix elaboration. These findings suggest that adequate distributions of insulin, ascorbate, and glucose, but not necessarily of transferrin and selenous acid, must be ensured within large engineered cartilage constructs to produce a viable substitute for joint tissue lost due to OA.
Introduction
Osteoarthritis (OA) is a pathological condition characterized by the breakdown of articular cartilage, resulting in pain, inflammation, and impaired joint mobility. Although currently no treatments for OA exist that restore the native joint surface, cartilage tissue engineering is a promising approach for replenishing lost native tissue with de novo tissue produced by culturing cells under controlled conditions within a suitable 3D scaffold. The culture of chondrocyte-seeded agarose constructs containing 30×106 cells/mL in a chemically defined medium under transient supplementation of human recombinant TGF-β3 has been shown to reproduce native levels of compressive Young's modulus (E-Y) and sulfated glycosaminoglycan (sGAG) content.2,3 This cell-seeding density is on the same order of magnitude as the native cell density in adult bovine articular cartilage (50–150×106 cells/mL).4
A major challenge in cartilage tissue engineering is the ability to make sufficiently large constructs for adequate repair of cartilage defects. Current strategies employ smaller constructs (∼20 mm2); however, OA generally does not become symptomatic until the defects reach ≥5 cm2, and therefore small constructs may not ultimately be clinically relevant for treatment of OA.5,6 As the construct size increases, inhomogeneous tissue properties tend to develop, as consumption by cells on the periphery results in poor nutrient availability toward the construct center.7–10 Various techniques have been developed to overcome transport limitations such as direct perfusion,11–13 dynamic loading,14–16 and the introduction of nutrient channels.7,17 A major goal of our research is to implement computational models to optimize the placement of channels for successful growth of large constructs. This technique necessitates an understanding of which nutrients are critical for tissue growth.
Agarose gels are often employed in cartilage tissue engineering applications, as agarose is known to stabilize the chondrocyte phenotype in vitro.18–20 Further, proteoglycans produced by chondrocytes in agarose have been shown to be most similar to those of native cartilage as compared to proteoglycans produced in fibrin, alginate, and PGA scaffolds.21 Chondrocyte-seeded agarose constructs have also performed well in an in vivo canine model, wherein the constructs exhibited excellent integration with the host tissue,22 as well as in phase II clinical trials for autologous chondrocyte implantation.23 A phase III clinical trial evaluating their efficacy is currently underway in France.24
As an alternative to the serum-based medium, which contains a complex profile of nutrients and growth factors that may vary batch to batch, many tissue engineering experiments employ a chemically defined medium, consisting of insulin-transferrin-selenium (ITS) additives. Insulin is required for cellular metabolism of glucose and is thus necessary for cell culture.25 Transferrin is an essential protein that is known to transport iron into cells, where iron plays a role in the hydroxylation of proline during collagen synthesis,26,27 and selenium is an essential trace element that boosts the activity of glutathione peroxidases in shielding cells from oxidative stress.28 ITS-supplemented media have been shown to viably support and promote matrix deposition within chondrocyte-seeded agarose constructs.29,30 The effects of relative concentrations of ITS on cell proliferation have been investigated in 2D monolayer culture of rat neuroblastoma cells31 and HeLa cells, but the specific effects of ITS concentrations on the functional properties of engineered cartilage have not been characterized.
Ascorbate is a requisite cofactor in the production of collagen II by chondrocytes32 and has also been observed to influence sGAG production,33 and thus is an essential medium supplement for cartilage tissue engineering. Ascorbate levels have been modulated over narrow ranges in short-term pellet culture,34 and comparisons have been made between chondrocyte-seeded agarose constructs that have been cultured in the presence or absence of ascorbate,33,35 yet the effects of its concentration over a broad range on tissue-engineered constructs remain to be uncovered.
Due to the avascular nature of mature cartilage and its resulting low oxygen tension, chondrocytes rely primarily upon glycolysis and lactic acid fermentation to generate ATP. Further, glucose is used by chondrocytes to form building blocks in the synthesis of sGAG.36 The chondrocytes require adequate glucose concentrations.37,38 Both chondrocyte density and metabolic consumption shape the gradient of glucose concentration throughout the depth of articular cartilage,39 and computational models predict that glucose gradients are also present in engineered cartilage.40,41 Therefore, it is important to also investigate the influences of various levels of glucose in our current culture system.
In this study, we characterized the response of chondrocyte-seeded agarose constructs to varying concentrations of several key nutrients in a chondrogenic medium (CM), to determine the relative importance of CM supplements in the growth of the engineered cartilage.1 This aim was achieved by subjecting chondrocyte–agarose constructs to varying supplementation levels of the three ITS constituents as well as ascorbate and glucose. The quality of the resulting engineered tissue constructs was evaluated by their mechanical properties and their content of the major cartilage matrix constituents, and the effects of nutrient levels on construct cellularity were examined.
Materials and Methods
Media were prepared, in which the concentrations of each of the nutrients of interest were individually modulated over a broad range for 6 weeks of cultivation time. Constructs were assessed for mechanical and transport properties (hydraulic permeability, k; equilibrium compressive Young's modulus, E-Y; and equilibrium tensile Young's modulus, E+Y) and subsequently evaluated for sGAG and collagen contents, as well as their cellularities (DNA contents).
Materials
Bovine wrists were obtained from Green Village Packing Co. (Green Village, NJ). High-glucose Dulbecco's Modified Eagle Medium (DMEM), glucose-free DMEM, penicillin, streptomycin, amphotericin B (PS/AM) antibiotic–antimycotic, fetal bovine serum (FBS), and PicoGreen dsDNA quantitation assay kit were purchased from Life Technologies (Grand Island, NY). d-glucose, l-proline, agarose type VII-A, ascorbic acid 2-phosphate (AAP), human recombinant insulin, phosphate-buffered saline, dexamethasone, sodium pyruvate, Tris–HCl, Trizma base, EDTA, diethylpyrocarbonate, collagenase, and sodium selenite were obtained from Sigma-Aldrich Corp. (St. Louis, MO). Human recombinant TGF-β3 was obtained from R&D Systems (Minneapolis, MN). ITS+ Premix, human holo transferrin, and bovine serum albumin/linoleic acid complex were obtained from BD Biosciences (San Jose, CA). Proteinase K was obtained from MP Biomedicals, LLC (Solon, OH); 12 N HCl, iodoacetamide, and pepstatin A were obtained from Fisher Scientific (Pittsburgh, PA).
