Abstract
Background
The contribution of albuminuria to the increased risk of incident end-stage renal disease (ESRD) in individuals with a family history of ESRD has not been well studied.
Study Design
Prospective cohort study.
Study Setting & Participants
We analyzed data for family history of ESRD collected from 19,409 participants of the Renal REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort study.
Predictor
Family history of ESRD was ascertained by asking “Has anyone in your immediate family ever been told that he or she had kidney failure? This would be someone who is on or had been on dialysis or someone who had a kidney transplant.”
Study Outcomes
Incidence rate for ESRD.
Measurements
Morning urine albumin-creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR). Incident cases of ESRD were identified through the US Renal Data System.
Results
A family history of ESRD was reported by 11.1% of participants. Mean eGFRs for those with and without a family history of ESRD were 87.5 ± 22.2 (SD) and 86.5 ± 19.3 mL/min/1.73 m2, respectively (P = 0.05) and the respective geometric mean ACRs were 12.2 and 9.7 mg/g (P < 0.001). ESRD incidence rates for those with and without a family history of ESRD were 244.3 and 106.1/100,000 person-years, respectively. After adjusting for age, sex, and race, the ESRD HR for those with versus those without a family history of ESRD was 2.13 (95% CI, 1.18-3.83). Adjustment for comorbid conditions and socioeconomic status attenuated this association (HR, 1.82; 95% CI, 1.00-3.28), and further adjustment for baseline eGFR and ACR completely attenuated the association between family history of ESRD and incident ESRD (HR, 1.12; 95% CI, 0.69-1.80).
Limitations
The report of a family history of ESRD was not validated.
Conclusion
Family history of ESRD is common in older Americans and the increased risk of ESRD associated with a family history reflects lower GFR, higher albuminuria, and comorbid conditions.
Keywords: Race, albuminuria, end-stage renal disease, chronic kidney disease
End-stage renal disease (ESRD) in a first-degree family member (subsequently referred to in this article as a family history of ESRD) is reported at the start of renal replacement therapy by as many as 1 in 5 patients in the United States.1-4 Little is known about the prevalence of risk factors for progressive kidney disease in these individuals. In particular, the prevalence of albuminuria, a major risk factor for progression of kidney disease, has not been well described in individuals with a family history of ESRD.5,6 There have been reports that albuminuria is associated with a family history of ESRD in small clinic-based populations7 and a European population,8 but this association is poorly described in family members in the US population. Further, there is limited information about the extent to which individuals in the general US population with a family history of ESRD progress to ESRD independently of albuminuria and other established risk factors for progressive kidney disease.
These issues are a matter of considerable clinical and public health importance. If individuals with a family history of ESRD have an increased risk of progressive kidney disease, they might benefit from efforts to identify early kidney disease and subsequent interventions to prevent or delay progression to ESRD.9,10 We address these issues by reporting the prevalence of albuminuria in participants with a family history of ESRD and the association between family history of ESRD, albuminuria, and incident ESRD in a large, population-based, biracial cohort study.
METHODS
Study Design
The Renal REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort study involves a population-based sample of white and black adults 45 years and older recruited throughout the continental United States between January 2003 and October 2007.11
Data
A family history of ESRD was ascertained by asking “Has anyone in your immediate family ever been told that he or she had kidney failure? This would be someone who is on or had been on dialysis or someone who had a kidney transplant.” Individuals answering yes were asked “What relative or relatives had or has kidney failure?” A family history of ESRD in a first-degree relative was defined as identification of a sibling, child, or parent as having ESRD.
We ascertained awareness of kidney disease by asking the participant “Has a doctor or other health professional ever told you that you had kidney disease?”
