Abstract
Systemic lupus erythematosus (SLE) is a prototype of systemic autoimmunity affecting many systems. Both antibodies and autoreactive T cells play significant roles in its pathogenesis. Experimental data and clinical observations indicate that autoimmunity and end organ damage are under separate genetic control and that there are significant interactions between these two pathways. Experimental evidence has been obtained to support the hypothesis that autoantibodies and autoreactive T effector cells may be initiated by environmental factors through molecular mimicry and the inherent polyreactive nature of antigen receptors. A unified hypothesis has been postulated for the pathogenesis of SLE that has practical implications.
Systemic lupus erythematosus (SLE) is characterized by the presence of autoantibodies of diverse specificities and protean clinical presentation. It is considered a prototype of systemic autoimmunity. In the recent past, significant progress has been made in our understanding of the pathogenesis of SLE (Tsokos, 2011; Crow, 2013) During the past several years, we have carried out genetic studies on a novel lupus prone mouse model, NZM2328 (Waters et al, 2001; Waters et al., 2004; Ge et al., 2009). We have also utilized mice expressing human HLA-DR antigens to explore the role of microbes in the initiation of autoantibody response to lupus-related antigens (Deshmukh et al., 2011). The results lead us to put forward the hypothesis that autoimmunity and end organ damage are under separate genetic controls. Although autoimmunity may be an inherent part of the immune response, it does not necessarily lead to end organ damage (Waters et al., 2004; Ge et al., 2009). HLA-DR as a susceptibility gene exerts its effect through presentation of microbial antigens to T cells that are also reactive to autoantigens (Deshmukh et al., 2011). The latter conclusion puts great emphasis on the influence of environmental factors on the pathogenesis of SLE. In addition, the initiation and expansion of lupus related autoantibodies may be mediated by responses to environmental antigens.
End Organ Damage vs Autoimmunity
In considering the pathogenesis of autoimmune diseases, autoimmunity either at the antibody or effector levels traditionally has been emphasized (Tsokos, 2011; Crow, 2013). The most recent GWAS studies on autoimmune disorders such as SLE, multiple sclerosis, type 1 diabetes mellitus and rheumatoid arthritis have identified a shared set of genes that contributes to immune responsiveness (Crow, 2013; Rose and Bell, 2012; Tsokos, 2011). Little attention has been devoted to the responsiveness of target organs to the effector antibodies or the cells. The underlying cause for this dichotomy is not apparent. Perhaps it is due to the inherent nature of the term, “autoimmune diseases”.
Experimental Model NZM2328 for Proliferative Lupus Nephritis
In experimental lupus glomerulonephritis, the (NZB/NZW) F1 has been a well-studied murine model (Thiofilopoulos and Dixon, 1985). In this model, the female mice develop anti-nuclear antibodies and immune complex mediated glomerulonephritis (GN) resulting in premature death. Recently, revived interest in the New Zealand strains of mice has been generated due to the availability of the New Zealand Mixed (NZM) mice (Rudofsky et al., 1999). The NZM mice are inbred strains of mice generated from extended intercrosses from NZB/NZW F1 with various contributions from NZB and NZW. It is of interest that these strains have various phenotypes including anti-nuclear antibodies (ANA) and varying clinical presentation such as immune complex mediated GN and neurological disorder. For example, NZM64 mice have positive ANA without any clinical symptoms while NZM2410 mice have positive ANA and chronic GN (cGN) with early death in both sexes. The data from NZM strains support the hypothesis that autoimmunity and end organ damage are under separate genetic control.
