Abstract
This study explored the possibility of producing transgenic cloned embryos by interspecies somatic cell nuclear transfer (iSCNT) of cattle, mice, and chicken donor cells into enucleated pig oocytes. Enhanced green florescent protein (EGFP)-expressing donor cells were used for the nuclear transfer. Results showed that the occurrence of first cleavage did not differ significantly when pig, cattle, mice, or chicken cells were used as donor nuclei (p>0.05). However, the rate of blastocyst formation was significantly higher in pig (14.9±2.1%; p<0.05) SCNT embryos than in cattle (6.3±2.5%), mice (4.2±1.4%), or chicken (5.1±2.4%) iSCNT embryos. The iSCNT embryos also contained a significantly less number of cells per blastocyst than those of SCNT pig embryos (p<0.05). All (100%) iSCNT embryos expressed the EGFP gene, as evidenced by the green florescence under ultraviolet (UV) illumination. Microinjection of purified mitochondria from cattle somatic cells into pig oocytes did not have any adverse effect on their postfertilization in vitro development and embryo quality (p>0.05). Moreover, NCSU23 medium, which was designed for in vitro culture of pig embryos, was able to support the in vitro development of cattle, mice, and chicken iSCNT embryos up to the blastocyst stage. Taken together, these data suggest that enucleated pig oocytes may be used as a universal cytoplast for production of transgenic cattle, mice, and chicken embryos by iSCNT. Furthermore, xenogenic transfer of mitochondria to the recipient cytoplast may not be the cause for poor embryonic development of cattle–pig iSCNT embryos.
Introduction
Interclass/intergenus/interspecies nuclear transfer (iSCNT) is valuable tool for studying nucleo-cytoplasmic interactions, rescuing endangered species whose oocytes are difficult to obtain, and generating stem cells in species in which authentic stem cells have not yet been established (Beyhan et al., 2007; Gomez et al., 2010; Hosseini et al., 2012; Sha et al., 2009). Introduction of genes into somatic cells prior to SCNT also offers opportunities for producing transgenic embryos and/or validating the success of iSCNT (Arat et al., 2003; Uhm et al., 2007; Uhm et al., 2009).
In recent years, successful cloned embryo production has been reported by iSCNT in several species (see Loi et al., 2011 for a detailed review). However, success of iSCNT appears to vary with the phylogenic differences between the donor karyoplast and the recipient cytoplast. In particular, mice somatic cells were found to be incompatible for iSCNT in the oocytes of nonrodent species such as cattle (Arat et al., 2003; Lagutina et al., 2011). Putative cloned embryos produced by iSCNT of mice somatic cells into nonrodent oocytes showed reduced embryonic development due to abnormalities in donor microtubule and centrosome organization (Zhong et al., 2005; Zhong et al., 2007), heterogeneous mitochondrial distribution (Zhong et al., 2008), chromosomal DNA loss, aberrant gene expression, and apoptosis (Jiang and St. John, 2008). Interestingly, phylogenically distant (at class level) chicken cells could be reprogrammed by rabbit (Liu et al., 2004) and cattle (Kim et al., 2004) oocytes. Thus, the reprogramming mechanism seems to be conserved across species, and phylogenic differences between the donor karyoplast and recipient cytoplast does not seem to be the sole cause of reduced embryonic development in iSCNT embryos (Amarnath et al., 2011a; Lagutina et al., 2011). Conversely, a universal recipient cytoplast may exist for use in iSCNT program.
Pig oocytes could reprogram somatic cells from rodents such as rabbit (Chen et al., 2006) and nonrodents such as dog (Lee et al., 2008; Sugimura et al., 2009), goat (Loi et al., 2011), tiger (Hashem et al., 2007), monkey (Qin et al., 2012), and cattle (Uhm et al., 2007). Pig oocytes were also shown to reprogram the somatic cells of rat that could not be reprogrammed by iSCNT into phylogenically closer mice oocytes (Sugawara et al., 2009). Chen et al. (2006) further observed that rabbit fibroblasts were better reprogrammed (demethylation of repetitive sequence) in pig oocytes than in rabbit oocytes themselves. Improved reprogramming of DNA methylation in repetitive DNA sequences was also observed in intraspecies pig SCNT embryos (Kang et al., 2001; Kang et al., 2003). Thus, it appears that pig oocytes may have a better reprogramming ability and, hence, may be a better candidate for being used as universal oocyte for iSCNT than other species.
Therefore, this study explored the possibility of using enucleated pig oocyte as a universal recipient cytoplast for production of transgenic cloned embryos by iSCNT. Donor cells, stably expressing the enhanced green fluorescent protein (EGFP) gene, from phylogenically distant cattle, mice, and chicken were used to test this possibility. We further investigated if xenogenic mitochondrial transfer from somatic cells to recipient cytoplast was a cause for the reduced embryonic development of iSCNT embryos.
Materials and Methods
All chemicals were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA) unless otherwise specifically indicated.
Preparation of somatic cells
Primary fibroblast cells from pig and cattle skin were prepared and transfected with EGFP by retroviral vector essentially as we described earlier (Uhm et al., 2007; Uhm et al., 2009). EGFP-expressing mouse fibroblast and bone marrow cells were obtained from transgenic mouse (a gift from Dr. Jin Young Ju, CHA Hospitals, South Korea) and transgenic chicken (Koo et al., 2006), as described elsewhere. Fibroblast cells were routinely passaged on 60-mm tissue culture dishes (Falcon BD, NJ, USA) in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Grand Island, NY, USA) supplemented with 10% (vol/vol) fetal bovine serum (FBS; Hyclone, Logan UT), 1% (vol/vol) minimum essential medium (MEM) nonessential amino acids (Gibco BRL), and 1% (vol/vol) penicillin-streptomycin (Gibco BRL) up to four to seven passages. Cells were synchronized to the G0/G1 stage of the cell cycle by confluence culture. Chicken bone marrow cells were used for SCNT without further cell culture.