Serum creatinine level was calibrated to a creatinine standard determined by isotope-dilution mass spectrometry, and estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease (CKD) Epidemiology Collaboration (CKD-EPI) estimating equation.12 A random morning urine sample was collected for creatinine and albumin measurement. Urinary albumin was measured using the BN ProSpec Nephelometer from Dade Behring (www.balticnordic.com/dade-behring-marburg-gmbhmarburg/company.html). Urinary creatinine was measured with a rate-blanked Jaffé procedure, using the Modular-P analyzer (Roche/Hitachi, www.gmi-inc.com/Roche-Hitachi-911-Chemistry-Analyzer.html). The observed assay range was 1-650 mg/dL on initial sampling. Results were expressed for each participant as urinary albumin-creatinine ratio (ACR) in milligrams per gram.
All-cause mortality was ascertained by proxy report during follow-up telephone surveys conducted semiannually. Death status was confirmed by cross-reference to the US Social Security Death Index. Incident cases of ESRD were identified through linkage of REGARDS Study participants with the US Renal Data System (USRDS), which records >90% of incident ESRD cases in the United States. A finder file containing unique individual identifiers (social security number, date of birth, and last and first name) was submitted for linkage with the USRDS database. Sequential matching was accomplished using different configurations of full and partial individual identifiers. Individual records that did not produce a match candidate were processed again in subsequent rounds. For individuals with a match, but who did not match on all identifiers, visual inspection of nonmatching variables was performed in the following rounds to confirm valid matches. Data from the USRDS included incident ESRD cases through August 2009. Patient follow-up started on the date of patient enrollment and continued until the first of the following: date of death, ESRD incidence, or September 1, 2009. We excluded individuals with ESRD by self-report or data from the USRDS indicating that the incidence of ESRD occurred prior to their enrollment into the REGARDS cohort.
Covariate information was ascertained during a computer-assisted telephone interview and subsequent in-home examination. Age, sex, race, marital status, educational attainment, household income, hypertension, and diabetes were included in our analyses. Diabetes was defined as self-reported use of diabetic medications or insulin, fasting glucose level ≥126 mg/dL or nonfasting glucose ≥200 mg/dL. Hypertension was defined as an in-home systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or self-reported treatment. Body mass index was calculated as weight in kilograms divided by height in meters squared.
Analysis
Baseline characteristics are described as mean values or percentages. We used t tests, analysis of variance, and χ2 tests to examine differences in clinical characteristics of participants with and without a family history of ESRD. ACR was log-transformed in these analyses and reported as geometric mean. Logistic regression models were used to examine the association between family history of ESRD and baseline characteristics.
The association between family history of ESRD and ESRD risk was examined using multivariable-adjusted Cox proportional hazards regression models. We initially controlled for demographic and clinical characteristics, including age in years, sex, race, hypertension and diabetes, household income <$20,000, and education less than high school. In subsequent models, we also included baseline eGFR and ACR, first separately and then in combination. All analyses were conducted using SAS, version 9.2 (SAS Institute Inc, www.sas.com).
RESULTS
Of 21,645 eligible REGARDS participants, participants were excluded if they were missing ACR (n = 1,199), eGFR (n = 630), or family history information (n = 196) or had prevalent ESRD (n = 53). We further excluded 158 persons who had missing data for one or more covariates. Participant characteristics are listed in Table 1. For the 19,409 (89.7%) participants included in the present analyses, mean age was 63.9 ± 9.7 (SD) years, 37.8% were men, and 39.9% were African American. There were 11.1% of participants with less than a high school education and 16.7% with an annual household income <$20,000. Hypertension was present in 57.6%, and diabetes, in 19.9%. Body mass index ≥35 kg/m2 was recorded for 16.7%. Mean eGFR was 86.6 ± 19.6 mL/min/1.73 m2 and geometric mean and median ACR values were 9.9 and 7.24 mg/g, respectively.
Table 1.