Our laboratory has been using NZM2328 as a model for genetic and cellular studies on lupus proliferative GN (Waters et al., 2001; Waters et al., 2004; Ge et al., 2009). In this strain, the majority of mice mice develop ANA and anti-dsDNA antibodies. cGN and severe proteinuria with associated early mortality are seen only in females. In males, despite the presence of circulating autoantibodies that are similar to the females in titers and kinetics of development, very few of them progress to cGN with end stage renal disease and premature death. In a backcross analysis of (NZM2328XC57L/J)F1XNZM2328 (Waters et al., 2001), a single locus, Adaz1 was identified to be associated with the production of ANA and anti-dsDNA antibodies on chromosome 4. In this backcross analysis, acute GN (aGN) that is characterized by cellular proliferation, focal necrosis and epithelial crescents with little tubular or interstitial changes is observed as a distinct phenotype from cGN that may have tubular dilatation and interstitial infiltrate and fibrosis in addition to all the characteristics of aGN. A single locus, Cgnz1 that associated with cGN was identified on the distal end of chromosome 1. Three loci were identified to be associated with aGN: Agnz1 on chromosome 1 distal to Cgnz1, the H-2-Tnf complex and Agnz2 distal to the H2-Tnf complex on chromosome 17. Two congenic lines NZM2328.Lc1(Lc1) and NZM2328.Lc4 (Lc4) were generated by introgression of a genetic segment of C57L/J from chromosome 1 or 4 containing Cgnz1 and Agnz1 or Adaz1 to NZM2328 (Waters et al., 2004). The phenotype of these two congenic lines confirmed the genetic data in that Lc1 females have markedly reduced incidence of proliferative GN with no early mortality and that Lc4 females have cGN and end stage of renal failure in a manner not distinguishable from NZM2328 despite lack of ANA and anti-dsDNA antibodies. These data support the thesis that autoimmune responses can be separated from end organ damage and that anti-ds DNA and ANA need not be a part of lupus nephritis.
Recently, we have generated multiple intrachromosomal 1 recombinant strains from Lc1. One of them named NZM2328.Lc1R27 (R27) in which an 8 Mb genetic segment from C57L/J that contains only Cgnz1, was introgressed to NZM2328 (Ge et al., 2009). The female mice of R27 have immune mediated GN without severe proteinuria. These female mice have normal renal function and normal life span. Additional experimental evidence has been obtained that support the thesis that Cgnz1 confers end organ resistance to damage. Thus it is firmly established that autoimmunity and end organ damage are under separate genetic control.
Clinical Observations
For clinical investigation, the American College of Rheumatology has published classification criteria for SLE (Tan et al., 1982; Hochberg, 1997). Among the proposed eleven criteria, nine address clinical presentations and the remaining two pertain to the presence of autoantibodies. In a previous review (Fu et al., 2011), these eleven criteria were classified into two broad categories: end organ damage and autoimmunity. The manifestations of end organ damage include malar rash, discoid rash, photosensitivity, oral ulcers, non-erosive arthritis, serositis, renal disorders with persistent proteinuria and cellular casts, neurologic disorders such as psychosis and seizure and hematologic disorders including hemolytic anemia, leukopenia, lymphopenia and thrombocytopenia. The two criteria for serologic evidence of autoimmunity are (A) antibodies to native DNA in abnormal titers, antibody to Sm nuclear antigen, and/or positive finding of antiphospholipid antibodies based on (a) an abnormal serum level of IgG or IgM anticardiolipin antibodies, (b) a positive test result for lupus anticoagulant using a standard method, and/or (c) a false-positive serologic test for syphilis and (B) an abnormal titer of ANA by immunofluorescence. The consensus recommendation was that SLE patients should have at least four of these 11 criteria either concurrently or sequentially during their illness. Implicit in these classification criteria is the scenario that individuals may have detectable levels of autoantibodies as commonly measured in clinical laboratories without any symptoms and that patients may have SLE without the presence of autoantibodies that are customarily tested in the clinical laboratories. Although not stated in the initial publication, the committee could not resolve the question as to the unique usefulness of autoimmune parameters as measured in clinical laboratories. None of the autoantibodies has been directly linked to specific end organ damage. Numerous clinical examples exist of individuals with positive autoantibody tests who never go on to develop manifestations of autoimmune disease further emphasizing that the presence of autoantibodies is not sufficient to lead to the development of end organ damage.
In clinical practice, the presentation and course of SLE are variable (Tsokos, 2011). In the cases of lupus nephritis, the responsiveness of patients to therapy is also variable. Nevertheless, despite the progress in therapy of lupus nephritis, the incidence of end stage renal disease in patients with lupus nephritis remains constant over the past two decades (Ward, 2009) suggesting that some patients are very susceptible to end organ damage while the majority of the cases are relatively resistant. Whether this difference is under genetic control is being investigated.
Recently, a familial form of SLE has been reported with six Arab families involved (Al-Mayouf et al., 2011). The inheritance represents a dominant recessive pattern. All six families have a history of consanguinity. The candidate gene was identified to be a loss-of-function variant in DNASE1L3. Of 27 offspring, 17 were diagnosed to have SLE. The clinical presentations of 17 affected individuals were of interest (table 1). Eleven of them have nephritis with hypocomplementemia. One of these patients with nephritis had no detectable anti-dsDNA antibodies. The other six individuals had positive ANA and anti-dsDNA antibodies along with hypocomplementemia. The intervals between the diagnosis of SLE and the time of the publication were 1. 1.5, 5.5, 7.5, 8 and 13 years. Thus despite consanguinity and the presence of lupus nephritis in at least one sibling and marked serological abnormalities, the phenotype of these six individuals suggest the presence of genes that are capable to confer resistance to kidney damage.