Cell harvest and culture
Bovine articular cartilage was harvested from 2-month-old calf carpometacarpal joints (n=18, male and female). The cartilage fragments were digested in high-glucose DMEM supplemented with 5% FBS and collagenase for 6 h at 37°C to release chondrocytes, and then passed through a 70-μm mesh to remove the debris. The freshly isolated chondrocytes were suspended in the CM at 60×106 cells/mL and mixed with equal parts 4% (w/v) low-gelling-temperature agarose in phosphate-buffered saline to yield a final concentration of 30×106 cells/mL in 2% agarose. The solution was poured between glass slides and allowed 15 min to set, and a biopsy punch was employed to form cylindrical constructs (ø4×2.34 mm). Constructs were cultured at 37°C, 5% CO2 in various media for 42 days; the media were changed three times weekly.
Medium compositions
All media were based upon the standard CM: high-glucose DMEM containing 100 nM dexamethasone, 100 μg/mL sodium pyruvate, 50 μg/mL l-proline, 1% ITS+ Premix (final medium concentrations: 6.25 μg/mL human recombinant insulin, 6.25 ng/mL selenous acid, 6.25 μg/mL human holotransferrin, 1.25 mg/mL bovine serum albumin, and 5.35 μg/mL linoleic acid), 1% PS/AM antibiotic–antimycotic, and 173 μM AAP. The concentrations of the five investigated CM constituents were individually varied relative to the 1× standard: insulin (INS; 0×, 0.3×, 3×, 10×, and 30×), transferrin (TR; 0×, 0.3×, 3×, 10×, and 30×), selenous acid (SA; 0×, 0.1×, and 10×), AAP (0×, 0.3×, 3×, 10×, and 30×), and glucose (GLU; 0×, 0.3×, and 3×). AAP groups and their 1×control were prepared from the standard CM containing 1% ITS+ Premix. For GLU groups and their 1× control, glucose was added to the standard CM that had instead been prepared with glucose-free DMEM. INS, TR, and SA groups shared a 1× ITS control prepared using the individual constituents of the ITS+ Premix at their standard concentrations. All media were supplemented with 10 ng/mL TGF-β3 for the first 2 weeks of culture.2 The samples were taken from all groups at days 0, 21, and 42 (n=5 per group).
Mechanical characterization
Constructs were tested under unconfined compression between impermeable platens in a CM bath. For each sample, a creep tare load of 0.005 N was first applied for 200 s, and subsequently a stress-relaxation test was conducted that comprised of a ramp displacement to 10% strain over 300 s and then held until equilibrium was reached (∼1800 s). Stress-relaxation data were curve-fitted as previously described.42 Briefly, finite element models were constructed (FEBio43) that incorporated the geometry of the specimens, and the engineered cartilage was modeled as a biphasic material containing intrinsically incompressible fluid and solid phases44; the composite porous solid phase consisted of a compressible neo-Hookean solid ground matrix45 reinforced by a continuous, random distribution of fibril bundles sustaining tension only, with a linear stress–strain response; the hydraulic permeability was assumed constant.42,46 A least-square parameter optimization routine in FEBio43 was used to curve-fit the stress-relaxation data and extract the specimen k, E-Y, and ξ (fibril tensile modulus) values. These values were substituted into a finite element analysis of equilibrium uniaxial tension to produce E+Y.
Biochemical characterization
Mechanically tested samples were lyophilized and digested in proteinase K solution (0.5 mg/mL proteinase K, 50 mM Tris-buffered saline with 1 mM EDTA, 1 mM iodoacetamide, and 10 μg/mL pepstatin A) overnight at 56°C.47,48 sGAG content was determined by 1,9-dimethylmethylene blue dye-binding assay.49 PicoGreen dsDNA quantitation assay was used to determine the DNA content.50 Collagen content was assessed by assaying orthohydroxyproline (OHP) as previously described47; briefly, proteinase K digests were mixed with equal parts 12 N HCl and hydrolyzed at 110°C for 16 h, then dried, and resuspended in an OHP assay buffer. The OHP content of the hydrolysis product was assessed via chloramine T and dimethylaminobenzaldehyde assay and a 1:7.64 OHP-to-collagen mass ratio was assumed48; in similar chondrocyte–agarose constructs, it has been shown that collagen measured by this method is predominantly type II.51 Contents of collagen and sGAG were normalized to sample wet weights (%/ww), while DNA contents were expressed in total mass per construct.
Statistics
Statistical significance was assessed using one-way ANOVA with Tukey's HSD post hoc tests, with α=0.05. E-Y, E+Y, ξ, k, sGAG, collagen, and DNA content served as dependent variables, while culture time or medium treatment served as the independent variable. Data are reported as mean±standard deviation (n=5).