Demographic Characteristics of Study Participants by Family History of ESRD
| Family History of ESRD |
OR (95% CI) for Family History of ESRD |
||||
|---|---|---|---|---|---|
| Overalla | Absenta | Presenta | Crude | Adjustedb | |
| Age category | |||||
| 45-54 y | 3,381 (17.4) | 2,934 (17.0) | 447 (20.8) | 1.00 (reference) | 1.00 (reference) |
| 55-64 y | 7,114 (36.6) | 6,238 (36.1) | 876 (40.8) | 0.91 (0.81-1.03) | 0.94 (0.83-1.06) |
| 65-74 y | 5,911 (30.4) | 5,314 (30.8) | 597 (27.8) | 0.73 (0.64-0.83) | 0.78 (0.68-0.89) |
| ≥75 y | 3,003 (15.5) | 2,775 (16.1) | 228 (10.6) | 0.54 (0.45-0.63) | 0.61 (0.51-0.72) |
| Sex | |||||
| Men | 7,333 (37.8) | 6,682 (38.7) | 651 (30.3) | 0.69 (0.63-0.76) | 0.75 (0.68-0.82) |
| Women | 12,076 (62.2) | 10,582 (61.3) | 1,494 (69.6) | 1.00 (reference) | 1.00 (reference) |
| Race | |||||
| African American | 7,746 (39.9) | 6,472 (37.5) | 1,274 (59.4) | 2.43 (2.22-2.66) | 2.34 (2.13-2.56) |
| White | 11,663 (60.1) | 10,792 (62.5) | 871 (40.6) | 1.00 (reference) | 1.00 (reference) |
| High BP | 11,177 (57.6) | 9,795 (56.7) | 1,382 (64.4) | 1.39 (1.50-1.84) | 1.23 (1.11-1.35) |
| Diabetes | 3,855 (19.9) | 3,257 (18.9) | 598 (27.9) | 1.66 (1.30-1.61) | 1.44 (1.30-1.61) |
| Education <HS | 2,153 (11.1) | 1,860 (10.8) | 293 (13.7) | 1.31 (1.15-1.50) | 1.11 (0.97-1.27) |
| Income <$20,000 | 3,236 (16.8) | 2,733 (15.8) | 503 (23.4) | 1.62 (1.46-1.81) | 1.36 (1.21-1.52) |
| ACR category | |||||
| <30 mg/g | 16,718 (86.1) | 14,985 (86.8) | 1,733 (80.8) | 1.00 (reference) | 1.00 (reference) |
| 30-299.9 mg/g | 2,228 (11.5) | 1,921 (11.1) | 307 (14.3) | 1.39 (1.22-1.56) | 1.35 (1.18-1.54) |
| ≥300 mg/g | 463 (2.4) | 358 (2.1) | 105 (4.9) | 2.57 (2.07-3.20) | 2.22 (1.76-2.79) |
| eGFR category | |||||
| ≥60 mL/min/1.73 m2 | 17,494 (90.1) | 15,594 (90.3) | 1,900 (88.6) | 1.00 (reference) | 1.00 (reference) |
| 30-59.9 mL/min/1.73 m2 | 1,772 (9.1) | 1,561 (9.0) | 211 (9.8) | 1.11 (0.95-1.29) | 1.32 (1.12-1.54) |
| 15-29.9 mL/min/1.73 m2 | 143 (0.7) | 109 (0.6) | 34 (1.6) | 2.56 (1.74-3.77) | 2.68 (1.79-3.99) |
Abbreviations: ACR, albumin-creatinine ratio; BP, blood pressure; CI, confidence interval; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HS, high school; OR, odds ratio.
Values shown are number (percentage).
All OR (95% CI) adjusted for region, age, race, and sex. Age is entered as a categorical variable.
A family history of ESRD was reported by 11.1% of participants. Individuals with a family history of ESRD were older on average than those without a family history of ESRD at 62.2 ± 9.3 versus 64.1 ± 9.7 years (P < 0.001), and the prevalence of a family history of ESRD decreased with increasing age (Table 1). Patient characteristics independently associated with family history of ESRD included female sex (odds ratio [OR], 1.33; 95% confidence interval [CI], 1.20-1.46), African American race (OR, 2.35; 95% CI, 2.13-2.56), those residing in a household with annual income <$20,000 (OR, 1.36; 95% CI, 1.21-1.52), and those with either hypertension (OR, 1.23; 95% CI, 1.11-1.35) or diabetes (OR, 1.44; 95% CI, 1.30-1.61; Table 1). Mean body mass index values for those with and without a family history of ESRD were 31.3 ± 7.04 and 29.1 ± 6.18 kg/m2, respectively (P < 0.001).