Table 1.
Characteristics of subjects with DNASE1L3-related SLE (Al–Mayouf et al., 2011)
| ID | Age (yrs) |
Age at onset (yrs) |
Gender | SLE Activity Index |
Nephritis | Serology Profile |
|---|---|---|---|---|---|---|
| F1-A | 3.5 | 2 | F | 13 | − | ANA, anti-dsDNA, ANCA, low c3 and c4 |
| F1-B | 9.5 | 4 | F | 8 | − | ANA, anti-dsDNA, ANCA, low c3 and c4 |
| F1-C | 11 | 5 | M | 14 | + | ANA, anti-dsDNA, ANCA, low c3 and c4 |
| F2-A | 11 | 2.5 | F | 11 | + | ANA, anti-dsDNA, ANCA, low c3 and c4, anti-card |
| F2-B | 5 | 4.5 | M | 18 | + | ANA, anti-dsDNA, ANCA, low c3 and c4, anti-card |
| F2-C | 10 | 6 | F | 9 | + | ANA, anti-dsDNA, ANCA, low c3 and c4, anti-card |
| F2-D | 6 | 4.5 | M | 22 | + | ANA, anti-dsDNA, ANCA, low c3 and c4, anti-card |
| F3-A | 4.5 | 3 | M | 18 | + | ANA, anti-dsDNA, ANCA, low c3 and c4 |
| F3-B | 15 | 2 | M | 8 | + | ANA, anti-dsDNA, ANCA, low c3 and c4 |
| F4-A | 12 | 4.5 | F | 13 | − | ANA, anti-dsDNA, ANCA, low c3 and c4 |
| F4-B | 9 | 8 | F | 14 | + | ANA, anti-dsDNA, ANCA, low c3 and c4 |
| F5-A | 13 | 3 | M | 14 | + | ANA, anti-dsDNA, low C3 and c4 |
| F5-B | 10 | 5 | M | 11 | + | ANA, low c3 and c4 |
| F5-C | 22 | 12 | M | 12 | + | ANA, anti-dsDNA, low C3 and C4 |
| F5-D | 24 | 11 | M | 10 | − | ANA, anti-dsDNA, low C3 and C4, anti-card |
| F6-A | 14 | 6 | M | 10 | − | ANA, anti-dsDNA, low C3 and C4 |
| F6-B | 12 | 11 | M | 9 | − | ANA, anti-dsDNA, low C3 and C4 |
−, negative; +, positive; ANA, anti-nuclear antibodies; anti-dsDNA, antibody recognizing double-stranded DNA; ANCA, anti-neutrophil cytoplasmic antibodies; anti-card, anti-cardiolipin antibody
On a population basis, a recent publication (Satoh et al., 2012) suggests that more than 32 million persons in the US population have positive ANA and the prevalence was 13.8% for individuals over 12. This prevalence remains relatively constant for each decade of age. These findings suggest that in the majority of individuals with positive ANA, SLE does not develop further suggesting that breaking tolerance to nuclear antigens as indicated by being ANA positive may not have clinical significance.