Oocyte retrieval and in vitro maturation
Retrieval and in vitro maturation (IVM) of pig oocytes from abattoir-derived ovaries were performed essentially as we described earlier (Uhm et al., 2010). Briefly, cumulus–oocyte complexes (COCs) were aspirated from medium-sized follicles (3- to 6-mm diameter) and matured in groups of 50 in 500 μL of Tissue Culture Medium-199 with Earle's salts (TCM-199; Gibco BRL, Grand Island, NY, USA) supplemented with 25 mM NaHCO3, 10% (vol/vol) pig follicular fluid, 0.57 mM cysteine, 0.22 μg/mL sodium pyruvate, 25 μg/mL gentamicin sulfate, 0.5 μg/mL follicle-stimulating hormone (FSH; Folltropin V; Vetrepharm, Canada), 1 μg/mL estradiol-17β, and 10 ng/mL epidermal growth factor (EGF) under mineral oil at 39°C in a humidified atmosphere of 5% CO2 in air for 38–40 h.
Somatic cell nuclear transfer
The intraspecies SCNT and iSCNT were performed as we described earlier (Uhm et al., 2007; Uhm et al., 2009) with modifications. Briefly, IVM oocytes were denuded of cumulus cells in Tyrode's lactate (TL)-HEPES medium supplemented with 0.1% (wt/vol) hyaluronidase and incubated for 15 min in TCM-199 medium supplemented with 10% (vol/vol) FBS, 7.5 μg/mL cytochalasin B (CB), and 5 μg/mL Hoechst 33342. The oocytes were then enucleated by aspirating the first polar body and adjacent cytoplasm (∼20% of ooplasm), using a beveled borosilicate pipette, in HEPES-buffered TCM (HTCM-199, Gibco BRL) medium supplemented with 10% (vol/vol) FBS. Successful enucleation was confirmed by UV-assisted visualization of fluorescent metaphase plate in the aspirated cytoplasm contained within the enucleation pipette. Enucleated oocytes were subsequently reconstructed by inserting a donor cell into the perivitelline space of each enucleated oocytes and electrofusion by a single DC pulse of 2.1 kv/cm for 30 μs delivered by a BTX Electro Cell Manipulator 2001 (BTX, San Diego, CA, USA). In the case of chicken–pig iSCNT, donor cells were directly microinjected into the cytoplasm of the enucleated oocytes by whole cell intracytoplasmic injection (WCICI), as we described earlier (Das et al., 2010).
Electroactivation of oocytes
Electroactivation of oocytes was performed in activation medium (0.3 M mannitol, 0.1 mM MgSO4, and 1.0 mM CaCl2) by a single DC pulse of 1.0 kV/cm for 30 μs delivered by a BTX Electro Cell Manipulator 2001 (BTX, San Diego, CA, USA), essentially as we described earlier (Gupta et al., 2007a). Activated oocytes were then cultured in North Carolina State University 23 (NCSU23) medium supplemented with 7.5 μg/mL CB for 4 h.
Isolation and microinjection of mitochondria into oocytes
Mitochondria were isolated from granulosa cells of pig or cattle COCs. Briefly, granulosa cells were stripped off COCs by vortexing for 90 s in 0.1% (vol/vol) hyaluronidase solution, washed three times with phosphate-buffered saline (PBS), homogenized with a glass homogenizer, and centrifuged at 1000×g for 3 min at 4°C. The pellet was then used for mitochondria isolation using a mitochondria isolation kit (Pierce, IL, USA) following the manufacturer's protocol. Microinjection of mitochondria into the cytoplasm of oocytes was undertaken using a constant flow system (Femtojet, Eppendorf, Cambridge, UK) attached to an inverted microscope (Olympus, Tokyo, Japan) with manipulators (Narishige, Tokyo, Japan). Oocytes to be microinjected were partially denuded by repeat pipetting and placed in an elongated drop of ∼40 μL HTCM-199 supplemented with 10% (vol/vol) FBS under mineral oil on a 60-mm Petri dish. Each oocyte was then injected with ∼5 pL of mitochondria that was equivalent to mitochondria from approximately five granulosa cells. The microscope stage was maintained at 35°C during the entire procedure, which took approximately 15–30 min, depending on the number of oocytes.
In vitro fertilization of pig oocytes
In vitro fertilization (IVF) of pig oocytes was performed essentially as we described earlier (Gupta et al., 2009). Briefly, oocytes were partially denuded of cumulus cells using 0.1% (wt/vol) hyaluronidase and were placed into groups of 10–15 oocytes per 50-μL droplets of the fertilization medium [modified Tris-buffered medium containing 1 mM caffeine sodium benzoate and 0.1% bovine serum albumin (BSA)]. Boar sperm, retrieved from the cauda epididymis of abattoir-derived boar testes, were subjected to swim-up in sperm culture (Sp-TALP) medium for 10 min and added to the fertilization droplet to obtain a final sperm concentration of 5×105 cells/mL. In each experimental replicate, the semen was derived from the same testis for all the experimental groups. Sperm and oocytes were co-incubated at 39°C in a humidified atmosphere of 5% CO2 in air for 6 h.
In vitro culture of embryos
Embryos were cultured in groups of 12–15 per 50-μL microdroplets of NCSU23 medium supplemented with 0.4% (wt/vol) essentially fatty acid–free BSA under mineral oil in a humidified atmosphere of 5% CO2 in air at 37°C (mouse), 38.5°C (cattle), or 39°C (pig and chicken) (Gupta et al., 2008; Uhm et al., 2007). Embryonic development was recorded daily for 7 days, and rates of cleavage and blastocyst formation were expressed as percentage of total number of karyoplast–cytoplast couplets. The SCNT efficiency index was calculated as SCNT blastocyst rate/parthenogenetically activated (PA) blastocyst rate×100 (Illmensee et al., 2006). The hatching ability of embryos was expressed as: Number of blastocysts hatched out of zona pellucida/total number of blastocysts cultured×100. The total nuclei number of blastocysts was counted by fluorescent Hoechst 33342 staining, essentially as we described earlier (Gupta et al., 2007b). Expression of the EGFP gene was examined by viewing the cells and embryos under fluorescence microscope using a standard fluroescein isothiocyantate (FITC) filter set.