Geometric mean ACR values for those with and without a family history of ESRD were 12.2 and 9.7 mg/g, respectively (P < 0.001). As albuminuria increased from <30 to ≥300 mg/g, the prevalence of a family history of ESRD increased more than 2-fold from 10.4% to 22.7% (OR, 2.22; 95% CI, 1.76-2.80; Table 1).
Mean eGFRs for those with and without a family history of ESRD were 87.5 ± 22.2 and 86.5 ± 19.3 mL/min/1.73 m2, respectively (P = 0.05). The prevalence of a family history of ESRD increased from 10.9% to 23.8% (OR, 2.68; 95% CI, 1.80-4.00) in those with eGFR ≥60 vs <30 mL/min/1.73 m2 (Table 1).
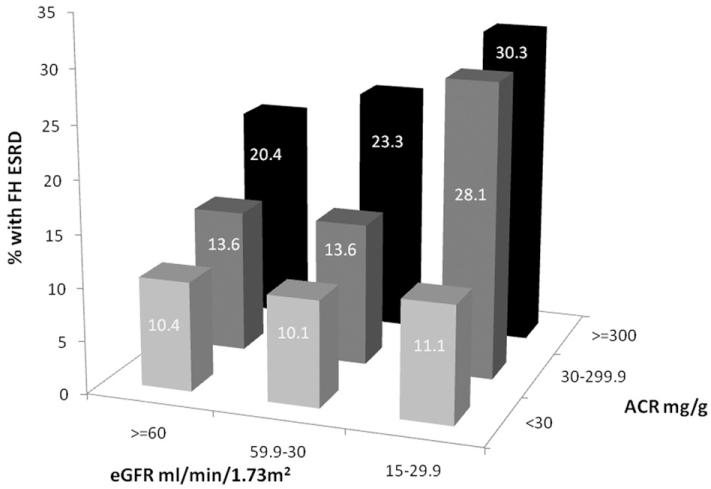
The prevalence of a family history of ESRD for the combined presence of albuminuria and GFR is shown in Fig 1. At every level of eGFR, the prevalence of a family history of ESRD increased as ACR increased. For example, for individuals with eGFR ≥60 mL/min/1.73 m2, the prevalence of a family history of ESRD increased from 10.4% in those with ACR <30 mg/g to 20.4% in participants with ACR ≥300 mg/g. The prevalence of a family history of ESRD was highest (30.3%) in those with eGFR of 15-29.9 mL/min/1.73 m2 and ACR ≥300 mg/g.
Figure 1.
Prevalence of a family history (FH) of end-stage renal disease (ESRD) by joint distribution of albumin-creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR).
Awareness of kidney disease was low in individuals both with and without a family history of ESRD. Although individuals with a family history of ESRD were more likely to be aware of their own kidney disease than those without a family history of ESRD (level of kidney disease awareness, 7.2% and 3.6%, respectively), awareness was low in both groups.
For individuals who might not otherwise have been screened based on current guidelines (did not have hypertension or diabetes), but who had a family history of ESRD, the prevalence of GFR <60 mL/min/1.73 m2 without albuminuria was 3.6%, ACR >30 mg/g without decreased kidney function was 6.2%, and both conditions together was 9.2%.
During a median 4.0 years of follow-up, 111 (0.58%) participants developed incident ESRD, including 1.25% (n = 29) and 0.49% (n = 82) of those with and without a family history of ESRD, respectively (P < 0.001; Table 2). The ESRD incidence rate was 202.4 persons/100,000 years of follow-up: 433.5 and 170.6 persons/100,000 years in those with and without a family history of ESRD, respectively. There were 1,266 (6.7%) deaths during follow-up, including 141 (6.1%) and 1,125 (6.7%) individuals with and without a family history of ESRD, respectively. Corresponding mortality rates were 2,288 deaths/100,000 years of follow-up: 2,107 deaths/100,000 years in those with and 2,312 deaths/100,000 years in those without a family history of ESRD. The risk of death was similar for individuals with and without a family history of ESRD, with crude and age-, sex-, and race-adjusted hazard ratios (HRs) of 0.92 (95% CI, 0.78-1.10) and 1.02 (95% CI, 0.86-1.22), respectively (Table 2).