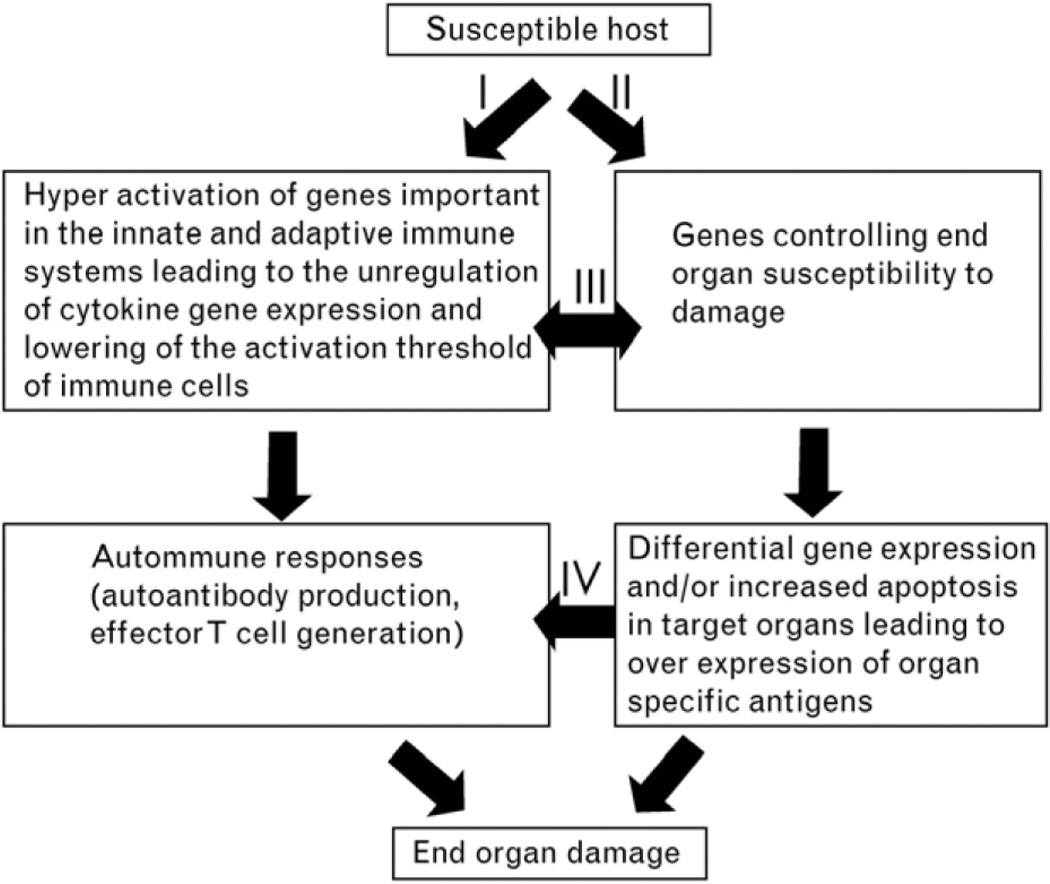
In summary, both experimental results and clinical data suggest that autoimmunity and end organ damage are under separate genetic control and that autoimmunity may not lead to end organ damage as depicted in figure 1. These conclusions suggest that SLE remains a clinical diagnosis and that the application of the ACR classification criteria should be flexible. In addition, individual variability should be taken into consideration in treating SLE patients.
Figure 1.
Proposed model for the pathogenesis of SLE describes the independent and yet interactive nature of the genes contributes to autoimmunity and end organ damage. I, Autoantibody production and activation of effector T cells and II, activation of susceptibility for end organ damage, can be initiated independently while they interact at different levels as indicated by pathways III and IV. Interaction of these pathways leads to end organ damage (Waters et al., 2004).
Origin of Autoantibodies to Lupus-Related Autoantibodies and Autoreactive T Cells in SLE
As reviewed by Crow (Crow, 2013), the etiology of SLE remains largely unknown. Similarly the causes of appearance of autoantibodies such as ANA in both patients and normal individuals remain to be determined. However, emergent evidence in the press and from our laboratory suggests that molecular mimicries at the T cell level should be considered seriously.
Despite the identification of more than 30 candidate lupus susceptibility genes, the MHC complex region remains the most important loci for SLE (Graham et al., 2007, Hom et al., 2008). Utilizing mice expressing human HLA-DR regions, we have shown that mice with human HLA-DR2 and DR3 are capable of generating a diverse immune response while those with human DR4 failed to do so (Paisansinsup et al., 2002; Jiang et al., 2010; Deshmukh et al., 2011). Most importantly mice with DR3 can make antibodies to proteins within the snRNP particles including the SmD, SmB, A and C proteins (Jiang et al., 2010). In addition, they make anti-dsDNA antibodies. About 1/3 of these anti-dsDNA antibodies could be absorbed with the immunogen SmD. These results are congruent with the observation by Reichlin et al that anti-dsDNA antibodies are cross-reactive with proteins within the snRNP particles (Reichlin et al., 1994). These data provide significant information regarding the role of HLA-D region in the pathogenesis of SLE and they support the thesis that the origin of the anti-dsDNA antibodies in humans is the result of the generation of antibodies to snRNP some of which are cross reactive with dsDNA.
