Abstract
Age-related priming of microglia and release of inflammatory cytokines, such as interleukin-1β (IL-1β) and interleuekin-6 (IL-6) have been associated with deficits in cognitive function. The present study assessed whether treatment with minocycline could improve spatial cognition in aged mice, and whether these improvements in behavior were associated with reduced microglia activation and an enhancement in hippocampal neurogenesis. Adult (3 months) and aged (22 months) male BALB/c mice received minocycline in their drinking water or control mice received distilled water for 20 days. Mice received BrdU to label dividing cells on days 8–17. Spatial learning was measured using the water maze. Immunohistochemistry was conducted to measure number of BrdU positive neurons and number and size of microglia by detection of Iba-1 in the dentate gyrus molecular layer. Further, hippocampal samples were collected to measure changes in IL-1β, IL-6, and CD74 expression. The data show that aged mice have increased hippocampal expression of IL-1β, IL-6, and CD74 relative to adults. Minocycline treatment significantly improved acquisition of the water maze in aged mice but not adults. Minocycline reduced the average size of Iba-1 positive cells and total Iba-1 counts, but did not affect hippocampal cytokine gene expression. Minocycline increased neurogenesis in adults but not aged mice. Collectively, the data indicate that treatment with minocycline may recover some aspects of cognitive decline associated with aging, but the effect appears to be unrelated to adult hippocampal neurogenesis.
Keywords: Microglia, water maze, hippocampus, aging, IL-1β, IL-6, CD74, Iba-1 cytokines
INTRODUCTION
Neuroinflammation has been proposed to contribute to the progression of neurodegenerative diseases and the occurrence of cognitive deficits associated with aging [1–4]. This age-related change in the inflammatory profile of the brain likely results from alterations in the activation status of the brain’s primary immune cell, microglia. Research has established that microglia from aged animals are primed to express an inflammatory phenotype [5–7]. Additionally, increased basal levels of the pro-inflammatory cytokines interleukin-1β (IL-1β) and interleukin-6 (IL-6) have been reported in aged animals [8, 9]. Together these data confirm that normal aging increases immune activation within the brain.
Correlational work in humans has shown a connection between inflammation and cognitive function. For instance, higher serum levels of IL-6 are associated with an increased risk of cognitive deficits [2]. A more recent study found that 50% of elderly patients with mild cognitive impairment had increased microglia activation compared to age-matched controls [3]. Moreover, individuals with metabolic syndrome that show increased inflammation have more pronounced cognitive deficits compared to those that have low levels of inflammation [1]. Though limited, research with animal models has shown similar results. Gemma et al. [6] report that administration of a caspase-1 inhibitor (prevents cleavage of IL-1β into mature form) improved contextual memory in aged rats. Additionally, chronic administration of a selective cycloxygenase-2 (COX-2) inhibitor, if given around middle-age, improves performance in the water maze [10]. Collectively, these data provide evidence that age-related changes in neuroinflammation may contribute to cognitive decline, but additional work is needed to confirm this link and determine the role microglial cells play in maintenance of these deficits.
Accompanying age-related cognitive deficits are deficiencies in measures of neural plasticity such as hippocampal neurogenesis. Aged subjects show decreases in both the proliferation and survival of new hippocampal cells, which may contribute to cognitive decline associated with aging as hippocampal neurogenesis has been suggested to play a role in certain forms of hippocampus-dependent memory [11–14]. The age-associated deficits in hippocampal neurogenesis have been proposed to, in part, result from increased inflammatory signaling in the brain. For instance, administration of a caspase-1 inhibitor increased production of new hippocampal neurons in aged subjects [15]. Similarly, Bachstetter et al. [16] reported that administration of fractalakine, a chemokine that inhibits microglia activation, increased hippocampal neurogenesis in aged rats. Chronic overexpression of transforming growth factor-β (TGF-β) has been reported to decrease hippocampal neurogenesis [17]. Existing data indicate that age-related changes in neuroinflammation may contribute to the reductions in neurogenesis. Whether there is a direct link between inflammation-induced reductions in neurogenesis and age-related cognitive decline remains unknown.
The present study investigated whether repeated administration of the anti-inflammatory drug minocycline, improves cognitive function and increases the survival and neural differentiation of new cells in aged mice. Minocycline is a tetracycline antibiotic that has been shown to inhibit microglial cell activation [18–20]. Therefore the present study tested the hypothesis that chronic administration of minocycline would improve spatial leaning in aged mice by increasing hippocampal neurogenesis and reducing microglial cell activation.
2. METHODS
2.1. Animals
Subjects were adult (3 months, n=19) and aged male (22 months, n=16) BALB/c mice purchased from Charles River Laboratories (Roanoke, IL). Aged mice were purchased at 7 months old and then aged in the AAALAC approved facility at the Beckman Institute at the University of Illinois. A second group of adult and aged male mice (adult n=12, aged n=10) were purchased from the National Institute of Aging colony (maintained by Charles River Laboratories) and used in Experiment 2. Mice were given ad libitum access to food and housed under a 12 hr light/dark cycle. Animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals and the experiments were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign (protocol number 09167 and Animal Welfare Assurance Number A3118-01).
