Abstract
This study investigates the inhibitory effects of Korean mistletoe extract (KME) on adipogenic factors in 3T3-L1 cells and obesity and nonalcoholic fatty liver disease (NAFLD) in mice fed a high-fat diet. Male C57Bl/6 mice fed a high-fat diet were treated with KME (3 g/kg/day) for 15 weeks for the antiobesity and NAFLD experiments. Body weight and daily food intake were measured regularly during the experimental period. The epididymal pad was measured and liver histology was observed. The effects of KME on thermogenesis and endurance capacity were measured. The effects of KME on adipogenic factors were examined in 3T3-L1 cells. Body and epididymal fat pad weights were reduced in KME-treated mice, and histological examination showed an amelioration of fatty liver in KME-treated mice, without an effect on food consumption. KME potently induces mitochondrial activity by activating thermogenesis and improving endurance capacity. KME also inhibited adipogenic factors in vitro. These results demonstrate the inhibitory effects of KME on obesity and NAFLD in mice fed a high-fat diet. The effects appear to be mediated through an enhanced mitochondrial activity. Therefore, KME may be an effective therapeutic candidate for treating obesity and fatty liver caused by a high-fat diet.
1. Introduction
Obesity is the most common metabolic disorder and results from the combined effects of excess energy intake and reduced energy expenditure [1]. It is one of the fastest growing major disorders throughout the developed nations [2]. It is associated with a variety of clinical disorders in developed nations [2]. Obesity is associated with a variety of clinical disorders, including hypertension, insulin resistance, glucose intolerance, and dyslipidemia. It is well known that an oversupply of fat is associated with the development of obesity in mice [3]. Long-term feeding with a high-fat diet can induce obesity with hyperlipidemia, insulin resistance, hyperphagia, and hypergluconemia [4, 5].
Nonalcoholic fatty liver disease (NAFLD) is present in up to one-third of the general population and affects all ages and ethnic groups. NAFLD is the second leading cause of death in the general population [6, 7]. At present, there is no pharmacological agent known to reverse NAFLD and effective medical interventions have focused on the modification of risk factors, such as weight reduction and diet [8].
Mistletoe is a hemiparasitic plant growing all over the world on various deciduous trees, such as oak. It has been used as a traditional herbal medicine, especially in Europe [9]. Korean mistletoe (KM, Viscum album cololatum) extract (KME) has been shown to have anticancer [10], antioxidant [11], antidiabetes [12] and antidementia [13] effects, and it enhances immune system function [14]. Recently, we reported that KME significantly increases mitochondrial function [15].
Mitochondria are known as “cellular power plants” because they generate most of the cellular supply of adenosine triphosphate (ATP) which is used as a source of chemical energy. Studies have implicated mitochondria in several human diseases, including metabolic diseases [16], cardiac dysfunction [17], mental disorders [18], and the aging process [19]. Decreased mitochondrial respiration rates [20] and reduced expression of genes involved in mitochondrial oxidative capacity [21] have been reported in diet-induced obese rats. Because KME improves mitochondrial function, we asked whether it has beneficial effects on obesity.
It is well known that adipocyte differentiation and the extent of subsequent fat accumulation are closely related to the occurrence and advancement of various diseases, such as coronary artery disease and obesity [1, 22, 23]. 3T3-L1 cells have served as a useful in vitro model for adipocyte differentiation and function [24]. The differentiation of preadipocytes into adipocytes requires a variety of effectors that activate a cascade of transcription factors, such as CCAAT/enhancer-binding protein-α (C/EBP-α), peroxisome proliferator-activated receptorγ (PPAR-γ), and sterol regulatory element binding element protein-1c (SREBP-1c). This cascade begins with the CCAAT enhancer-binding protein- (C/EBP-) β and δ, which induces the expression of C/EBP-α and PPAR-γ [25–27]. These transcription factors coordinate the expression of genes involved in creating and maintaining the adipocyte phenotype, including genes for adipocyte fatty acid-binding protein, glucose transporter 4, lipoprotein lipase (LPL) and leptin [28, 29]. SREBP-1c is strongly associated with SREBP cleavage-activating protein (SCAP). Activated SREBP-1c accelerates adipogenesis through the overexpression of adipogenic enzymes, such as fatty acid synthase(FAS), acyl-CoA synthase (ACC), and acyl-CoA carboxylase (ACS). LPL is the major enzyme that hydrolyzes triglyceride (TG) molecules of chylomicrons and VLDL particles. The released free fatty acids are either oxidized to generate ATP in muscle or stored in adipose tissue [30].
We measured the inhibitory effect of KME on adipogenic factors in 3T3-L1 cells and on the development of obesity and NAFLD in mice fed a high-fat diet. Our data show that KME suppresses adipocyte differentiation through down-regulation of adipogenic factors and could ameliorate obesity and NAFLD in mice fed a high-fat diet. Our results indicate the great potential of KME as a potential metabolic regulator of adipocyte differentiation and a potential therapeutic agent for preventing or treating obesity and NAFLD.
2. Materials and Methods
2.1. Preparation of KME
Mistletoe growing on oak was harvested from Gangwon-do, Republic of Korea, in February. The mistletoe was 1 or 2 years old, and the leaves, stems, and fruits were cut into 2 joints from the end of a branch, washed with distilled water (DW), and dried. The vacuum-packed mistletoe was stored at −80°C until extracted. Then mistletoe leaves, fruits, and stems were freeze-dried, crushed and ground in approximately 10 volumes of DW for 30 seconds. After being washed, they were ground in a mixer for 2 minutes and stirred for 16 hours at 4°C. To obtain fine mistletoe extract, the homogenized mistletoe was centrifuged at 8,000 rpm for 30 minutes at 4°C, and the resulting supernatant was successively filtered through different pore sizes (0.9 and 0.45 μm). The mistletoe extract was freeze-dried and resuspended in DW at an appropriate dilution factor.