Results
Mechanical characterization
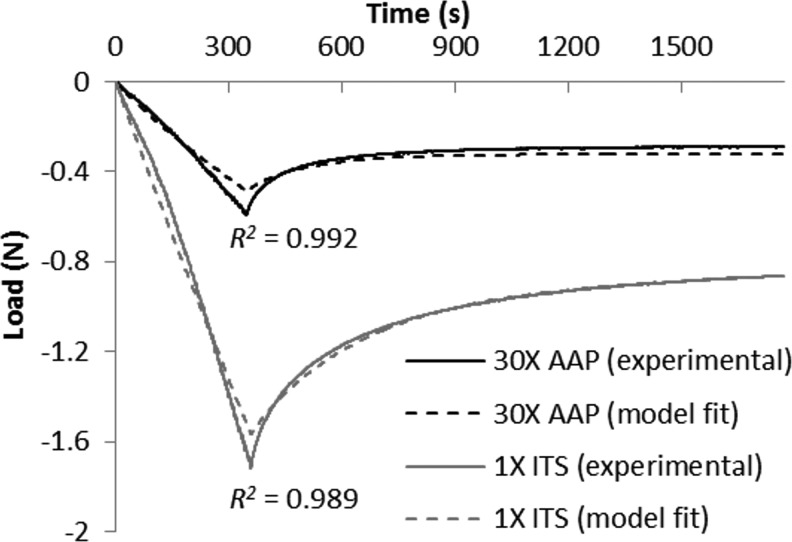
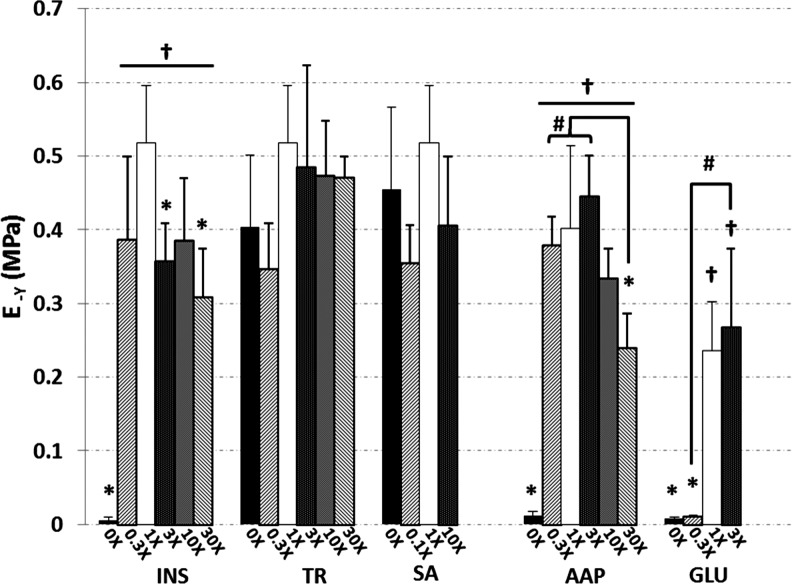
Curve fits were generally faithful to the experimental data (Fig. 1), producing near-unity coefficients of determination (R2=0.968±0.050, Table 1). After 42 days in culture, the 1× ITS control exhibited k=2.3±0.8×10−3 mm4/N·s, E-Y=518±78 kPa, and E+Y=1420±430 kPa; statistically on par with the 1× AAP control values (k=5.4±7.0×10−3 mm4/N·s, E-Y=401±113 kPa, E+Y=1140±500 kPa). The 1× GLU control had k=3.3±1.7×10−3 mm4/N·s, E-Y=236±67 kPa, and E+Y=1240±280 kPa. Complete removal of insulin or ascorbate or reduction of glucose resulted in significantly higher k (Table 1) and significantly reduced E-Y (Fig. 2) and E+Y (Table 1) as compared to their respective controls (p<0.005); in fact, 0× INS, 0× AAP, 0× GLU, and 0.3× GLU possessed mechanical properties similar to day-0 values (k ∼0.10 mm4/N·s, E-Y ∼9 kPa, E+Y ∼80 kPa). The high doses 30× INS and 30× AAP also exhibited significantly lower E-Y than controls (p<0.005), and 30× INS yielded lower E+Y (p<0.05). Conversely, modulations in TR or SA dosages had no significant effect on E-Y or E+Y (p>0.07), and only 0.3× TR exhibited a heightened k (p<0.05). Similar day-42 trends were observed at day 21, with the exception of several TR and SA groups that showed reduced E-Y versus 1× control (p<0.05, data not shown).
FIG. 1.
Representative curve fits for constructs with high E-Y (1× ITS) and with low E-Y (30× AAP) during stress-relaxation test of ramp to 10% strain, followed by 1500-s relaxation. ITS, insulin-transferrin-selenium; AAP, ascorbic acid 2-phosphate.
Table 1.
Hydraulic Permeabilities (k), Fibril Tensile Moduli (ξ), Equilibrium Tensile Young's Moduli (E+Y), and Coefficients of Determination of Curve Fits (R2) of Day-42 Constructs
| k (10−3 mm4/N·s) | p | ξ (kPa) | p | E+Y (kPa) | p | R2 | |
|---|---|---|---|---|---|---|---|
| 0× INS | 60±13 | <0.0005 | 17±5 | NS | 170±40 | <0.05 | 0.978±0.011 |
| 30× INS | 5.2±2.7 | NS | 24±13 | NS | 590±210 | <0.05 | 0.975±0.010 |
| Other INS | 3.1±1.5 | — | 53±31 | — | 1250±500 | — | 0.982±0.016 |
| 0.3× TR | 4.7±1.5 | <0.05 | 38±13 | NS | 980±190 | NS | 0.990±0.003 |
| Other TR | 2.3±0.8 | — | 66±30 | — | 1530±500 | — | 0.990±0.06 |
| All SA | 2.7±1.0 | — | 52±24 | — | 1280±430 | — | 0.983±0.010 |
| 0× AAP | 36.7±22.8 | <0.001 | 12±6 | NS | 150±50 | <0.01 | 0.897±0.077 |
| Other AAP | 4.0±4.3 | — | 50±20 | — | 1160±350 | — | 0.982±0.016 |
| 0× GLU | 80.0±21.5 | <0.0005 | 12±10 | <0.0005 | 130±90 | <0.0005 | 0.827±0.111 |
| 0.3× GLU | 40.7±11.4 | <0.005 | 15±7 | <0.0005 | 170±60 | <0.0005 | 0.843±0.135 |
| 1× GLU | 3.3±1.3 | — | 70±18 | — | 1240±280 | — | 0.981±0.008 |
| 3× GLU | 3.4±1.7 | NS | 41±9 | <0.05 | 920±170 | NS | 0.981±0.011 |
Statistically indistinct groups for each nutrient treatment have been condensed; p-values indicate statistical significance versus corresponding 1× control or condensed group.