Although there has been a considerable amount of literature regarding the role of molecular mimicry in multiple sclerosis, experimental autoimmune encephalitis and other autoimmune diseases such as type 1 diabetes (Hemmer et al., 1999; Martin et al., 2001; Wucherpfenning et al., 2007; Wucherpfennig and Sethi, 2011), little has been published in this area regarding SLE. In view of the observation that lupus autoantibody response is antigen driven and T cell dependent (Peng and Craft, 1996), we have devised an experimental system to identify microbial mimics that can stimulate T cells autoreactive to SLE related autoantigens. Some of these mimics have the capability to induce lupus related autoantibodies (Deshmukh et al., 2011). In this system, we have taken advantage of the availability of mice that do not express endogenous class II antigen but express the HLA-DR3 transgene. We were able to demonstrate that these mice make antibodies to snRNP, nuclear antigens and dsDNA in response to immunization with SmD (Jiang et al., 2010). The choice of SmD as the immunogen is based on the observation that the amino acid sequences of mouse and human SmD are identical. The T cell epitopes in the context of presentation by DR3 were identified. One of the dominant epitopes was identified to be located within SmD79–83 (Deshmukh et al., 2011). A T-T hybridoma, C1P2 reactive to SmD79–83 was generated following immunization with SmD and used for further experiments. By alanine substitution, aa81-aa88 were found to be involved in either binding DR3 or TCR. By homology search with allowable amino acid substitutions, 20 peptides were identified to be of human or microbial origin. In addition to reactivity to SmD and SmD79–83, the T-T hybridomas, C1P2 was found to be reactive with P20, a peptide from the galactoside transporter aa145–159 of Vibrio cholera. With P20 as the immunogen, another T-T hybridoma, P20P1 was generated. This hybridoma was polyreactive in that it was reactive to SmD, SmD79–83, P20, P17 (TcmP methyltransferase aa215-aa229 from Streptococcus agalactiae), and P11 (La related protein 1 aa926-aa229 from Homo sapien). Immunization with P20, P17 and P11 revealed that P20 is immunogenic in that the immunized mice made antibodies to the immunogen as well as to A protein within the snRNP. Some of these observations are summarized in table 2.
Table 2.
Hybridomas C1P2 and P20P1 react with different antigens and use different TCRs (modified from Deshmukh et al., 2011)
| Hybridoma | Immunogen | Reactivity | Vα | Vβ |
|---|---|---|---|---|
| C1P2 | SmD | SmD, aSmD79e93, bP20 | TRAV6D-7*04; TRAJ43*01 | TRBV13-1*02; TRBD1*01; TRBJ1-1*01 |
| P20P1 | P20 | SmD, SmD79e93, P20, cP11, dP17 | TRAV12D-2*02; TRAJ16*01 | TRBV13-3*01; TRBD2*01; TRBJ2-5*01 |
SmD79–93 LLVDVEPKVKSKKRE SmD aa79-aa93 (Homo sapiens)
P20 LD I D IDPKVKVATLS Galactoside ABC transporter aa145-aa159 (V. cholera)
P11 KNLD I DPKLQEYLGK La-related protein 1 aa926-aa940 (Homo sapien)
P17 EI VDLDPKLKQ I NL I TcmP methyltransferase aa215-aa229 (S. agalactiae)
It is apparent that many of the autoreactive T cells are polyreactive. They utilize multiple TCR. It is also important to recognize that these T cell epitopes have diverse chemical structures. The observation that autoreactive T cells can readily identified from the immunization with a microbial peptide such as P20 supports the thesis that autoreactive T cells and autoantibodies are frequently generated from infections. This thesis is supported by the detection of lupus related autoantibodies such as ANA, anti-phospholipid antibodies in patients with subacute endocarditis (Bonaci-Nikolic et al., 2007), a condition with constant antigenic stimulation resembling that in an autoimmune response. Additionally, this is supported by the clinical observation of lupus flares in SLE patients following infections.