Statistical analyses
Statistical analyses were carried out using SPSS statistical software version 17.0 (SPSS Inc., Chicago, IL, USA). Embryo development data was analyzed by a chi-squared test or analysis of variance (ANOVA), and the means of cell number were compared by ANOVA followed by Bonferroni multiple pairwise comparison after testing for normality. Arc-sine transformation was performed before analyzing the percentage data. Data are presented as mean±standard error of the mean SEM. Differences at p<0.05 were considered significant.
Results
Experiment 1: In vitro development of transgenic cattle, mice, and chicken embryos produced by iSCNT into enucleated pig oocytes
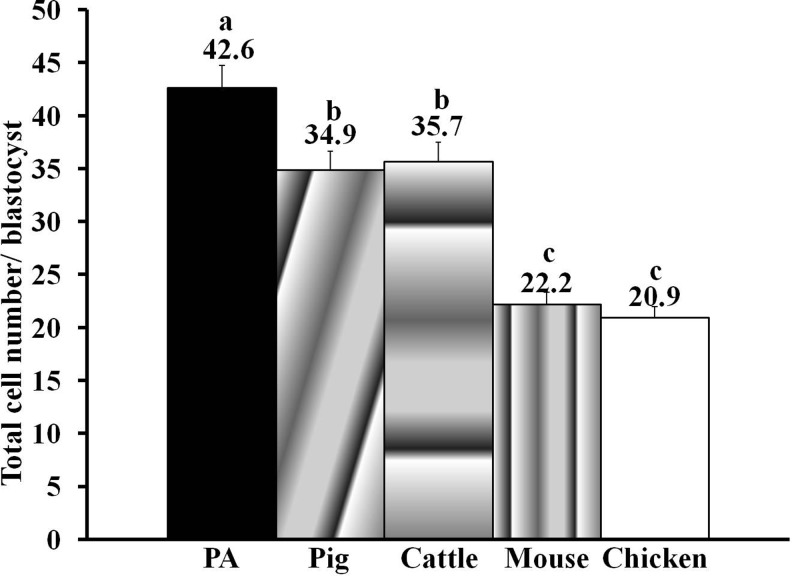
We first evaluated the in vitro development, blastocyst quality, and transgene expression in cattle, mice, and chicken iSCNT blastocysts. Because EGFP expression is easy to monitor under ultraviolet (UV) illumination in an epifluorescent microscope, EGFP-expressing cells from cattle, mice, and chicken were used as donor cells. EGFP-expressing pig fibroblasts were used for intraspecies SCNT in enucleated pig oocytes as control for comparison. Results showed that, cattle (75.0±2.8%), mice (74.0±3.7%), and pig (77.2±4.9%) donor cells fused with enucleated pig oocytes with similar efficiency (p>0.05; Table 1). However, chicken cells did not fuse well with the enucleated pig oocytes and, hence, the WCICI approach was used for the iSCNT of chicken cells. Results further showed that the occurrence of first cleavage and the rate of blastocyst formation in iSCNT embryos were significantly lower in iSCNT embryos than intraspecies SCNT embryos (Table 1). However, the SCNT efficiency index was similar for cattle, mice, and chicken iSCNT embryos (p>0.05). The total cell number per blastocyst in mice (22.2±4.7) and chicken (20.9±4.8) iSCNT embryos was significantly lower whereas that of cattle iSCNT embryos (35.7±4.6) was similar (p>0.05) to pig–pig intraspecies SCNT embryos (34.9±4.4) (Fig. 1).
Table 1.
In Vitro Development (Mean±SEM) and EGFP Expression in Cloned Embryos Produced by Somatic Cell Nuclear Transfer of Pig, Cattle, Mouse, or Chicken Cell into Enucleated Pig Oocytes
| |
|
|
No. (%) of embryos |
|
|
|
|---|---|---|---|---|---|---|
| Donor cell | No. of oocytes | No. (%) of couplets fused | Cleaved | Blastocyst | SCNT efficiency index (%)* | EGFP expression (%) |
| PA | 207 | — | 159 (76.8±2.8)a | 56 (27.1±2.2)a | — | — |
| PF | 240 | 186 (77.2±4.9)a | 129 (69.6±3.8)a | 28 (14.9±2.1)b | 55.6±5.5a | 100 (28) |
| CF | 189 | 142 (75.0±2.8)a | 82 (57.6±4.6)b | 9 (6.3±2.5)c | 20.2±4.9b | 100 (7) |
| MF | 158 | 177 (74.0±3.7)a | 65 (55.5±3.1)b | 5 (4.2±1.4)c | 15.8±3.0b | 100 (5) |
| CBC** | 134 | — | 51 (37.9±4.6)c | 7 (5.1±2.4)c | 15.6±3.3b | 100 (7) |
Development rates were calculated on the basis of number of fused couplets.
SCNT efficiency index=SCNT blastocyst rate/PA blastocyst rate×100.
WCICI method was used for iSCNT of CBC into the enucleated pig oocytes.
Values with different superscripts (a, b, c) within a column denote a statistical difference (p<0.05).
SEM, standard error of the mean; EGFP, enhanced green fluorescent protein; SCNT, somatic cell nuclear transfer; PA, parthenogenetic activation; PF, pig fibroblast; CF, cattle fibroblast; MF, mouse fibroblast; CBC, chicken bone marrow cell.
FIG. 1.
Total cell number [mean±standard error of the mean (SEM)] in blastocysts produced by somatic cell nuclear transfer of pig, cattle, mouse, or chicken cell into enucleated pig oocytes. PA, parthenogenetically activated pig blastocyst. Letters (a, b, c, d) over the bar denote statistical difference (p<0.05).
