Abstract
Objective
Disrupted amygdala activity in depressed adolescents and adults while viewing facial expressions of emotion has been reported. However, little data is available to inform the developmental nature of this phenomenon, an issue that studies of the earliest known forms of depression might elucidate. The current study addressed this question by examining functional brain activity and its relationships to emotion regulation in depressed 4–6 year old children and their healthy peers.
Method
Fifty-four medication-naïve 4–6 year olds (23 depressed/31 healthy) participated in a case-control study using functional magnetic resonance imaging (fMRI). Imaging data were used to compare functional brain activity in children with and without depression during emotion face processing.
Results
A right-lateralized pattern of elevated amygdala, thalamus, inferior frontal gyrus, and angular gyrus activity during face processing was found in depressed 4–6 year olds. Additionally, relationships between increased amygdala activity during face processing and disruptions in parent reported emotion regulation and negative affect were found. No between group differences specific to emotion face type were identified.
Conclusion
To our knowledge, this is the earliest evidence of alterations in functional brain activity in depression using fMRI. Results suggest that, similar to findings in older depressed groups, depression at this age is associated with disrupted amygdala functioning during face processing. They also raise the intriguing possibility that disrupted amygdala function is a depression related biomarker that spans development. Additional studies will be needed to clarify whether the current findings are a precursor or a consequence of very early childhood depression.
Keywords: amygdala, depression, face processing, functional magnetic resonance imaging (fMRI), preschool depression
INTRODUCTION
The amygdala, a subcortical structure highly sensitive to affectively valenced stimuli, has been consistently implicated in the pathogenesis of depression.1 Studies examining amygdala function in depression have frequently included the use of facial expressions of emotion given their well-established relationship with activity within this structure.2 In adults with depression or at increased risk for depression, this approach has generally given rise to reports of elevated amygdala reactivity to facial expressions of negative affect.2, 3 Similarly, studies in depressed children and adolescents have also consistently reported altered amygdala reactivity to facial expressions of emotion, but with the nature of these differences more mixed.4 For example, increased amygdala reactivity has been reported across multiple expression types in some studies5, 6 but not others.7 Nevertheless, despite a growing body of research suggesting that disrupted amygdala function may be a common feature shared by the pediatric and adult forms of this disorder, there is still very little known about the developmental trajectory of this alteration. Studies of the earliest validated forms of depression, such as Preschool-Onset Depression (PO-MDD)8, are likely to provide unique insight into this question and lay critical groundwork for our understanding of disrupted amygdala function as a potential biomarker of depression across the lifespan.
To date, there have been 2 studies examining functional brain activity during face processing in PO-MDD. In the first, Gaffrey et al.9 examined emotion face processing in a small sample of currently depressed preschoolers. Results from this study indicated that higher levels of depression severity were associated with greater right amygdala activity, especially while viewing sad faces. However, the lack of a healthy comparison group prevented any conclusions about whether amygdala function in PO-MDD was deviant from anticipated normative levels, information that is critical for a more fully informed developmental model of depression. In a more recent study of face processing in school age children with a known history of PO-MDD and their healthy peers, depression severity measured during the preschool period was again positively related to increased activity in the right amygdala when viewing sad faces.10 Interestingly, this relationship remained significant when multiple indicators of current (e.g., current diagnosis of depression) and previous (e.g., history of other internalizing disorders) symptoms were controlled, suggesting a specific association between level of depression severity experienced during the preschool period (PO-MDD) and current amygdala reactivity at school age. While these initial findings raise the possibility that disrupted amygdala functioning may already be evident in preschoolers with PO-MDD, the absence of any data directly informing this represents a critically important gap in the literature.
The goal of the current study was to begin to fill this gap by reporting on a case-control comparison of functional brain activity in currently depressed preschoolers and their age matched healthy peers. To our knowledge, this is the first case-control comparison of any psychiatric condition using functional magnetic resonance imaging (fMRI) during this early developmental period. A face-processing task was chosen given its established use in neuroimaging studies of depression and recognized utility for eliciting amygdala activity as young as 5 years of age.4, 11, 12 The use of this paradigm also ensured that study findings could be interpreted within the context of previous research in older depressed samples while still meeting the pragmatic constraints of imaging very young children. Based on our previous findings in PO-MDD noted above, we established a priori hypotheses predicting increased right amygdala activity during face processing in depressed preschoolers when compared to their healthy peers. We also hypothesized that amygdala function would be positively associated with higher levels of negative affect, and negatively related to child emotion regulation abilities (both obtained by parent report), based on our previous work and that of others.e.g., 6 Given that mixed findings have been reported in older depressed pediatric groups, hypotheses about specific facial expressions were not made.
