Abstract
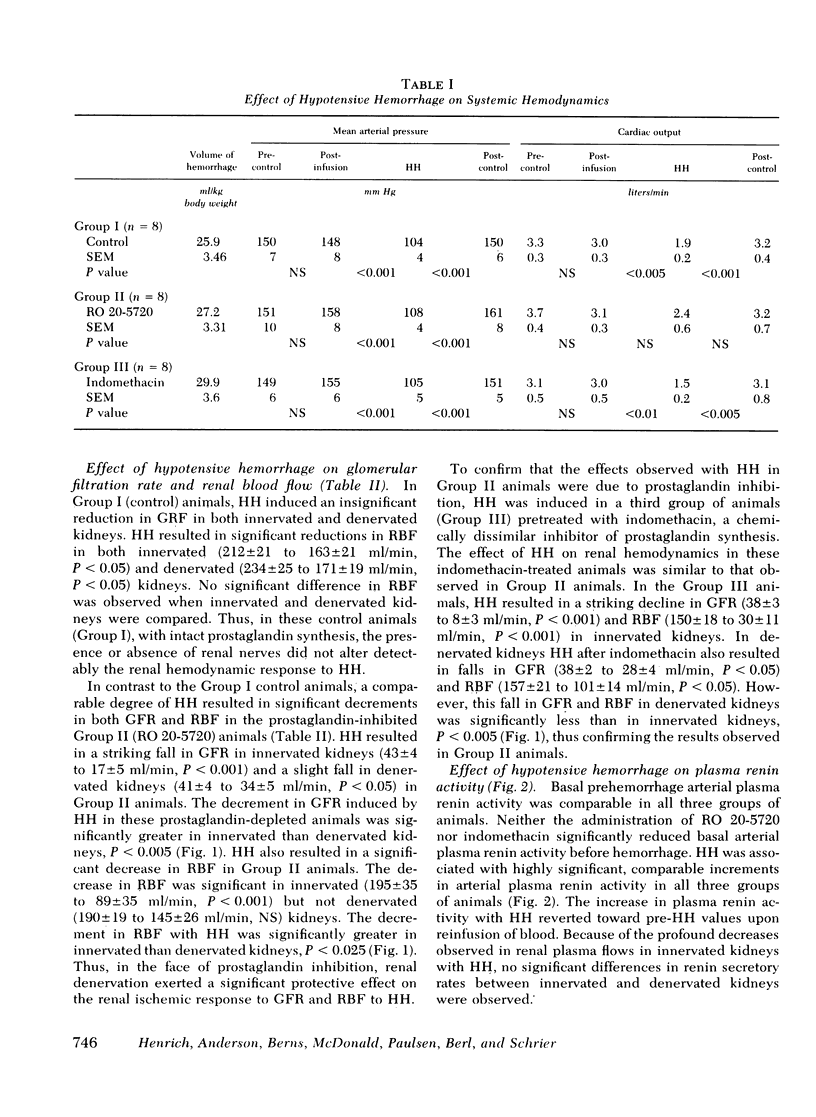
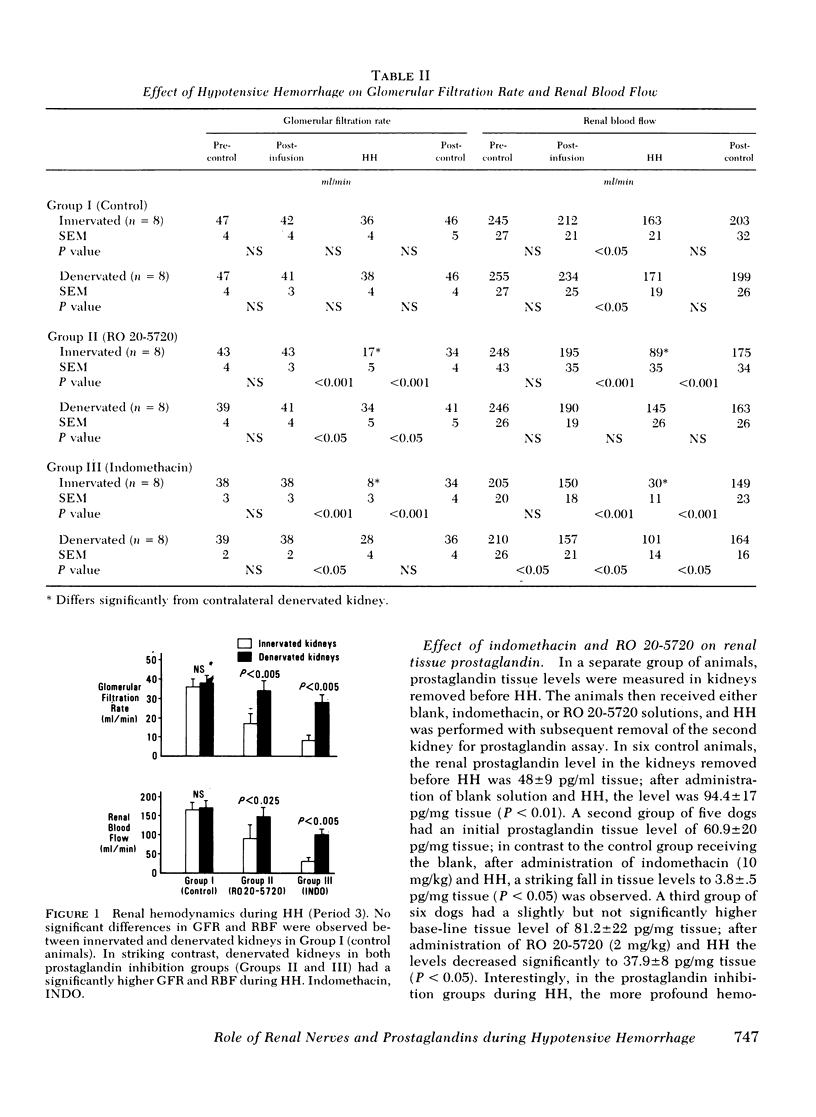
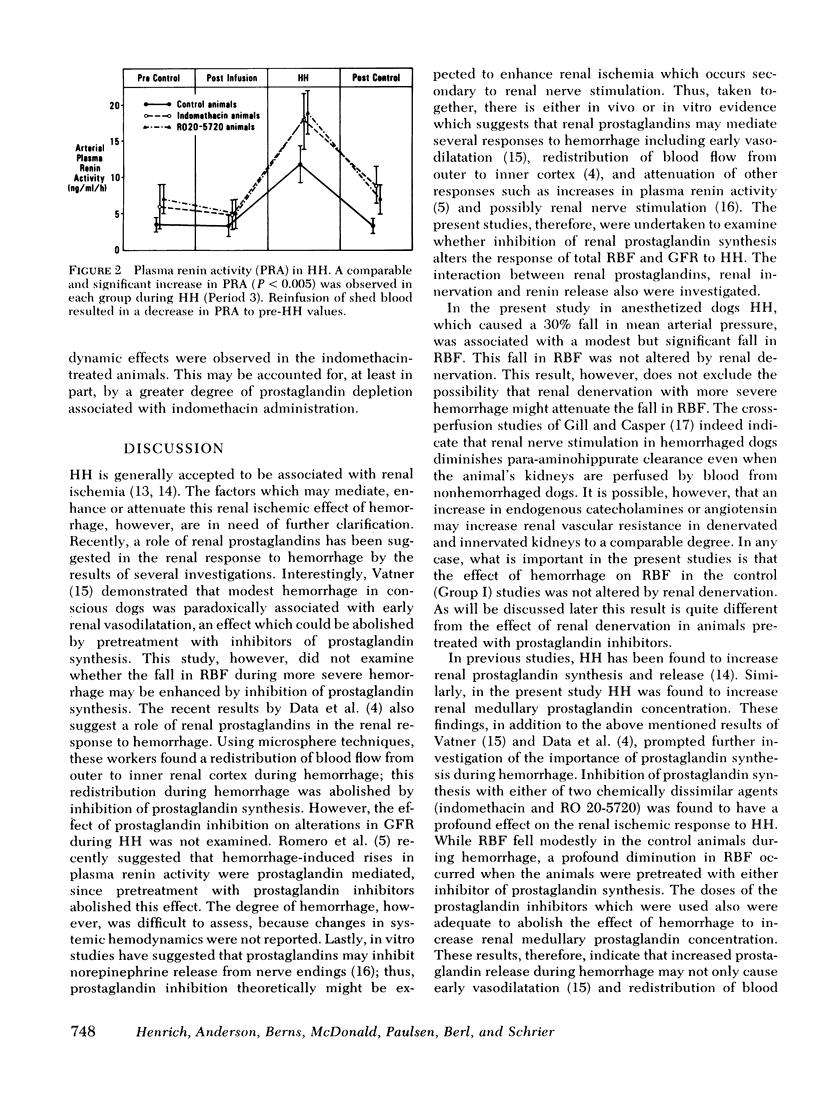
The effects of hypotensive hemorrhage (HH) on renal hemodynamics and plasma renin activity (PRA) during prostaglandin (PG) synthesis inhibition were examined in three groups of dogs. In each group of animals arterial blood pressure was lowered by a 30% decrement. In the first group of eight control animals, HH was not associated with a significant change in glomerular filtration rate (GFR, 42-36 ml/min, NS); renal blood flow (RBF) declined significantly, from 234 to 171 ml/min, P < 0.05. In the second group of eight animals, pretreated with RO 20-5720 (RO, 2 mg/kg), a competitive inhibitor of PG synthesis, HH was associated with a significant fall in GFR (43-17 ml/min, P < 0.001) and RBF (195-89 ml/min, P < 0.001). In the third group of eight animals, pretreatment with indomethacin (IN, 10 mg/kg), a chemically dissimilar PG inhibitor, HH was also associated with a significant fall in GFR (38-8 ml/min, P < 0.001) and RBF (150-30 ml/min, P < 0.001). Renal denervation attenuated this renal ischemic effect of HH in the presence of PG inhibition. In the RO group, GFR (34 vs. 17 ml/min, P < 0.005) and RBF (145 vs. 89 ml/min, P < 0.025) were significantly greater in denervated vs. innervated kidneys during HH. Similarly, in animals treated with IN, a significantly higher GFR (28 vs. 8 ml/min, P < 0.005) and RBF (101 vs. 30 ml/min, P < 0.005) occurred in denervated as compared to innervated kidneys during HH. With HH, the increase in PRA in the control group (3.34-11.68 ng/ml per h, P < 0.005) was no different than that observed in the RO group (4.96-18.9 ng/ml per h, P < 0.001) or IN group (4.71-17.8 ng/ml per h, P < 0.001). In summary, the present results indicate that renal PG significantly attenuate the effect of HH to decrease GFR and RBF. Furthermore, renal denervation exerts a protective effect against the enhanced renal ischemic effects which occur in the presence of PG inhibition during HH. Finally, PG inhibition does not alter the effect of HH to cause an increase in PRA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. J., Taher M. S., Cronin R. E., McDonald K. M., Schrier R. W. Effect of beta-adrenergic blockade and inhibitors of angiotensin II and prostaglandins on renal autoregulation. Am J Physiol. 1975 Sep;229(3):731–736. doi: 10.1152/ajplegacy.1975.229.3.731. [DOI] [PubMed] [Google Scholar]
- Data J. L., Chang L. C., Nies A. S. Alteration of canine renal vascular response to hemorrhage by inhibitors of prostaglandin synthesis. Am J Physiol. 1976 Apr;230(4):940–945. doi: 10.1152/ajplegacy.1976.230.4.940. [DOI] [PubMed] [Google Scholar]
- Dunham E. W., Zimmerman B. G. Release of prostaglandin-like material from dog kidney during nerve stimulation. Am J Physiol. 1970 Nov;219(5):1279–1285. doi: 10.1152/ajplegacy.1970.219.5.1279. [DOI] [PubMed] [Google Scholar]
- Gill J. R., Jr, Casper A. G. Role of the sympathetic nervous system in the renal response to hemorrhage. J Clin Invest. 1969 May;48(5):915–922. doi: 10.1172/JCI106050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp A., Veyrat R., Rosset E., Scherrer J. R., Truniger B. Relationship between renin and intrarenal hemodynamics in hemorrhagic hypotension. J Clin Invest. 1971 May;50(5):970–978. doi: 10.1172/JCI106590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry D. P., Starman B. J., Johnson D. G., Williams R. H. A sensitive radioenzymatic assay for norepinephrine in tissues and plasma. Life Sci. 1975 Feb 1;16(3):375–384. doi: 10.1016/0024-3205(75)90258-1. [DOI] [PubMed] [Google Scholar]