The timing of the first two cleavage divisions did not differ with the source of the donor cell. However, blastocyst formation could be observed after day 7 for cattle iSCNT, day 4 for mice iSCNT, and day 6 for chicken iSCNT. All iSCNT blastocysts expressed the EGFP protein, which, thereby, confirms their donor cell origin (Fig. S1) (Supplementary Data are available at www.liebertpub.com/cell/). Becuase previous studies have reported the developmental arrest of mice–pig iSCNT embryos, we further confirmed their donor cell origin by karyotyping. Indeed, mice–pig iSCNT embryos contained normal diploid 40 numbers of chromosomes (Fig. S2). Furthermore, mice–pig iSCNT embryos had XY chromosomes originating from the male donor cells used for the iSCNT. These data confirm the donor cell origin of the mice–pig iSCNT embryos.
Experiment 2: Effect of xenogenic mitochondrial transfer on postfertilization in vitro development of pig oocytes
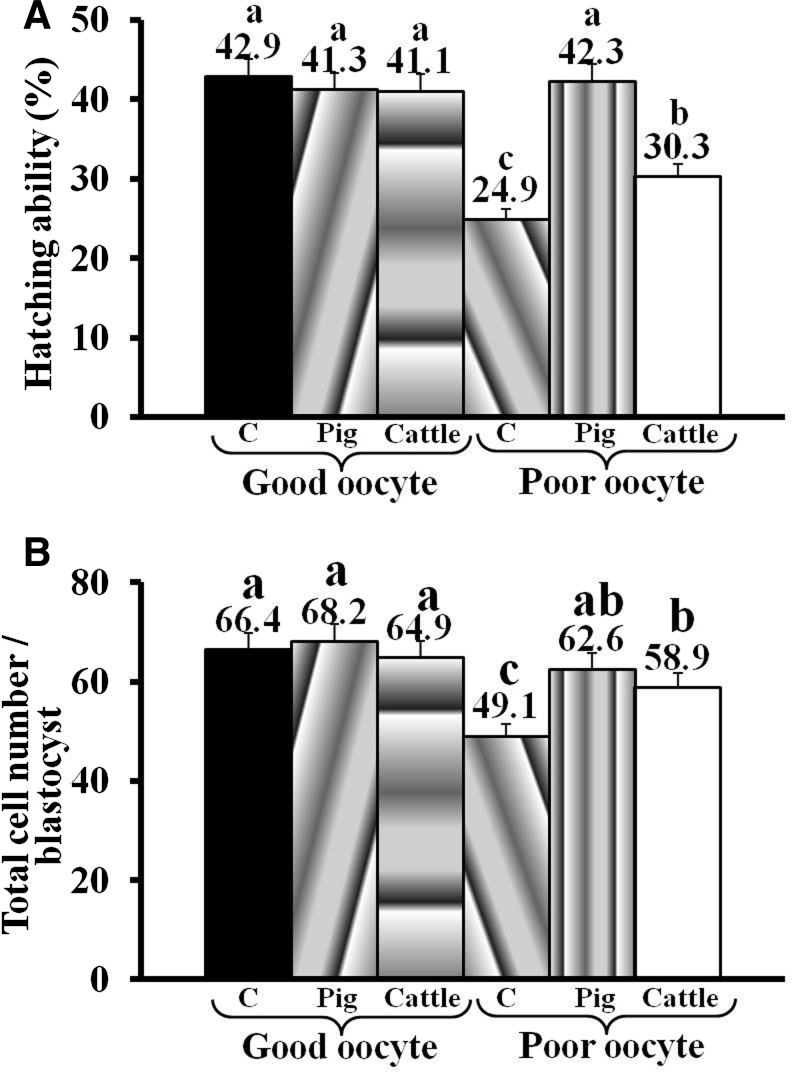
To investigate whether xenogenic mitochondrial transfer was the cause for lower in vitro development of iSCNT embryos, we microinjected the cattle mitochondria into pig oocytes and evaluated their postfertilization in vitro development. Because mitochondrial content of oocytes can have a direct effect on their embryonic development potential, we morphologically classified the pig oocytes as good quality (oocytes with more than five layers of cumulus cells and homogeneously spread granular cytoplasm) or poor quality (oocytes with less than five layers of cumulus cells and nonhomogeneously spread granular/agranular cytoplasm) oocytes, which were then confirmed to contain high and low amounts of mitochondrial DNA (mtDNA), respectively (Fig. S3). As shown in Table 2, good-quality oocytes had a significantly higher ability to cleave and form blastocysts than did poor-quality oocytes. Microinjection of purified mitochondria from pig or cattle granulosa cells had no significant effect on the in vitro development (Table 1; p>0.05) or blastocyst quality (Fig. 2; p>0.05) of good-quality pig oocytes. However, microinjection of pig mitochondria into poor-quality oocytes improved (p<0.05) their developmental ability and blastocyst quality, which was then comparable to those of noninjected good-quality oocytes. Nonetheless, microinjection of cattle mitochondria into poor-quality pig oocytes had no adverse effect (p>0.05) on the occurrence of cleavage (64.2±1.0 vs. 67.5±1.5%) and the rate of blastocyst formation (27.9±2.5 vs. 24.4±1.7%). In fact, microinjection of cattle mitochondria into poor-quality pig oocytes significantly improved the blastocyst quality in terms of their hatching ability (30.3±3.5 vs. 24.9±1.7%; p<0.05) and total cell number per blastocyst (58.7±3.5 vs. 49.1±3.4; p<0.05) (Fig. 2).
Table 2.