METHOD
PARTICIPANTS
Participants were recruited from pediatrician’s offices, daycares, and other community resources (e.g., booths at science fairs) throughout the greater St. Louis metropolitan area. A screening checklist (Preschool Feelings Checklist13 [PFC]) was used to identify preschoolers with depressive symptoms as well as a healthy control group. More specifically, caregivers indicating that their preschoolers were at “low” (≤1 items PFC endorsed) or “high” (≥3 PFC items endorsed) risk for depression related difficulties were contacted and invited to complete additional phone screening steps assessing for the presence of neurological disorders (e.g., seizure disorder, closed head injury, etc.), autism spectrum disorders or developmental delays, premature birth (<36 weeks gestation), and psychotropic medication use. Endorsement of any of these conditions acted as exclusionary for all children. Low risk children passing the exclusion criteria were invited to enroll in the full study. Primary caregivers of high-risk preschoolers were additionally asked to complete the Major Depressive Disorder (MDD) module of the Preschool Age Psychiatric Assessment (PAPA).14 If the PAPA indicated PO-MDD (see Diagnostic Assessment section below), families were invited to participate in the full study. Using these screening criteria, 68 children were recruited into the current study. Of these 67 children, 47 passed our fMRI quality control (QC) measures (see Functional Imaging Data Acquisition and Preprocessing section). Of the remaining children, fMRI data was lost due to failed QC (18), equipment failure (1), and discontinuation of scan per child request (1). Parent report and neuroimaging data from a small subsample of PO-MDD children (n = 7) previously reported9 were also included. Thus, 54 preschoolers between 4–6 years of age with (n = 23) and without (n= 31) PO-MDD were included in the final sample. Parental written consent and child verbal assent were obtained for all subjects. The Institutional Review Board at Washington University in St. Louis approved all experimental procedures.
DIAGNOSTIC ASSESSMENT
Diagnostic assessments were conducted using the Preschool Age Psychiatric Assessment (PAPA)14, a developmentally appropriate interviewer-based instrument designed for use with the primary caregivers of children between 2 to 6 years of age. The PAPA includes all relevant DSM-IV15 criteria and their age appropriate manifestations, has established test-retest reliability16, and is widely used to assess for DSM-IV Axis I disorders in preschoolers. Detailed training and calibration methods have been previously described.17 Following completion of the PAPA by trained research assistants, relevant symptom, impairment, and duration criteria gathered during the interview were used to generate diagnoses, including PO-MDD. Inter-rater reliability for PO-MDD was assessed in 20% of the cases, with excellent reliability for both diagnosis (κ = 1) and symptom endorsement (ICC = 0.98) found. The 2-week episode duration criterion for MDD was not required for PO-MDD given that our previous work has suggested its strict application fails to identify many preschoolers experiencing clinically significant depressive symptoms and impairment.18-20 Children placed into the Control group did not meet criteria for any DSM-IV Axis I disorder according to parent report on the PAPA (see Table 1).
Table 1.
Characteristics of Study Groups
| PO-MDD | Healthy | t/x2 | p | ||
|---|---|---|---|---|---|
| Characteristic | (n = 23) | (n = 31) | value | value | |
| Age (years) | 5.04 (.76) | 5.06 (.89) | −0.09 | .92 | |
| Gender | Female | 10 | 16 | 0.35 | .55 |
| Male | 13 | 15 | |||
| Handedness | Right | 23 | 28 | 2.35 | .12 |
| Left | 0 | 3 | |||
| Ethnicity | White | 17 | 26 | 1.88 | .39 |
| African American |
3 | 1 | |||
| Other | 3 | 3 | |||
| Family Income ($/n per group) |
≤ 5,000 | 1 | 1 | 7.03 | .53 |
| 5,001–10,000 | 1 | 1 | |||
| 15,001–20,000 | 3 | 1 | |||
| 20,001–25,000 | 0 | 1 | |||
| 35,001–40,000 | 2 | 0 | |||
| 45,001–50,000 | 1 | 2 | |||
| 50,001–55,000 | 1 | 2 | |||
| 55,001–60,000 | 0 | 2 | |||
| ≥ 60,000 | 14 | 21 | |||
| Response Rate (%)a | 90 (16) | 96 (7) | −1.7 | .09 | |
| Emotion Identificationb | 10.6(2.5) | 11.4(2.7) | −1 | .301 | |
| Comorbidityc | |||||
| None | 10 | 31 | |||
| Internalizing | 7 | NA | |||
| Externalizing | 2 | NA | |||
| Int. and Ext. | 4 | NA | |||
| Emotion Regulation Checklistd | |||||
| Negativity | 34.7(5.3) | 25.7(5.2) | 6.17 | <.001 | |
| Emotion Regulation | 19.3(3.8) | 26(3.7) | −6.97 | <.001 |
Note: NA = not applicable.
Percentage of total possible button presses completed (32 possible presses per run); Unavailable for 2 children with preschool depression (PO-MDD).
Average raw score out of 17 possible expressions reported; groups did not differ at level of individual face types (p>.05).
Internalizing (n): Generalized Anxiety Disorder (GAD) (2), Separation Anxiety Disorder (SAD) (3); GAD/SAD (2); Externalizing (n): Attention-Deficit/Hyperactivity Disorder (ADHD) (1); ADHD/Oppositional Defiant Disorder (1); Both (n): ODD/SAD (3), ADHD/SAD (1).
Raw scores.