2.2. Minocycline treatment
Minocycline was administered in the animal’s drinking water. Minocycline was dissolved in distilled water at different concentrations for adult and aged mice. Since aged mice weigh more than adults they received minocycline at a concentration of 0.533 mg/ml and adult were given minocycline at a concentration of 0.416 mg/ml. These different concentrations were used to ensure that mice would receive the same dosage based on body weight, for instance if an adult (approximately 25 g) or an aged (approximately 32 g) mouse drank 6 ml within a day they would receive a dose of 100 mg/kg/day. Control mice received distilled water. All mice were individually housed in standard mouse cages. Mice received minocycline in their drinking water for a total of 20 days. The amount of liquid intake was measured daily.
2.3. Experiment 1: Effects of minocycline on spatial learning and hippocampus neurogenesis
Mice were divided within an age group to the minocycline or control group. All mice received daily intraperitoneal injections of 5-Bromodeoxyuridine (BrdU: 50 mg/kg), a thymidine analogue that incorporates into dividing cells. Injections began one week after the onset of minocycline or control treatment and continued for ten consecutive days.
2.3.1 Spatial learning
After two weeks of minocycline or control treatment, mice were tested in the water maze to assess spatial learning. The maze consisted of a circular tub (100 cm diameter) and a clear mesh plastic square platform (8.5 cm). The platform was submerged 1 cm under the surface of the water. The water was made opaque with white tempera paint to conceal the platform. Water temperature was maintained at 19° ± 1° C throughout testing. Extra-maze cues were located around the maze. Mice received three trials (up to 60 sec) per day from different start locations for five consecutive days. If a mouse failed to locate the platform within the 60 sec they were gently guided to the platform. Prior to the first trial of Day 1, all mice were placed on the platform and remained there for 10 sec. All mice remained on the platform for 10 seconds at the end of each trial. A video tracking system (Topscan, CleverSystems, Reston, VA) was used to measure distance swam, latency to locate the platform and swim speed. A single 60 s probe trial was conducted approximately two hours after the subjects last trial on day 5. The platform was removed and the number of times the animal crossed the original location of the platform was recorded by the tracking system.
2.3.2. Perfusions and immunohistochemistry
Mice in Experiment 1 were euthanized by transcardial perfusion with 4% paraformaldehyde in phosphate buffer solution after 20 days of drug treatment to assess hippocampal neurogenesis and microglia size and number via immunohistochemistry. Following perfusion, brains were fixed overnight in 4% paraformaldehyde and then transferred into 30% sucrose solution. Brains were sectioned at 40 micrometers using a cryostat. A one-in-six series was stained for BrdU to identify newly divided cells. Briefly, free floating sections were rinsed in tissue buffering solution (TBS) and then treated with 0.6% hydrogen peroxide for 30 min. To denature DNA, sections were placed in a solution of 50% de-ionized formamide and 10% 20x SCC buffer for 120 min at 65 °C. Followed by 10% 20x SCC buffer for 15 min, then 2 N hydrochloric acid for 30 min at 37 °C, then 0.1 M boric acid (pH 8.5) for 10 min. Sections were blocked with a solution of 0.3% Triton-X and 3% goat serum in TBS (TBS-X plus) for 30 min, and then incubated with primary rat anti-BrdU antibody (1:200; AbD Serotec, Raleigh, NC, USA) at a dilution of 1:200 in TBS-X plus for 72 h at 4 °C. After washing with TBS, sections were treated with TBS-X plus for 30 min and then incubated with a biotinylated goat anti-rat secondary antibody (1:250) in TBS-X plus for 100 min at room temperature. Sections were then treated with the ABC system (Vector, Burlingame, CA, USA) and stained using a diaminobenzidine kit (DAB; Sigma, St. Louis, MO, USA). To measure the number of new cells that differentiated into new neurons a separate one-in-six series was double-labeled with BrdU and NeuN (mature neuron marker). Free-floating sections were handled as described above with the exception of the use of a cocktail of primary antibodies: rat anti-BrdU (1:200) and mouse anti-neuronal nuclear protein (NeuN; 1:50; Millipore, Billerica, MA, USA). Fluorescent markers were conjugated to secondary antibodies, made in goat, at a dilution of 1:200 and also delivered as a cocktail, anti-rat and anti-mouse. ABC and DAB steps were omitted.
A separate one-in-six series was used to measure Iba-1 positive cells in the hippocampus. Free-floating sections were handled as described above with the exception of excluding the DNA denaturing step (i.e., incubations in formamide, SCC buffer, hydrochloric acid, and boric acid) and the use of rabbit anti-mouse Iba-1 (1:500;Wako Chemicals, Richmond VA) as the primary antibody and a biotinylated goat anti-rabbit (1:250) as the secondary antibody.
2.3.3. Image analysis
BrdU positive cells (DAB)
The entire granule layer (bilateral), from a one-in-six series, was imaged by an individual blind to the experimental conditions by systematically advancing the field of view of a Zeiss brightfield light microscope, and taking multiple photographs, via axiocam interfaced to computer, under 10x (total 100x) magnification. Images were analyzed by ImageJ software. For each image, the granule layer was outlined, and BrdU-positive nuclei were automatically counted by setting a fixed threshold to remove the background. The threshold selected was validated by comparing automated counts to hands counts. In addition, the area (pixels) within the trace was recorded. We reported the total number of BrdU positive cells counted in the granular cell layer rather than density due to differential tissue shrinkage between aged and adult animals. Our objective was to assess relative differences between treatment groups which would be influenced by differences in tissue shrinkage across groups. Though precautions were taken to eliminate bias in counts, by using the automated counting software ImageJ and an individual blinded to the treatment conditions the cell counts were not confirmed by unbiased stereology.