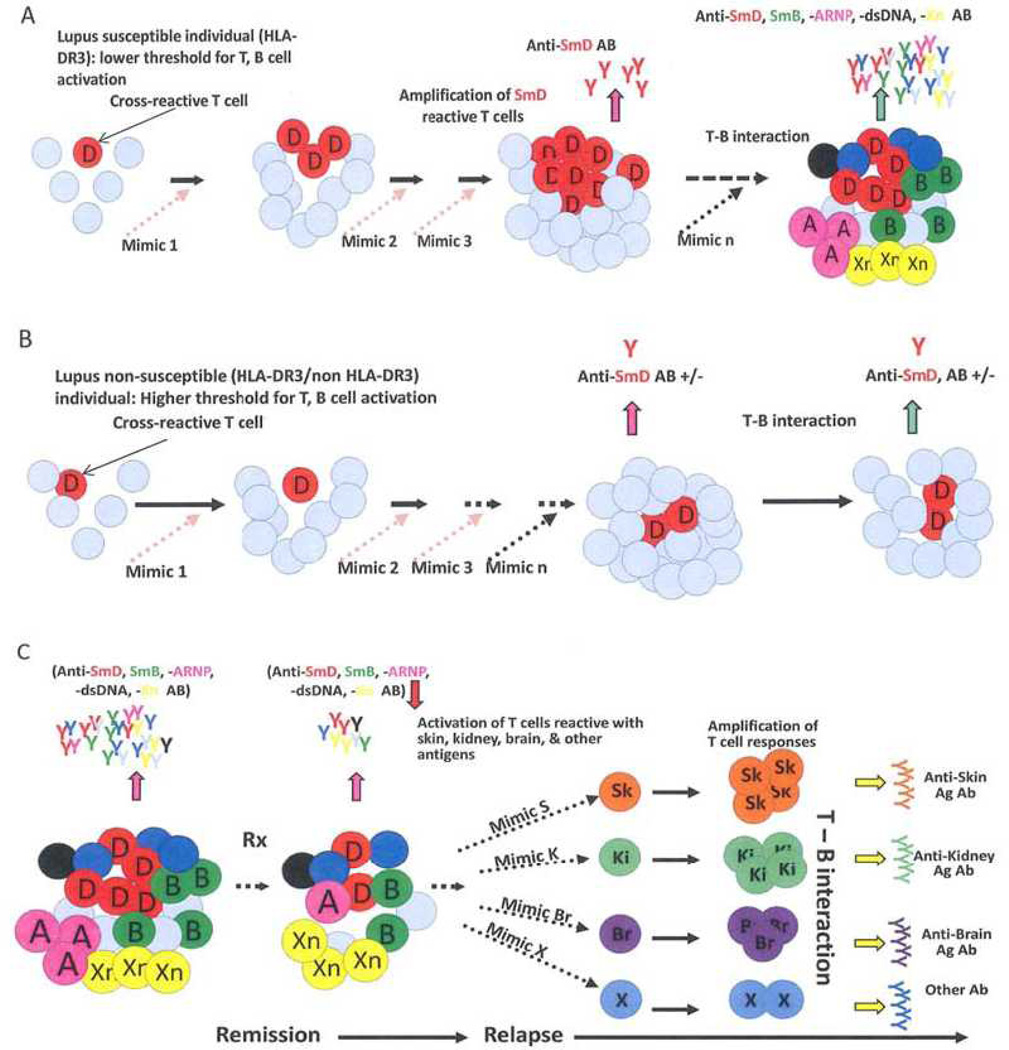
The fact that microbial antigens can initiate an autoimmune response should not be surprising in view of the well-established paradigm that positive selection of T cells are based on self class II molecules for CD4+ T cells and on self class I molecules for CD8+ T cells. This suggests that all T cells have the potential of being autoreactive. It is peripheral regulation that keeps these T cells at bay. These circulating autoreactive T cell are likely to have intermediate and low affinity for autoantigens. Through negative selection, autoreactive T cells with high affinity are deleted. Thus it is likely that lupus-related autoantibody responses can be initiated by multiple mimics in a DR restricted manner. Once initiated, autoantigens and innate immune mechanisms can amplify the autoimmune response leading to epitope spreading. This process results in the generation of autoantibodies of complex reactivity and effector T cell again various organs. This process is depicted in figure 2.
Figure 2.
Generation of autoreactive Abs and effector T cells in SLE by environmental T cell epitope mimics. Accumulation of cross-reactive T cells is a consequence of responses to environmental mimics in hosts withsusceptibility genes as depicted in A but not in hosts without these genes as depicted in B. In C, the accumulation of diverse autoreactive Abs and T cells as a response to these mimic results in varied SLE clinical presentation. After therapy, the complexity of these autoantibodies and autoreactive T cells are reduced, leading to remission. Over a period of time after discontinuing therapy, the complexity of autoantibodies and effector T cells returns, leading to a protean clinical presentation in relapses. The mimics reside on a diverse array of environmental antigens and the chances for exposure to these mimics are random, providing a scenario in that SLE is not caused by a single pathogen. This mechanism has the flavor of a stochastic process (Fu et al., 2011).