PARENT REPORT MEASURE OF CHILD’S EMOTION REGULATION AND COMPETENCE
The Emotion Regulation Checklist (ERC)21 is a parent report measure of children’s self-regulation and emotionality and includes both positively and negatively weighted items to be rated on a 4-point Likert scale. The ERC provides dimensional subscale scores measuring a parent’s perception of their child’s ability to successfully self-regulate their emotions (Emotion Regulation) as well as their dysregulated expression of negative affect (Negativity). The Emotion Regulation (Cronbach’s alpha = .75) and Negativity (Cronbach’s alpha = .89) subscales were of particular interest and therefore were used in the brain-behavior analyses described below.
CHILD FACE EMOTION LABELING ACCURACY
In order to assess each child’s ability to identify facial expressions of emotion, the Facial Affect Comprehensive Evaluation, Emotion Labeling subtest (FACE-EL) was administered.22 The FACE-EL requires each child to identify which emotion an individual is displaying from seven different possible choices (happy, sad, anger, fear, surprise, disgust, shame). In line with our in-scanner task (see Facial Emotion Viewing Task section below), accuracy in identifying happy (n=5), sad (n=7), and fear (n=5) faces (Total N=17 expressions) was examined.
PROCEDURE
Facial Emotion Viewing Task
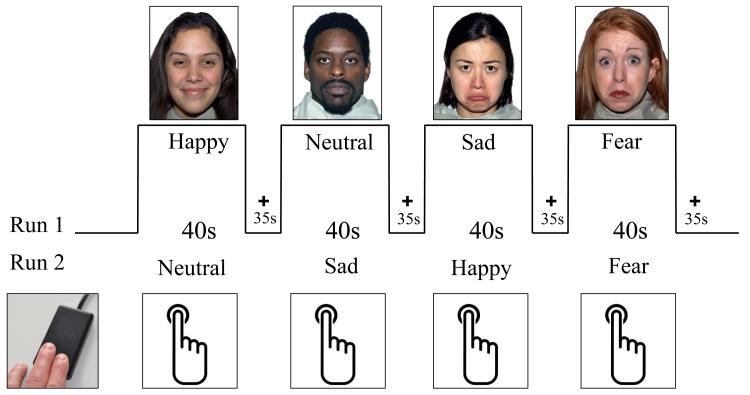
Children participated in a modified version of a common face emotion-viewing task used in depression neuroimaging research.2 As in these prior studies, children were presented with a series of faces varying in affective content (see Figure 1) and asked to complete a simple button press each time a face appeared. A less constrained response was chosen given the young age of our child participants and previous research suggesting that heightened amygdala responses associated with depression may be more apparent during such tasks.23, 24 Of the possible 43 unique individuals in the Nimstim Set of Facial Expressions (NimStim; http://www.macbrain.org/resources.htm), 21 were used and counterbalanced for gender and ethnicity. Preschoolers were shown neutral, happy, sad, and fearful facial expressions. In the subset of PO-MDD children who were included from our previous report (n = 7), pictures of their mother were displayed instead of fear faces. As such, the current study focuses on the sad, happy, and neutral face conditions. Faces were shown for 3.5 seconds followed by a 1.5-second ITI. Each block contained 8 faces of the same emotion type (40 seconds total) and the 4 blocks in each run (neutral, happy, sad, and fear[mother]) were interleaved with 35-second fixation blocks (fear and mother faces were always presented at the end of a run; see Figure 1). Thus, each run was 5.3 minutes. Two runs were presented during each scan session for an approximate total of 11 minutes functional scanning time.
FIGURE 1.
Face processing task used in preschoolers with and without preschool depression. Note: Please see Method for greater detail. The bottom left image in the figure illustrates the child-friendly response device used in the current study.
Functional Imaging Data Acquisition and Preprocessing
Imaging data were collected using a 3T TIM TRIO Siemens whole body system. To create familiarity and comfort with study procedures, each child was provided with a child friendly video introducing the fMRI experience prior to their visit, introduced to the scanning environment using a mock scanner training protocol during their initial in-person assessment, allowed to watch a movie of their choice during structural scans, and rewarded with small prizes following scan completion.
Image acquisition included an initial low-resolution 3D sagittal T1-weighted MP-RAGE rapidly warped to Talairach space.25 This image was then used to provide online slice localization for the functional images, placing them as close as possible to the target template. T1 images were acquired as part of the structural imaging protocol and used in the transformation of images to a common template space optimized for preschool children.25 The accuracy and validity of this transformation for preschool age children has been demonstrated in previous research26 and was confirmed through visual inspection for distortions and the accuracy of alignment for key cortical and subcortical landmarks. The functional images were collected with a 12-channel head coil using an asymmetric spin-echo echo-planar sequence sensitive to blood oxygen level dependent (BOLD) contrast (T2*) (repetition time [TR]=2500ms, echo time [TE]=27ms, field of view [FOV]=256mm, flip=90°). During each functional run, sets of 32 contiguous axial images with isotropic voxels (4mm3) were acquired parallel to the anterior-posterior commissure plane. Stimuli were presented using PsyScope X on an Intel Macintosh computer, with the start of each run directly triggered by a pulse from the scanner.