Iba positive cells (DAB)
We opted to analyze the molecular layer of the hippocampus for changes in the number and size of Iba-1 positive cells since it would provide a larger number of cells than the granular layer and changes in the molecular layer would likely influence the environment in the granule cell layer given its proximity. The molecular layer (bilateral), from a one-in-six series, was imaged by an individual blind to the experimental conditions by systematically advancing the field of view of a Zeiss brightfield light microscope, and taking multiple photographs, via axiocam interfaced to computer, under 10x (total 100x) magnification. Similar to the BrdU analysis, Iba-positive cells were automatically counted with a fixed threshold. The total number of Iba-1 positive cells counted and the density of Iba-1 positive cells are expressed by per cubic micrometer molecular layer sampled. In addition, average size of individual cells stained with Iba-1 within the trace was recorded. The average cell size is automatically calculated by ImageJ by dividing the total area occupied by Iba-1 staining, as determined by the background setting, by the number of cells. Importantly, the average size of the area imaged was not different between groups (data not shown). Therefore the difference in Iba-1 staining cannot be attributed to measuring a larger or smaller area.
Double labeled BrdU and NeuN cells
BrdU-positive cells in the dentate gyrus were micro-analyzed by an individual blinded to the treatment condition by confocal microscopy to estimate the proportion of BrdU positive cells that co-express NeuN (neuronal nuclear marker). Number of new neurons per cubic micrometer per mouse was calculated as number of BrdU cells per cubic micrometer (from above) multiplied by average proportion of BrdU cells co-expressing NeuN for a given experimental group.
Experiment 2.4: Effects of minocycline on hippocampal expression of IL-1β, IL-6, and CD74
Adult (n=12) and aged (n=10) male mice were divided within an age group to the minocycline or control group. Consistent with Experiment 1, mice received distilled water or minocycline in their drinking water for a total of 20 days.
2.4.1. qRT-PCR
Mice in Experiment 2 were sacrificed by rapid decapitation without anesthesia after the 20 days of minocycline or control treatment. Hippocampal samples were dissected on a chilled glass dish and immediately placed into RNAlater solution (Qiagen, Valencia, CA) and stored at −20°C until RNA isolation. Hippocampal samples were homogenized and RNA purified by the RNeasy Mini kit (Qiagen, Valencia, CA), then quantified and assessed for purity using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). The average RNA yield was 903 ng/μl per hippocampus sample with a 260/280 purity ratio of 1.9 or higher. Conversion of RNA into cDNA was completed by following the instructions of the High-capacity cDNA reverse transcription kit that included an RNase inhibitor step (Applied Biosystems, Foster City, CA) using the following cycling conditions: 10 min at 25°C, 120 min at 37°C and 5 min at 85°C. After reverse transcription, cDNA was held at 2–8° C in a refrigerator overnight before conducting two-step quantitative real-time reverse-transcription polymerase chain reaction (qRT_PCR) to determine the amount of specific mRNA transcript present in each hippocampal sample. Hippocampal samples were analyzed for expression levels of IL-1β (Mm00434228_m1), IL-6 (Mm00446190_m1), and CD74 (invariant polypeptide of MHC II, Mm00658576_m1). β-actin (Mm00607939_s1) served as the endogenous control gene. Levels of β-actin expression did not differ across age or treatment groups. Each sample was run in triplicate for each gene in a 10μl reaction that contained 80ng of cDNA and 0.5μl of a 20X probe/primer mix in one 384 well plate. The amount of specific mRNA present was determined by utilizing TaqMan™ probe and primer chemistry (Applied Biosystems, Foster City, CA) specifically designed to bind to reverse-transcribed cDNA of the genes of interest using an Applied Biosystems 7900HT PCR instrument (Applied Biosystems, Foster City, CA) using the following cycle parameters: 2 min at 50°C, 10 min at 95°, followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C. The amplification efficiency was equal across all wells for a given gene. Florescence data (ΔRn) was exported from the Applied Biosystems SDS software and analyzed by DART (Data Analysis for RT-PCR) [21]. Gene expression data were normalized by dividing the Ro values of the target genes by the Ro values of the endogenous control gene β-actin.
2.5. Statistical analyses
All data met the basic requirements to perform an ANOVA analysis with the exception of the number of BrdU cells and IL-6 gene expression data. The BrdU cell counts required a power transformation to normalize the distribution and one outlier was removed from the IL-6 data to comply with the ANOVA requirements. The number of BrdU and Iba-1 positive cells, the number of BrdU cells that co-labeled with NeuN, and RT-PCR gene expression data were analyzed by ANOVA with Age (adult or aged) and Treatment (minocycline or control) as the between-subjects factors. Body weight, consumption of minocycline/water and water maze data were analyzed by repeated measures ANOVA with Age and Treatment as the between-subjects variables and Day as the within-subjects (i.e., repeated-measures) variable. When the overall F was significant Fisher’s LSD was used to perform post hoc comparisons when appropriate. Correlations between Iba-1 staining intensities or cell counts and average path length across the five days of testing were conducted using Pearon’s r. An alpha level of p<0.05 was considered statistically significant.