Table 2.
ESRD and Mortality by Family History of ESRD
| Events | Follow-up Timea | Rateb | Crude HR (95% CI) | Adjusted HR (95% CI)c | |
|---|---|---|---|---|---|
| ESRD | |||||
| All | 111 | 73,113 | 202.4 | — | — |
| No family history of ESRD |
82 | 64,243 | 170.6 | 1.00 (reference) | 1.00 (reference) |
| Family history of ESRD | 29 | 8,870 | 433.5 | 2.58 (1.69-3.94) | 2.04 (1.33-3.15) |
| Mortality | |||||
| All | 1,266 | 73,113 | 2,288 | — | — |
| No family history of ESRD |
1125 | 64,243 | 2,312 | 1.00 (reference) | 1.00 (reference) |
| Family history of ESRD | 141 | 8,870 | 2,107 | 0.92 (0.78-1.10) | 1.02 (0.86-1.22) |
Abbreviations: CI, confidence interval; ESRD, end-stage renal disease; HR, hazard ratio.
Follow-up time is in years.
Rate per 100,000 person-years.
Adjusted for age, race, and sex.
In comparison with individuals lacking a family history of ESRD, the crude and age-, sex-, and race-adjusted HRs for developing ESRD in individuals with a family history of ESRD were 2.58 (95% CI, 1.69-3.94) and 2.04 (95% CI, 1.33-3.15), respectively (Table 2). After further adjustment of the age, sex, and race model for additional covariate (hypertension, diabetes, family income, and educational status), the association between family history of ESRD and incident ESRD persisted (HR, 1.93; 95% CI, 1.22-3.07; Table 3). Risk factors associated with incident ESRD in this model were black race (HR, 3.04; 95% CI, 1.82-5.06), hypertension (HR, 4.08; 95% CI, 2.20-10.52), and diabetes (HR, 4.12; 95% CI, 2.67-6.36).
Table 3.
Association Between Family History of ESRD and Incident ESRD
| HR (95% CI) for Incident ESRD |
||||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Family history of ESRD | 2.04 (1.33-3.15) | 1.80 (1.17-2.78) | 1.93 (1.22-3.07) | 1.12 (0.69-1.80) |
| Age (/1-y older) | 1.07 (0.88-1.30) | 1.003 (0.98-1.02) | 1.002 (0.98-1.02) | 0.97 (0.94-0.99) |
| Female | 1.51 (1.04-2.20) | 1.44 (0.98-2.11) | 1.50 (0.99-2.29) | 1.45 (0.95-2.21) |
| African American race | 5.60 (3.49-8.99) | 3.60 (2.22-5.83) | 3.04 (1.82-5.06) | 2.30 (1.37-3.88) |
| High BP present | 4.97 (2.40-10.34) | 4.08 (2.20-10.52) | 2.05 (0.87-4.86) | |
| Diabetes present | 4.15 (2.78-6.17) | 4.12 (2.67-6.36) | 1.73 (1.10-2.73) | |
| Education <HS | 1.18 (0.71-1.97) | 1.18 (0.69-2.02) | ||
| Income <$20,000 | 1.22 (0.77-1.94) | 1.27 (0.76-2.03) | ||
| eGFR <60 mL/min/1.73 m2 | 14.8 (7.6-28.8) | |||
| ACR >30 mg/g | 17.7 (10.3-30.4) | |||
Abbreviations: ACR, albumin-creatinine ratio; BP, blood pressure; CI, confidence interval; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HR, hazard ratio; HS, high school.