- Johnston P. A., Selkurt E. E. Effect of hemorrhagic shock on renal release of prostaglandin E. Am J Physiol. 1976 Mar;230(3):831–838. doi: 10.1152/ajplegacy.1976.230.3.831. [DOI] [PubMed] [Google Scholar]
- Lum G. M., Aisenbrey G. A., Dunn M. J., Berl T., Schrier R. W., McDonald K. M. In vivo effect of indomethacin to potentiate the renal medullary cyclic AMP response to vasopressin. J Clin Invest. 1977 Jan;59(1):8–13. doi: 10.1172/JCI108624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGiff J. C., Crowshaw K., Itskovitz H. D. Prostaglandins and renal function. Fed Proc. 1974 Jan;33(1):39–47. [PubMed] [Google Scholar]
- Needleman P., Douglas J. R., Jr, Jakschik B., Stoecklein P. B., Johnson E. M., Jr Release of renal prostaglandin by catecholamines: relationship to renal endocrine function. J Pharmacol Exp Ther. 1974 Feb;188(2):453–460. [PubMed] [Google Scholar]
- Satoh S., Zimmerman B. G. Influence of the renin-angiotensin system on the effect of prostaglandin synthesis inhibitors in the renal vasculature. Circ Res. 1975 Jun;36(6 Suppl 1):89–96. doi: 10.1161/01.res.36.6.89. [DOI] [PubMed] [Google Scholar]
- Vatner S. F. Effects of hemorrhage on regional blood flow distribution in dogs and primates. J Clin Invest. 1974 Aug;54(2):225–235. doi: 10.1172/JCI107757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTON R. P., RICHARDSON J. A., WALTON R. P., Jr, THOMPSON W. L. Sympathetic influences during hemorrhagic hypotension. Am J Physiol. 1959 Jul;197(1):223–230. doi: 10.1152/ajplegacy.1959.197.1.223. [DOI] [PubMed] [Google Scholar]



