Abstract
Our goal was to assess a nonhuman primate diet that mimicked the Western-type diet of humans with regard to palatability and the diet's effects on plasma lipid concentrations and other cardiometabolic risk factors. We evaluated male (n = 8) and female (n = 11) African green monkeys (vervets; Chlorocebus aethiops sabaeus) that initially were fed a standard diet. Each cohort then was divided into 2 groups, which received either standard chow or the Western diet. Food consumption and fecal quality were measured weekly. Body weight, waist circumference, and body–mass index were measured every 2 wk. CBC and clinical chemistry analyses were performed at baseline and 4 wk after the diet change. Plasma lipid concentrations, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, glucose, insulin, and fructosamine were measured at baseline and at 4, 8, and 12 wk after the diet change. Isoflavones were measured in the male monkeys at 6 wk after diet change, and lipid particle size was measured in the female monkeys at the 12-wk point. Green monkeys readily ate the Western diet and maintained baseline body weight and morphometric measures, with no adverse effects on fecal quality or clinical measures. Total plasma cholesterol was higher in monkeys fed the Western diet compared with standard chow. Isoflavones were higher in male monkeys fed standard chow compared with the Western diet, but lipid particle size did not differ by diet in female monkeys. Our data indicate that the Western diet led to changes in various biomedical risk factors of green monkeys to become similar to those of humans in the United States.
Almost all nonhuman primates used for biomedical research are fed commercially prepared, biscuit diets commonly referred to as ‘monkey chow.’ Although these diets have some advantages—including widespread availability, easy storage, high palatability, and low cost— they have critical disadvantages for use in translational research. The primary disadvantage of these diets is that they typically rely on soy as the almost-exclusive source of protein. Soy protein contains high quantities of isoflavones, often referred to as phytoestrogens. The isoflavones of soy consist primarily of genistein, daidzein, and glycetin—all of which are biologically active compounds with robust effects particularly on the cardiovascular and reproductive systems. Studies at our own institution have identified a variety of effects on the cardiovascular system of Old World monkeys. Specifically, reports have shown that postmenopausal female macaques fed a diet rich in soy proteins had fewer atherosclerotic plaques than did control animals4 and decreases in inflammatory markers.14 Similar studies in male monkeys found a significant reduction in atherosclerotic plaque size when animals consumed moderate amounts of dietary soy.1 In addition, soy isoflavones diminish the proliferative effects of mammalian estrogens, whether produced endogenously or exogenously, on the breast and endometrium.19 Isoflavones reduce the rate of ovarian follicle depletion in cynomolgus monkeys.2 Furthermore, soy isoflavones increased the aggressive behavior of male cynomolgus monkeys and reduced their affiliative behavior.17
Previously, a group at our institution, in collaboration with the Yerkes National Primate Research Center, compared plasma concentrations of soy isoflavones in rhesus macaques fed typical monkey chow with those of macaques fed an experimental diet in which the source of protein was primarily isolated soy protein.18 Plasma concentrations of isoflavones were higher among animals eating the usual monkey chow than in animals fed a diet with a purified isolated soy protein that provided an amount of total isoflavones that was equivalent to a human consumption of 130 mg daily. From a translational point of view, such plasma concentrations are of considerable concern, given that the average isoflavone consumption in women in the United States is strikingly lower, at 3.2 mg daily.13
Several recent studies have emphasized the importance of various aspects of diet when studying nonhuman primate models of human disease. These include the effect of a high-sugar, high-fat diet on adiposity and cardiometabolic syndrome,9,12 the effect of carbohydrate levels on metabolism,6 and the influence of maternal nutrient reduction on development of insulin resistance.3
Because traditional monkey chow diets induce high plasma concentrations of isoflavone and given the robust effects of isoflavones on nonhuman primates, we sought to develop a diet comparable to that consumed by adults in the United States. We reason that such a diet will increase the translational value of studies addressing age and age-related chronic diseases. Here we report our efforts to devise such a Western-type diet, how the monkeys responded to the newly formulated diet, and the finding that the diet led to changes in various biomedical risk factors, making them more like those of humans in the United States.
