Abstract
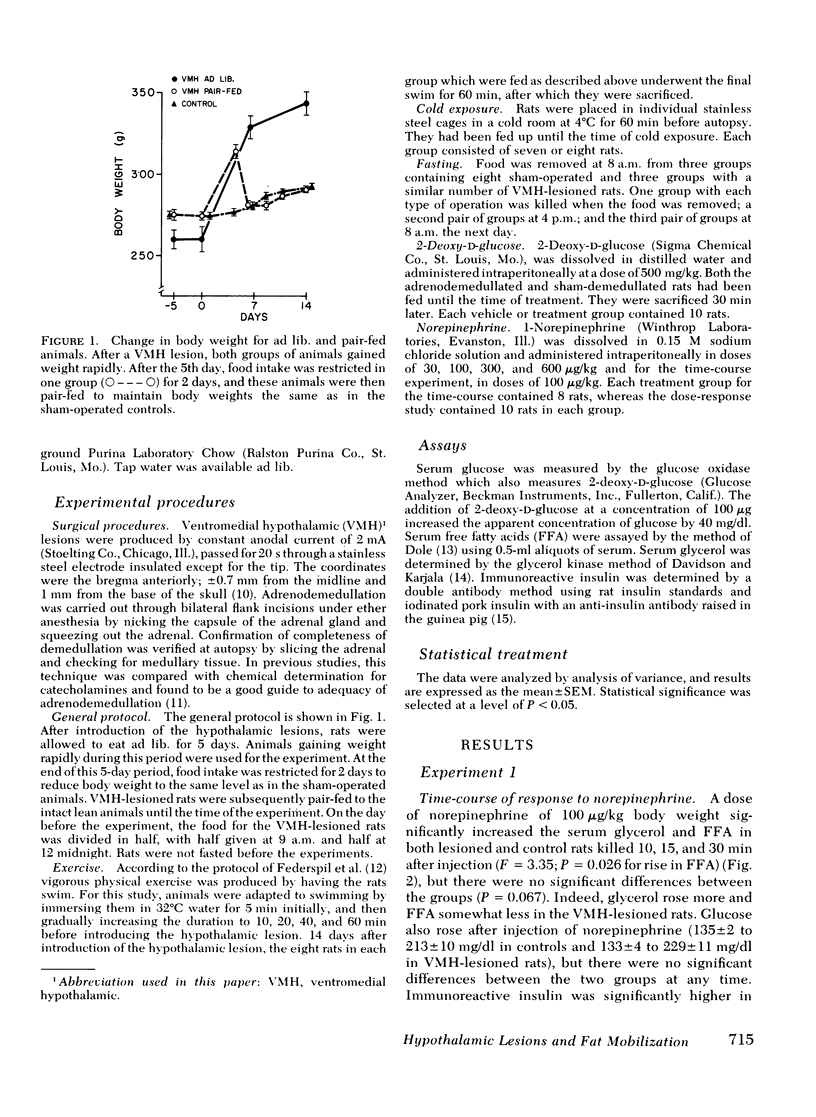
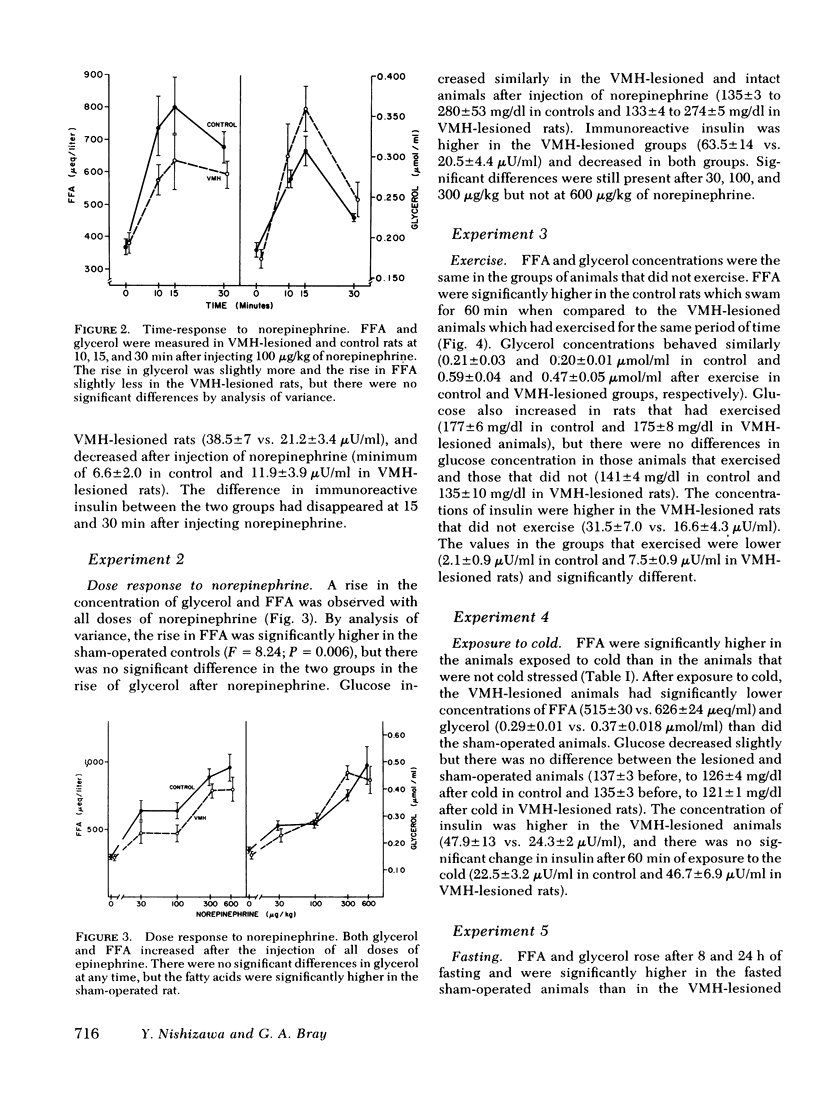
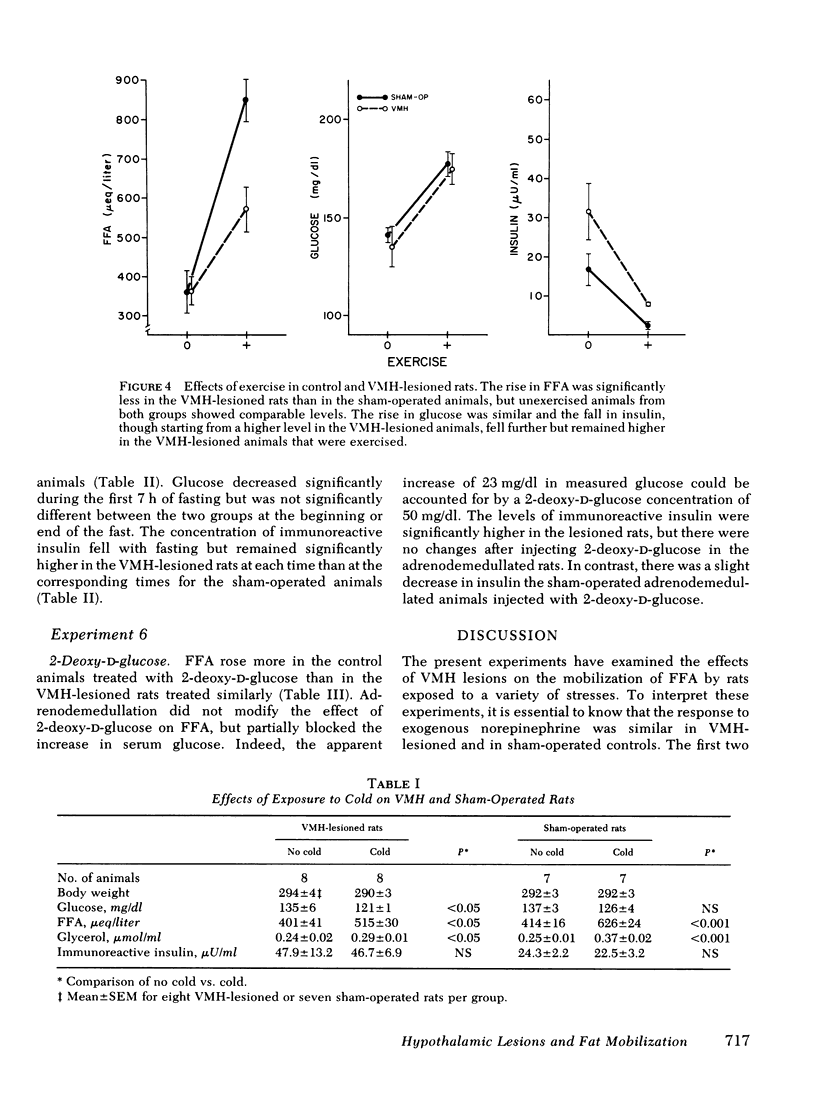
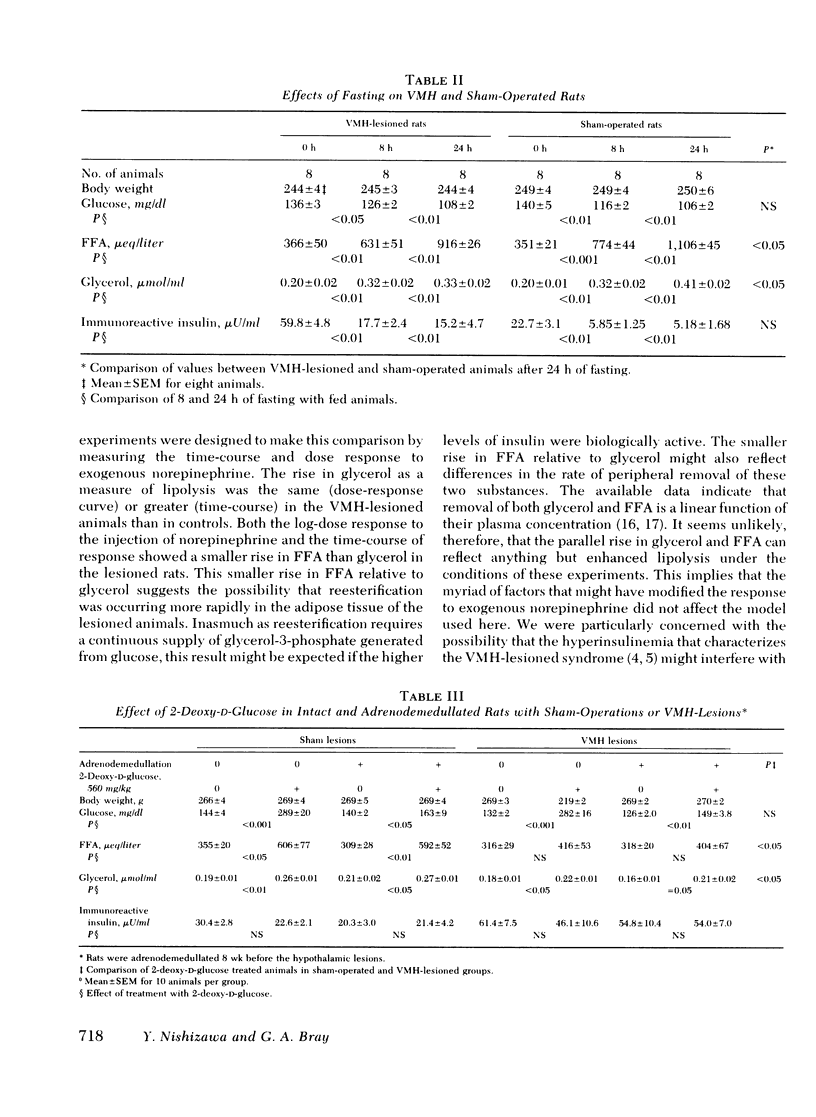
We have explored the effects of ventromedial hypothalamic lesions on the mobilization of free fatty acids in rats exposed to several stresses. The rise in free fatty acids and glycerol in response to norepinephrine had the same time-course and dose-response characteristics in the sham-operated and lesioned animals, indicating comparable degrees of peripheral responsiveness to this hormone. Forced swimming significantly lowered insulin and increased glycerol and free fatty acids more in control than in ventromedial hypothalamic-lesioned rats. During fasting, the rise in glycerol and free fatty acids was smaller in the lesioned rats, but the fall in insulin was greater. Exposure to cold raised fatty acids and glycerol more in the control than in the sham-operated animals, but had no significant effect on plasma insulin or glucose concentration. Injection of 2-deoxyglucose was done on lesioned or control rats with intact or removed adrenal medullas. The rise in free fatty acids and glycerol was less in the lesioned rats than in the controls, and was not affected by adrenodemedullation. The rise in glucose, however, was completely blocked in the adrenodemedullated rats. Changes in insulin were small and not statistically significant. The reduced mobilization of fatty acids from adipose tissue depots after ventromedial hypothalamic injury is consistent with the hypothesis that