3. RESULTS
3.1. Body weight
As expected, aged mice (28.9 grams) in Experiment 1 weighted significantly more than adults (23.23 grams), as shown by a main effect of Age (F(1,31)=177.92;p<0.0001; see Figure 1A). A significant main effect of Treatment condition showed that minocycline treated mice weighed more than control mice (F(1,31)=8.67;p<0.01). A significant Age x Day interaction showed that aged mice tended to lose weight across the 20 days, regardless of their treatment condition, and adult mice tended to gain weight (F(3,93)=24.92;p<0.0001). Overall, aged mice lost an average of 0.87 grams and adult mice gained an average of 0.60 grams. No significant interaction between minocycline treatment and age was observed.
Figure 1.

Body weight and consumption. Aged mice weighed more than adult mice (A). Minocycline treatment had no effect on body weight (A). Regardless of treatment, aged mice drank less than adult mice (B). Minocycline-treated adults drank significantly more than adult controls (B). * indicates a significant difference from age-matched control group. + indicates a significant difference from treatment-matched adult mice. Bars represent means ± standard error of the means (SEMs).
In agreement, aged mice in Experiment 2 weighed more than adults, as shown by a main effect of Age (F(1,18)=64.91;p<0.0001; data not shown). Additionally, aged mice lost weight over the 20 days whereas adult mice gained weight, regardless of their treatment condition, as shown by a significant Age x Day interaction (F(3,54)=3.21;p<0.05). No significant interaction between minocycline treatment and age was observed.
3.2. Consumption of minocycline and water
Overall adult mice in Experiment 1 drank more than aged mice regardless of the animal’s treatment condition, as shown by a significant main effect of Age (F(1,31)=23.00;p<0.0001; see Figure 1B). On average adult mice drank 5.2 ml/day which translates to an average daily minocycline dose of 86.50 mg/kg/day and aged mice drank 4 ml/day which averages out to a daily minocycline dose of 73.55 mg/kg/day with an average body weight of approximately 29 grams. No significant main effect or interaction with minocycline treatment was observed.
In Experiment 2, there was a similar trend for adult mice to drink more than aged mice regardless of the treatment condition, as shown by a significant main effect of Age (F(1,18)=4.92;p<0.05; data not shown). On average adult mice drank 5.81 ml/day and aged mice drank 5.03 ml/day. No significant main effect or interaction with minocycline treatment was observed.
3.3. Effects of minocycline on spatial learning
Analysis of distance swam (path length) to the platform showed that all of the mice acquired the task as distance swam decreased across the five days of testing (F(4,124)=44.50;p<0.0001; see Figure 2A). Additionally there was a significant main effect of Age and a significant Age x Treatment x Day interaction (F(1,31)=7.37;p<0.01;F(4,124)=4.17;p<0.005, respectively). Adult mice overall swam a shorter distance to locate the platform than aged mice. On day 2 of testing, aged mice administered minocycline swam a shorter distance than aged control mice (p<0.05; see Figure 2A). Minocycline administration did not significantly alter the distance adult mice swam to the platform. Minimal effects of minocycline administration were observed on swim speed. Adult mice given minocycline swam faster than adult controls, particularly on Day 1 of testing, as shown by a significant Age x Treatment interaction (F(1,31)=3.93;p<0.05; see Figure 2B). Additionally, swim speed decreased across the five days of testing (F(4,124)=38.79; p<0.0001; see Figure 2B). For the probe trial, a significant main effect of Age showed that adult mice crossed that original platform location more often than the aged mice (F(1,31)=15.34;p<0.0005; see Figure 2C). No significant main effect or interaction with minocycline treatment was observed.
Figure 2.

Spatial learning. Overall all adults had a shorter path length than aged mice, but aged mice treated with minocycline showed a significantly shorter path to the platform compared to aged controls on day 2 (A). Minocycline treatment had no effect on swim speed in aged mice, but increased swim speed in adult mice on day 1 (B). During the probe trial, adult mice crossed the original location of the platform more often than aged mice, but no effect of minocycline treatment was observed (C). * indicates a significant difference from age-matched control group. + indicates a significant difference from treatment-matched adult mice. Bars represent means ± SEMs.