NS, no significance; INS, insulin; TR, transferrin; SA, selenous acid; AAP, ascorbic acid phosphate; GLU, glucose.
FIG. 2.
Compressive Young's moduli (E-Y) of constructs at day 42. Asterisks (*) denote p<0.05 versus corresponding 1× control; daggers (†) denote p<0.05 versus corresponding 0× group; hash marks (#) denote other statistical differences (p<0.05). INS, insulin; TR, transferrin; SA, selenous acid; GLU, glucose.
Sulfated GAG content
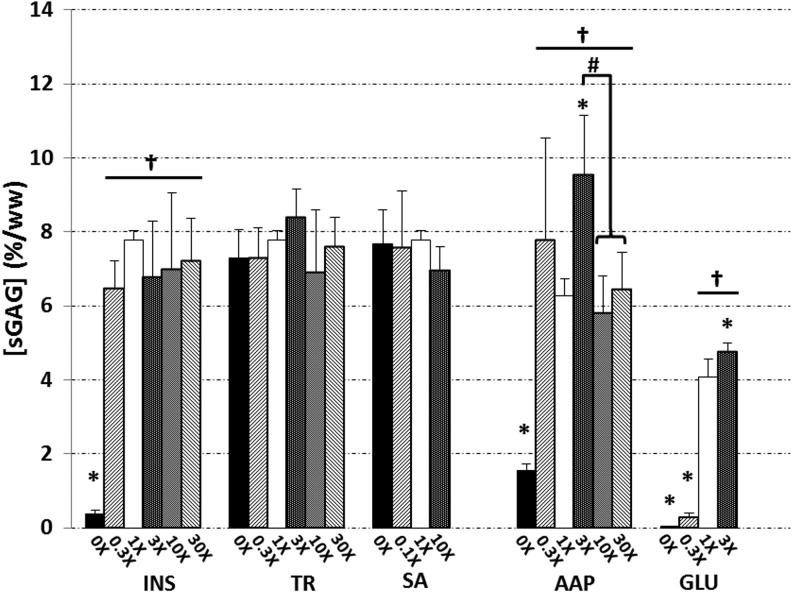
sGAG contents ultimately reached 7.78±0.26 and 6.28±0.44%/ww in 1× ITS and 1× AAP controls, respectively (Fig. 3). Within the treatment groups of ITS and AAP, only 3× AAP exhibited a significantly elevated sGAG content of 9.54±1.60%/ww versus control (p<0.05). Subtraction of insulin or ascorbate yielded dramatically reduced sGAG contents (p<0.0005), 4-fold in the absence of ascorbate and 20-fold in that of insulin. Elaboration of sGAG appeared to be insensitive to the changes in concentrations of transferrin (p=0.25) or selenous acid (p=0.56), as well as to nonzero variations of insulin (p>0.5). The 1× GLU control's content reached 4.07±0.49%/ww; groups 0× GLU and 0.3× GLU were significantly lower (p<0.0005), and 3× GLU was significantly higher (p<0.01). Data from the day-21 timepoint revealed the same trends, with the exception of the 3× AAP group, which had not yet significantly exceeded its 1× control (p=0.22).
FIG. 3.
sGAG contents of constructs at day 42. Asterisks (*) denote p<0.05 versus corresponding 1× control; daggers (†) denote p<0.05 versus corresponding 0× group; hash marks (#) denote other statistical differences (p<0.05). sGAG, sulfated glycosaminoglycan.
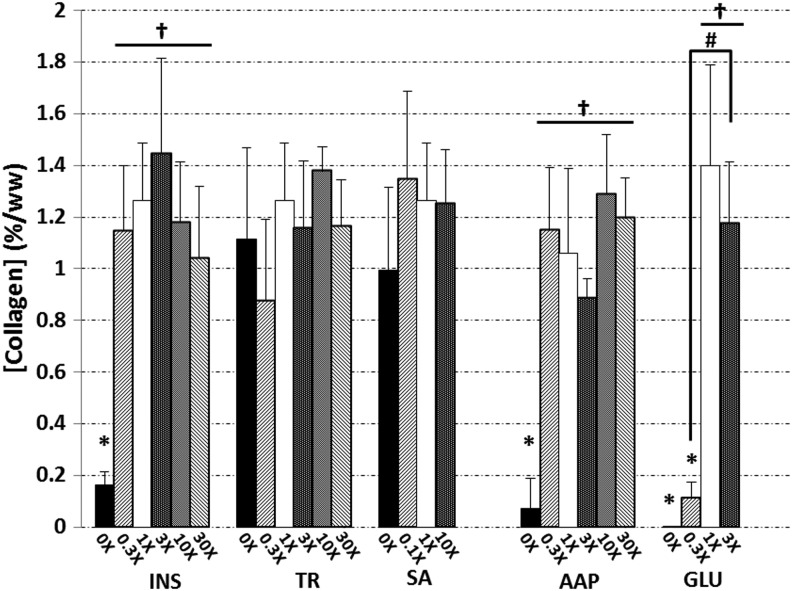
Collagen content
Collagen levels reached 1.27%±0.22%, 1.06%±0.33%, and 1.40%±0.39% by wet weight in the 1× ITS control, 1× AAP control, and 1× GLU control, respectively (Fig. 4). Significant decreases in the collagen content were elicited by complete removal of insulin, ascorbate, or glucose (p<0.0005), or in the case of reduced glucose (0.3× GLU, p<0.0005). In all of these groups, the measured collagen contents were extremely low (<0.2%/ww). Similar behavior was observed in day-21 collagen contents that became more pronounced over time (data not shown). At this earlier time, the absence of selenous acid appeared to have detrimental effects on collagen production versus 1× control (p<0.005), but by the end of the study, the concentrations of selenous acid and transferrin had no effect on collagen (p=0.26 and p=0.085, respectively).
FIG. 4.
Collagen contents of constructs at day 42. Asterisks (*) denote p<0.05 versus corresponding 1× control; daggers (†) denote p<0.05 versus corresponding 0× group; hash marks (#) denote other statistical differences (p<0.05).