the ventromedial hypothalamic region serves to modulate activation of the sympathetic nervous system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG D. T., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S., DE BODO R. C. Regulation of plasma free fatty acid turnover. Am J Physiol. 1961 Jul;201:9–15. doi: 10.1152/ajplegacy.1961.201.1.9. [DOI] [PubMed] [Google Scholar]
- BROWN J., BACHRACH H. L. Effects of 2-deoxyglucose on blood glucose levels in the rat. Proc Soc Exp Biol Med. 1959 Mar;100(3):641–643. doi: 10.3181/00379727-100-24728. [DOI] [PubMed] [Google Scholar]
- Balasse E. Influence of norepinephrine, growth hormone and fasting on FFA mobilization and glucose metabolism in lean and obese subjects. Diabetologia. 1968 Jan;4(1):20–25. doi: 10.1007/BF01241029. [DOI] [PubMed] [Google Scholar]
- Boninsegna A., Federspil G., De Palo C. The effect of muscular exercise on free fatty acids, acetoacetate and 3-hydroxybutyrate blood levels. Horm Metab Res. 1974 Nov;6(6):488–491. doi: 10.1055/s-0028-1093809. [DOI] [PubMed] [Google Scholar]
- Bray G. A., Gallagher T. F., Jr Manifestations of hypothalamic obesity in man: a comprehensive investigation of eight patients and a reveiw of the literature. Medicine (Baltimore) 1975 Jul;54(4):301–330. doi: 10.1097/00005792-197507000-00002. [DOI] [PubMed] [Google Scholar]
- Bray G. A., York D. A. Genetically transmitted obesity in rodents. Physiol Rev. 1971 Jul;51(3):598–646. doi: 10.1152/physrev.1971.51.3.598. [DOI] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M. B., Karjala R. Simplified fluorometric method for the determination of plasma glycerol. J Lipid Res. 1970 Nov;11(6):609–612. [PubMed] [Google Scholar]
