Abstract
The aortas of 11 pigs (aged 1-3 yr) with homozygous von Willebrand's disease (vWd) were compared with those of 11 normal pigs of the same ages. Six of the controls exhibited multiple arteriosclerotic plaques with intimal thickening of 63-130 μm. In contrast, none of the pigs with vWd had multiple plaques, and only one had a lesion >2 mm in diameter.
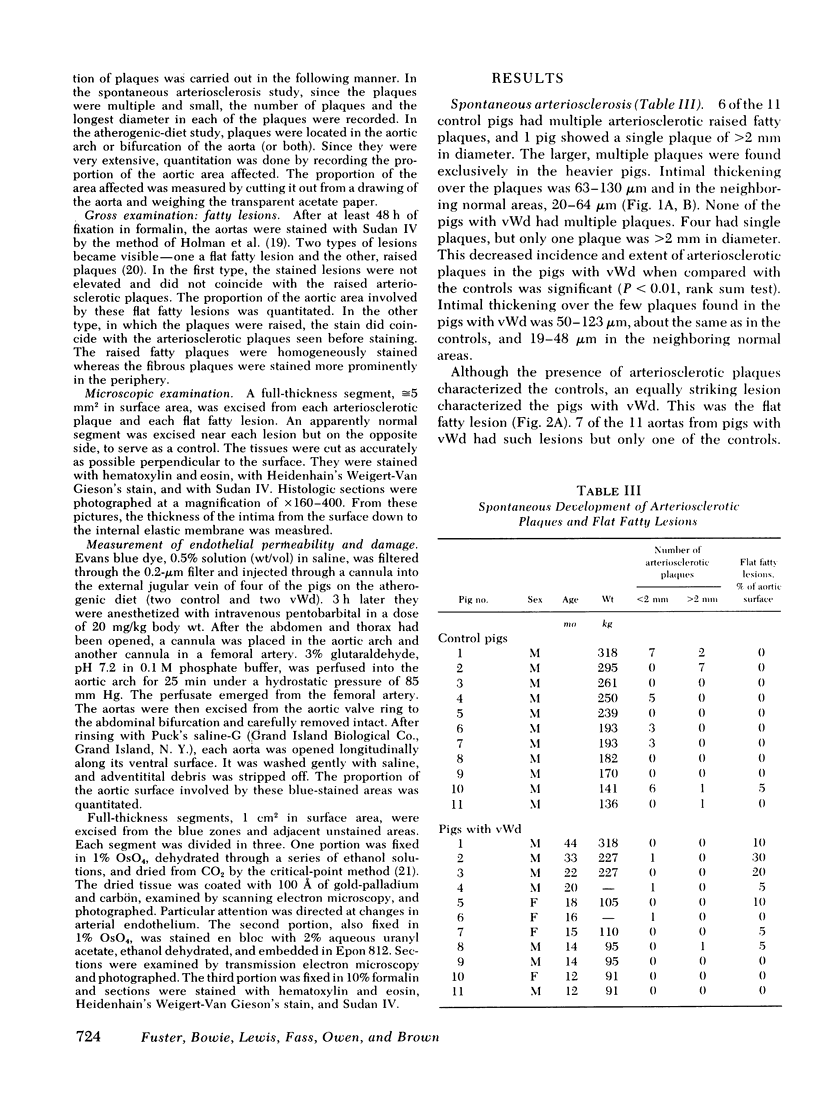
In a subsequent study, 3-mo-old pigs (11 controls and 7 with homozygous vWd) were placed on a 2% cholesterol diet for up to 6 mo. All of the controls developed arteriosclerotic plaques in the aorta, and in nine of the controls, at least 13% of the entire surface was involved. Intimal thickness ranged up to 390 μm. In contrast, four of the pigs with vWd did not develop such lesions, two developed arteriosclerotic lesions affecting 6 and 7% of the aortic surface, and the seventh had 13% of the aortic surface involved.