Conclusions
In this review, we have outlined our views on the pathogenesis of SLE. Our hypothesis places an emphasis on the dissociation of autoimmunity from end organ damage and the likelihood that environmental antigens play a major role in the initiation of autoantibodies and the generation of autoreactive T effector cells that may cause end organ damage. The hypothesis explains the protean clinical presentation in SLE, the dominant role of HLA-D region in SLE susceptibility and the remitting/relapsing nature of the illness. It offers a logical approach in therapy in that serological abnormality without end organ damage should not be treated with immunosuppressive drugs and that inflammation of any cause should be suppressed as a part of therapy and prevention of relapses.
Acknowledgements
We thank Ms Lena Garrison for her help in preparing this manuscript. This work was supported in part by NIH grants P50-AR04522, R01-AR047988, R01-AR049449 and R01-AI079621 and grants from Alliance for Lupus Research.
Footnotes
Disclosure Statement
Authors do not have any known or potential conflicts of interest.
References
- Al-Mayouf SM, Sunker A, Abdwani R, Abrawi SA, Almurshedi F, Alhashmi N, Sonbul A, Sewairi W, Qari A, Abdallah E, Al-Owain M, Al Motywee S, Al-Rayes H, Hashem M, Khalak H, Al-Jebali L, Alkuraya FS. Loss-of-function variant in DNASE1L3 causes a familial form of systemic lupus erythematosus. Nat Genet. 2011;43:1186–1188. doi: 10.1038/ng.975. [DOI] [PubMed] [Google Scholar]
- Bonaci-Nikolic B, Andrejevic S, Pavlovic M, Dimcic Z, Ivanovic B, Nikolic M. Prolonged infections associated with antineutrophil cytoplasmic antibodies specific to proteinase 3 and myeloperoxidase: diagnostic and therapeutic challenge. Clin Rheumatol. 2010;29:893–904. doi: 10.1007/s10067-010-1424-4. [DOI] [PubMed] [Google Scholar]
- Crow MK. Etiology and Pathogenesis of Systemic Lupus Erythematosus. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O'Dell JR, editors. Kelly's Textbook of Rheumatology. Philadelphia PA: Elsevier Saunders; 2013. pp. 1269–1282. [Google Scholar]
- Deshmukh US, Sim DL, Dai C, Kannapell CJ, Gaskin F, Rajagopalan G, David CS, Fu SM. HLA-DR3 restricted T cell epitope mimicry in induction of autoimmune response to lupusassociated antigen SmD. J Autoimmun. 2011;37:254–262. doi: 10.1016/j.jaut.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SM, Deshmukh US, Gaskin F. Pathogenesis of systemic lupus erythematosus revisited 2011: end organ resistance to damage, autoantibody initiation and diversification, and HLA-DR. J Autoimmun. 2011;37:104–112. doi: 10.1016/j.jaut.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Jiang C, Gaskin F, Sung SJ, Bagavant H, Fu SM. Pathogenesis of proliferative lupus nephritis: different genetic control for acute and chronic glomerulonephritis and new insight into the mechanism of immune complex mediated nephritis. Arthritis Rheum. 60:S755. abstract, 2009. [Google Scholar]
- Graham RR, Ortmann WA, Langefeld CD, Jawaheer D, Selby SA, Rodine PR, Baechler EC, Rohlf KE, Shark KB, Espe KJ, Green LE, Nair RP, Stuart PE, Elder JT, King RA, Moser KL, Gaffney PM, Bugawan TL, Erlich HA, Rich SS, et al. Visualizing human leukocyte antigen class II risk haplotypes in human systemic lupus erythematosus. Am J Hum Genet. 2002;71:543–553. doi: 10.1086/342290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmer B, Gran B, Zhao Y, Marques A, Pascal J, Tzou A, Kondo T, Cortese I, Bieleko va B, Straus SE, McFarland HF, Houghten R, Simon R, Pinilla C, Martin R. Identification of candidate T-cell epitopes and molecular mimics in chronic Lyme disease. Nat Med. 1999;5:1375–1382. doi: 10.1038/70946. [DOI] [PubMed] [Google Scholar]
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725–1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- Hom G, Graham RR, Modrek B, Taylor KE, Ortmann W, Garnier S, Lee AT, Chung SA, Ferreira RC, Pant PV, Ballinger DG, Kosoy R, Demirci FY, Kamboh MI, Kao AH, Tian C, Gunnarsson I, Bengtsson AA, Rantapaa-Dahlqvist S, Petri M, et al. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. N Engl J Med. 2008;358:900–909. doi: 10.1056/NEJMoa0707865. [DOI] [PubMed] [Google Scholar]