Materials and Methods
Subjects.
The subjects in this study consisted of 2 cohorts of African green monkeys (vervets; Chlorocebus aethiops sabaeus): 8 males (at the start of the study: age, 4.2 to 10.8 y; weight, 5.12 to 9.01 kg) and 11 females (age, 4.5 to 24.9 y; weight, 3.90 to 7.03 kg). There were initially 12 female monkeys, but one died during the baseline period for reasons not associated with the study. All animals were captive-born, of known age, mother-reared, and were part of the Vervet Research Colony, a multigenerational breeding colony of Caribbean-origin vervets housed at Wake Forest Primate Center. This colony is a genomically characterized, NIH-supported biomedical research resource that was transferred from its original location in California to Wake Forest in 2008.11
Housing.
All animals were originally housed in large, indoor-outdoor matrilineal breeding groups. Prior to the initiation of this study, all animals were moved to indoor housing and were housed in pairs in standard primate quad cages. All caging had perches and were supplied with enrichment devices including toys, mirrors, and foraging devices. Animals were maintained on a 12:12-h light:dark schedule. The pair-mate of the female monkey that died was single-housed after the baseline period.
Control and experimental groups.
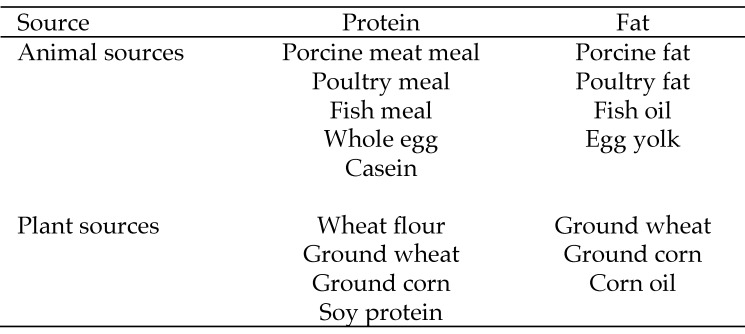
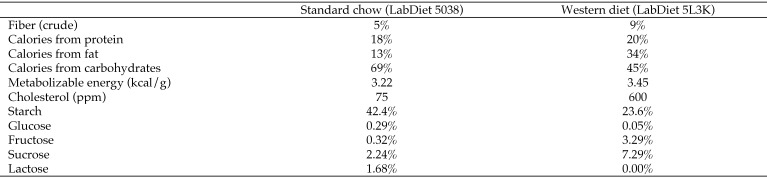
Prior to the initiation of the study, and during the baseline period, all animals were fed a standard commercial monkey chow (LabDiet 5038, Purina Mills, St Louis, MO). Animals in both the male and female cohorts were pseudorandomly assigned to either a control (males, n = 4; females, n = 4) or an experimental diet (males, n = 4; females, n = 7) group. Pair-mates were assigned to the same diet group. Controls were maintained on standard monkey chow for the duration of the study. Experimental animals were fed standard chow at baseline and then switched to a Western-type diet (LabDiet 5L3K, Purina Mills) over the course of a 3-d acclimation period. This diet was formulated to mimic a typical American diet and was higher in fat (34% of calories) and lower in carbohydrates (45%) than was the standard monkey chow. In addition, the source of protein in the Western diet was mostly from animal sources, whereas the protein in the standard chow was primarily from soy. We tried to balance the sources of protein and fat in the Western diet to be comparable to those eaten by humans in the United States (Figures 1 and 2). Both diets meet the nutritional guidelines of the National Research Council. Diet specification sheets are available from LabDiet's webpage (http://labdiet.com/) or on request.
Figure 1.
Sources of protein and fat in the Western diet (LabDiet 5L3K).
Figure 2.
Comparison of the standard and Western diets.