Prior to preprocessing, the first 4 frames of each run were discarded to allow for signal stabilization. The fMRI data were preprocessed and analyzed using in-house Washington University software. Data were reconstructed into images and normalized across runs by scaling whole-brain signal intensity to a fixed value and removing the linear slope on a voxel-by-voxel basis to counteract effects of drift.27 Data were also corrected for head motion using rigid-body rotation and translation correction algorithms28-30, co-registered to Talairach space using a 12 parameter linear (affine) transformation that included resampling to 3mm cubic, and smoothed using a 6mm FWHM Gaussian filter. Within scan head movement was assessed using output from the rigid-body rotation and translation algorithm. After measuring the translations and rotations in the x, y, and z planes across frames, total root mean square (RMS) linear and angular measures were calculated and used to obtain the average amount of movement in millimeters from frame-to-frame (i.e., TR-to-TR) in a given run for each subject (RMS/frame). Face-processing runs with greater than 0.15mm RMS/frame were excluded from further data analysis. Using this criterion, 7 children in each of the experimental groups provided usable face-processing data from only 1 of the 2 possible runs. Groups did not differ in terms of movement (PO-MDD RMS/frame = .09[.03]mm; Control RMS/frame = .08[.03]mm; p > .05). To further reduce any potential effects of head movement on data quality, custom Matlab (The Mathworks, Natwick, MA) code was used to identify frames with greater than 0.7mm absolute movement.31 The identified frames, as well as the frames before and after them, were removed from further data analysis. Groups did not differ in terms of the mean percentage of frames removed (PO-MDD = ~6(3)%; Control = ~5(2)%; p > .05).
Estimates of functional activation during each condition were obtained using block-design analyses. This included the use of a general linear model (GLM) incorporating regressors for linear trend and baseline shift to estimate the hemodynamic response function for each stimulus type (i.e., facial expression). Within the GLM, a hemodynamic response shape was assumed (Boynton function) and used to derive magnitude estimates for each stimulus type relative to baseline fixation, which were then used in all subsequent statistical analyses.
Functional Imaging Data Analysis
The present study used both a region of interest (ROI) and whole-brain approach. The more conservative ROI analysis focused on cortico-limbic regions thought to be important for emotion processing and regulation in depression1, 10, 24, 32-38 and used in our previous study of school age children with a known history of PO-MDD, including the amygdala, hippocampus, striatum, dorsolateral prefrontal cortex, dorsal anterior cingulate, pregenual anterior cingulate, and subgenual cingulate. Both ROI and whole-brain analyses used a repeated measures analysis of variance (ANOVA) with diagnostic group as the between subject factor and emotion face type as the within subject factor. ROI and whole brain analyses were corrected for multiple comparisons using combined p-value/cluster size thresholds determined using Monte Carlo simulations.39, 40 Thresholds were z = 2.58 (p<.005) and 16 voxels within our emotion regulation mask (correcting for all ROIs simultaneously; false positive rate of p<.05 for the whole ROI mask) and z = 3 (p<.001) and 13 voxels for whole-brain analyses (whole-brain false positive rate of p<.05).
Following the identification of group differences, magnitude estimates were obtained for each region and subsequently examined in separate correlational analyses using the ERC Emotion Regulation and Negativity subscales (Pearson r; IBM SPSS Statistics version 19; SPSS Inc., Chicago, IL, USA). Given our a priori hypotheses regarding right amygdala activity and emotion regulation and negative affect, these relationships were of primary interest. Additional post-hoc analyses examining the relationship between emotion regulation and negative affect with other identified regions of difference were subsequently conducted and corrected for multiple comparisons.
RESULTS
DEMOGRAPHIC, CLINICAL, AND BEHAVIORAL CHARACTERISTICS
Demographic and clinical characteristics including rates of comorbidity are summarized in Table 1. Groups did not differ in age, gender, ethnicity, family income, task response rate, or handedness. Accuracy in labeling facial expressions of emotion did not differ between groups. As expected, parents of PO-MDD children endorsed significantly higher scores on the ERC Negativity subscale and significantly lower scores on the ERC Emotion Regulation subscale (see Table 1).