The association between incident ESRD and family history of ESRD was no longer significant when either baseline eGFR (HR, 1.56; 95% CI, 0.97-2.50), ACR (HR, 1.40; 95% CI, 0.88-2.24), or both (HR, 1.12; 95% CI, 0.69-1.80) were added to the model. In the final model controlling for all covariates including ACR and GFR, the only risk factors that remained independently associated with ESRD were younger age (HR for each increased year, 0.97; 95% CI, 0.94-0.99), ACR >30 mg/g (HR, 17.7; 95% CI, 10.3-30.4), and eGFR <60 mL/min/1.73 m2 (HR, 14.8; 95% CI, 7.6-28.8). Other covariates that were associated with incident ESRD in this model included female sex (HR, 1.27; 95% CI, 1.13-1.42) and black race (HR, 1.92; 95% CI, 1.72-2.14).
DISCUSSION
Our study extends to individuals in the general population observations of increased risk of ESRD in individuals with a family history of ESRD. Further, we have shown that the increased risk of ESRD in those with a family history of ESRD is independent of demographic factors, comorbid conditions, and socio-economic factors. Finally, we have shown that the increased prevalence of low eGFR and increased albuminuria in individuals with a family history of ESRD accounts for the residual risk of ESRD in these individuals.
We have previously reported that Renal REGARDS participants with a family history of ESRD are characterized by lower eGFRs.13 The increased albumin excretion rates in Renal REGARDS participants with a family history of ESRD have not been reported previously. For older REGARDS participants, the prevalence of ACR ≥30 mg/g was 13.3% for those without a family history of ESRD and 19.5% for those with a family history of ESRD. The observations stand in contrast to those from the Third National Health and Nutrition Examination Survey (NHANES III).14 The prevalence of ACR ≥30 mg/g in the NHANES III population was 7.8% of the population.14 Prevalences of ACR ≥300 mg/g in NHANES III participants were 1.4% and 1.5% for men and women, respectively, which was substantially lower in those with and without a family history of ESRD (5.2% and 2.2%, respectively) in REGARDS. The difference in prevalence in REGARDS may reflect the higher proportion of African Americans and older adults in the REGARDS sample.
The increased prevalence of proteinuria with family history of ESRD is consistent with earlier reports by Bergman et al.7 Bello et al8 drew a random sample of all patients with ESRD in a population-based registry in the United Kingdom. These individuals were contacted and asked to identify first-degree relatives interested in participating in a study of the familial association of kidney disease. For the 274 relatives identified, the prevalence of microalbuminuria was 9.5%, significantly greater than the 1.4% prevalence in the age- and sex-matched controls and greater than that in the general registry population (7.0%). First-degree relatives of patients with ESRD in the Bello et al8 study were younger than the general registry population and more likely to be women, have less educational attainment, be obese, have a family history of either hypertension or diabetes, and have histories of hypertension and cardiovascular disease8; the present study is consistent with these findings.
Tsai et al3 compared the prevalence of markers of kidney disease (ACR ≥30 mg/g or eGFR <60 mL/min/1.73 m2) in 196 first- and second-degree relatives and 95 spouses of Taiwanese hemodialysis patients. Both albuminuria (10.7% vs 4.1%) and low eGFR (5.1% vs 3.4%) were more frequent in family members, although only the difference in prevalence of ACR ≥30 mg/g was statistically significant (P = 0.01). Of note, spouses of patients with ESRD, used as an environmental control group, were concordant with the index ESRD cases for increased prevalence of diabetes and hypertension, smoking, and increased prevalence of albuminuria and low eGFR.3
The clinical significance of the increased prevalence of albuminuria in individuals with a family history of ESRD is evident from the associated increased risk of ESRD we observed in REGARDS participants. Most importantly, our results suggest that physicians and other health care providers, when interviewing patients, should ask about a family history of ESRD in the same manner as a family history of cardiovascular disease or cancer. These individuals are unlikely to be aware of their kidney disease despite their higher risk of CKD and ESRD, and targeted screening of ACR and eGFR seem warranted. Finally, our results provide additional support for ongoing population-based education and screening for CKD in family members, such as those currently conducted by the National Kidney Disease Education Program’s Family Reunion Initiative15 and the National Kidney Foundation’s Kidney Disease Early Evaluation Program (KEEP).16
Individuals with a positive family history were nearly 2.6 times more likely to develop incident ESRD compared with other REGARDS participants. This increased risk was attenuated, but not fully explained, by controlling for other risk factors associated with progressive kidney disease.17 In contrast, after additional control for either baseline eGFR or ACR alone or both eGFR and ACR, the association between family history of ESRD and incident ESRD did not persist. It is not surprising that additional adjustment for ACR, eGFR, or both substantially attenuated the residual risk of ESRD in family members because both measures of kidney function are in the pathway of progressive kidney failure.