3.4. Effect of minocycline on new cell survival and neuronal differentiation
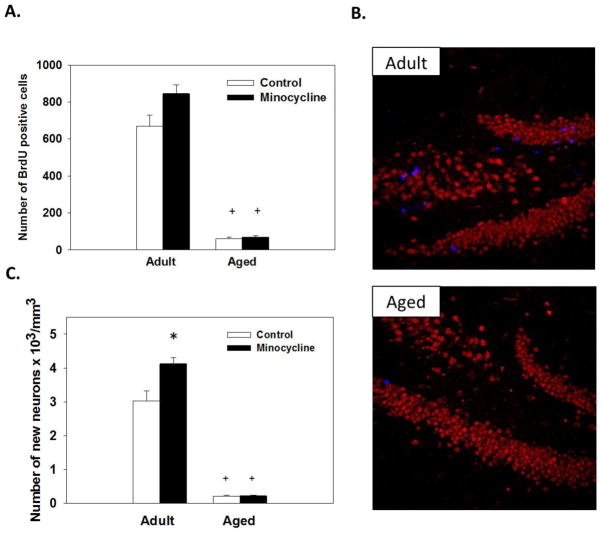
As expected, aged mice showed significantly fewer BrdU positive cells in the granular layer of the hippocampus compared to adults, as shown by a main effect of Age (F(1,31)=72.27;p<0.0001; see Figure 3A). No significant main effect or interaction with minocycline was observed. Additionally, assessment of the estimated number of BrdU-positive cells co-expressing NeuN (i.e., new neurons) in the granular cell layer showed significant main effects of Age and Treatment and a significant Age x Treatment interaction (F(1,31)=339.83;p<0.0001; (F(1,31)=9.08;p<0.05; (F(1,31)=8.91;p<0.05, respectively, see Figure 3C). Aged mice had significantly fewer new neurons than adults. Administration of minocycline increased the number of new neurons in adult mice compared to controls (p<0.05) but not in aged mice (see Figure 3C).
Figure 3.
Hippocampal neurogenesis. Aged mice showed decrease new cell survival as indicated by reduction in the number of BrdU positive cells in the granular layer compared to adults. Minocycline treatment had no effect on the number of BrdU positive cells in adult or aged mice (A). Representative hippocampal sections double labeled with antibodies against NeuN (mature neuron; red) and BrdU (new cell; blue) from adult and aged mice (B). Minocycline treatment increased neuronal differentiation in adult mice, but had no effect in aged mice (C). + indicates a significant difference from treatment-matched adult mice. * indicates a significant difference from age-matched control group. Bars represent mean number of cell ± SEMs.
3.5. Effect of minocycline on Iba-1 positive cells
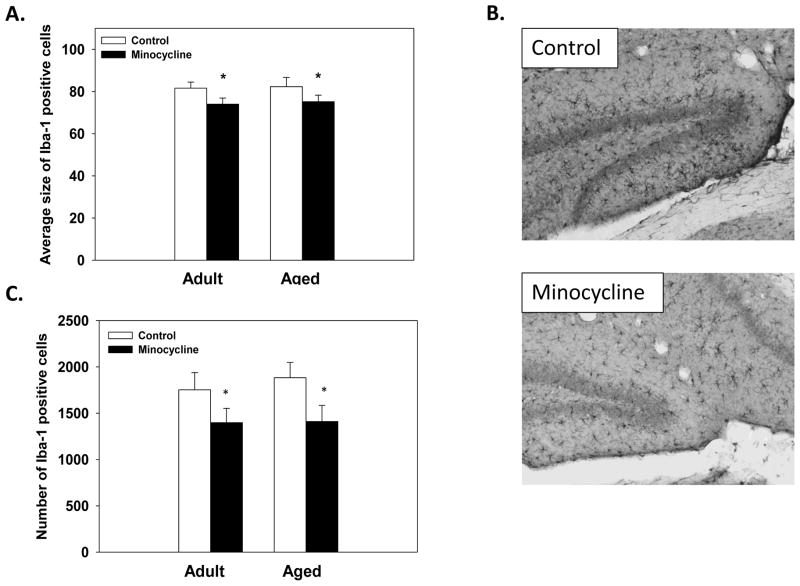
Assessment of the of Iba-1 positive cells in the molecular layer of the dentate gyrus showed that minocycline administration did not alter the density of Iba-1 positive cells (F(1,31)=3.12;p<0.08ns). However, there was a significant main effect of Treatment for the total number of Iba-1 positive cells counted (F(1,31)=5.73;p<0.05, see Figure 4C), that showed minocycline treatment decreased the total number of cells counted in the molecular layer. Additionally, minocycline administration significantly decreased the average size of Iba-1 positive cell compared to control mice, regardless of age (F(1,31)=5.11;p<0.05, see Figure 4A). No significant interaction between minocycline treatment and age was observed.
Figure 4.
Iba-1 positive cells. (A) Regardless of age minocycline treatment significantly reduced the average size of Iba-1 positive cells. (B) Sample images of Iba-1 labeled cells in the molecular layer of the dentate gyrus of aged control and minocycline-treated mice. (C) Minocycline treatment significantly reduced the number of Iba-1 positive cells counted in the molecular layer of the hippocampus. + indicates a significant difference from treatment-matched adult mice. * indicates a significant difference from aged-matched control mice. Bars represent means ± SEMs.
3.6. Effect of minocycline on hippocampal gene expression
Analysis of the endogenous control gene, β-actin, showed no significant changes in expression across the age or treatment groups. Aged mice showed a significant increase in hippocampal expression of IL-1β, IL-6 and CD74 compared to adults (F(1,18)=5.85;p<0.05; (F(1,17)=22.18;p<0.005; (F(1,18)=27.41;p<0.0001, respectively; see Figure 5A–C). However, no significant main effects or interactions with minocycline treatment were observed for any of the genes measured.
Figure 5.