Effect (Mean±SEM) of Interspecies Mitochondrial Transfer on Postfertilization In Vitro Development of Pig Oocytes
| Quality of oocyte | Source of mitochondria | No. of oocytes | No. (%) of embryo cleaved | No. (%) of blastocyst |
|---|---|---|---|---|
| Good | Control | 250 | 190 (76.0±1.2)a | 77 (40.5±2.3)a |
| Pig | 232 | 183 (78.9±2.3)a | 80 (43.7±0.8)a | |
| Cattle | 246 | 185 (75.2±1.7)a | 68 (36.6±2.0)a | |
| Poor | Control | 164 | 164 (67.5±1.5)b | 40 (24.4±1.7)b |
| Pig | 188 | 188 (74.0±1.8)a | 71 (37.8±1.3)a | |
| Cattle | 154 | 154 (64.2±1.0)b | 43 (27.9±2.5)b |
Each oocyte was then injected with approximately 5 pL of mitochondria that was equivalent to mitochondria from approximately five granulosa cells.
Values with different superscripts (a, b) within a column denote a statistical difference (p<0.05).
SEM, standard error of the mean.
FIG. 2.
Hatching ability (A) and total cell number per blastocyst (B) in in vitro–fertilized pig blastocysts produced from good- or poor-quality oocytes microinjected with pig or cattle mitochondria. (C) Noninjected controls. Letters (a, b, c) over the bar denote statistical difference (p<0.05).
Discussion
Use of genetically modified donor cells for iSCNT not only offers a method of transgenic animal production but also provides solid evidence for donor cell origin of the resultant cloned embryos. The latter is particularly important because iSCNT embryos in several species are known to show normal chromatin remodeling and embryonic development beyond the embryonic genomic activation (EGA) stage, but they fail to develop further (Arat et al., 2003; Lee et al., 2008) due to aberrant nuclear reprogramming and gene expression (Arat et al., 2003; Chung et al., 2009; Lagutina et al., 2010; Lagutina et al., 2011; Ostrup et al., 2012; Wang et al., 2009; Wang et al., 2011). In this study, using EGFP-expressing donor cells, we have shown that pig oocytes are capable of supporting the early embryonic development of cattle, mice, and chicken nuclei at least up to the blastocyst stage. Consistent with previous reports (Loi et al., 2011), the in vitro development of iSCNT embryos was lower than that of intraspecies SCNT embryos. However, the lower development was possibly not related to xenogenic mitochondrial transfer from donor cells to recipient cytoplasts.
Pig oocytes have previously been reported to reprogram the donor cell nuclei from cattle (Uhm et al., 2007). However, they were inefficient in reprogramming the somatic cell nuclei from mice (Amarnath et al., 2011a; Arat et al., 2003; Jiang and St. John 2008; Lagutina et al., 2011). The cause for developmental failure of mice–pig embryos is not known but are believed to be due to removal of microtubule-organizing centers (MTOCs) from enucleated oocytes during the iSCNT procedure (Zhong et al., 2007). Unlike many nonrodent species, MTOCs in mice embryos are inherited from the maternal side; thus, their absence in the enucleated oocyte might contribute to developmental failure (Zhong et al., 2005; Zhong et al., 2007). The successful generation of transgenic mice–pig iSCNT embryos in our study may likely be reminiscent of pig MTOCs in the enucleated oocyte and their subsequent incorporation into the donor cell MTOCs. This seems likely because components of donor cell centrosomes involved in spindle assembly are not species-specific and may contribute to the formation of a normal functional mitotic spindle along with the reminiscent centrosomal materials in the recipient cytoplast (Zhong et al., 2005). Further experiments are, however, required to test this possibility.
Pig oocytes could also reprogram the bone marrow cells from chicken to successfully produce the transgenic chicken iSCNT blastocyst that morphologically resembled mammalian blastocysts. However, under the electrofusion conditions used in this study, chicken cells did not fuse well with the enucleated pig oocytes. Species-specific differences in fusion of donor cells with recipient cytoplasts has been reported earlier, and it is likely that changes in fusion parameters might improve the fusion efficiency of chicken cells with pig oocytes. However, we preferred to use the WCICI approach for iSCNT of chicken cells into pig oocytes, as we and other have described earlier (Das et al., 2010; Lee et al., 2003). Indeed, the WCICI approach successfully resulted in the generation of chicken iSCNT embryos. This result is similar to two previous reports that documented the successful generation of chicken blastocysts by iSCNT of chicken fibroblast into enucleated rabbit (Liu et al., 2004) and cattle (Kim et al., 2004) oocytes. We further noted that, irrespective of the method of nuclear transfer, the SCNT efficiency index was similar for cattle, mice, and chicken iSCNT embryos. Thus, the reprogramming mechanism appears to be conserved among pig, cattle, mice, and chicken. However, similar to previous reports, the efficiency of reprogramming was lower in iSCNT than in intraspecies SCNT (Jiang and St. John 2008; Lagutina et al., 2011; Loi et al., 2011; Uhm et al., 2007). Furthermore, the number of embryonic cell divisions supported by the pig cytoplasm seems to be a limiting factor, because total cell number per blastocyst in cattle–pig and mice–pig iSCNT embryos was lower than those reported for intraspecies SCNT embryos or IVF embryos (Boiani et al., 2003; Dindot et al., 2004).
The cause for low development of iSCNT embryos is not clear at present, but is believed to be a cumulative result of multiple factors, including defective nucleologenesis (Song et al., 2009), partial EGA (Lagutina et al., 2010; Wang et al., 2009; Wang et al., 2011), abnormalities in donor microtubule and centrosome organization (Zhong et al., 2007), mitochondrial heteroplasmy (Takeda et al., 2012; Zhong et al., 2008), poor nucleocytoplasmic compatibility (Amarnath et al., 2011a; Lagutina et al., 2010; Lagutina et al., 2011), chromosomal DNA loss, aberrant gene expression, and apoptosis (Jiang and St. John 2008). Recent studies have also suggested that the presence of donor cell cytoplasm and/or mitochondria can negatively influence the embryonic development of embryos by disrupting the normal cellular processes, including metabolic function and cell division (Amarnath et al., 2011a; Hua et al., 2012; Sansinena et al., 2011; Van Thuan et al., 2006). Thus, to address the possible nucleocytoplasmic incompatibility due to xenogenic mitochondrial transfer from donor cell to recipient cytoplast, we microinjected the purified mitochondria from cattle somatic cells to pig oocytes and investigated their embryonic development in vitro. Interestingly, we did not observe any adverse effect of xenogenic mitochondrial transfer on in vitro development and embryo quality of pig oocytes. Our results are consistent with those of Chiaratti et al. (2010), who observed that xeno-ooplasmic transfer of buffalo ooplasm into bovine zygotes did not affect the blastocyst development. Takeda et al. (2012) also observed that microinjection of mtDNA from mice fibroblasts did not affect the in vitro development of cattle oocytes. Interestingly, we also observed that microinjection of purified mitochondria into poor-quality pig oocytes (having low mtDNA content) had a beneficial effect on improving the embryo quality in terms of total cell number per blastocyst and hatching ability. Thus, xenogenic mitochondrial transfer may, in fact, offer a strategy for improving the developmental ability of poor-quality oocytes having low mtDNA content.