Throughout the study, all animals had 24-h ad libitum access to water and were given food enrichment, consisting of fruits, vegetables, and seeds on a rotating basis, 3 times each week. Enrichment foods made up only a small percentage (less than 5%) of the animals’ total caloric intake.
To facilitate the measurement of food intake, during the baseline period and the first 8 wk after the diet change, the study animals were fed their daily ration once each day and were separated from their pair-mates during feeding. Pairs were separated in the morning, and each monkey received 100 kcal of diet per kilogram of prestudy body weight. All remaining food was removed after 4 h, and the pair-mates were reunited. Enrichment foods were provided in the afternoons. After 8 wk, pairs were no longer separated during feeding, and the same quantity of diet was available to each pair, ad libitum, for the entire day. During the entire study, diet amounts were maintained at 100 kcal/kg according to the prestudy body weights of the individual subjects or pairs.
Procedures.
Animals were acclimated to the feeding procedures for at least 4 wk prior to the diet change. All monkeys were fed standard chow during this acclimation period. Food consumption was measured once each week by recording the weight of food (in g) fed to each animal and the weight of food remaining in the cage after the 4-h feeding period. The percentage of food consumed was calculated. Once each week, fecal quality was rated according to a 5-point Likert scale that ranged from 1 (solid, normal stool) to 5 (diarrhea, extremely runny stool).
Once during baseline and once every 2 wk thereafter for the duration of the 12 wk study, monkeys were anesthetized with ketamine (10 to 15 mg/kg IM) for the collection of blood samples, body weights, and other morphometric measurements. Animals were fasted prior to each collection unless otherwise noted. Body weight and morphometric measures including waist circumference and crown–rump and trunk lengths (in cm) were measured every 2 wk; body mass indices (in kg/cm2) were derived by using these 2 length measurements.
Blood samples were collected via femoral venipuncture according to standard techniques. CBC and blood chemistry assays were run by a commercial laboratory (Idexx Laboratories, Greensboro, NC; www.idexx.com). Fasted blood samples were collected for lipid and glycemic assays. Unfasted (4 h after feeding) blood samples for isoflavone assays were collected from the male cohort only. At 12 wk after the diet change, blood samples for lipid particle-size assays were collected from the female cohort only.
All procedures involving animals were reviewed and approved by the Wake Forest University Animal Care and Use Committee. Wake Forest is an AAALAC-accredited institution, and all research was conducted in compliance with the eighth edition of the Guide for the Care and Use of Laboratory Animals.10
Assays.
Plasma levels of total plasma cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides were measured. Lipoprotein distributions were measured via gel filtration chromatography,8 and all measures were recorded in ng/dL. Hydrodynamic radius determinations (in nm) were done by using purified lipoprotein preparations, and a multiangle light-scattering detector was used in conjunction with gel filtration chromatography of purified lipoprotein preparations to determine the hydrodynamic radius of LDL and HDL. Fasting blood glucose (in mg/dL), fructosamine, and insulin levels were assayed by using enzymatic colorimetric methods. Isoflavones were assayed by using liquid chromatography and mass spectrometry.1,7 The major isoflavones, genistein and daidzein, as well as the daidzein metabolite equol were measured (in nm/L).
Statistics.
All results are reported as mean ± SEM. Data were analyzed by using repeated-measures ANOVA, independent-group t tests, correlations, and ANCOVA. Analyses were performed by using raw data or percentage change from baseline. Experimental group (standard or Western diet) was used as a between-subjects independent variable and, when applicable, time point (baseline, 4 wk, 8 wk, 12 wk) was used as a within-group independent variable. All variables were tested for normality and homogeneity of variance. All statistical tests were performed separately by sex, and all analyses were performed by using SAS (version 9.1, SAS Institute, Cary, NC).