- Federspil G., Lefebvre P., Luyckx A. Effets d'un exercice musculaire continu (nage forcée) sur la glycémie et le taux plasmatique des acides gras libres, de l'insuline et de la corticostérone chez le rat. Arch Int Physiol Biochim. 1969 Oct;77(4):778–786. doi: 10.3109/13813456909059790. [DOI] [PubMed] [Google Scholar]
- Federspil G., Udeschini G., De Palo C., Sicolo N. Role of growth hormone in lipid mobilization stimulated by prolonged muscular exercise in the rat. Horm Metab Res. 1975 Nov;7(6):484–488. doi: 10.1055/s-0028-1093709. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Bernardis L. L. Effect of hypothalamic stimulation on plasma glucose, insulin, and glucagon levels. Am J Physiol. 1971 Dec;221(6):1596–1603. doi: 10.1152/ajplegacy.1971.221.6.1596. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Bernardis L. L. Growth hormone and insulin levels in weanling rats with ventromedial hypothalamic lesions. Endocrinology. 1968 Jun;82(6):1125–1132. doi: 10.1210/endo-82-6-1125. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Muller E. E., Cocchi D. Central nervous system mediated inhibition of insulin secretion due to 2-deoxyglucose. Horm Metab Res. 1973 Jan;5(1):21–26. doi: 10.1055/s-0028-1093995. [DOI] [PubMed] [Google Scholar]