The increased risk of ESRD in African Americans in our study persisted after controlling for family history of ESRD, sex, and age. Controlling for comorbid factors and socioeconomic status attenuated but did not fully explain racial disparities in ESRD, whereas additional control for ACR and eGFR fully accounted for differences in the occurrence of ESRD. These observations suggest that the increased prevalence of albuminuria and lower eGFR account for racial differences in ESRD in individuals with a family history of ESRD. There are multiple factors that may be related to familial differences in albuminuria and eGFR that warrant exploration. In particular, recently described genetic polymorphisms18-20 are strong candidates to explain the persistence of racial disparities after controlling for family history of ESRD.
The limitations and strengths of our study should be noted. First, both ACR and eGFR were estimated using a single measurement; therefore, we may have misclassified some individuals with transient abnormalities as having CKD. Additionally, follow-up for incident ESRD was available through only August 2009. Therefore, our inferences on the role of ACR in mediating the increased risk of ESRD with family history of ESRD may apply to only short-term incidence. Finally, our conclusions are based on observational data and thus cannot be construed as reflecting causal associations. Although we cannot be sure that the higher prevalence of a family history of ESRD that we observed at more severe degrees of baseline kidney disease, measured by either eGFR or ACR, does not reflect recall bias, we believe this is unlikely in the face of the low levels of awareness of individual CKD in REGARDS participants.
Despite these limitations, the present analysis maintains several strengths. Our findings are based on a very large population-based sample of older US adults.11 Participants were sampled within 3 geographic strata, 2 race strata, and age strata, with the goal of recruiting 20% of the sample from the coastal plain of the Southeastern United States, 30% from the rest of the Southern United States, and 50% from the remaining contiguous United States. Within each geographic stratum, we sought to recruit 50% blacks, and within each race, 50% men. This sampling strategy was unlikely to introduce sampling bias with respect to family history of ESRD. Additionally, study data were collected following a standardized protocol with strict quality-control procedures.
Also, it is unlikely that differential mortality rates in individuals with and without a family history of ESRD might have contributed to our findings in that death rates were similar in the 2 groups. Finally, case ascertainment used the USRDS, which identifies >90% of incident patients with ESRD, and it is unlikely that differences in case ascertainment biased our observations.
ACKNOWLEDGEMENTS
We thank the other investigators, the staff, and participants in the REGARDS study for valuable contributions. A full list of participating REGARDS investigators and institutions can be found at www.regardsstudy.org.