Results
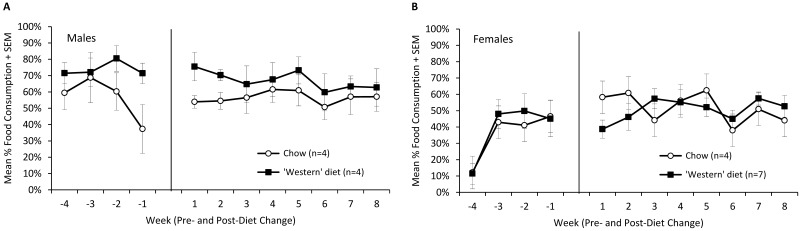
Overall, both male and female green monkeys tolerated the Western diet well. Weekly food consumption was comparable between diet groups and remained stable across the first 8 wk of the study (Figure 3). The male monkeys fed the Western diet tended to consume more food than did those eating standard chow, but this difference in consumption was mirrored during the baseline period and remained stable during the study. The low level of food consumption by female monkeys during the first baseline measurement period was attributed to lack of habituation to the temporary separation during feeding, given that food consumption increased to a stable level after that first week. In addition, fecal quality ratings remained normal throughout the study and showed no significant differences over the course of the study for either male or female monkeys. The only alteration observed was a slight softening of the stool in both groups immediately after the diet change. Fecal ratings averaged between 1.00 and 1.85 for female monkeys and 1.00 and 1.33 for male monkeys, with no significant differences by diet group or time point.
Figure 3.
(A) Percentage food consumption (mean ± SEM) in male green monkeys by diet type and week of study. Food consumption data represent the percentage of food consumed during the daily 4-h individual feeding session, when animals were separated temporarily from cagemates and given ad libitum access to diet (100 kcal/kg). (B) Percentage food consumption (mean ± SEM) in female green monkeys by diet type and week of study. Additional experimental details were as those for male monkeys.
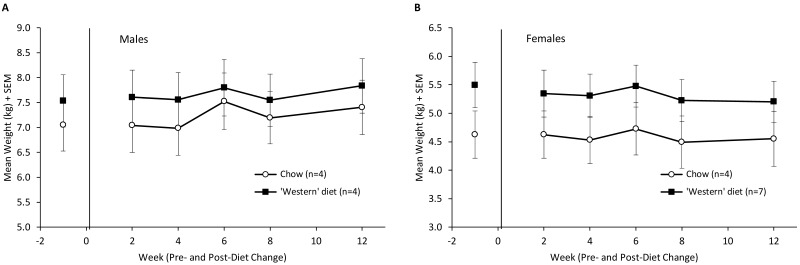
Body weight in male monkeys did not differ significantly by diet group, time period, or their interaction (F3,18 = 0.16, nonsignificant; Figure 4). For female monkeys, there was a significant main effect of time period (F3,27 = 7.80, P < 0.001), but the 2 diet groups did not differ at baseline or at any of the postdiet change time periods. There were no significant differences for waist circumference or body–mass index over the course of the study (Table 1).
Figure 4.
(A) Body weight (kg, mean ± SEM) of male green monkeys by diet type and time point. (A) Body weight (kg, mean ± SEM) of female green monkeys by diet type and time point.
Table 1.
Summary of percentage changes (mean ± SEM) in evaluated parameters from baseline to 12 wk after diet change
| Male monkeys |
Female monkeys |
|||
| Chow (n = 4) | Western diet (n = 4) | Chow (n = 4) | Western diet (n = 7) | |
| Body weight | 6% ± 5% | 4% ± 5% | −2% ± 2% | −5% ± 1% |
| Waist circumference | 0% ± 1% | 3% ± 6% | −3% ± 3% | −5% ± 1% |
| Body mass index | 5% ± 3% | −1% ± 2% | −6% ± 5% | −6% ± 2% |
| Total plasma cholesterol | 9% ± 7% | 47% ± 8%a | −1% ± 3% | 22% ± 3%a |
| HDL cholesterol | 10% ± 12% | 37% ± 7% | −20% ± 3% | 11% ± 4%a |
| LDL cholesterol | 12% ± 10% | 56% ± 9%a | 9% ± 3% | 30% ± 5%a |
| Triglycerides | 47% ± 19% | 18% ± 20%a | 4% ± 12% | −15% ± 7% |
| Glucose | −21% ± 5% | 7% ± 8% | −15% ± 6% | −10% ± 5% |
| Insulin | 180% ± 113% | 36% ± 33% | 149% ± 98% | 29% ± 26% |
| Fructosamine | −11% ± 4% | −25% ± 5% | −2% ± 4% | −14% ± 8% |
Significant (t test, P < 0.05) difference between animals fed standard chow compared with Western diet after baseline values were obtained.