2.2. Animals and Diets
Lean, male C57BL/6 mice (7 weeks old) were purchased from Central Lab Animal Inc. (Seoul, Republic of Korea). All mice were housed for 1 week under a 12/12-hour light/dark cycle in a temperature—(22 ± 1°C) and humidity—(55 ± 5%) controlled room and fed standard laboratory chow and water ad libitum. KME was mixed with either powdered chow (M07, Feedlab, Republic of Korea) or high-fat (D12327, Research Diets, Inc., New Brunswick, NJ, USA) feed at a concentration of 4 g/kg of food to provide a 3000 mg/kg/day (mpk) dose. The powdered chow was then made into pellets. Control groups received pellets without KME. These dietary amounts represent the maximum amount of chow that these animals were able to consume during a 24-hour period. Seven C57BL/6 mice on the above ad libitum diets had their daily average caloric intake and weekly body weight measurement during the course of the study. At the conclusion of the in vivo experiment, the mice were sacrificed and the epididymal pads were collected and weighed.
2.3. Cold Test
The adaptive thermogenic response was measured in a cold test, during which the animals were individually housed at 4°C for 6 hours [31].
2.4. Liver Histology
Liver tissues were isolated immediately after sacrifice. For hematoxylin and eosin (HE) staining, tissues were fixed in 10% formalin and processed and embedded in paraffin prior to sectioning (10 μm.) and staining. Liver biopsies for electron microscopy were cut into 1 mm pieces, fixed immediately after collection in Karnovsky fixative (glutaraldehyde in cacodylate buffer), and kept at 4°C. The second step involved postfixation with 1% osmium tetraoxide in 0.1 M cacodylate buffer for 1 hour at 4°C. Tissues were then dehydrated through successive baths of graded alcohol, followed by a propylene oxide bath and a treatment with propylene oxide and resin mix before being embedded in a pure epoxy resin (araldite, Epon 812) that solidified after 48 h at 60°C. Semithin sections were cut at 2 μm, stained with toluidine blue, and then analyzed by light microscopy. Ultrathin sections were cut at 70 nm and examined with an electron microscope (BX 50, Olympus, Japan). Electron micrographs (400x magnification) were digitized and analyzed using ACD SEE, version 4.0. The liver of 1 mouse from each group (control, high-fat diet (HFD), HFD + KME 3000 mg/kg) was measured. In brief, the following criteria were used for scoring hepatic steatosis: grade 0, grade 1, hepatocytes occupying <33% of the hepatic parenchyma; grade 2, fatty hepatocytes occupying 33–66% of the hepatic parenchyma; and grade 3, fatty hepatocytes occupying >66% of the hepatic parenchyma.
2.5. Endurance Test
Mice were subjected to an endurance test using a variable-speed belt treadmill enclosed in a plexiglass chamber with a stimulus device consisting of a shock grid attached to the rear of the belt (Columbus Instruments Oxymax System, Columbus, OH, USA). The shock grid was set to deliver 0.2 mA, which caused an uncomfortable shock but did not physically harm or injure the animals. Animals were acclimatized to the test using a habituation protocol. Mice were run at 16.2 meter/minutes for 10 minutes with a 5° incline. For high-fat- (HF-) fed animals, the experiment was initiated at 10.8 meter/minutes at a 0° incline. The speed was gradually increased from 10.8 to 24.6 meter/minutes and then maintained until exhaustion [15]. Exhaustion was determined to have been reached if the mice were unable to run for 10 seconds [32], at which point the electric shock was discontinued. Figure 1 shows the running test protocol.
Figure 1.
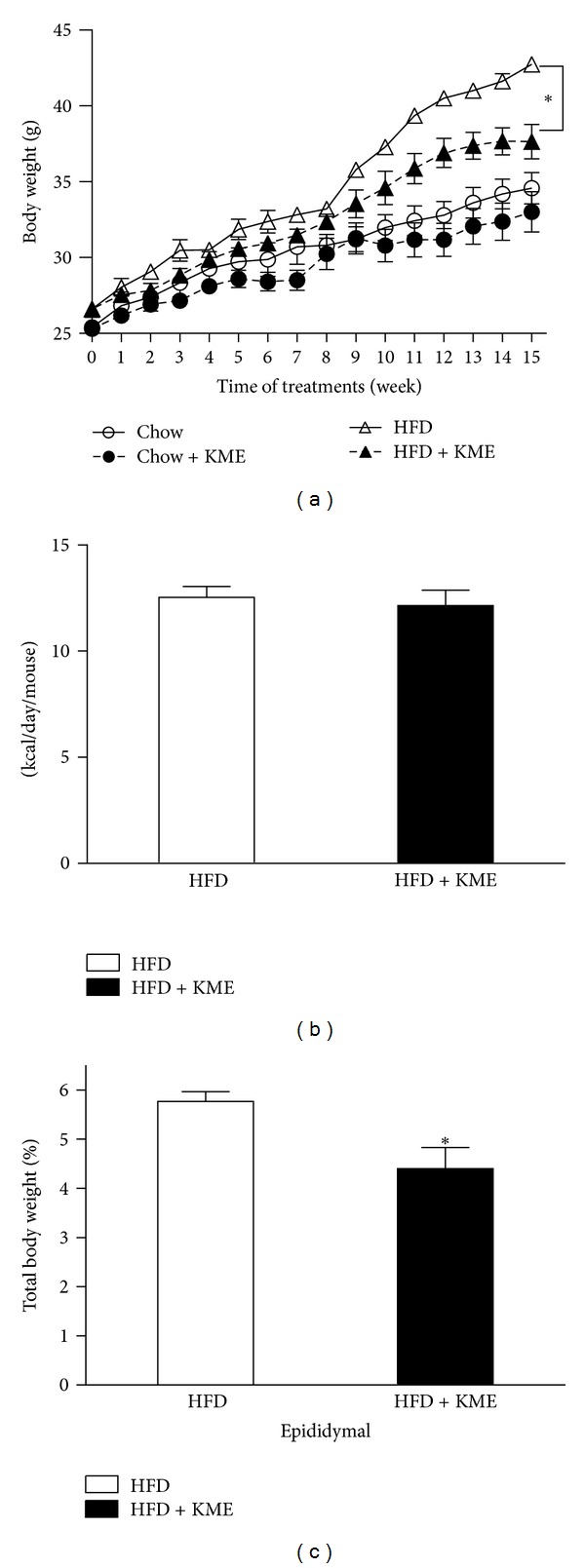
Effect of KME on body weight and food intake in mice fed a high-fat diet for 15 weeks. (a) Changes in body weight gain at each treatment period are shown. (○) C: chow diet; (●) C + KME: chow diet plus KME 3000 mg/kg; (△) HFD: high-fat diet; (▲) HFD + KME: high-fat diet plus KME 3000 mg/kg. (b) Average food intake expressed as kcal/mouse/day. (c) Epididymal fat weight expressed as percentage of total body weight. Values represent mean ± standard error of mean (SEM; *P < 0.05, n = 7 per group).