Support: This research project is supported by cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or NIH. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data.2 Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Inc to Dr Warnock.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
REFERENCES
- 1.Freedman BI, Volkova NV, Satko SG, et al. Population-based screening for family history of end-stage renal disease among incident dialysis patients. Am J Nephrol. 2005;25(6):529–535. doi: 10.1159/000088491. [DOI] [PubMed] [Google Scholar]
- 2.Freedman BI, Soucie JM, McClellan WM. Family history of end-stage renal disease among incident dialysis patients. J Am Soc Nephrol. 1997;8(12):1942–1945. doi: 10.1681/ASN.V8121942. [DOI] [PubMed] [Google Scholar]
- 3.Tsai JC, Chen SC, Hwang SJ, Chang JM, Lin MY, Chen HC. Prevalence and risk factors for CKD in spouses and relatives of hemodialysis patients. Am J Kidney Dis. 2010;55(5):856–866. doi: 10.1053/j.ajkd.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson R, Grim CE, Opgenorth TJ. A familial risk of chronic renal-failure among blacks on dialysis. J Clin Epidemiol. 1988;41(12):1189–1196. doi: 10.1016/0895-4356(88)90023-6. [DOI] [PubMed] [Google Scholar]
- 5.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20(5):1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iseki K, Ikemiya Y, Iseki C, Takishita S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003;63(4):1468–1474. doi: 10.1046/j.1523-1755.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- 7.Bergman S, Key BO, Kirk KA, Warnock DG, Rostand SG. Kidney disease in the first-degree relatives of African-Americans with hypertensive end-stage renal disease. Am J Kidney Dis. 1996;27(3):341–346. doi: 10.1016/s0272-6386(96)90356-x. [DOI] [PubMed] [Google Scholar]
- 8.Bello AK, Peters J, Wight J, de Zeeuw D, El Nahas M, Inst EK. A population-based screening for microalbuminuria among relatives of CKD patients: the Kidney Evaluation and Awareness Program in Sheffield (KEAPS) Am J Kidney Dis. 2008;52(3):434–443. doi: 10.1053/j.ajkd.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Schoolwerth AC, Burrows NR, Williams DE, Stith KR, McClellan W. Comprehensive public health strategies for preventing the development, progression, and complications of CKD: report of an expert panel convened by the Centers for Disease Control and Prevention. Am J Kidney Dis. 2009;53(3):522–535. doi: 10.1053/j.ajkd.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Black C, Sharma P, Scotland G, et al. Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol Asses. 2010;14(21):1–184. doi: 10.3310/hta14210. [DOI] [PubMed] [Google Scholar]
- 11.Howard VJ, Cushman M, Pulley L, et al. The Reasons for Geographic and Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClellan WM, Satko SG, Gladstone E, Krisher JO, Narva AS, Freedman BI. Individuals with a family history of ESRD are a high-risk population for CKD: implications for targeted surveillance and intervention activities. Am J Kidney Dis. 2009;53(3):S100–S106. doi: 10.1053/j.ajkd.2008.07.059. suppl 3. [DOI] [PubMed] [Google Scholar]
- 14.Jones CA, Francis ME, Eberhardt MS, et al. Microalbuminuria in the US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002;39(3):445–459. doi: 10.1053/ajkd.2002.31388. [DOI] [PubMed] [Google Scholar]
- 15.Narva AS, Briggs M. The National Kidney Disease Education Program: improving understanding, detection, and management of CKD. Am J Kidney Dis. 2009;53(3):S115–S120. doi: 10.1053/j.ajkd.2008.05.038. suppl 3. [DOI] [PubMed] [Google Scholar]
- 16.Brown WW, Collins A, Chen SC, et al. Identification of persons at high risk for kidney disease via targeted screening: the NKF Kidney Early Evaluation Program. Kidney Int. 2003;63:50–55. doi: 10.1046/j.1523-1755.63.s83.11.x. [DOI] [PubMed] [Google Scholar]
- 17.McClellan WM. Epidemiology and risk factors for chronic kidney disease. Med Clin North Am. 2005;89(3):419–445. doi: 10.1016/j.mcna.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol. 2010;21(9):1422–1426. doi: 10.1681/ASN.2010070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ned RM, Yesupriya A, Imperatore G, et al. Inflammation gene variants and susceptibility to albuminuria in the U.S. population: analysis in the Third National Health and Nutrition Examination Survey (NHANES III), 1991-1994. BMC Med Genet. 2010;11:155. doi: 10.1186/1471-2350-11-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bostrom MA, Freedman BI. The spectrum of MYH9-associated nephropathy. Clin J Am Soc Nephrol. 2010;5(6):1107–1113. doi: 10.2215/CJN.08721209. [DOI] [PMC free article] [PubMed] [Google Scholar]