- Goldman J. K., Bernardis L. L., Frohman L. A. Food intake in hypothalamic obesity. Am J Physiol. 1974 Jul;227(1):88–91. doi: 10.1152/ajplegacy.1974.227.1.88. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D. Exercise, adrenergic blockage, and free fatty acid mobilization. Am J Physiol. 1967 Sep;213(3):734–738. doi: 10.1152/ajplegacy.1967.213.3.734. [DOI] [PubMed] [Google Scholar]
- Han P. W., Frohman L. A. Hyperinsulinemia in tube-fed hypophysectomized rats bearing hypothalamic lesions. Am J Physiol. 1970 Dec;219(6):1632–1636. doi: 10.1152/ajplegacy.1970.219.6.1632. [DOI] [PubMed] [Google Scholar]
- Hustvedt B. E., Lovo A. Correlation between hyperinsulinemia and hyperphagia in rats with ventromedial hypothalamic lesions. Acta Physiol Scand. 1972 Jan;84(1):29–33. doi: 10.1111/j.1748-1716.1972.tb05152.x. [DOI] [PubMed] [Google Scholar]
- JUNGAS R. L., BALL E. G. Studies on the metabolism of adipose tissue. XII. The effects of insulin and epinephrine on free fatty acid and glycerol production in the presence and absence of glucose. Biochemistry. 1963 Mar-Apr;2:383–388. doi: 10.1021/bi00902a035. [DOI] [PubMed] [Google Scholar]
- Koivisto V. A., Akerblom H. K., Kiviluoto M. K. Metabolic and hormonal effects of exercise in the severely streptozotocin-diabetic rat. Diabetologia. 1974 Aug;10(4):329–335. doi: 10.1007/BF02627735. [DOI] [PubMed] [Google Scholar]
- Kumon A., Takahashi A., Hara T., Shimazu T. Mechanism of lipolysis induced by electrical stimulation of the hypothalamus in the rabbit. J Lipid Res. 1976 Nov;17(6):551–558. [PubMed] [Google Scholar]
- Lefebvre P. J., Luyckx A. S., Federspil G. Muscular exercise and pancreatic function in rats. Isr J Med Sci. 1972 Mar;8(3):390–398. [PubMed] [Google Scholar]