2.6. Cell Culture and Induction of Adipocyte Differentiation
3T3-L1 preadipocytes purchased from ATCC (American Type Culture Collection, Manassas, VA, USA) were cultured in Dulbecco's-modified Eagle's medium (DMEM, Hyclone, Logan, UT, USA) containing 10% bovine calf serum (BCS, Hyclone, Logan, Utah, USA) at 37°C in a humidified atmosphere of 5% CO2. After 3 or 4 days, the cells had reached 90% confluence and were collected with 0.05% trypsin/0.53 mM EDTA treatment. After centrifugation (1300 rpm, 5 minutes), the cells were plated in 6-well plates at a concentration of 3 × 104 cell/well. One day after confluence (designated “day 0”), cell differentiation was induced with a mixture of methylisobutylxanthine (0.5 mM), dexamethasone (0.25 mM), and insulin (5 mg/mL) in DMEM containing 10% fetal bovine serum (FBS). On day 2 and thereafter, DMEM supplemented with 10% FBS and 5 mg/mL insulin was replaced every 2 days. 3T3-L1 adipocytes, 7 to 8 days after differentiation, were treated with KME 6 μg/μL or phosphate-buffered saline (PBS) for 24 hours.
2.7. RNA Preparation and Real-Time RT-PCR
Total RNA was isolated from 3T3-L1 adipocytes using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Cells were homogenized by 4-5 passages through a 23-gauge needle. After a 5-minute reaction, samples were mixed with chloroform by vortex machine for 15 seconds. Samples were then centrifuged at 12,000 rpm for 10 minutes at 4°C. The colorless supernatant was transferred into new tubes. Total RNA was precipitated by mixing with isopropyl alcohol and centrifuging at 12,000 rpm. The resulting RNA pellets were washed with 75% ethanol and dissolved in RNase-free water. RNA was quantified by spectrophotometry at A260 and then stored at −80°C. Total extracted RNA (1 μg) mixed with annealing oligo dT primer and RNase-free water was incubated in a thermocycler for denaturing RNA. Reactant was mixed with 5X first strand buffer, 20 mM DTT, 10 mM dNTP mix, RNase-free water, and Superscript II reverse transcriptase. The mixtures were incubated in a thermocycler (42°C for 1 hour and 72°C for 7 minutes) to generate cDNA. Real-time RT-PCR analysis was performed with an AB 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA). Samples containing 2X SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 0.5 μmol each of appropriate primers, and cDNA were incubated in the AB 7500 real-time PCR system for an initial denaturation at 94°C for 10 minutes, followed by 40 PCR cycles. Each cycle was run at 95°C for 15 seconds and at 60°C for 1 minute. The oligonucleotide primers for the experiment are listed in Table 1. β-actin was used as an internal housekeeping control. To confirm amplification of specific transcripts, melting curve profiles were produced at the end of each PCR cycle by cooling the sample to 65°C for 15 seconds and heating slowly to 95°C, with continuous fluorescence measurement.
Table 1.
| Gene name | Accession number | Sequence | |
|---|---|---|---|
| ACC | AY451393 | Forward | 5′-GAG TGA CTG CCG AAA CAT CTC TG-3′ |
| Reverse | 5′-GCC TCT TCC TGA CAA ACG AGT-3′ | ||
| ACS | NM_007981 | Forward | 5′-TGA CCT CTC CAT GCA GTC AG-3′ |
| Reverse | 5′-GAG CCT ATG CAC TCA GCC AGT-3′ | ||
| FAS | NM_007988 | Forward | 5′-TGG GTT CTA GCC AGC AGA GT-3′ |
| Reverse | 5′-TAC CAC CAG AGA CCG TTA TGC-3′ | ||
| LPL | BC003305 | Forward | 5′-GCC CAG CAA CAT TAT CCA GT-3′ |
| Reverse | 5′-GGTCAG ACT TCC TGC TAC GC-3′ | ||
| SREBP-1c | 8C056922 | Forward | 5′-AAT GGT CCA GGC AAG TTC TGG GT-3′ |
| Reverse | 5′-TCC CTC TCA GCT GTG GTG GTG AA-3′ | ||
| C/EBP-α | M62362 | Forward | 5′-TGC TGG AGT TGA CCA GTG AC-3′ |
| Reverse | 5′-AAA CCA TCC TCT GGG TCT CC-3′ | ||
| PPAR-γ | NM_011146 | Forward | 5′-TTT TCA AGG GTG CCA GTT TCA ATC C-3′ |
| Reverse | 5′-AAT CCT TGG CCC TCT GAG AT-3′ | ||
| UCP1 | NM_009463 | Forward | 5′-GGC CCT TGT AAA CAA CAA AAT AC-3′ |
| Reverse | 5′-GGC AAC AAG AGC TGA CAG TAA AT-3′ | ||
| β-Actin | NM_007393 | Forward | 5′-GAC TAC CTC ATG AAG ATC-3′ |
| Reverse | 5′-GAT CCA CAT CTG CTG GAA-3′ |
ACD: acyl-coenzyme A dehydrogenase; ACO: acyl-coenzyme A oxidase; ACS: acetyl-coenzyme A synthetase; COX-2: cyclooxygenase 2; CPT-1: carnitine palmitoyltransferase 1; FAS: fatty acid synthase; HSL: hormone-sensitive lipase; IL-6: interleukin-6; LPL: lipoprotein lipase; MCP1: monocyte chemotactic protein 1; SREBP-1c: sterol regulatory element binding protein-1.