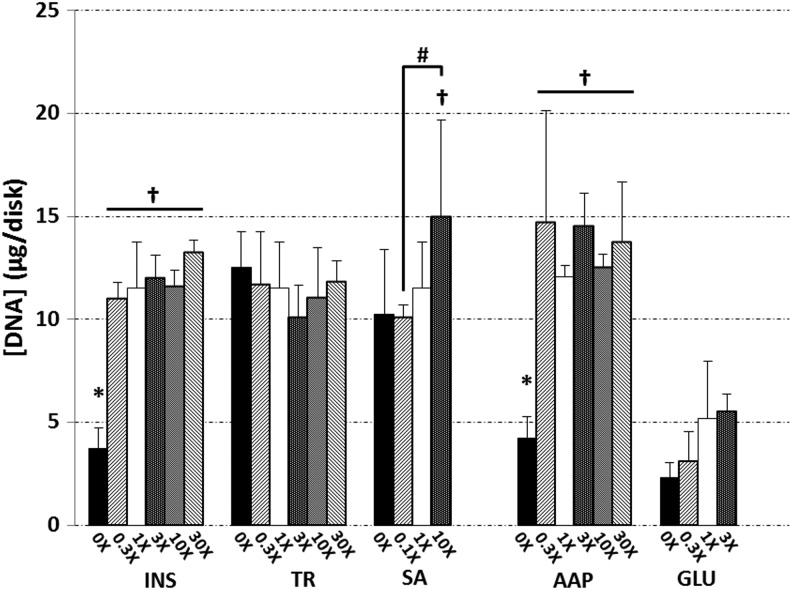
DNA content
After 42 days in culture, DNA content in all the INS, TR, SA, and AAP groups had increased roughly 4-fold from day-0 levels (2.90±0.34 μg/disk for the INS, TR, SA, and AAP groups; 4.85±0.35 μg/disk for GLU groups), except the GLU groups and those lacking ascorbate or insulin. The control DNA contents were 12.06±0.56 and 11.53±2.22 μg/disk for 1× AAP and 1× ITS controls, respectively (Fig. 5); both 0× INS and 0× AAP were significantly lower (p<0.005). The 1× GLU control ultimately reached 5.19±2.77 μg/disk. ANOVA detected a significant influence of the glucose concentration on DNA content at day 42 (p=0.03), though the Tukey's HSD post hoc analysis did not detect these specific differences. The overall DNA trends confirmed the results at day 21, though at that earlier time, the lower DNA contents in groups 0× INS and 0× AAP were not yet appreciable, and the 30× AAP group had temporarily exceeded the 1× AAP control by threefold (p<0.005, data not shown).
FIG. 5.
DNA contents of constructs at day 42. Asterisks (*) denote p<0.05 versus corresponding 1× control; daggers (†) denote p<0.05 versus corresponding 0× group; hash marks (#) denote other statistical differences (p<0.05).
Discussion
At the conclusion of the 6-week culture period, ITS and AAP controls possessed compressive Young's moduli approximately equal to the native values from similar bovine sources (∼550 kPa)52 and sGAG contents on par with that of native cartilage (∼4%/ww)53; the GLU control exhibited native values of sGAG and ∼1/2 of native E-Y. Native levels of type II collagen (∼10%/ww),53 k (∼5×10−4 mm4/N·s),52 and E+Y (∼15–20×103 kPa)46,54 were not reached by any groups. The absence of insulin, glucose, or ascorbate in the culture medium corresponded to the lack of mechanical stiffening and accretion of the matrix products within the constructs (Figs. 2–4 and Table 1). Considering the roles of glucose (anaerobic ATP production), insulin (cellular uptake of glucose), and ascorbate (collagen synthesis), it is not surprising to see the resulting poor functional properties. However, such a dramatic a drop across the board in the mechanical and biochemical properties places a strong emphasis upon the importance of transport and distribution of insulin, glucose, and ascorbate within the construct. These data suggest that it is vital to maintain sufficient concentrations of these nutrients throughout the tissue volume if there is to be matrix production at the center as well as the periphery.
While engineered cartilage constructs of the type and size implemented in this study typically produce a more-homogenous matrix,3,7,9 large heterogeneities of the elaborated matrix are often observed in cultures of larger constructs.7,8 The observations in the current study give rise to consideration as to whether deficiencies of these critical nutrients could be responsible for this behavior. Based upon the lack of significant differences between 0.3× and controls for insulin and ascorbate (Figs. 2–5 and Table 1), it is possible that dose dependencies could exist at lower concentrations than those implemented herein, and that elucidating these dose dependencies could merit further study. When glucose was reduced to 0.3× of the 25 mM concentration present in high-glucose DMEM—slightly higher than the standard glucose supplementation of 5.52 mM—dramatic reductions in tissue mechanical integrity (Fig. 2 and Table 1) and matrix deposition (Figs. 3 and 4) were observed. This reinforces the utilization of a high-glucose medium in place of standard DMEM in cartilage tissue engineering studies, and also suggests that diminishing glucose concentrations within constructs may impair tissue growth.
The media used during the culture phase of this study were based upon a CM formulation, including ITS, as a substitute for serum. It should be noted that although an FBS-containing medium formulation was used during the 24-h cartilage digestion step of the cell isolation procedure, it is unlikely that this initial exposure to serum before construct casting could have confounded the results of the study. The ITS medium contains what are believed to be essential supplements for proliferation of many cell types in a long-term culture.31 While our results corroborate the importance of insulin in culture, no detrimental effects were observed after 42 days in the absence of either transferrin or selenous acid (Figs. 2–5 and Table 1). This would suggest that the role of transferrin is not vital for chondrocyte survival or matrix synthesis, and perhaps that uptake of non-transferrin-bound iron, which has been observed in various other cell types,55,56 also occurs in chondrocytes. Similarly, selenous acid was found to be unnecessary for developing constructs, suggesting that either oxidative stress is not a threat in this culture system, or the cells are able to compensate for it. Further, cytotoxic effects that have previously resulted from exposure to high selenium concentrations31 were not observed. These results are valuable for resolving transport limitations in cartilage tissue engineering systems, as they suggest that concentrations of neither transferrin nor selenous acid need be considered as a limiting factor in tissue growth. Although their absence does not seem to elicit detrimental effects by day 42, it would be prudent to continue the usage of both these constituents in future studies, since day-21 results show some delays in matrix elaboration in the absence of these constituents.