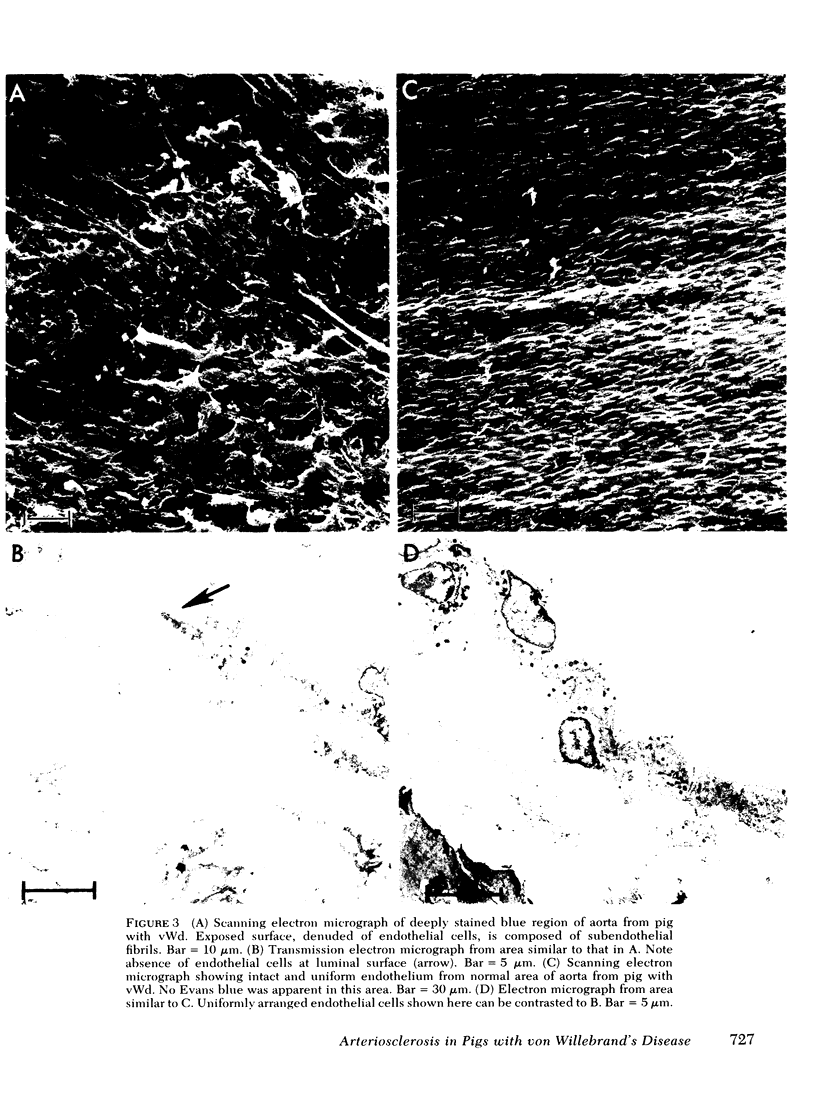
Most of the pigs with vWd, however, developed flat fatty lesions in contrast to the normal pigs whether on the normal or the high cholesterol diet. There was blue staining of the flat fatty lesions when two pigs with vWd were injected with Evans blue dye antemortem. By electron microscopy, severe endothelial damage was apparent, but there was no intimal proliferation. The coincidence of the impaired platelet-arterial wall interaction and lack of arteriosclerosis in this bleeding disease is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowie E. J., Owen C. A., Jr The value of measuring platelet "adhesiveness" in the diagnosis of bleeding diseases. Am J Clin Pathol. 1973 Sep;60(3):302–308. doi: 10.1093/ajcp/60.3.302. [DOI] [PubMed] [Google Scholar]
- Bowie E. J., Owen C. A., Jr, Zollman P. E., Thompson J. H., Jr, Fass D. N. Tests of hemostasis in swine: normal values and values in pigs affected with von Willebrand's disease. Am J Vet Res. 1973 Nov;34(11):1405–1407. [PubMed] [Google Scholar]
- Cohen P., McCombs H. L. Platelets and atherogenesis. 2. Amelioration of cholesterol atherogenesis in rabbits with reduced platelet counts as the result of 32P administration. J Atheroscler Res. 1968 May-Jun;8(3):389–398. doi: 10.1016/s0368-1319(68)80096-1. [DOI] [PubMed] [Google Scholar]
- Cohen P., McCombs H. L. Platelets and atherogenesis. I. Augmentation of cholesterol atherogenesis in the rabbit by a phlebotomy programme designed to induce thrombocytosis. Br J Exp Pathol. 1967 Jun;48(3):346–356. [PMC free article] [PubMed] [Google Scholar]
- Davies P. F., Reidy M. A., Goode T. B., Bowyer D. E. Scanning electron microscopy in the evaluation of endothelial integrity of the fatty lesion in atherosclerosis. Atherosclerosis. 1976 Oct;25(1):125–130. doi: 10.1016/0021-9150(76)90054-x. [DOI] [PubMed] [Google Scholar]
- Fass D. N., Brockway W. J., Owen C. A., Jr, Bowie E. J. Factor VIII (Willebrand) antigen and ristocetin-Willebrand factor in pigs with von Willebrand's disease. Thromb Res. 1976 Mar;8(3):319–327. doi: 10.1016/0049-3848(76)90025-6. [DOI] [PubMed] [Google Scholar]
- Florentin R. A., Nam S. C., Daoud A. S., Jones R., Scott R. F., Morrison E. S., Kim D. N., Lee K. T., Thomas W. A., Dodds W. J. Dietary-induced atherosclerosis in miniature swine. Exp Mol Pathol. 1968 Jun;8(3):263–301. doi: 10.1016/s0014-4800(68)80001-2. [DOI] [PubMed] [Google Scholar]
