Abstract
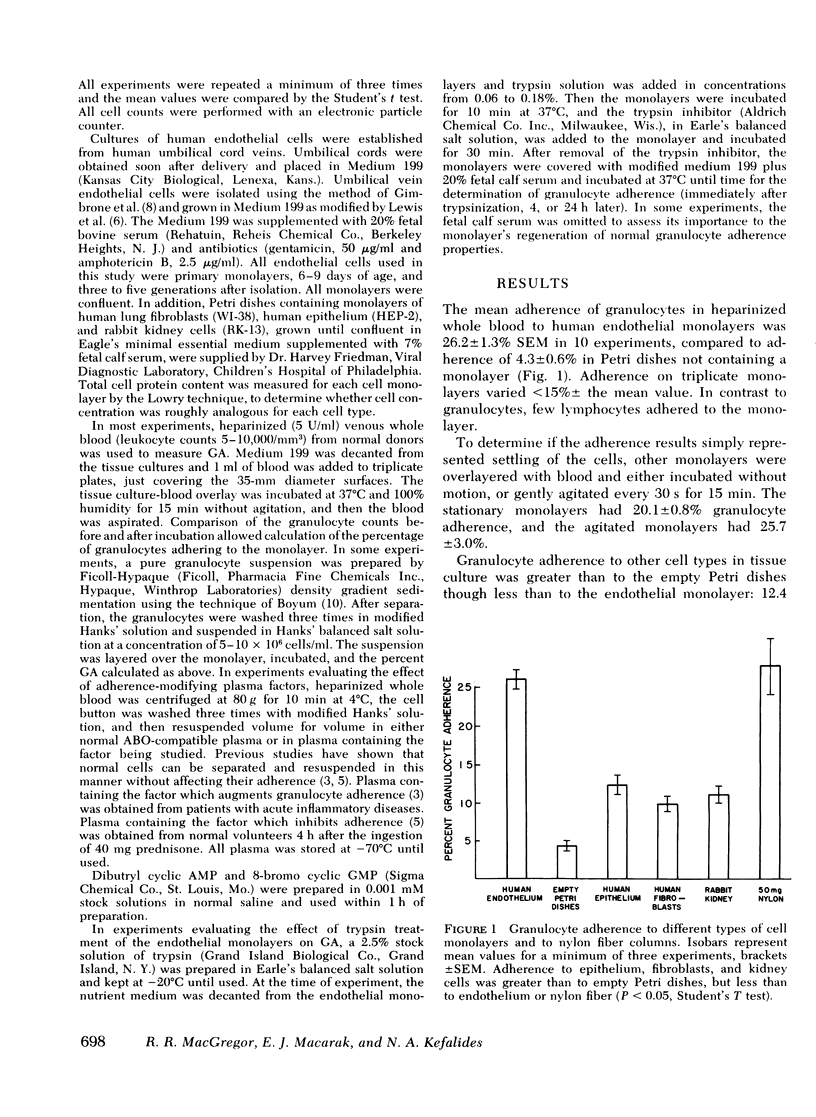
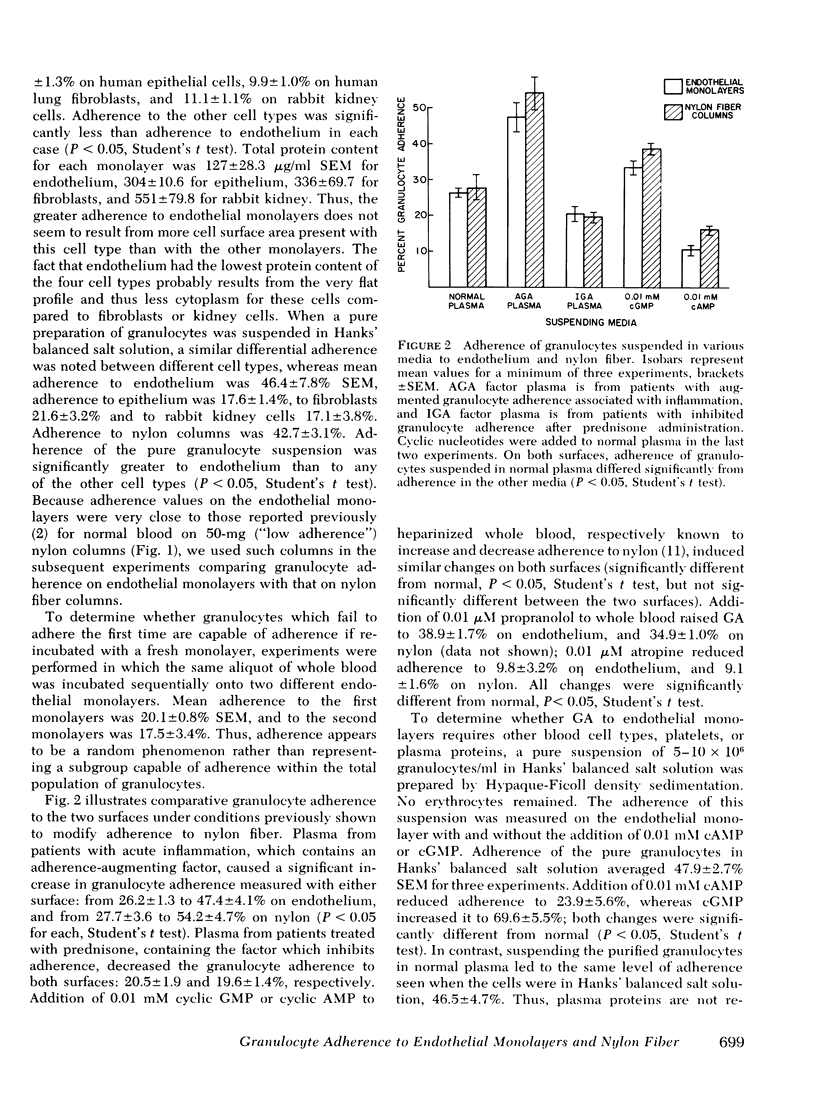
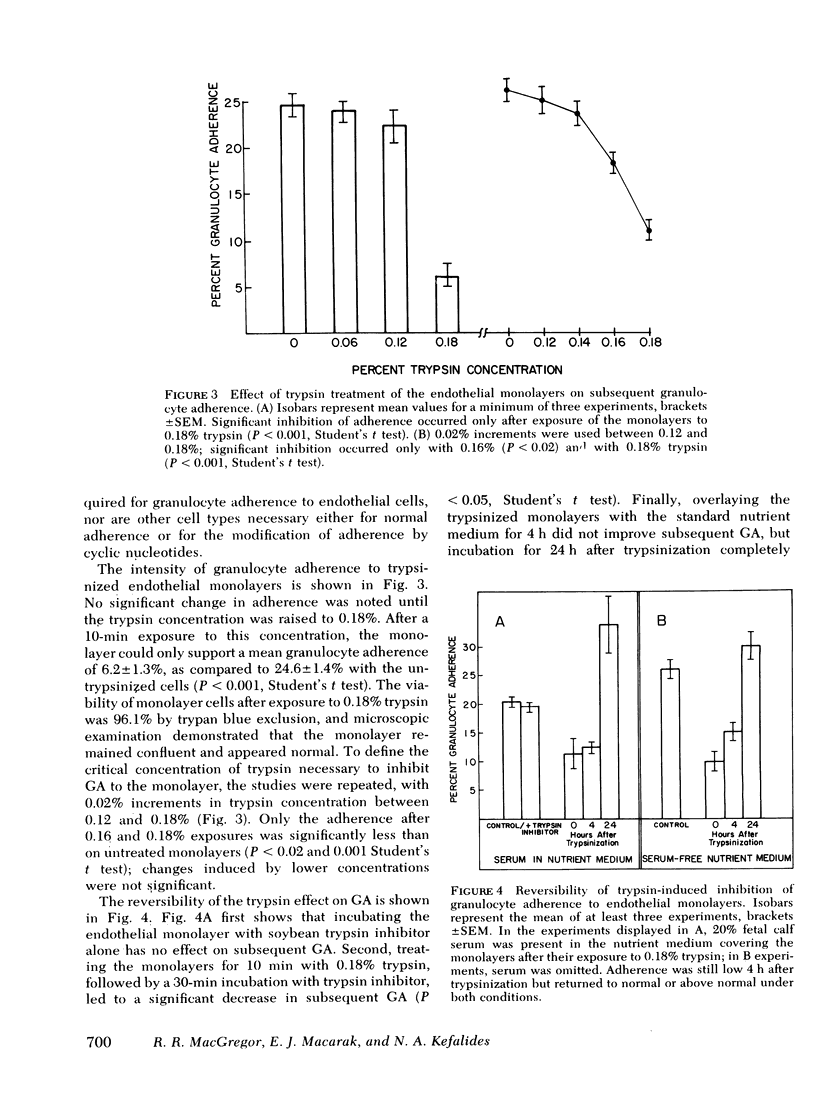
Adherence of granulocytes to tissue culture monolayers of endothelium averaged 26.2 +/- 1.3% SEM, which was similar to their adherence on 50-mg nylon fiber columns (27.7 +/- 3.6%). In contrast, adherence to epithelial cells, fibroblasts, kidney cells, and plastic Petri dishes without monolayers was only 12.4, 9.9, 11.1, and 4.3%, respectively. Cyclic nucleotides and adherence-modifying plasma factors induced changes of adherence to endothelium similar to those in nylon fiber columns. Adherence of granulocytes in whole blood was the same as for purified granulocytes in Hank's balanced salt solution. Exposure of endothelial monolayers to 0.18% trypsin for 10 min reduced subsequent granulocyte adherence to 25.2% of control values. Incubation of trypsin-treated monolayers with nutrient medium for 4 h did not improve adherence, but values returned to normal or above by 24 h, with or without serum proteins present in the nutrient medium. The similarity of granulocyte adherence to nylon fiber and to endothelial monolayers in vitro suggests that results with the nylon fiber assay reflect in vivo granulocyte-endothelium interaction. Furthermore, the endothelial monolayer offers a new model for studying this cell-cell relationship in vitro.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Codington J. F., Sanford B. H., Jeanloz R. W. Glycoprotein coat of the TA 3 cell. I. Removal of carbohydrate and protein material from viable cells. J Natl Cancer Inst. 1970 Oct;45(4):637–647. [PubMed] [Google Scholar]