The in vitro development of iSCNT embryos was also reported to vary with the in vitro culture (IVC) medium used for culturing the embryos (Liu et al., 2004). It still remains uncertain whether donor-specific or recipient-specific embryo culture medium may be suitable for optimal in vitro development of iSCNT embryos (Chang et al., 2003; Yamochi et al., 2012; Yang et al., 2003; Zhao et al., 2007). Amaranth et al. (2011b) observed that pig embryo culture medium could support in vitro development of mouse embryos. In our study, NCSU23 medium, which was designed for in vitro culture of pig embryos (Gupta et al., 2008; Petters et al., 1990), was able to support the in vitro development of cattle, mice, and chicken iSCNT embryos up to the blastocyst stage. The expression of EGFP protein was observed in all iSCNT blastocysts; this not only indicates successful transgenesis via iSCNT but also confirms the donor cell origin of the embryos.
In conclusion, our data suggest that the enucleated pig oocyte provides a universal cytoplast for production of transgenic cattle, mice, and chicken embryos by iSCNT and supports the notion that reprogramming factors in the ooplasm may be conserved across species. Furthermore, xenogenic transfer of mitochondria to the recipient cytoplast may not be the cause for poor embryonic development of cattle–pig iSCNT embryos.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 program (#PJ009046) of RDA, Republic of Korea.
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- Amarnath D. Choi I. Moawad A.R., et al. Nuclear-cytoplasmic incompatibility and inefficient development of pig–mouse cytoplasmic hybrid embryos. Reproduction. 2011a;142:295–307. doi: 10.1530/REP-11-0044. [DOI] [PubMed] [Google Scholar]
- Amarnath D. Wakayama S. Zhu J., et al. The novel use of modified pig zygotic medium for the efficient culture of the preimplantation mouse embryos. Theriogenology. 2011b;76:1639–1646. doi: 10.1016/j.theriogenology.2011.06.028. [DOI] [PubMed] [Google Scholar]
- Arat S. Rzucidlo S.J. Stice S.L. Gene expression and in vitro development of inter-species nuclear transfer embryos. Mol. Reprod. Dev. 2003;66:334–342. doi: 10.1002/mrd.10362. [DOI] [PubMed] [Google Scholar]
- Beyhan Z. Iager A.E. Cibelli J.B. Interspecies nuclear transfer: Implications for embryonic stem cell biology. Cell Stem Cell. 2007;1:502–512. doi: 10.1016/j.stem.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Boiani M. Eckardt S. Leu N.A., et al. Pluripotency deficit in clones overcome by clone-clone aggregation: Epigenetic complementation? EMBO J. 2003;22:5304–5312. doi: 10.1093/emboj/cdg507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.H. Lim J.M. Kang S.K., et al. Blastocyst formation, karyotype, and mitochondrial DNA of interspecies embryos derived from nuclear transfer of human cord fibroblasts into enucleated bovine oocytes. Fertil. Steril. 2003;80:1380–1387. doi: 10.1016/j.fertnstert.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Chen T. Zhang Y.L. Jiang Y., et al. Interspecies nuclear transfer reveals that demethylation of specific repetitive sequences is determined by recipient ooplasm but not by donor intrinsic property in cloned embryos. Mol. Reprod. Dev. 2006;73:313–317. doi: 10.1002/mrd.20421. [DOI] [PubMed] [Google Scholar]
- Chiaratti M.R. Ferreira C.R. Meirelles F.V., et al. Xenooplasmic transfer between buffalo and bovine enables development of homoplasmic offspring. Cell. Reprogram. 2010;12:231–236. doi: 10.1089/cell.2009.0076. [DOI] [PubMed] [Google Scholar]
- Chung Y. Bishop C.E. Treff N.R., et al. Reprogramming of human somatic cells using human and animal oocytes. Cloning Stem Cells. 2009;11:213–223. doi: 10.1089/clo.2009.0004. [DOI] [PubMed] [Google Scholar]
- Das Z.C. Gupta M.K. Uhm S.J., et al. Lyophilized somatic cells direct embryonic development after whole cell intracytoplasmic injection into pig oocytes. Cryobiology. 2010;61:220–224. doi: 10.1016/j.cryobiol.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Dindot S.V. Farin P.W. Farin C.E., et al. Epigenetic and genomic imprinting analysis in nuclear transfer derived Bos gaurus/Bos taurus hybrid fetuses. Biol. Reprod. 2004;71:470–478. doi: 10.1095/biolreprod.103.025775. [DOI] [PubMed] [Google Scholar]
- Gomez M.C. Serrano M.A. Pope C.E., et al. Derivation of cat embryonic stem-like cells from in vitro-produced blastocysts on homologous and heterologous feeder cells. Theriogenology. 2010;74:498–515. doi: 10.1016/j.theriogenology.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Gupta M.K. Uhm S.J. Han D.W., et al. Embryo quality and production efficiency of porcine parthenotes is improved by phytohemagglutinin. Mol. Reprod. Dev. 2007a;74:435–444. doi: 10.1002/mrd.20547. [DOI] [PubMed] [Google Scholar]