The glucose groups, which along with their control were cast separately from ITS and ascorbate treatment groups, exhibited lower E-Y (Fig. 2), sGAG contents (Fig. 3), and DNA contents (Fig. 5) than the ITS and ascorbate groups. While the cause of these differences is not entirely clear, it is likely the result of variability intrinsic to the harvest and casting processes. We have observed that constructs from different casts may develop disparate properties, such as E-Y and DNA, despite sharing similar casting conditions. Because the aim of this study was to modulate each constituent separately, comparisons were only made relative to the corresponding control for any given group.
While modulating glucose concentration affects the medium osmolarity, and osmotic environment changes have been shown to have an impact on chondrocyte metabolism,57,58 the osmolarity change elicited in this study by reducing glucose was low compared to osmolarities previously studied, and therefore the resulting changes in tissue properties are likely attributed to the concentration of glucose. Due to their lower medium concentrations, altering the concentrations of insulin, transferrin, selenous acid, or ascorbate does not elicit substantial changes in the medium osmolarity.
The chondrocytes used were isolated from bovine calf cartilage. Due to their availability for harvesting in large numbers and their ability to readily produce the cartilage matrix, these cells constitute an ideal model system and are most practical for large-scale studies such as this (∼250 constructs). For clinical tissue engineering applications where the cell source may be diseased adult human chondrocytes or stem cells, it may be necessary to verify whether the influences of the critical nutrients persist at similar concentrations. For example, diseased cells may exhibit lower metabolic requirements, while the converse may be true for stem cells.
With the exception of the highest dosage of selenium (10×), no treatments were found to increase the DNA content of the constructs; lack of insulin or ascorbate yielded significantly less DNA. Although it was not corroborated by post hoc analysis, a significant effect of glucose concentration on DNA was revealed by ANOVA, and therefore glucose levels should be a consideration in related studies in which a specific level of cell proliferation is desired. All other groups increased similarly throughout the culture duration, suggesting that the proliferation of chondrocytes within this culture system is nearly independent from concentration variations of ITS and ascorbate, so long as the insulin and ascorbate levels remain above 0.3×. It should be noted that while the DNA assay provides an estimate of the total cell number, it does not examine the state of the cells. For the purposes of this study, in which the most desired attributes of the constructs were their mechanical properties and matrix compositions, this simple estimate of the cell number was employed. Further experiments would be required to characterize specific cellular behavior such as cell fate processes.
The findings of this study have allowed us to identify several nutrients in the CM that could potentially be limiting for the growth of engineered cartilage: The presence of insulin and ascorbate is essential, but their concentrations may drop as low as 0.3×without detrimental effects; in contrast, excessive supplementation of insulin or ascorbate can be detrimental. The matrix elaboration was significantly dependent on glucose concentration, with loss of functional properties observed at 0.3× or less; however, excessive glucose supplementation (up to 3×) shows no detrimental effects. In contrast, transferrin and selenous acid appear to have no long-term influence on matrix elaboration in this culture system. Future work can now focus on transport and consumption of these critical nutrients within constructs and to what extent their availabilities influence construct inhomogeneity, especially as the construct dimensions increase. This information will in turn help to strengthen the finite element model predictions of transport and tissue growth, an invaluable tool in developing strategies for growing cartilage constructs of clinically relevant size.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers R01AR060361, R01AR046568, and T32AR059038. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1.Cigan A.D. Nims R.J. Albro M.B. Breves S.L. Hung C.T. Ateshian G.A. Insulin and ascorbate have a much greater influence than transferrin and selenous acid on the growth of engineered cartilage in chondrogenic media. Abstract presented at ASME 2012 Summer Bioengineering Conference; Fajardo, PR. 2012. Abstract No. SBC2012-80572. [Google Scholar]
- 2.Byers B.A. Mauck R.L. Chiang I.E. Tuan R.S. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;11:1821. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima E.G. Bian L. Ng K.W. Mauck R.L. Byers B.A. Tuan R.S. Ateshian G.A. Hung C.T. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jadin K.D. Wong B.L. Bae W.C. Li K.W. Williamson A.K. Schumacher B.L. Price J.H. Sah R.L. Depth-varying density and organization of chondrocytes in immature and mature bovine articular cartilage assessed by 3d imaging and analysis. J Histochem Cytochem. 2005;53:1109. doi: 10.1369/jhc.4A6511.2005. [DOI] [PubMed] [Google Scholar]
- 5.Cohen Z.A. Mow V.C. Henry J.H. Levine W.N. Ateshian G.A. Templates of the cartilage layers of the patellofemoral joint and their use in the assessment of osteoarthritic cartilage damage. Osteoarthritis Cartilage. 2003;11:569. doi: 10.1016/s1063-4584(03)00091-8. [DOI] [PubMed] [Google Scholar]