NEUROIMAGING FINDINGS
Categorical Group Comparison
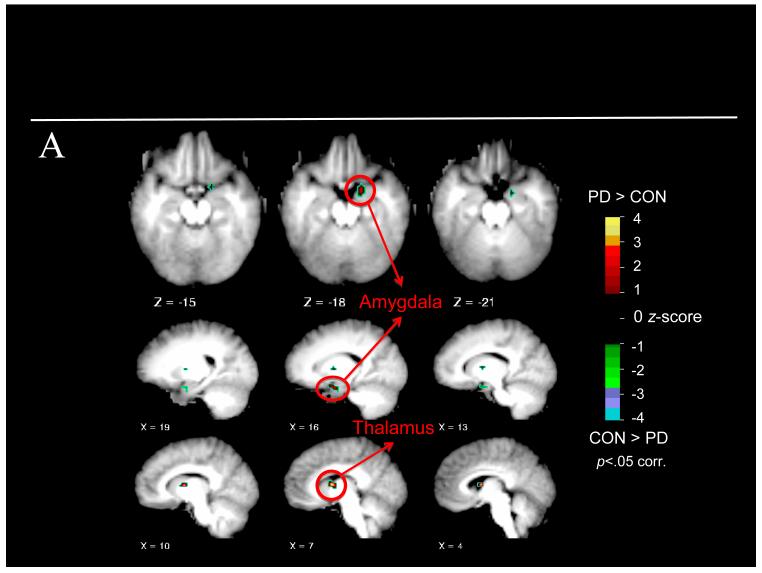
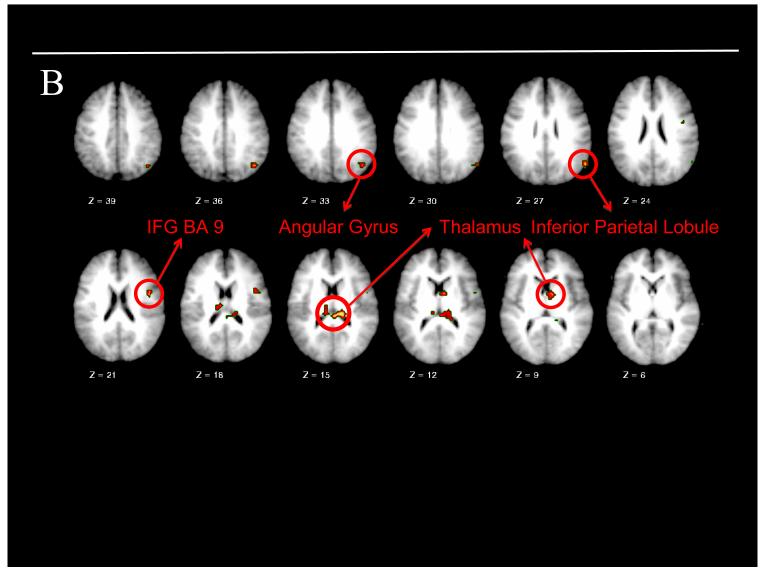
The voxel-wise ANOVA using our a priori ROI mask revealed a significant main effect of group in the right amygdala and right anterior thalamus, with greater activation present in both regions for children with PO-MDD (see Table 2 and Figure 2A). Neither a main effect of emotion nor a group X emotion interaction was found. At the whole-brain level, a similar ANOVA revealed a main effect of group with greater right inferior frontal gyrus, anterior thalamus, pulvinar, right angular gyrus, and right inferior parietal lobule activity present in PO-MDD children (see Table 2 and Figure 2B). All group differences remained significant after comorbidity was included as a covariate. In addition, a main effect of emotion was found in the right posterior cingulate gyrus, lingual gyrus, and thalamus (see Table 2 for details of directionality in each region). No group × emotion interaction was found. Follow-up analyses of the right amygdala confirmed greater activity in this region for the PO-MDD subgroup who saw mother faces (n=7) as well as the PO-MDD subgroup who saw fear (n=16) faces (all p<.05), and that the PO-MDD groups did not differ from each other (t[21] = .429, p = .672, d = .16).
Table 2.
Regions identified as demonstrating a main effect of group or face type
| Region | Hemisphere | BA | X | Y | Z | Cluster(voxels) |
|---|---|---|---|---|---|---|
| Main Effect of Group | ROI MASK |
|||||
| PO-MDD > Control | ||||||
| Amygdala | R | 16 | −3 | −18 | 21 | |
| Anterior Thalamus | R | 10 | −2 | 9 | 40 | |
|
| ||||||
| Main Effect of Group | WHOLE BRAIN |
|||||
| PO-MDD > Control | ||||||
| Anterior Thalamus | R | 6 | −2 | 10 | 24 | |
| Pulvinar | R | 10 | −31 | 14 | 59 | |
| Inferior Frontal Gyrus | R | 9 | 44 | 0 | 19 | 24 |
| Posterior Thalamus | L | −9 | −23 | 15 | 27 | |
| Angular Gyrus | R | 39 | 45 | −65 | 35 | 59 |
| Inferior Parietal Lobule | R | 40 | 53 | −50 | 49 | 21 |
|
| ||||||
| Main Effect of Face Typea |
WHOLE BRAIN |
|||||
| Lingual Gyrus: S > H,N | R | 19 | 17 | −66 | 5 | 28 |
| Thalamus: H > N,S | R | 1 | −11 | 13 | 19 | |
| Posterior Cingulate Gyrus: S > N > H |
R | 31 | 9 | −55 | 23 | 157 |
Note: BA = Brodmann Area; L = left; H = happy face; N = neutral face; PO-MDD = Preschool Depression; ROI = Region of Interest; R = right; S = sad face.
Direction of difference between face types is indicated following region name.
FIGURE 2.
Results of a priori region of interest and whole brain analyses. Note: Preschoolers with depression (PD) were found to have (A) clusters of increased functional activity within the right amygdala and right thalamus when our a priori mask of interest was used and (B) clusters of increased functional activity within the inferior frontal gyrus (IFG), angular gyrus, inferior parietal lobule, and thalamus when the whole-brain was examined. Warmer colors indicate increased functional activity in preschoolers with depression during face processing. BA=Brodmann area; CON=control.