- GARVIN J. E. Factors affecting the adhesiveness of human leucocytes and platelets in vitro. J Exp Med. 1961 Jul 1;114:51–73. doi: 10.1084/jem.114.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974 Mar;60(3):673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentnek A. L., Schreiber A. D., MacGregor R. R. The induction of augmented granulocyte adherence by inflammation. Mediation by a plasma factor. J Clin Invest. 1976 Apr;57(4):1098–1103. doi: 10.1172/JCI108354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis L. J., Hoak J. C., Maca R. D., Fry G. L. Replication of human endothelial cells in culture. Science. 1973 Aug 3;181(4098):453–454. doi: 10.1126/science.181.4098.453. [DOI] [PubMed] [Google Scholar]
- MARCHESI V. T., FLOREY H. W. Electron micrographic observations on the emigration of leucocytes. Q J Exp Physiol Cogn Med Sci. 1960 Oct;45:343–348. doi: 10.1113/expphysiol.1960.sp001489. [DOI] [PubMed] [Google Scholar]
- MOSCONA A. Effect of temperature on adhesion to glass and histogenetic cohesion of dissociated cells. Nature. 1961 Apr 29;190:408–409. doi: 10.1038/190408a0. [DOI] [PubMed] [Google Scholar]
- Mac Gregor R. R. Granulocyte adherence changes induced by hemodialysis, endotoxin, epinephrine, and glucocorticoids. Ann Intern Med. 1977 Jan;86(1):35–39. doi: 10.7326/0003-4819-86-1-35. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Spagnuolo P. J., Lentnek A. L. Inhibition of granulocyte adherence by ethanol, prednisone, and aspirin, measured with an assay system. N Engl J Med. 1974 Sep 26;291(13):642–646. doi: 10.1056/NEJM197409262911302. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R. The effect of anti-inflammatory agents and inflammation on granulocyte adherence. Evidence for regulation by plasma factors. Am J Med. 1976 Nov;61(5):597–607. doi: 10.1016/0002-9343(76)90137-6. [DOI] [PubMed] [Google Scholar]
- Macarak E. J., Howard B. V., Kefalides N. A. Biosynthesis of collagen and metabolism of lipids by endothelial cells in culture. Ann N Y Acad Sci. 1976;275:104–113. doi: 10.1111/j.1749-6632.1976.tb43344.x. [DOI] [PubMed] [Google Scholar]
- Macarak E. J., Howard B. V., Kefalides N. A. Properties of calf endothelial cells in culture. Lab Invest. 1977 Jan;36(1):62–67. [PubMed] [Google Scholar]
- Revel J. P., Hoch P., Ho D. Adhesion of culture cells to their substratum. Exp Cell Res. 1974 Mar 15;84(1):207–218. doi: 10.1016/0014-4827(74)90398-x. [DOI] [PubMed] [Google Scholar]
- Snow C., Allen A. The release of radioactive nucleic acids and mucoproteins by trypsin and ethylenediaminetetra-acetate treatment of baby-hamster cells in tissue culture. Biochem J. 1970 Oct;119(4):707–714. doi: 10.1042/bj1190707. [DOI] [PMC free article] [PubMed] [Google Scholar]