2.8. Statistical Analysis
Data (means ± SE) were analyzed using GraphPad Prism (version 5.04, GraphPad Software, USA). Unpaired two-tailed Student's t-tests were used to evaluate differences between means, as indicated. P values < 0.05 were considered statistically significant.
3. Results
3.1. KME-Induced Body Weight, Dietary Intake, and Adiposity Changes in HFD-Fed Mice
During the 15-week experiment, body weight and food intake were measured weekly. As shown in Figure 1, the KME-treated group had a slight decrease in body weight compared to the control group. However, this effect was significantly pronounced in animals that were fed an HF diet with KME. The difference between the KME-treated and control animals became significant at week 9. At the end of the experiment, the body weight of mice fed KME was 20% lower (P < 0.05) than that of the control group, whereas HF diet plus KME mice weighed almost the same as the mice that ate normal chow (Figure 1(a)). These effects of KME on body weight were not due to decreased food intake, as the amount of kcal consumed per mouse over a 24-hour period remained unchanged (Figure 1(b)). The data indicate that KME might have antiobesity effects in vivo, without affecting food intake.
To test whether body weight loss was caused by decreased adiposity, animals were sacrificed and epididymal white adipose tissue was dissected and weighed. This analysis revealed that epididymal white adipose tissue was significantly reduced in the HF diet plus KME group (Figure 1(c)).
3.2. Effect of KME on Cold Exposure in HFD-Fed Mice
We performed a cold test to assess the effect of KME on adaptive thermogenesis capacity. KME-treated animals maintained higher body temperatures than nontreated animals (Figure 2(a)), suggesting that it improved this capacity. To test whether thermogenesis is accompanied by changes in the expression of genes involved in thermogenesis, total RNA was prepared from BAT. We examined mRNA levels of UCP1, the major contributor to heat production, by Real-time RT-PCR. Figure 2(b) shows that the mRNA level of UCP1 was remarkably increased in KME-treated mice.
Figure 2.
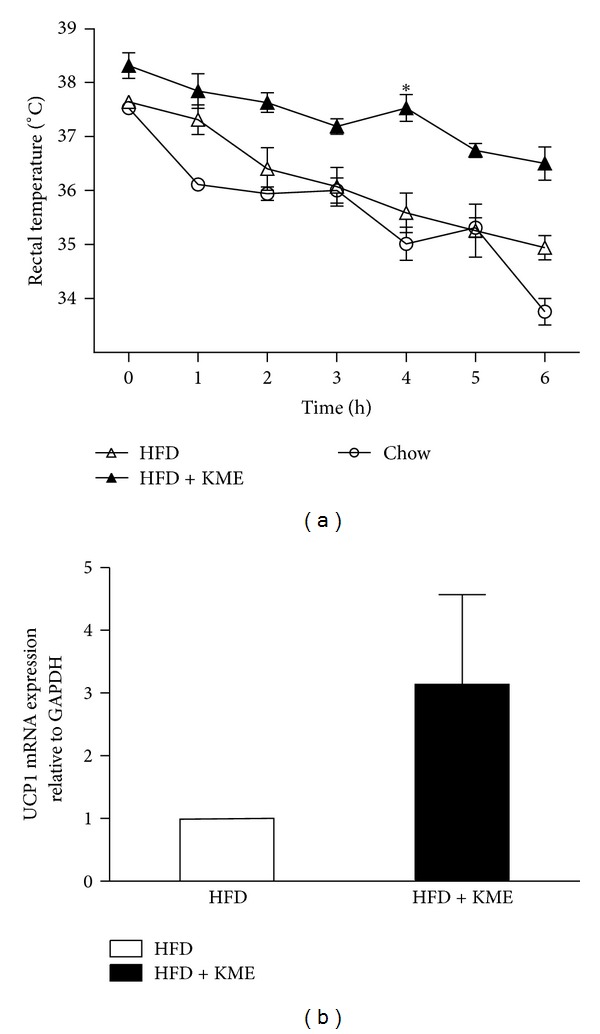
effect of KME on body temperature during the cold test (4°C for 6 h) and on UCP1 mRNA expression in BAT. (a) Hourly changes in body temperature are shown. (△) HFD: high-fat diet; (▲) HFD + KME: high-fat diet plus KME 3000 mg/kg (*P < 0.05, n = 7 per group). (b) The relative UCP1 mRNA expression level in BAT of animals treated with HFD or HFD + KME (high-fat diet plus KME 3000 mg/kg) as quantified by real-time PCR. Values represent mean ± SEM (*P < 0.05, n = 3).