- Gupta M.K. Uhm S.J. Lee H.T. Cryopreservation of immature and in vitro matured porcine oocytes by solid surface vitrification. Theriogenology. 2007b;67:238–248. doi: 10.1016/j.theriogenology.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Gupta M.K. Uhm S.J. Lee S.H., et al. Role of nonessential amino acids on porcine embryos produced by parthenogenesis or somatic cell nuclear transfer. Mol. Reprod. Dev. 2008;75:588–597. doi: 10.1002/mrd.20789. [DOI] [PubMed] [Google Scholar]
- Gupta M.K. Jang J.M. Jung J.W., et al. Proteomic analysis of parthenogenetic and in vitro fertilized porcine embryos. Proteomics. 2009;9:2846–2860. doi: 10.1002/pmic.200800700. [DOI] [PubMed] [Google Scholar]
- Hashem M.A. Bhandari D.P. Kang S.K., et al. Cell cycle analysis and interspecies nuclear transfer of in vitro cultured skin fibroblasts of the Siberian tiger (Panthera tigris Altaica) Mol. Reprod. Dev. 2007;74:403–411. doi: 10.1002/mrd.20528. [DOI] [PubMed] [Google Scholar]
- Hosseini S.M. Hajian M. Forouzanfar M., et al. Enucleated ovine oocyte supports human somatic cells reprogramming back to the embryonic stage. Cell. Reprogram. 2012;14:155–163. doi: 10.1089/cell.2011.0061. [DOI] [PubMed] [Google Scholar]
- Hua S. Lu C. Song Y., et al. High levels of mitochondrial heteroplasmy modify the development of ovine-bovine interspecies nuclear transferred embryos. Reprod. Fertil. Dev. 2012;24:501–509. doi: 10.1071/RD11091. [DOI] [PubMed] [Google Scholar]
- Illmensee K. Levanduski M. Zavos P.M. Evaluation of the embryonic preimplantation potential of human adult somatic cells via an embryo interspecies bioassay using bovine oocytes. Fertil. Steril. 2006;85(Suppl 1):1248–1260. doi: 10.1016/j.fertnstert.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Jiang Y. St. John J. Development of interspecies cloned mouse embryos reconstructed with porcine enucleated oocytes. Reprod. Fertil. Dev. 2008;21:117–118. [Google Scholar]
- Kang Y.K. Koo D.B. Park J.S., et al. Typical demethylation events in cloned pig embryos. Clues on species-specific differences in epigenetic reprogramming of a cloned donor genome. J. Biol. 2001;76:39980–39984. doi: 10.1074/jbc.M106516200. [DOI] [PubMed] [Google Scholar]
- Kang Y.K. Lee K.K. Han Y.M. Reprogramming DNA methylation in the preimplantation stage: peeping with Dolly's eyes. Curr. Opin. Cell Biol. 2003;15:290–295. doi: 10.1016/s0955-0674(03)00031-0. [DOI] [PubMed] [Google Scholar]
- Kim T.M. Park T.S. Shin S.S., et al. An interclass nuclear transfer between fowl and mammal: in vitro development of chicken-to-cattle interclass embryos and the detection of chicken genetic complements. Fertil. Steril. 2004;82:957–959. doi: 10.1016/j.fertnstert.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Koo B.C. Kwon M.S. Choi B.R., et al. Production of germline transgenic chickens expressing enhanced green fluorescent protein using a MoMLV-based retrovirus vector. FASEB J. 2006;20:2251–2260. doi: 10.1096/fj.06-5866com. [DOI] [PubMed] [Google Scholar]
- Lagutina I. Fulka H. Brevini T.A., et al. Development, embryonic genome activity and mitochondrial characteristics of bovine-pig inter-family nuclear transfer embryos. Reproduction. 2010;140:273–285. doi: 10.1530/REP-09-0578. [DOI] [PubMed] [Google Scholar]
- Lagutina I. Zakhartchenko V. Fulka H., et al. Formation of nucleoli in interspecies nuclear transfer embryos derived from bovine, porcine, and rabbit oocytes and nuclear donor cells of various species. Reproduction. 2011;141:453–465. doi: 10.1530/REP-10-0266. [DOI] [PubMed] [Google Scholar]
- Lee E. Kim J.H. Park S.M., et al. The analysis of chromatin remodeling and the staining for DNA methylation and histone acetylation do not provide definitive indicators of the developmental ability of inter-species cloned embryos. Anim. Reprod. Sci. 2008;105:438–450. doi: 10.1016/j.anireprosci.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Lee J.W. Wu S.C. Tian X.C., et al. Production of cloned pigs by whole-cell intracytoplasmic microinjection. Biol. Reprod. 2003;69:995–1001. doi: 10.1095/biolreprod.103.015917. [DOI] [PubMed] [Google Scholar]
- Liu S.Z. Zhou Z.M. Chen T., et al. Blastocysts produced by nuclear transfer between chicken blastodermal cells and rabbit oocytes. Mol. Reprod. Dev. 2004;69:296–302. doi: 10.1002/mrd.20091. [DOI] [PubMed] [Google Scholar]
- Loi P. Modlinski J.A. Ptak G. Interspecies somatic cell nuclear transfer: A salvage tool seeking first aid. Theriogenology. 2011;76:217–228. doi: 10.1016/j.theriogenology.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Ostrup O. Strejcek F. Petrovicova I., et al. Role of ooplasm in nuclear and nucleolar remodeling of intergeneric somatic cell nuclear transfer embryos during the first cell cycle. Cell. Reprogram. 2012;13:145–155. doi: 10.1089/cell.2010.0061. [DOI] [PubMed] [Google Scholar]
- Petters R.M. Johnson B.H. Reed M.L., et al. Glucose, glutamine and inorganic phosphate in early development of the pig embryo in vitro. J. Reprod. Fertil. 1990;89:269–275. doi: 10.1530/jrf.0.0890269. [DOI] [PubMed] [Google Scholar]