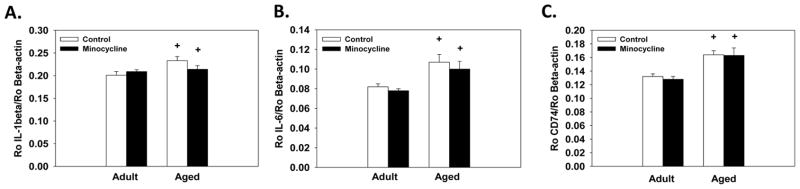
Hippocampal gene expression. Regardless of treatment condition aged mice showed higher expression of IL-1β (A), IL-6 (B) and CD74 (C) in the hippocampus compared to adult mice. + indicates a significant difference from treatment-matched adult mice. Bars represent means ± SEMs.
3.7. Correlations between performance in the water maze and Iba-1 cells
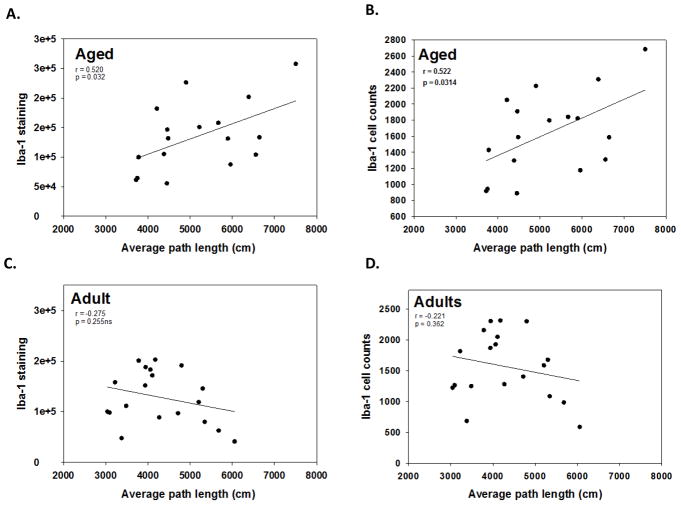
The average distance swam across the five days of testing (i.e., path length) was calculated for each individual mouse in Experiment 1 and correlated with their corresponding measurements of Iba-1 positive cells. For aged mice, there was a significant positive correlation between average path length and total Iba-1 staining in the molecular layer of the hippocampus (r = 0.52, p<0.05 see Figure 6A), in which higher levels of Iba-1 staining were associated with longer path lengths in aged mice. Additionally, aged mice showed a significant positive correlation between the average path length and the number of Iba-1 positive cells (r = 0.52, p<0.05, see Figure 6B), indicating that higher cell counts were associated with longer path lengths. Adult mice showed no correlation between path length and Iba-1 staining or total Iba-1 positive cell counts (see Figure 6C and 6D, respectively).
Figure 6.
Correlation between the overall average path length across the 5 days of water maze testing and Iba-1 staining. There are significant positive correlations between path length in the water maze and total Iba-1 staining (A) and Iba-1 cell counts (B) in aged mice. Path length did not correlate with Iba-1 staining (C) or Iba-1 cell counts (D) in adult mice. Individual graphs list the Pearson’s correlation coefficient (i.e., r value) and p value.
DISCUSSION
The present data indicate that repeated treatment with minocycline improves spatial learning in aged mice. To the best of our knowledge this is the first report that shows minocycline treatment can attenuate age-related cognitive deficits. Minocycline treatment also reduced the average size of Iba-1 positive cells which correlated with the distance swam in the water maze, potentially indicating a link between the improvements in spatial learning and microglial cell activity.
Performance of the aged mice treated with minocycline in the water maze was comparable to the adult mice during the initial days of testing, as minocycline treatment lead to faster acquisition compared to the aged controls. These findings complement prior reports that have shown minocycline improves cognitive performance in animal models of Alzheimer’s disease [22, 23]. For instance, spatial memory impairments induced by transgenic overexpression of the human β-amyloid precursor protein are attenuated by minocycline treatment [24]. Similarly, minocycline reduced the number of working and reference memory errors in the radial arm water maze in the Ts65Dn mouse model of down-syndrome that show increased microglia activation relative to wild-type mice [23]. These studies indicate that minocycline attenuates cognitive deficits in animal models of neurodegenerative diseases. The present data extend this existing work by demonstrating that minocycline treatment can attenuate learning deficits that develop with normal aging in the absence of a disease state or damage.
The minocycline-induced learning improvements in the age mice may be related to inhibition of microglial cells. We observed a significant reduction in the average the size and total number of Iba-1 positive cells in the molecular layer of the dentate gyrus following minocycline treatment. The reduction in size may indicate reduced activation as alterations in the morphological features are associated with changes in microglia activation, though changes in morphology alone are insufficient to determine the phenotype the cells are expressing [25]. Additionally, significant correlations in the aged, but not adult, mice revealed that higher levels of Iba-1 staining and higher numbers of Iba-1 positive cells were associated with increased path length in the water maze. Assessment of hippocampal expression of the proinflammatory cytokines IL-1β and IL-6 and expression of CD74 confirmed that aged mice showed increased basal expression of these inflammatory genes compared to adults in agreement with prior reports that show aging primes microglial cells [8, 9]. As shown in Figure 5, we saw a modest, but non-significant, reduction in IL-1β expression in the aged minocycline-treated mice compared to aged controls. Whether minocycline treatment reduced protein levels of these proinflammatory cytokines was not assessed in the present study, leaving the possibility that post-translation effects of minocycline may occur. An additional possibility is that assessment of microglia expression levels of proinflammatory cytokines rather than expression in the entire hippocampus would have resulted in different findings given that other cells in the hippocampus may be releasing inflammatory cytokines as well [26]. Even in the absence of alterations in cytokine expression, our data indicate that minocycline is influencing microglia as shown by a reduction in the number and cell size.