3.3. Effect of KME on Endurance Capacity of HFD-Fed Mice
Because KME reduced body weight (Figure 1(a)) and increased thermogenesis (Figure 3), we evaluated the effect of KME administration on a treadmill endurance test. The mice were trained to run at 16.2 meter/minutes for 10 minutes at a 5° incline the day before the running test, according to the procedure used for our previous report [15]. The experiment was initiated at 10 meter/minutes at a 0° incline with a gradual increase in speed. The mice were run until exhaustion, which was defined as remaining on the shock grid for longer than 10 consecutive seconds. Surprisingly, KME-treated mice ran twice as far as high-fat diet mice (Figure 3).
Figure 3.
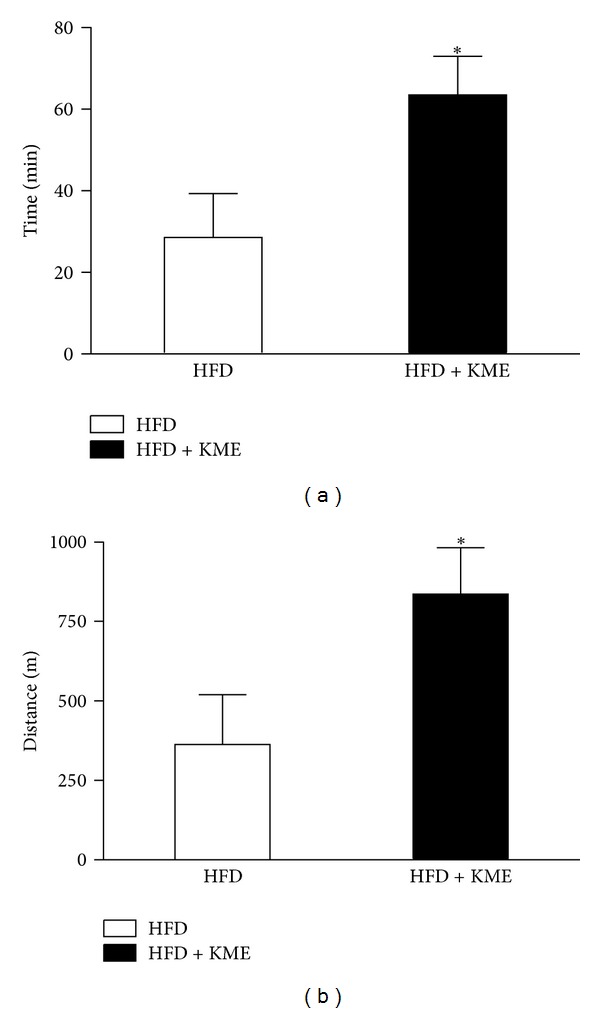
Endurance capacity was enhanced in KME-treated mice over 15 weeks. Average times (a) and distance (b) run until exhaustion are presented for animals treated with HF or HF + KME at 3000 mpk. Values represent the mean ± SEM (*P < 0.05, n = 7 per group).
3.4. Effect of KME on Hepatic Histology of HFD-Fed C57BL/6
One of the most common characteristics among people with obesity is the development of fatty liver [33, 34]. Therefore, we also analyzed the effect of KME on fatty liver development. Histologic evaluation is regarded as the “gold standard” for assessing the presence and severity of NAFLD [35]. We histologically evaluated liver sections to determine the extent to which KME attenuated hepatic steatosis development. As shown in Figure 4, the control group exhibited little histologic evidence of hepatic steatosis. Severe steatosis was observed in mice fed a high-fat diet without KME. However, a marked reduction in the degree of steatosis was seen in livers from high-fat diet mice treated with KME. Furthermore, hepatic steatosis scores were dramatically lower in the high-fat diet mice fed KME (grade 1).
Figure 4.
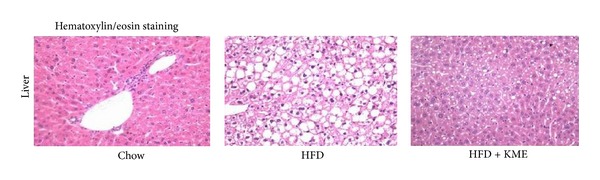
Histologic evaluation of hepatic steatosis. Hematoxylin and eosin staining of liver from mice fed chow diet (c), high-fat diet (HFD), or high-fat diet supplemented with KME at 3000 mpk (HFD + KME) (original magnification 400x). High-fat diet mice had severe hepatic steatosis. High-fat diet-induced mice fed KME at 3000 mpk had significantly reduced evidence of hepatic steatosis.
3.5. Effect of KME on mRNA Levels of Adipogenic Factors in 3T3-L1 Adipocytes
To demonstrate the inhibitory mechanism of KME on adipocyte differentiation, we measured the expression levels of the transcription factors PPAR-γ, C/EBP-α, and SREBP-1c in 3T3-L1 cells treated with 6 μg/μL of KME. Our results show that PPAR-γ, C/EBP-α, and SREBP-1c mRNA levels were decreased by 64%, 60%, and 32%, respectively, (Figure 5(a)). We also measured the mRNA expression levels of adipogenic enzymes, such as FAS, ACS, and ACC. We found that they were reduced by 69%, 55%, and 22%, respectively (Figure 5(b)). LPL expression is not directly associated with lipid synthesis but is strongly associated with adipogenesis. Therefore, we measured the expression level of LPL mRNA and found that the level of expression in KME-treated 3T3-L1 cells was decreased by 89% compared to that of untreated cells (Figure 5(a)).
Figure 5.