- French J. E., Jennings M. A., Florey H. W. Morphological studies on atherosclerosis in swine. Ann N Y Acad Sci. 1965 Sep 8;127(1):780–799. doi: 10.1111/j.1749-6632.1965.tb49444.x. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Aster R. H., Cotran R. S., Corkery J., Jandl J. H., Folkman J. Preservation of vascular integrity in organs perfused in vitro with a platelet-rich medium. Nature. 1969 Apr 5;222(5188):33–36. doi: 10.1038/222033a0. [DOI] [PubMed] [Google Scholar]
- Glagov S. Mechanical stresses on vessels and the non-uniform distribution of atherosclerosis. Med Clin North Am. 1973 Jan;57(1):63–77. doi: 10.1016/s0025-7125(16)32302-1. [DOI] [PubMed] [Google Scholar]
- HOLMAN R. L., McGILL H. C., Jr, STRONG J. P., GEER J. C. Technics for studying atherosclerotic lesions. Lab Invest. 1958 Jan-Feb;7(1):42–47. [PubMed] [Google Scholar]
- Harker L. A., Ross R., Slichter S. J., Scott C. R. Homocystine-induced arteriosclerosis. The role of endothelial cell injury and platelet response in its genesis. J Clin Invest. 1976 Sep;58(3):731–741. doi: 10.1172/JCI108520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E. G., Lundberg W. O., Titus J. L. Experimental atherosclerosis in swine. II. Effects of methionine and menhaden oil on an atherogenic diet containing tallow and cholesterol. Mayo Clin Proc. 1971 Sep;46(9):621–625. [PubMed] [Google Scholar]
- Imai H., Thomas W. A. Cerebral atherosclerosis in swine: role of necrosis in progression of diet-induced lesions from proliferative to atheromatous stage. Exp Mol Pathol. 1968 Jun;8(3):330–357. doi: 10.1016/s0014-4800(68)80004-8. [DOI] [PubMed] [Google Scholar]
- LEVINE J. B., ZAK B. AUTOMATED DETERMINATION OF SERUM TOTAL CHOLESTEROL. Clin Chim Acta. 1964 Oct;10:381–384. doi: 10.1016/0009-8981(64)90073-7. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Lewis J. C., Kottke B. A. Endothelial damage and thrombocyte adhesion in pigeon atherosclerosis. Science. 1977 May 27;196(4293):1007–1009. doi: 10.1126/science.860128. [DOI] [PubMed] [Google Scholar]
- Moore S., Friedman R. J., Singal D. P., Gauldie J., Blajchman M. A., Roberts R. S. Inhibition of injury induced thromboatherosclerotic lesions by anti-platelet serum in rabbits. Thromb Haemost. 1976 Feb 29;35(1):70–81. [PubMed] [Google Scholar]
- Olson J. D., Brockway W. J., Fass D. N., Magnuson M. A., Bowie E. J. Evaluation of ristocetin-Willebrand factor assay and ristocetin-induced platelet aggregation. Am J Clin Pathol. 1975 Feb;63(2):210–218. doi: 10.1093/ajcp/63.2.210. [DOI] [PubMed] [Google Scholar]
- ROSKAM J. DU R OLE DE LA PAROI VASCULAIRE DANS L'H'EMOSTASE SPONTAN'EE ET LA PATHOG'ENIE DES 'ETATS H'EMORRAGIQUES. Thromb Diath Haemorrh. 1964 Oct 15;12:338–352. [PubMed] [Google Scholar]