- Luyckx A. S., Dresse A., Cession-Fossion A., Lefebvre P. J. Catecholamines and exercise-induced glucagon and fatty acid mobilization in the rat. Am J Physiol. 1975 Aug;229(2):376–383. doi: 10.1152/ajplegacy.1975.229.2.376. [DOI] [PubMed] [Google Scholar]
- Luyckx A. S., Lefebvre P. J. Mechanisms involved in the exercise-induced increase in glucagon secretion in rats. Diabetes. 1974 Feb;23(2):81–93. doi: 10.2337/diab.23.2.81. [DOI] [PubMed] [Google Scholar]
- Salvador R. A., Colville K. I., Burns J. J. Adrenergic mechanisms and lipid mobilization. Ann N Y Acad Sci. 1965 Oct 8;131(1):113–118. doi: 10.1111/j.1749-6632.1965.tb34783.x. [DOI] [PubMed] [Google Scholar]
- Schemmel R., Mickelsen O., Gill J. L. Dietary obesity in rats: Body weight and body fat accretion in seven strains of rats. J Nutr. 1970 Sep;100(9):1041–1048. doi: 10.1093/jn/100.9.1041. [DOI] [PubMed] [Google Scholar]
- Shaw W. A., Issekutz T. B., Issekutz B., Jr Interrelationship of FFA and glycerol turnovers in resting and exercising dogs. J Appl Physiol. 1975 Jul;39(1):30–36. doi: 10.1152/jappl.1975.39.1.30. [DOI] [PubMed] [Google Scholar]
- Shimazu T., Ogasawara S. Effects of hypothalamic stimulation on gluconeogenesis and glycolysis in rat liver. Am J Physiol. 1975 Jun;228(6):1787–1793. doi: 10.1152/ajplegacy.1975.228.6.1787. [DOI] [PubMed] [Google Scholar]
- Teixeira V. L., Antunes-Rodrigues J., Migliorini R. H. Evidence for centers in the central nervous system that selectively regulate fat mobilization in the rat. J Lipid Res. 1973 Nov;14(6):672–677. [PubMed] [Google Scholar]