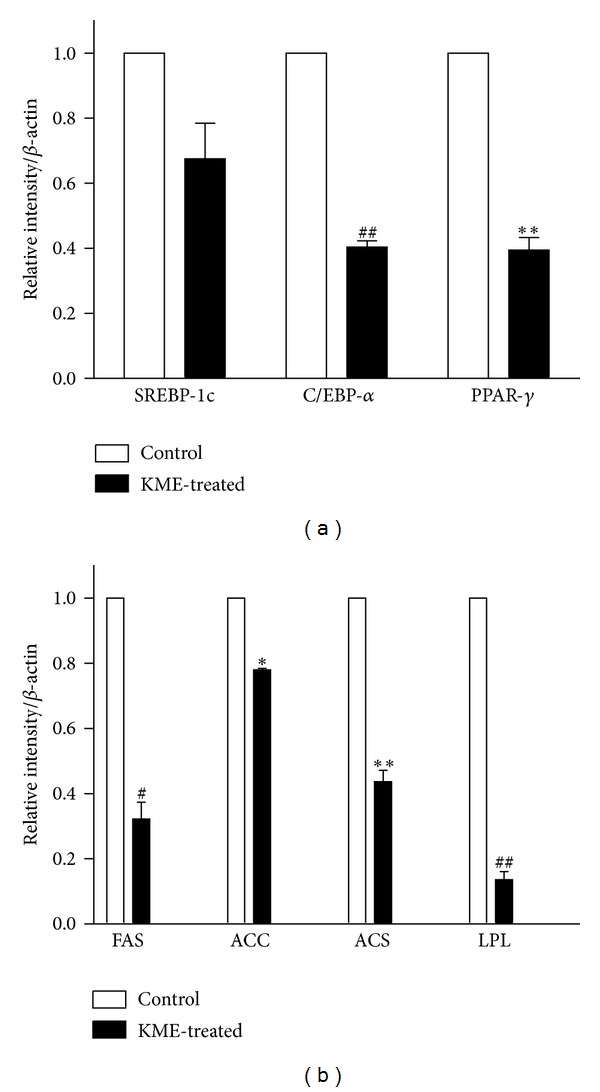
mRNA expressions of adipogenic enzymes and transcription factors in KME-treated 3T3-L1 adipocytes. On day 8 after differentiation, adipocytes were cultured in 6-well plates with or without KME (6 μg/μL) for 18 hours. Graphs represent mRNA expression of (a) adipogenic enzymes ACC, ACS, FAS, and LPL and (b) adipogenic transcription factors SREBP-1c, C/EBP-α, and PPAR-γ. Data are expressed relative to untreated control cells and represent the mean ± SEM (*P < 0.05, # P < 0.01, **P < 0.005, ## P < 0.001, n = 3).
4. Discussion
Our study is the first to demonstrate that KME prevents weight gain in mice. We examined the effects of KME on HFD-induced obesity in C57Bl/6 mice. Our results showed that body weight gain in groups fed a diet supplemented with KME was reduced compared to control HFD mice. This effect was more distinct in animals fed a high-fat diet. Epididymal WAT in C57Bl/6 mice was significantly reduced by KME supplementation. This study also provides evidence that dietary supplementation of KME protects against hepatic steatosis development. We have considered the possibility that the effect of KME may be mediated by food intake because decreased food intake would be expected to significantly affect body weight, which influences hepatic steatosis. In this study, however, food intake did not differ between the groups. This suggests that KME directly protected against obesity and hepatic steatosis independent of food intake.
Brown adipose tissues (BATs) use stored triacylglycerols (TG) to maintain body temperature. These cells produce energy from fatty acid metabolism to generate heat through the action of uncoupling protein 1 (UCP1), a mitochondrial protein found only in brown adipose tissue. Brown adipocytes contain less TG and more mitochondria than white adipocytes, resulting in their unique color. KME treatment increased UCP1 expression levels in BAT, which poised the mitochondria for respiration uncoupling [36]. This effect is consistent with the observed enhancement of cold tolerance and helps explain the increase in energy expenditure and resistance to weight gain.
Mitochondrial function can affect total body metabolism. This is most evident in muscle, a metabolically flexible tissue that switches between lipid and carbohydrate substrates to fulfill energy requirements [37]. One study found that mitochondrial OXPHOS activity within the oxidative soleus muscle, which is resistant to fatigue and is dependent on mitochondrial activity for ATP production, was more affected than in the glycolytic muscle tibialis anterior. The main changes included a reduction in respiratory chain activity with a concomitant decrease in mitochondrial ATP production [38]. In line with this finding and previously [15], KME significantly improved muscle oxidative capacity, resulting in enhancing the endurance.
To further consolidate our hypothesis that KME inhibits obesity, we evaluated the effects of KME treatment in vitro using an adipocyte differentiation development system, which is regulated by transcriptional activators such as PPAR-γ, C/EBP-α, and some transcriptional regulators at the molecular level [39, 40]. PPAR-γ is a member of the nuclear receptor superfamily of transcription factors and is dominantly expressed in adipose tissue. These transcription factors appear to be the main activators of adipocyte differentiation [41].
The C/EBPs belong to a large family of leucine zipper transcription factors [42]. C/EBP-α is a promising candidate transcription factor for directly controlling adipocyte differentiation [43].
SREBP1 is known to critically cross-activate a ligand binding domain of PPAR-γ and activate the production of an endogenous PPAR-γ ligand [27]. SREBP1 also regulates the expression of enzymes involved in fatty acid desaturation and lipogenesis.
Adipocyte differentiation involves a series of programmed changes in the expression of specific genes. Adipogenesis can be induced through the action of some enzymes, such as FAS, ACC, and acyl-CoA synthetase (ACS). The expressions of these genes are regulated by transcription factors, including PPAR-γ, C/EBP-α, and SREBP1, which are known to be crucial activators for adipogenesis and showed early changes in gene expression during adipocyte differentiation [44, 45]. In this study, we showed that KME treatment significantly decreased SREBP-1c, C/EBP-α, and PPAR-γ mRNA expression in cultured 3T3-L1 adipocytes and inhibited expression of adipocyte-specific proteins (FAS, ACC, ACS, and LPL). Collectively, these observations suggest that KME suppresses adipocyte differentiation by regulating the expression levels of adipogenic transcription factors.