- 6.Moisio K. Eckstein F. Chmiel J.S. Guermazi A. Prasad P. Almagor O. Song J. Dunlop D. Hudelmaier M. Kothari A. Sharma L. Denuded subchondral bone and knee pain in persons with knee osteoarthritis. Arthritis Rheum. 2009;50:3703. doi: 10.1002/art.25014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bian L. Angione S.L. Ng K.W. Lima E.G. Williams D.Y. Mao D.Q. Ateshian G.A. Hung C.T. Influence of decreasing nutrient path length on the development of engineered cartilage. Osteoarthritis Cartilage. 2009;17:677. doi: 10.1016/j.joca.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung C.T. Lima E.G. Mauck R.L. Takai E. LeRoux M.A. Lu H.H. Stark R.G. Guo X.E. Ateshian G.A. Anatomically shaped osteochondral constructs for articular cartilage repair. J Biomech. 2003;36:1853. doi: 10.1016/s0021-9290(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 9.Hung C.T. Mauck R.L. Wang C.C. Lima E.G. Ateshian G.A. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 10.Kelly T.A. Ng K.W. Ateshian G.A. Hung C.T. Analysis of radial variations in material properties and matrix composition of condrocyte-seeded agarose hydrogel constructs. Osteoarthritis Cartilage. 2009;17:73. doi: 10.1016/j.joca.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cartmell S.H. Porter B.D. Garcia A.J. Guldberg R.E. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003;9:1197. doi: 10.1089/10763270360728107. [DOI] [PubMed] [Google Scholar]
- 12.Maidhof R. Marsano A. Lee E.J. Vunjak-Novakovic G. Perfusion seeding of channeled elastomeric scaffolds with myocytes and endothelial cells for cardiac tissue engineering. Biotechnol Prog. 2010;26:565. doi: 10.1002/btpr.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsano A. Maidhof R. Tandon N. Gao J. Wang Y. Vunjak-Novakovic G. Engineering of functional contractile cardiac tissues cultured in a perfusion system. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:3590. doi: 10.1109/IEMBS.2008.4649982. [DOI] [PubMed] [Google Scholar]
- 14.Mauck R.L. Hung C.T. Ateshian G.A. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering. J Biomech Eng. 2003;125:602. doi: 10.1115/1.1611512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albro M.B. Chahine N.O. Li R. Yeager K. Hung C.T. Ateshian G.A. Dynamic loading of deformable porous media can induce active solute transport. J Biomech. 2008;41:3152. doi: 10.1016/j.jbiomech.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chahine N.O. Albro M.B. Lima E.G. Wei V.I. Dubois C.R. Hung C.T. Ateshian G.A. Effect of dynamic loading on the transport of solutes into agarose hydrogels. Biophys J. 2009;97:968. doi: 10.1016/j.bpj.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckley C.T. Thorpe S.D. Kelly D.J. Engineering of large cartilaginous tissues through the use of microchanneled hydrogels and rotational culture. Tissue Eng Part A. 2009;15:3213. doi: 10.1089/ten.TEA.2008.0531. [DOI] [PubMed] [Google Scholar]
- 18.Benya P.D. Shaffer J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 19.Buschmann M.D. Gluzband Y.A. Grodzinsky A.J. Kimura J.H. Hunziker E.B. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 20.Mauck R.L. Soltz M.A. Wang C.C. Wong D.D. Chao P.H. Valhmu W.B. Hung C.T. Ateshian G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 21.Mouw J.K. Case N.D. Guldberg R.E. Plaas A.H. Levenston M.E. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthritis Cartilage. 2005;13:828. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Ng K.W. Lima E.G. Bian L. O'Conor C.J. Jayabalan P.S. Stoker A.M. Kuroki K. Cook C.R. Ateshian G.A. Cook J.L. Hung C.T. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A. 2009;16:1041. doi: 10.1089/ten.tea.2009.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selmi T.A. Verdonk P. Chambat P. Dubrana F. Potel J.F. Barnouin L. Neyret P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br. 2008;90:597. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 24.Comparison of autologous chondrocyte implantation versus mosaicplasty: a randomized trial (Cartipatch) ClinicalTrials.Gov. 2007. http://clinicaltrials.gov/ct2/show/record/NCT00560664?spons=%22Institut+National+de+la+Sant%C3%A9+Et+de+la+Recherche+M%C3%A9dicale%2C+France%22&sponsex=Y ClinicalTrials.Govhttp://clinicaltrials.gov/ct2/show/record/NCT00560664?spons=%22Institut+National+de+la+Sant%C3%A9+Et+de+la+Recherche+M%C3%A9dicale%2C+France%22&sponsex=Y
- 25.Gey G.O. Thalhimer W. Observations on the effects of insulin introduced into the medium of tissue cultures. JAMA. 1924;82:1609. [Google Scholar]
- 26.Bjare U. Serum-free cell culture. Pharmacol Ther. 1992;53:355. doi: 10.1016/0163-7258(92)90056-6. [DOI] [PubMed] [Google Scholar]
- 27.Hutton J.J., Jr. Trappel A.L. Udenfriend S. Requirements for alpha-ketoglutarate, ferrous ion and ascorbate by collagen proline hydroxylase. Biochem Biophys Res Commun. 1966;24:179. doi: 10.1016/0006-291x(66)90716-9. [DOI] [PubMed] [Google Scholar]
- 28.Helmy M.H. Ismail S.S. Fayed H. El-Bassiouni E.A. Effect of selenium supplementation on the activities of glutathione metabolizing enzymes in human hepatoma Hep G2 cell line. Toxicology. 2000;144:57. doi: 10.1016/s0300-483x(99)00190-0. [DOI] [PubMed] [Google Scholar]
- 29.Kisiday J.D. Kurz B. DiMicco M.A. Grodzinsky A.J. Evaluation of medium supplemented with insulin-transferrin-selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaffolds. Tissue Eng. 2005;11:141. doi: 10.1089/ten.2005.11.141. [DOI] [PubMed] [Google Scholar]
- 30.Kelly T.N. Fisher M.B. Oswald E.S. Tai T. Mauck R.L. Ateshian G.A. Hung C.T. Low-serum media and dynamic deformational loading in tissue engineering of articular cartilage. Ann Biomed Eng. 2008;36:769. doi: 10.1007/s10439-008-9476-1. [DOI] [PubMed] [Google Scholar]