- Qin Z.X. Huang G.B. Luo J., et al. Effect of TSA and VPA treatment on long-tailed macaque (Macaca fascicularis)-pig interspecies somatic cell nuclear transfer. Yi Chuan. 2012;34:342–347. doi: 10.3724/sp.j.1005.2012.00342. [DOI] [PubMed] [Google Scholar]
- Sansinena M.J. Lynn J. Bondioli K.R., et al. Ooplasm transfer and interspecies somatic cell nuclear transfer: Heteroplasmy, pattern of mitochondrial migration and effect on embryo development. Zygote. 2011;19:147–156. doi: 10.1017/S0967199410000419. [DOI] [PubMed] [Google Scholar]
- Sha H.Y. Chen J.Q. Chen J., et al. Fates of donor and recipient mitochondrial DNA during generation of interspecies SCNT-derived human ES-like cells. Cloning Stem Cells. 2009;11:497–507. doi: 10.1089/clo.2009.0021. [DOI] [PubMed] [Google Scholar]
- Song B.S. Lee S.H. Kim S.U., et al. Nucleologenesis and embryonic genome activation are defective in interspecies cloned embryos between bovine ooplasm and rhesus monkey somatic cells. BMC Dev. Biol. 2009;9:44. doi: 10.1186/1471-213X-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara A. Sugimura S. Hoshino Y., et al. Development and spindle formation in rat somatic cell nuclear transfer (SCNT) embryos in vitro using porcine recipient oocytes. Zygote. 2009;17:195–202. doi: 10.1017/S0967199409005322. [DOI] [PubMed] [Google Scholar]
- Sugimura S. Narita K. Yamashiro H., et al. Interspecies somatic cell nucleus transfer with porcine oocytes as recipients: A novel bioassay system for assessing the competence of canine somatic cells to develop into embryos. Theriogenology. 2009;72:549–559. doi: 10.1016/j.theriogenology.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Takeda K. Srirattana K. Matsukawa K., et al. Influence of intergeneric/interspecies mitochondrial injection; parthenogenetic development of bovine oocytes after injection of mitochondria derived from somatic cells. J. Reprod. Dev. 2012;58:323–329. doi: 10.1262/jrd.2011-013. [DOI] [PubMed] [Google Scholar]
- Uhm S.J. Gupta M.K. Kim T., et al. Expression of enhanced green fluorescent protein in porcine- and bovine-cloned embryos following interspecies somatic cell nuclear transfer of fibroblasts transfected by retrovirus vector. Mol. Reprod. Dev. 2007;74:1538–1547. doi: 10.1002/mrd.20755. [DOI] [PubMed] [Google Scholar]
- Uhm S.J. Gupta M.K. Das Z.C., et al. Effect of transgene introduction and recloning on efficiency of porcine transgenic cloned embryo production in vitro. Reprod. Domest. Anim. 2009;44:106–115. doi: 10.1111/j.1439-0531.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- Uhm S.J. Gupta M.K. Yang J.H., et al. Epidermal growth factor can be used in lieu of follicle-stimulating hormone for nuclear maturation of porcine oocytes in vitro. Theriogenology. 2010;73:1024–1036. doi: 10.1016/j.theriogenology.2009.11.029. [DOI] [PubMed] [Google Scholar]
- Van Thuan N. Wakayama S. Kishigami S., et al. Injection of somatic cell cytoplasm into oocytes before intracytoplasmic sperm injection impairs full-term development and increases placental weight in mice. Biol. Reprod. 2006;74:865–873. doi: 10.1095/biolreprod.105.047803. [DOI] [PubMed] [Google Scholar]
- Wang K. Beyhan Z. Rodriguez R.M., et al. Bovine ooplasm partially remodels primate somatic nuclei following somatic cell nuclear transfer. Cloning Stem Cells. 2009;11:187–202. doi: 10.1089/clo.2008.0061. [DOI] [PubMed] [Google Scholar]
- Wang K. Otu H.H. Chen Y., et al. Reprogrammed transcriptome in rhesus-bovine interspecies somatic cell nuclear transfer embryos. PLoS One. 2011;6:e22197. doi: 10.1371/journal.pone.0022197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamochi T. Kida Y. Oh N., et al. Development of interspecies cloned embryos reconstructed with rabbit (Oryctolagus cuniculus) oocytes and cynomolgus monkey (Macaca fascicularis) fibroblast cell nuclei. Zygote Apr. 2012;5:1–9. doi: 10.1017/S0967199412000019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Yang C.X. Han Z.M. Wen D.C., et al. In vitro development and mitochondrial fate of macaca-rabbit cloned embryos. Mol. Reprod. Dev. 2003;65:396–401. doi: 10.1002/mrd.10320. [DOI] [PubMed] [Google Scholar]
- Zhao Z.J. Li R.C. Cao H.H., et al. Interspecies nuclear transfer of Tibetan antelope using caprine oocyte as recipient. Mol. Reprod. Dev. 2007;74:412–419. doi: 10.1002/mrd.20608. [DOI] [PubMed] [Google Scholar]
- Zhong Z. Spate L. Hao Y., et al. Remodeling of centrosomes in intraspecies and interspecies nuclear transfer porcine embryos. Cell Cycle. 2007;6:1510–1520. [PubMed] [Google Scholar]
- Zhong Z. Hao Y. Li R., et al. Analysis of heterogeneous mitochondria distribution in somatic cell nuclear transfer porcine embryos. Microsc. Microanal. 2008;14:418–432. doi: 10.1017/S1431927608080896. [DOI] [PubMed] [Google Scholar]
- Zhong Z.S. Zhang G. Meng X.Q., et al. Function of donor cell centrosome in intraspecies and interspecies nuclear transfer embryos. Exp. Cell Res. 2005;306:35–46. doi: 10.1016/j.yexcr.2005.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.