An alternative possibility is that minocycline treatment is improving cognitive function independent of its anti-inflammatory effects. Minocycline has also been shown to have anti-apoptotic effects through inhibition of capases and to inhibit several enzymes including matrix metalloproteinases (MMPs) which play a role in neural plasticity and intracellular signaling [27, 28]. Animal models of Fragile-x syndrome have indicated that minocycline, through inhibition of MMP-9, improves cognitive function and promotes maturation of dendritic spines in vivo and in vitro [28]. Recent work by Lee et al. [29] has shown that MMP-9 levels are elevated in the brain of aged mice compared to adults. Though currently unknown, the age-related increase in MMP-9 may contribute to cognitive deficits that may be attenuated by minocycline treatment. Further minocycline may influence cognitive function by enhancing protein translation. Prior work has shown that minocycline can reduce phosphorylation of eukaryotic initiation translation factor 2α (eIF2α) [30]. Phosphorylation of eIF2α reduces protein production by preventing eIF2α from forming a pre-initiating complex that is required to initiate translation. Increased phosphorylation of eIF2α is associated with impairments in memory consolidation and synaptic plasticity [31, 32]. Normal aging increases the phosphorylated form of eIF2α [31]. Potentially, minocycline may improve cognitive performance in aged animals by attenuating eIF2α phosphorylation, but additional work is needed to test this possibility.
Prior reports have implicated neurogenesis in hippocampal-dependent cognitive performance [12–14]. Aging is associated with a substantial decline in the production of new cells relative to adults [11], as confirmed in the current study, potentially indicating that age-related reductions in neurogenesis contribute to cognitive decline. Therefore we wanted to assess whether improvements in spatial learning were associated with increases in neurogenesis. Prior reports have shown that reducing production of IL-1β or increasing levels of fractalakine, a neuroimmune modulatory protein, in aged subjects increases neurogenesis [15, 16]. In the current report, we found that minocycline had no effect on new cell numbers or neural differentiation of new granular cells in aged mice. The divergence from prior reports may result the differences in the compounds, the route of administration, and duration of the treatments. The inability of minocycline to restore levels of neurogenesis in the aged mice is not completely unexpected, as although age-related increases in neuroinflammation may contribute to the decline in neurogenesis several other factors, such as an age-related decline in the of number viable stem cells and slowing of the cell cycle, likely play a larger role in suppressing neurogenesis in aged subjects compared to inflammation [33, 34]. The data indicate that the minocycline-induced improvements in spatial learning in the aged subjects were independent of alterations in neurogenesis. In adult mice, minocycline had no effect on survival of BrdU positive cells, but interestingly, we observed an increase in the proportion of new cells that differentiated into neurons in the adult subjects following minocycline treatment. The present study did not investigate the potential causes for this increase in new neurons, but we can speculate that minocycline may reduce microglia phagocytosis of new neurons or potentially microglia may encourage new cells to differentiate into astrocytes and that minocycline treatment blocks this bias. Further studies will need to determine whether these or other mechanisms of minocycline mediate the increase in new neurons in adult mice.
In summary, these findings indicate that prolonged treatment with minocycline improves acquisition of a spatial learning task in aged animals. One possibility is that the age-related changes in microglia activation contribute to the development of cognitive deficits, as performance of aged mice in the water maze was correlated with Iba-1 staining levels and cell counts, as lower counts and staining levels were associated with a shorter overall path length to locate the platform. However, minocycline may improve cognition through inflammation independent mechanisms such as inhibition of MMP and/or improving translation. Further work is needed to elucidate the primary pathways through which minocycline enhances cognitive performance in aged subjects, as such knowledge will facilitate the development of novel treatments to ensure the preservation of cognitive function though the lifespan.
Highlights.
Minocycline attenuates age-related spatial learning deficits.
Treatment with minocycline reduces the size of Iba-1 positive cells.
Minocycline increases neuronal differentiation of new granular cells in adults.
Acknowledgments
Funding source: This work was supported by grants from National Institute of Health, MH083807 and DA027487 to J.S.R and from National Institute on Aging K99AG0404184 to R.A.K. Funding sources had no involvement in the experimental design or interpretation of the results.