Further investigation is necessary to define specific mechanisms by which KME protects against obesity-mediated hepatic steatosis. To elucidate the mechanism associated with the antiobesity effects of KME, additional studies should be conducted with the most abundant component in KME. Sung [46] reported that pentacyclic triterpenoid oleanolic acid has antiobesity activity. We have shown that KME contains oleanolic acid, which has anticancer activity [47]. It is possible that oleanolic acid might be the leading antiobesity component in KME.
5. Conclusion
KME had marked inhibitory effects on the development of obesity and NAFLD in mice fed high-fat diets. Activating endurance capacity and increasing thermogenesis are two possible mechanisms for the antiobesity effect of KME. This finding suggests that KME may be a potential dietary strategy for preventing obesity and NAFLD.
Acknowledgments
The authors thank Dr. Chun-Hyung Kim, Kyoung-Shim Kim, and Baek-Soo Han for critical evaluation of this paper. We would like to extend our appreciation to Sung-Min Park for his technical support and also like to thank An-Na Lee for her assistance in animal care and data analysis. This research was supported by Grant no. 112084-2 from High Value-added Food Technology Development Program funded by the Ministry for Food, Agriculture, Forestry, and Fisheries, Republic of Korea.
References
- 1.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104(4):531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 2.Allan JD. Rampant obesity: what you can do. Sexuality, Reproduction and Menopause. 2004;2(4):195–198. [Google Scholar]
- 3.Rebuffe-Scrive M, Surwit R, Feinglos M, Kuhn C, Rodin J. Regional fat distribution and metabolism in a new mouse model (C57BL/6J) of non-insulin-dependent diabetes mellitus. Metabolism. 1993;42(11):1405–1409. doi: 10.1016/0026-0495(93)90190-y. [DOI] [PubMed] [Google Scholar]
- 4.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37(9):1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 5.Widdowson PS, Upton R, Buckingham R, Arch J, Williams G. Inhibition of food response to intracerebroventricular injection of leptin is attenuated in rats with diet-induced obesity. Diabetes. 1997;46(11):1782–1785. doi: 10.2337/diab.46.11.1782. [DOI] [PubMed] [Google Scholar]
- 6.Younossi ZM, Diehl AM, Ong JP. Nonalcoholic fatty liver disease: an agenda for clinical research. Hepatology. 2002;35(4):746–752. doi: 10.1053/jhep.2002.32483. [DOI] [PubMed] [Google Scholar]
- 7.Franzese A, Vajro P, Argenziano A, et al. Liver involvement in obese children: ultrasonography and liver enzyme levels at diagnosis and during follow-up in an Italian population. Digestive Diseases and Sciences. 1997;42(7):1428–1432. doi: 10.1023/a:1018850223495. [DOI] [PubMed] [Google Scholar]
- 8.Medina J, García-Buey L, Fernández-Salazar LI, Moreno-Otero R. Approach to the pathogenesis and treatment of nonalcoholic steatohepatitis. Diabetes Care. 2004;27(8):2057–2066. doi: 10.2337/diacare.27.8.2057. [DOI] [PubMed] [Google Scholar]
- 9.Molassiotis A, Fernadez-Ortega P, Pud D, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Annals of Oncology. 2005;16:655–663. doi: 10.1093/annonc/mdi110. [DOI] [PubMed] [Google Scholar]
- 10.Yoon TJ, Yoo YC, Choi OB, et al. Inhibitory effect of Korean mistletoe (Viscum album coloratum) extract on tumour angiogenesis and metastasis of haematogenous and non-haematogenous tumour cells in mice. Cancer Letters. 1995;97(1):83–91. doi: 10.1016/0304-3835(95)03956-w. [DOI] [PubMed] [Google Scholar]
- 11.Yao H, Liao ZX, Wu Q, et al. Antioxidative flavanone glycosides from the branches and leaves of Viscum coloratum . Chemical and Pharmaceutical Bulletin. 2006;54(1):133–135. doi: 10.1248/cpb.54.133. [DOI] [PubMed] [Google Scholar]
- 12.Gray AM, Flatt PR. Insulin-secreting activity of the traditional antidiabetic plant Viscum album (mistletoe) Journal of Endocrinology. 1999;160(3):409–414. doi: 10.1677/joe.0.1600409. [DOI] [PubMed] [Google Scholar]
- 13.Kim JB. Mystery of Mistletoe: Biological Activities of Korean Mistletoe and Application (Korean Version) 3rd edition. Seoul, Korea: Choziilgwan Press; 2008. [Google Scholar]
- 14.Song SK, Moldoveanu Z, Nguyen HH, et al. Intranasal immunization with influenza virus and Korean mistletoe lectin C (KML-C) induces heterosubtypic immunity in mice. Vaccine. 2007;25(34):6359–6366. doi: 10.1016/j.vaccine.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Jung HY, Lee AN, Song TJ, et al. Korean mistletoe (Viscum album coloratum) extract improves endurance capacity in mice by stimulating mitochondrial activity. Journal of Medicinal Food. 2012;15(7):621–628. doi: 10.1089/jmf.2010.1469. [DOI] [PubMed] [Google Scholar]
- 16.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia—reperfusion, aging, and heart failure. Journal of Molecular and Cellular Cardiology. 2001;33(6):1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 18.Gardner A, Boles RG. Current Psychiatry Reviews. 3rd edition. Vol. 1. Oak Park, Ill, USA: Bentham Science; 2005. Is a mitochondrial psychiatry in the future? A review; pp. 255–271. [Google Scholar]
- 19.Conley KE, Marcinek DJ, Villarin J. Mitochondrial dysfunction and age. Current Opinion in Clinical Nutrition and Metabolic Care. 2007;10(6):688–692. doi: 10.1097/MCO.0b013e3282f0dbfb. [DOI] [PubMed] [Google Scholar]
- 20.Iossa S, Lionetti L, Mollica MP, et al. Effect of high-fat feeding on metabolic efficiency and mitochondrial oxidative capacity in adult rats. British Journal of Nutrition. 2003;90(5):953–960. doi: 10.1079/bjn2003000968. [DOI] [PubMed] [Google Scholar]