Dimensional Analyses
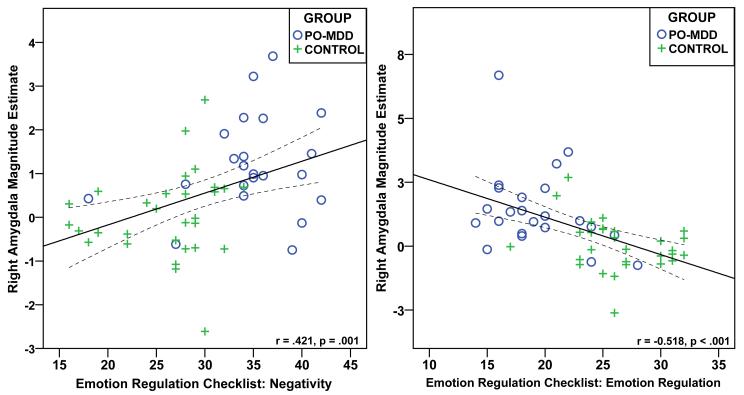
A multivariate approach to identifying potential outliers using Mahalanobis D2 was conducted prior to carrying out a priori correlational analyses including the amygdala and ERC subscales. This revealed one outlier in the Control group for the ERC Emotion Regulation analysis and two outliers, one in each group, for the ERC Negativity analysis. The identified outliers were removed and not used in subsequent analyses. Correlational analyses using the whole sample revealed a positive relationship between ERC Negativity scores and right amygdala activity during face processing (r[52] = .42 [95% CI: .17 to .63], p=.001 [one-tailed]; see Figure 3). Conversely, a similar analysis revealed a negative relationship between ERC Emotion Regulation scores and right amygdala activity during face processing (r[53] = −0.52 [95% CI: −0.29 to −0.69], p<.001 [one-tailed]). Controlling for comorbidity did not change the results (all p < .05). These relationships did not reach significance (p > .05) in the Control (r[30] = .15 [Negativity] and −0.29 [Emotion Regulation]) or PO-MDD (r[22] = .18 [Negativity] and r[23] = −0.32 [Emotion Regulation]) groups alone, although the directionality in each group was similar to that for the combined sample. Post hoc analyses corrected for multiple comparisons (.05/7 = p<.007 [two-tailed]) revealed additional relationships. Specifically, ERC Negativity scores were positively associated with activity in the right pulvinar (r[54] = .43) and angular gyrus (r[54] = .37). ERC Emotion Regulation scores were negatively associated with activity in the right anterior thalamus (ROI mask: r[54] = −0.42; whole brain: r[54] = −0.39), right angular gyrus (r[54] = −0.47), and right inferior parietal lobule (r[54] = −0.37).
FIGURE 3.
Scatter plot illustrating the relationship between right amygdala activity and the Emotion Regulation Checklist subscales of Negativity and Emotion Regulation. Note: Circles indicate preschoolers with depression and crosses indicate healthy control preschoolers. PO-MDD=preschool depression.
DISCUSSION
As hypothesized, we found increased right amygdala activity during face viewing in depressed preschoolers when compared to their healthy peers. Consistent with previous reports of disrupted amygdala function in older depressed children and adults, this finding suggests disrupted amygdala functioning in depression may occur as early as the preschool period. Importantly, this finding also raises the intriguing possibility that disrupted amygdala functioning may be a neural biomarker for depression across the life span and evident early in life. The detection of such a marker as early as the preschool period of development is potentially important for early identification of this chronic and relapsing disorder. It may also provide key targets for early intervention. However, future longitudinal studies examining the specificity of disrupted amygdala reactivity in PO-MDD (e.g., versus anxiety) as well as its relationship to future episodes of depression will be necessary to address this possibility.
The current finding of increased amygdala reactivity across multiple face emotion types, including sad, happy, and neutral, is consistent with some5, 6, 41 but not all previous research in older depressed children and adolescents.e.g.,7 However, with no other fMRI studies of face processing in depressed preschoolers available, determining whether the current results are consistent with previous work in the same age range is not possible. Also, with a near absence of data available to inform the normative developmental trajectory of amygdala reactivity to facial expressions of emotion, determining if disrupted amygdala function in PO-MDD reflects an exaggeration of an expected response at this age (i.e., indiscriminant amygdala reactivity to all emotion types) remains unknown. Nevertheless, previous research has suggested the right amygdala is preferentially involved in the rapid detection of (i.e., directing attention towards) emotionally relevant stimuli while the left amygdala is more closely tied to subsequent stimulus evaluation.42 In light of this, the right lateralized amygdala finding in the PO-MDD group raises the possibility of disrupted amygdala functioning early in the visual processing of faces (i.e., detecting faces) and, potentially, provides a feasible explanation of its increased reactivity across face emotion types. However, the current study cannot inform this functional distinction (rapid detection versus stimulus evaluation) and future research will be needed to disentangle the potential influence of PO-MDD on these processes.
In line with our previous reports9, 43, greater right amygdala reactivity was found to be associated with increased levels of negative affect when the entire study sample was examined. Extending this finding to the construct of emotion regulation, right amygdala activity was found to be negatively associated with emotion regulation ability in the entire sample as well. Possibly due to small sample sizes and reduced statistical power, the relationship between ERC subscale scores and amygdala reactivity did not remain significant at the subgroup level, although they were in the same direction. Nevertheless, while preliminary, these findings match those reported in older depressed individuals6, 44 and indicate a similarly important relationship between amygdala reactivity to faces and disorder relevant behavior in depressed preschoolers.