Footnotes
Conflicts of interests: All authors declare that there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–42. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 2.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–8. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 3.Okello A, Edison P, Archer HA, Turkheimer FE, Kennedy J, Bullock R, Walker Z, Kennedy A, Fox N, Rossor M, Brooks DJ. Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology. 2009;72:56–62. doi: 10.1212/01.wnl.0000338622.27876.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elwan O, Madkour O, Elwan F, Mostafa M, Abbas Helmy A, Abdel-Naseer M, Abdel Shafy S, El Faiuomy N. Brain aging in normal Egyptians: cognition, education, personality, genetic and immunological study. J Neurol Sci. 2003;211:15–22. doi: 10.1016/s0022-510x(03)00032-7. [DOI] [PubMed] [Google Scholar]
- 5.Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–22. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Gemma C, Fister M, Hudson C, Bickford PC. Improvement of memory for context by inhibition of caspase-1 in aged rats. Eur J Neurosci. 2005;22:1751–6. doi: 10.1111/j.1460-9568.2005.04334.x. [DOI] [PubMed] [Google Scholar]
- 7.Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–24. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- 8.Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–48. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- 9.McLinden KA, Kranjac D, Deodati LE, Kahn M, Chumley MJ, Boehm GW. Age exacerbates sickness behavior following exposure to a viral mimetic. Physiol Behav. 2012;105:1219–25. doi: 10.1016/j.physbeh.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Casolini P, Catalani A, Zuena AR, Angelucci L. Inhibition of COX-2 reduces the age-dependent increase of hippocampal inflammatory markers, corticosterone secretion, and behavioral impairments in the rat. J Neurosci Res. 2002;68:337–43. doi: 10.1002/jnr.10192. [DOI] [PubMed] [Google Scholar]
- 11.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–54. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–58. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 15.Gemma C, Bachstetter AD, Cole MJ, Fister M, Hudson C, Bickford PC. Blockade of caspase-1 increases neurogenesis in the aged hippocampus. Eur J Neurosci. 2007;26:2795–803. doi: 10.1111/j.1460-9568.2007.05875.x. [DOI] [PubMed] [Google Scholar]
- 16.Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32:2030–44. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckwalter MS, Yamane M, Coleman BS, Ormerod BK, Chin JT, Palmer T, Wyss-Coray T. Chronically increased transforming growth factor-beta1 strongly inhibits hippocampal neurogenesis in aged mice. Am J Pathol. 2006;169:154–64. doi: 10.2353/ajpath.2006.051272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon SY, Patel D, Dougherty PM. Minocycline blocks lipopolysaccharide induced hyperalgesia by suppression of microglia but not astrocytes. Neuroscience. 2012;221:214–24. doi: 10.1016/j.neuroscience.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–72. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- 20.Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist. 2005;11:308–22. doi: 10.1177/1073858405275175. [DOI] [PubMed] [Google Scholar]
- 21.Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31:e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parachikova A, Nichol KE, Cotman CW. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol Dis. 2008;30:121–9. doi: 10.1016/j.nbd.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter CL, Bachman D, Granholm AC. Minocycline prevents cholinergic loss in a mouse model of Down’s syndrome. Ann Neurol. 2004;56:675–88. doi: 10.1002/ana.20250. [DOI] [PubMed] [Google Scholar]
- 24.Fan R, Xu F, Previti ML, Davis J, Grande AM, Robinson JK, Van Nostrand WE. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci. 2007;27:3057–63. doi: 10.1523/JNEUROSCI.4371-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol. 2009;85:352–70. doi: 10.1189/jlb.0608385. [DOI] [PubMed] [Google Scholar]
- 26.Ruan L, Kang Z, Pei G, Le Y. Amyloid deposition and inflammation in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Curr Alzheimer Res. 2009;6:531–40. doi: 10.2174/156720509790147070. [DOI] [PubMed] [Google Scholar]
- 27.Garrido-Mesa N, Zarzuelo A, Galvez J. What is behind the non-antibiotic properties of minocycline? Pharmacol Res. 2012;67:18–30. doi: 10.1016/j.phrs.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Siller SS, Broadie K. Matrix metalloproteinases and minocycline: therapeutic avenues for fragile X syndrome. Neural Plast. 2012:124548. doi: 10.1155/2012/124548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee P, Kim J, Williams R, Sandhir R, Gregory E, Brooks WM, Berman NE. Effects of aging on blood brain barrier and matrix metalloproteases following controlled cortical impact in mice. Exp Neurol. 2012;234:50–61. doi: 10.1016/j.expneurol.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi Y, Kim HS, Shin KY, Kim EM, Kim M, Park CH, Jeong YH, Yoo J, Lee JP, Chang KA, Kim S, Suh YH. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease models. Neuropsychopharmacology. 2007;32:2393–404. doi: 10.1038/sj.npp.1301377. [DOI] [PubMed] [Google Scholar]
- 31.Segev Y, Michaelson DM, Rosenblum K. ApoE epsilon4 is associated with eIF2alpha phosphorylation and impaired learning in young mice. Neurobiol Aging. 2012 doi: 10.1016/j.neurobiolaging.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 32.Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, Krnjevic K, Lacaille JC, Nader K, Sonenberg N. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackmore DG, Golmohammadi MG, Large B, Waters MJ, Rietze RL. Exercise increases neural stem cell number in a growth hormone-dependent manner, augmenting the regenerative response in aged mice. Stem Cells. 2009;27:2044–52. doi: 10.1002/stem.120. [DOI] [PubMed] [Google Scholar]
- 34.Werry EL, Enjeti S, Halliday GM, Sachdev PS, Double KL. Effect of age on proliferation-regulating factors in human adult neurogenic regions. J Neurochem. 2010;115:956–64. doi: 10.1111/j.1471-4159.2010.06992.x. [DOI] [PubMed] [Google Scholar]