- Jiang C, Deshmukh US, Gaskin F, Bagavant H, Hanson J, David CS, Fu SM. Differential responses to Smith D autoantigen by mice with HLA-DR and HLA-DQ transgenes: dominant responses by HLA-DR3 transgenic mice with diversification of autoantibodies to small nuclear ribonucleoprotein, double-stranded DNA, and nuclear antigens. J Immunol. 2010;184:1085–1091. doi: 10.4049/jimmunol.0902670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Gran B, Zhao Y, Markovic-Plese S, Bielekova B, Marques A, Sung MH, Hemmer B, Simon R, McFarland HF, Pinilla C. Molecular mimicry and antigen-specific T cell responses in multiple sclerosis and chronic CNS Lyme disease. J Autoimmun. 2001;16:187–192. doi: 10.1006/jaut.2000.0501. [DOI] [PubMed] [Google Scholar]
- Paisansinsup T, Deshmukh US, Chowdhary VR, Luthra HS, Fu SM, David CS. HLA class II influences the immune response and antibody diversification to Ro60/Sjogren's syndrome-A: heightened antibody responses and epitope spreading in mice expressing HLA-DR molecules. J Immunol. 2002;168:5876–5884. doi: 10.4049/jimmunol.168.11.5876. [DOI] [PubMed] [Google Scholar]
- Peng SL, Craft J. T cells in murine lupus: propagation and regulation of disease. Mol Biol Rep. 1996;23:247–251. doi: 10.1007/BF00351176. [DOI] [PubMed] [Google Scholar]
- Reichlin M, Martin A, Taylor-Albert E, Tsuzaka K, Zhang W, Reichlin MW, Koren E, Ebling FM, Tsao B, Hahn BH. Lupus autoantibodies to native DNA cross-react with the A and D SnRNP polypeptides. J Clin Invest. 1994;93:443–449. doi: 10.1172/JCI116980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AM, Bell LC. Epistasis and immunity: the role of genetic interactions in autoimmune diseases. Immunology. 2012;137:131–138. doi: 10.1111/j.1365-2567.2012.03623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudofsky UH, Evans BD, Balaban SL, Mottironi VD, Gabrielsen AE. Differences in expression of lupus nephritis in New Zealand mixed H-2z homozygous inbred strains of mice derived from New Zealand black and New Zealand white mice. Origins and initial characterization. Lab Invest. 1993;68:419–426. [PubMed] [Google Scholar]
- Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, Jusko TA, Walker NJ, Germolec DR, Whitt IZ, Crockett PW, Pauley BA, Chan JY, Ross SJ, Birnbaum LS, Zeldin DC, Miller FW. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012;64:2319–2327. doi: 10.1002/art.34380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- Ward MM. Changes in the incidence of endstage renal disease due to lupus nephritis in the United States, 1996–2004. J Rheumatol. 2009;36:63–67. doi: 10.3899/jrheum.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters ST, Fu SM, Gaskin F, Deshmukh US, Sung SS-J, Kannapell CC, Tung KS, McEwen SB, McDuffie M. NZM2328: a new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci. Clin Immunol. 2001;100:372–383. doi: 10.1006/clim.2001.5079. [DOI] [PubMed] [Google Scholar]
- Waters ST, McDuffie M, Bagavant H, Deshmukh US, Gaskin F, Jiang C, Tung KS, Fu SM. Breaking tolerance to double stranded DNA, nucleosome, and other nuclear antigens is not required for the pathogenesis of lupus glomerulonephritis. J Exp Med. 2004;199:255–264. doi: 10.1084/jem.20031519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig KW, Allen PM, Celada F, Cohen IR, DeBoer R, Garcia KC, Goldstein B, Greenspan R, Hafler D, Hodgkin P, Huseby ES, Krakauer DC, Nemazee D, Perelson AS, Pinilla C, Strong Rk, Sercarz EE. Polyspecificity of T cell and B cell receptor recognition. Semin Immunol. 2007;19:216–224. doi: 10.1016/j.smim.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig KW, Sethi D. T cell receptor recognition of self and foreign antigens in the induction of autoimmunity. Semin Immunol. 2011;23:84–91. doi: 10.1016/j.smim.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]