No differences within the fusiform gyrus or other lower level face processing regions (e.g., inferior occipital gyrus) were found, suggesting at the level of brain function the groups did not differ in general face processing. However, whole-brain and ROI comparisons revealed increased activity during face processing in the thalamus, pulvinar, right inferior frontal gyrus, right angular gyrus and right parietal lobule when children with PO-MDD were compared to their healthy peers. Previous research has suggested the pulvinar, amygdala, and superior colliculus form a subcortical network that rapidly responds to the presence of emotionally relevant stimuli.45 As suggested for the amygdala, increased activity in the pulvinar and other identified thalamic regions could be associated with heightened reactivity during early face processing in PO-MDD. Alternatively, activity in these regions may be the result of increased reactivity to the novelty, rather than content, of the stimuli (i.e., strange adults). The frontal and parietal regions with greater activity in PO-MDD have frequently been associated with attention related processes in models of face processing46, including the right lateralized ventral attention network believed to be important for reorienting attention towards salient stimuli.47 Future studies of PO-MDD examining these regions (including the amygdala) and their interactions will be important for identifying how early occurring depression affects the ongoing integration and development of brain circuits related to social cognition, attention, and emotion regulation. Such information is also likely to be important for further clarifying the exact role of the amygdala in depression (e.g., event specificity) as well as its unfolding relationships with other disorder relevant brain regions and networks over the course of development.
Several limitations should be mentioned. First, our examination of individual differences would have benefited from a larger sample size, especially at the subgroup level. However, we believe this study provides the largest sample of depressed preschoolers yet studied using task based fMRI. Second, PO-MDD children taking medications were excluded from participating. This limits generalization of the current findings to preschoolers with PO-MDD and no history of psychotropic medication use; however this limitation is mitigated by the fact that the vast majority of children with PO-MDD do not take medications.48 In addition, adding groups at increased risk for depression or with other related disorders (e.g., anxiety) will be necessary to further clarify whether amygdala hyper-reactivity to facial expressions is specific to PO-MDD and whether it precedes or follows the onset of this disorder. Lastly, though a mock scanner was used to acclimate each child to the fMRI environment prior to their scan, the potential effects of this environment (i.e., increased anxiety) cannot be definitively ruled out.
To our knowledge, this is the first study to compare preschool age children with a known depressive disorder to a healthy comparison group using fMRI. Consistent with previous reports in older depressed groups and school age children with a known history of PO-MDD, we found evidence of disrupted amygdala function during the processing of facial expressions of emotion. As such, the current findings provide the earliest known findings of disrupted brain function in depression and uniquely add to our understanding of brain development in this disorder.
Acknowledgments
The Klingenstein Third Generation Foundation (M.S.G.) and the Communities Healing Adolescent Depression and Suicide (CHADS) Coalition for Mental Health (J.L.L., D.M.B.) provided funding for this study. This work was supported by grant K23 MH098176 (M.S.G.) from the National Institute of Mental Health (NIMH).
The authors thank the children and their families who participated in this study.
Disclosures: Dr. Gaffrey has received funding from the McDonnell Center for Systems Neuroscience (MCSN). Dr. Barch has received grants from the NIMH, National Institute on Aging, the National Alliance for Research on Schizophrenia and Depression (NARSAD), the Sydney R. Baer Foundation, Allon, Novartis, and MCSN. Dr. Luby has received grants from NIMH, the Sydney R. Baer Foundation, and NARSAD. Ms. Singer and Ms. Shenoy report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–44. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 2.Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord. 2011;1(1):10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan SW, Norbury R, Goodwin GM, Harmer CJ. Risk for depression and neural responses to fearful facial expressions of emotion. British Journal of Psychiatry. 2009;194(2):139–45. doi: 10.1192/bjp.bp.107.047993. [DOI] [PubMed] [Google Scholar]
- 4.Gaffrey MS, Luby JL, Barch DM. Towards the study of functional brain development in depression: An Interactive Specialization approach. Neurobiology of Disease. 2013;52:38–48. doi: 10.1016/j.nbd.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau JY, et al. Amygdala function and 5-HTT gene variants in adolescent anxiety and major depressive disorder. Biological Psychiatry. 2009;65(4):349–55. doi: 10.1016/j.biopsych.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang TT, et al. Adolescents with major depression demonstrate increased amygdala activation. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(1):42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monk CS, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. American Journal of Psychiatry. 2008;165(1):90–8. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 8.Luby JL, Si X, Belden AC, Tandon M, Spitznagel E. Preschool depression: homotypic continuity and course over 24 months. Archives of General Psychiatry. 2009;66(8):897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaffrey MS, Luby JL, Belden AC, Hirshberg JS, Volsch J, Barch DM. Association between depression severity and amygdala reactivity during sad face viewing in depressed preschoolers: an fMRI study. Journal of Affective Disorders. 2011;129(1-3):364–70. doi: 10.1016/j.jad.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barch DM, Gaffrey MS, Botteron KN, Belden AC, Luby JL. Functional brain activation to emotionally valenced faces in school-aged children with a history of preschool-onset major depression. Biological Psychiatry. 2012;72(12):1035–42. doi: 10.1016/j.biopsych.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fusar-Poli P, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience. 2009;34(6):418–32. [PMC free article] [PubMed] [Google Scholar]