- 31.Bottenstein J.E. Sato G.H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979;76:514. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeffrey J.J. Martin G.R. The role of ascorbic acid in the biosynthesis of collagen. II. Site and nature of ascorbic acid participation. Biochim Biophys Acta. 1966;121:281. doi: 10.1016/0304-4165(66)90117-6. [DOI] [PubMed] [Google Scholar]
- 33.Priddy N.H.n. Cook J.L. Kreeger J.M. Tomlinson J.L. Steffen D.J. Effect of ascorbate and two different media on canine chondrocytes in three-dimensional culture. Vet Ther. 2001;2:70. [PubMed] [Google Scholar]
- 34.Ibold Y. Lubke C. Pelz S. Augst H. Kaps C. Ringe J. Sittinger M. Effect of different ascorbate supplementations on in vitro cartilage formation in porcine high-density pellet cultures. Tissue Cell. 2009;41:249. doi: 10.1016/j.tice.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Bounelis P. Daniel J.C. The effect of ascorbate on embryonic chick sternal chondrocytes cultured in agarose. Tissue Cell. 1983;15:683. doi: 10.1016/0040-8166(83)90043-5. [DOI] [PubMed] [Google Scholar]
- 36.Stockwell R.A. Biology of Cartilage Cells. Cambridge: Cambridge University Press; 1979. [Google Scholar]
- 37.Otte P. Basic cell metabolism of articular cartilage. Manometric studies. Z Rheumatol. 1991;50:304. [PubMed] [Google Scholar]
- 38.Heywood H.K. Bader D.L. Lee D.A. Glucose concentration and medium volume influence cell viability and glycosaminoglycan synthesis in chondrocyte-seeded alginate constructs. Tissue Eng. 2006;12:3487. doi: 10.1089/ten.2006.12.3487. [DOI] [PubMed] [Google Scholar]
- 39.Mobasheri A. Vannucci S.J. Bondy C.A. Carter S.D. Innes J.F. Arteaga M.F. Trujillo E. Ferraz I. Shakibaei M. Martin-Vasallo P. Glucose transport and metabolism in chondrocytes: a key to understanding chondrogenesis, skeletal development and cartilage degradation in osteoarthritis. Histol Histopathol. 2002;17:1239. doi: 10.14670/HH-17.1239. [DOI] [PubMed] [Google Scholar]
- 40.Zhou S. Cui Z. Urban J.P.G. Nutrient gradients in engineered cartilage: metabolic kinetics measurement and mass transfer modeling. Biotechnol Bioeng. 2008;101:408. doi: 10.1002/bit.21887. [DOI] [PubMed] [Google Scholar]
- 41.Sengers B.G. Heywood H.K. Lee D.A. Oomens C.W.J. Bader D.L. Nutrient utilization by bovine articular chondrocytes: a combined experimental and theoretical approach. J Biomech Eng. 2005;127:758. doi: 10.1115/1.1993664. [DOI] [PubMed] [Google Scholar]
- 42.Huang A.H. Baker B.M. Ateshian G.A. Mauck R.L. Sliding contact loading enhances the tensile properties of mesenchymal stem cell-seeded hydrogels. Eur Cell Mater. 2012;24:29. doi: 10.22203/ecm.v024a03. [DOI] [PubMed] [Google Scholar]
- 43.Maas S.A. Ellis B.J. Ateshian G.A. Weiss J.A. FEBio: finite elements for biomechanics. J Biomech Eng. 2012;134:011005. doi: 10.1115/1.4005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mow V.C. Kuei S.C. Lai W.M. Armstrong C.G. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102:73. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 45.Bonet J. Wood R.D. New York: Cambridge University Press; 1997. Nonlinear Continuum Mechanics for Finite Element Analysis; pp. 117–143. [Google Scholar]
- 46.Ateshian G.A. Rajan V. Chahine N.O. Canal C.E. Hung C.T. Modeling the matrix of articular cartilage using a continuous fiber angular distribution predicts many observed phenomena. J Biomech Eng. 2009;131:061003. doi: 10.1115/1.3118773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly T.A. Ng K.W. Wang C.C. Ateshian G.A. Hung C.T. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39:1489. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 48.Hollander A.P. Heathfield T.F. Webber C. Iwata Y. Bourne R. Rorabeck C. Poole A.R. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 50.McGowan K.B. Kurtis M.S. Lottman L.M. Watson D. Sah R.L. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258. Osteoarthritis Cartilage. 2002;10:580. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 51.Ng K.W. Kugler L.E. Doty S.B. Ateshian G.A. Hung C.T. Scaffold degradation elevates the collagen content and dynamic compressive modulus in engineered articular cartilage. Osteoarthritis Cartilage. 2009;17:220. doi: 10.1016/j.joca.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soltz M.A. Ateshian G.A. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 53.Basalo I.M. Mauck R.L. Kelly T.A. Nicoll S.B. Chen F.H. Hung C.T. Ateshian G.A. Cartilage interstitial fluid load support in unconfined compression following enzymatic digestion. J Biomech Eng. 2004;126:779. doi: 10.1115/1.1824123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park S. Ateshian G.A. Dynamic response of immature bovine articular cartilage in tension and compression, and nonlinear viscoelastic modeling of the tensile response. J Biomech Eng. 2006;128:623. doi: 10.1115/1.2206201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson G.J. Vulpe C.D. Mammalian iron transport. Cell Mol Life Sci. 2009;66:3241. doi: 10.1007/s00018-009-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Latunde-Dada G.O. Simpson R.J. Regulation of iron absorption and distribution. In: Yehuda S., editor; Mostofsky D.I., editor. Iron Deficiency and Overload. New York: Humana Press; 2010. pp. 31–49. [Google Scholar]
- 57.Oswald E.S. Ahmed H.S. Kramer S.P. Bulinski J.C. Ateshian G.A. Hung C.T. Effects of hypertonic (NaCl) two-dimensional and three-dimensional culture conditions on the properties of cartilage tissue engineered from an expanded mature bovine chondrocyte source. Tissue Eng Part C. 2011;17:1041. doi: 10.1089/ten.tec.2011.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu X. Urban J.P.G. Tirlapur U.K. Cui Z. Osmolarity effects on bovine articular chondrocytes during three-dimensional culture in alginate beads. Osteoarthritis Cartilage. 2010;18:433. doi: 10.1016/j.joca.2009.10.003. [DOI] [PubMed] [Google Scholar]