- 21.Obici S, Wang J, Chowdury R, et al. Identification of a biochemical link between energy intake and energy expenditure. Journal of Clinical Investigation. 2002;109(12):1599–1605. doi: 10.1172/JCI15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawada T, Takahashi N, Fushiki T. Biochemical and physiological characteristics of fat cell. Journal of Nutritional Science and Vitaminology. 2001;47(1):1–12. doi: 10.3177/jnsv.47.1. [DOI] [PubMed] [Google Scholar]
- 23.Sharma AM. Adipose tissue: a mediator of cardiovascular risk. International Journal of Obesity and Related Metabolic Disorders. 2002;26(supplement 4):S5–S7. doi: 10.1038/sj.ijo.0802210. [DOI] [PubMed] [Google Scholar]
- 24.Green H, Meuth M. An established pre adipose cell line and its differentiation in culture. Cell. 1974;3(2):127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 25.Morrison RF, Farmer SR. Insights into the transcriptional control of adipocyte differentiation. Journal of Cellular Biochemistry. 1999;32-33(supplement 1):59–67. doi: 10.1002/(sici)1097-4644(1999)75:32+<59::aid-jcb8>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- 26.Rosen ED, Spiegelman BM. Peroxisome proliferator-activated receptor γ ligands and atherosclerosis: ending the heartache. Journal of Clinical Investigation. 2000;106(5):629–631. doi: 10.1172/JCI10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes and Development. 2000;14(11):1293–1307. [PubMed] [Google Scholar]
- 28.Tontonoz P, Graves RA, Budavari AI, et al. Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPAR gamma and RXR alpha. Nucleic Acids Research. 1994;22:5628–5634. doi: 10.1093/nar/22.25.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes and Development. 1994;8(10):1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 30.Auwerx J, Leroy P, Schoonjans K. Lipoprotein lipase: recent contributions from molecular biology. Critical Reviews in Clinical Laboratory Sciences. 1992;29(3-4):243–268. doi: 10.3109/10408369209114602. [DOI] [PubMed] [Google Scholar]
- 31.Picard F, Géhin M, Annicotte JS, et al. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell. 2002;111(7):931–941. doi: 10.1016/s0092-8674(02)01169-8. [DOI] [PubMed] [Google Scholar]
- 32.Hakimi P, Yang J, Casadesus G, et al. Overexpression of the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) in skeletal muscle repatterns energy metabolism in the mouse. Journal of Biological Chemistry. 2007;282(45):32844–32855. doi: 10.1074/jbc.M706127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YX, Lee CH, Tiep S, et al. PPARδactivates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 34.Schaffer JE. Lipotoxicity: when tissues overeat. Current Opinion in Lipidology. 2003;14(3):281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Brunt EM. Pathology of nonalcoholic steatohepatitis. Hepatology Research. 2005;33(2):68–71. doi: 10.1016/j.hepres.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 37.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes and Development. 2004;18(4):357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 38.Chanseaume E, Malpuech-Brugère C, Patrac V, et al. Diets high in sugar, fat, and energy induce muscle type-specific adaptations in mitochondrial functions in rats. Journal of Nutrition. 2006;136(8):2194–2200. doi: 10.1093/jn/136.8.2194. [DOI] [PubMed] [Google Scholar]
- 39.MacDougald OA, Lane MD. Transcriptional regulation of gene expression during adipocyte differentiation. Annual Review of Biochemistry. 1995;64:345–373. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- 40.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiological Reviews. 1998;78(3):783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 41.Cornelius P, MacDougald OA, Lane MD. Regulation of adipocyte development. Annual Review of Nutrition. 1994;14:99–129. doi: 10.1146/annurev.nu.14.070194.000531. [DOI] [PubMed] [Google Scholar]
- 42.Jeon T, Hwang SG, Hirai S, et al. Red yeast rice extracts suppress adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 cells. Life Sciences. 2004;75(26):3195–3203. doi: 10.1016/j.lfs.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 43.Wu Z, Y Xie, Bucher NL, Farmer SR. Conditional ectopic expression of C/EBP beta in NIH-3T3 cells induces PPAR gamma and stimulates adipogenesis. Genes and Development. 1995;9:2350–2363. doi: 10.1101/gad.9.19.2350. [DOI] [PubMed] [Google Scholar]
- 44.Latasa MJ, Moon YS, Kim KH, Sul HS. Nutritional regulation of the fatty acid synthase promoter in vivo: sterol regulatory element binding protein functions through an upstream region containing a sterol regulatory element. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(19):10619–10624. doi: 10.1073/pnas.180306597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luong A, Hannah VC, Brown MS, Goldstein JL. Molecular characterization of human acetyl-CoA synthetase, an enzyme regulated by sterol regulatory element-binding proteins. Journal of Biological Chemistry. 2000;275(34):26458–26466. doi: 10.1074/jbc.M004160200. [DOI] [PubMed] [Google Scholar]
- 46.Sung HY, Kang SW, Kim JL, et al. Oleanolic acid reduces markers of differentiation in 3T3-L1 adipocytes. Nutrition Research. 2010;30(12):831–839. doi: 10.1016/j.nutres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Jung MJ, Yoo YC, Lee KB, Kim JB, Song KS. Isolation of epi-oleanolic acid from Korean mistletoe and its apoptosis-lnducing activity in tumor cells. Archives of Pharmacal Research. 2004;27(8):840–844. doi: 10.1007/BF02980176. [DOI] [PubMed] [Google Scholar]