- 12.Hoehl S, Brauer J, Brasse G, Striano T, Friederici AD. Children’s processing of emotions expressed by peers and adults: an fMRI study. Soc Neurosci. 2010;5(5-6):543–59. doi: 10.1080/17470911003708206. [DOI] [PubMed] [Google Scholar]
- 13.Luby J, Heffelfinger A, Koenig-McNaught A, Brown K, Spitznagel E. The preschool feelings checklist: A brief and sensitive screening measure for depression in young children. J Am Acad Child Adolesc Psychiatry. 2004;43(6):708–717. doi: 10.1097/01.chi.0000121066.29744.08. [DOI] [PubMed] [Google Scholar]
- 14.Egger HL, Ascher B, Angold A. The Preschool Age Psychiatric Assessment: Version 1.4, in Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences. Duke University Medical Center; Durham, NC: 2003. 1999. [Google Scholar]
- 15.APA . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. American Psychiatric Association; Washington, D.C.: 2000. [Google Scholar]
- 16.Egger HL, Erkanli A, Keeler G, Potts E, Walter B, Angold A. Test-Retest Reliability of the Preschool Age Psychiatric Assessment (PAPA) J Am Acad Child Adolesc. Psychiatry. 2006;45(5):538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- 17.Luby JL, Si X, Belden AC, Tandon M, Spitznagel E. Preschool depression: homotypic continuity and course over 24 months. Arch Gen Psychiatry. 2009;66(8):897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Hessler M, Spitznagel E. Modification of DSM-IV criteria for depressed preschool children. Am J Psychiatry. 2003;160(6):1169–72. doi: 10.1176/appi.ajp.160.6.1169. [DOI] [PubMed] [Google Scholar]
- 19.Luby JL, Belden AC, Pautsch J, Si X, Spitznagel E. The clinical significance of preschool depression: Impairment in functioning and clinical markers of the disorder. Journal of Affective Disorders. 2009;112(1-3):111–119. doi: 10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaffrey MS, Belden AC, Luby JL. The 2-week duration criterion and severity and course of early childhood depression: implications for nosology. Journal of Affective Disorders. 2011;133(3):537–45. doi: 10.1016/j.jad.2011.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shields A, Cicchetti D. Emotion regulation among school-age children: the development and validation of a new criterion Q-sort scale. Dev Psychol. 1997;33(6):906–16. doi: 10.1037//0012-1649.33.6.906. [DOI] [PubMed] [Google Scholar]
- 22.Mrakeotsky C. Visual perception, spatial cognition and affect recognition in preschool depressive syndromes. University of Vienna/Washington University; 2001. [Google Scholar]
- 23.Monk CS, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165(1):90–8. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 24.Fales CL, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63(4):377–84. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme Medical Publishers; Stuttgart: 1988. [Google Scholar]
- 26.Ghosh SS, et al. Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11 years of age. Neuroimage. 2010;53(1):85–93. doi: 10.1016/j.neuroimage.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandettini PA, Jesmanowiz J, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magnetic Resonance in Medicine. 1993;30(2):161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- 28.Friston KJ, Jezzard P, Turner R. The analysis of functional MRI time series. Human Brain Mapping. 1994;1(2):153–171. [Google Scholar]
- 29.Snyder AZ. In: Difference image versus ratio image error function forms in PET-PET realignment, in Quantification of Brain Function Using PET. Myer VJCR, Bailey DL, Jones T, editors. Academic Press; San Diego: 1996. pp. 131–137. [Google Scholar]
- 30.Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography. 1992;16(4):620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 31.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13(9):829, 833–57. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 34.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1-2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13(8):663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant threatment: an fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 37.Sheline YI, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fales CL, et al. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord. 2009;112(1-3):206–11. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 40.McAvoy MP, Ollinger JM, Buckner RL. Cluster size thresholds for assessment of significant activation in fMRI. NeuroImage. 2001;13:S198. [Google Scholar]
- 41.Beesdo K, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry. 2009;66(3):275–85. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Barch DM, Gaffrey MS, Botteron KN, Belden AC, Luby JL. Functional brain activation to emotionally valenced faces in school aged children with a history of preschool onset major depression. Biological Psychiatry. 2012;72(12):1035–42. doi: 10.1016/j.biopsych.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Canli T, et al. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport. 2005;16(12):1267–70. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- 45.Johnson MH. Subcortical face processing. Nat Rev Neurosci. 2005;6(10):766–74. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- 46.Palermo R, Rhodes G. Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia. 2007;45(1):75–92. doi: 10.1016/j.neuropsychologia.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 47.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(26):10046–51. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luby J, Stalets M, Belden A. Psychotropic prescriptions in a sample of healthy mood and disruptive disordered preschoolers: relationships to diagnosis impairment, prescriber type and assessment methods. Journal of Child and Adolescent Psychopharmacology. 2007;17(2):205–216. doi: 10.1089/cap.2007.0023. [DOI] [PubMed] [Google Scholar]