- Ratcliffe H. L., Luginbühl H., Pivnik L. Coronary, aortic and cerebral atherosclerosis in swine of 3 age-groups: implications. Bull World Health Organ. 1970;42(2):225–234. [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silwer J., Cronberg S., Nilsson I. M. Occurrence of arteriosclerosis in von Willebrand's disease. Acta Med Scand. 1966 Oct;180(4):475–484. doi: 10.1111/j.0954-6820.1966.tb02860.x. [DOI] [PubMed] [Google Scholar]
- Somer J. B., Evans G., Schwartz C. J. Influence of experimental aortic coarctation on the pattern of aortic Evans blue uptake in vivo. Atherosclerosis. 1972 Jul-Aug;16(1):127–133. doi: 10.1016/0021-9150(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B., Ross R. Experimental arteriosclerosis. I. Fibrous plaque formation in primates, an electron microscope study. J Exp Med. 1972 Oct 1;136(4):769–789. doi: 10.1084/jem.136.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESOLOWSKI S. A., FRIES C. C., SABINI A. M., SAWYER P. N. THE SIGNIFICANCE OF TURBULENCE IN HEMIC SYSTEMS AND IN THE DISTRIBUTION OF THE ATHEROSCLEROTIC LESION. Surgery. 1965 Jan;57:155–162. [PubMed] [Google Scholar]
- Weiss H. J., Hoyer L. W., Rickles F. R., Varma A., Rogers J. Quantitative assay of a plasma factor deficient in von Willebrand's disease that is necessary for platelet aggregation. Relationship to factor VIII procoagulant activity and antigen content. J Clin Invest. 1973 Nov;52(11):2708–2716. doi: 10.1172/JCI107465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H. J., Tschopp T. B., Baumgartner H. R. Impaired interaction (adhesion-aggregation) of platelets with the subendothelium in storage-pool disease and after aspirin ingestion. A comparison with von Willebrand's disease. N Engl J Med. 1975 Sep 25;293(13):619–623. doi: 10.1056/NEJM197509252931301. [DOI] [PubMed] [Google Scholar]
- Wojcik J. D., Van Horn D. L., Webber A. J., Johnson S. A. Mechanism whereby platelets support the endothelium. Transfusion. 1969 Nov-Dec;9(6):324–335. doi: 10.1111/j.1537-2995.1969.tb04945.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. S., Ratnoff O. D., Powell A. E. Immunologic differentiation of classic hemophilia (factor 8 deficiency) and von Willebrand's dissase, with observations on combined deficiencies of antihemophilic factor and proaccelerin (factor V) and on an acquired circulating anticoagulant against antihemophilic factor. J Clin Invest. 1971 Jan;50(1):244–254. doi: 10.1172/JCI106480. [DOI] [PMC free article] [PubMed] [Google Scholar]