CBC and blood chemistry results indicated that all animals remained in good health and showed no adverse effects of the Western diet after 4 wk. Although various parameters in both the blood chemistry panel and CBC analysis did change for each animal over time, no consistent changes were noted across groups, with the exception of total plasma cholesterol. In addition, values in all animals remained either within reference ranges for each analyte or were sufficiently consistent between baseline and the 4-wk sample that they prompted no clinical concern.
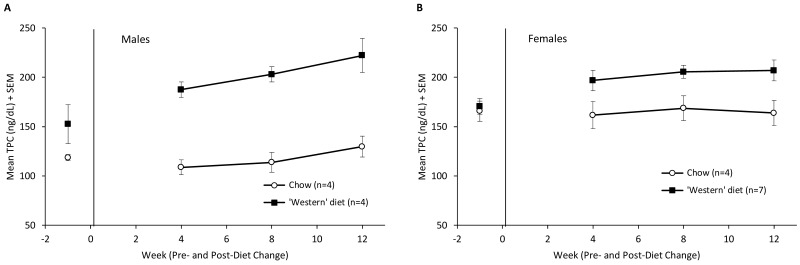
Concentrations of total plasma cholesterol were higher in both male and female monkeys that were fed the Western diet (Figure 5). Male monkeys showed a baseline difference in total plasma cholesterol concentrations, even when both diet groups were eating monkey chow (t6 = 3.30, P < 0.05). However, the magnitude of this difference was much higher after the change in diet, and there was a significant diet×time period interaction (F3,18 = 13.12, P < 0.0001). Total plasma cholesterol in female monkeys was comparable between diet groups at baseline (t9 = 0.36, nonsignificant) but, like male monkeys, showed a diet×time interaction (F3,27 = 5.83, P < 0.01). By the end of the 12-wk study, the monkeys eating the Western diet had higher total cholesterol concentrations (male, 222 ± 9 mg/dL; female, 207 ± 11 mg/dL) than did those eating chow (male: 130 ± 11 mg/dL, t6 = 6.73, P < 0.001; female: 164 ± 13 mg/dL, t9 = 2.51, P < 0.05). Total plasma cholesterol among animals fed the Western diet increased 47% in male monkeys and 22% in females (Table 1). All 4 of the male monkeys and 3 of the 7 females that were fed the Western diet had total plasma cholesterol concentrations that exceeded 200 mg/dL by the end of 12-wk period.
Figure 5.
(A) Total plasma cholesterol (mg/dL, mean ± SEM) of male green monkeys by diet type and time point. (B) Total plasma cholesterol (mg/dL, mean ± SEM) of female green monkeys by diet type and time point.
In addition, significant differences were identified between the standard-chow and Western-diet groups for HDL cholesterol and LDL cholesterol. The results for HDL cholesterol paralleled those for total plasma cholesterol: male and female monkeys fed the Western diet had higher HDL cholesterol concentrations (male, 97.6 ± 6 mg/dL; female, 79 ± 4 mg/dL) than did monkeys fed standard chow (male: 56 ± 4 mg/dL, t6 = 5.88, P < 0.01; female: 47 ± 4 mg/dL, t9 = 5.15, P < 0.001). For LDL cholesterol, only the male monkeys showed a difference (Western diet, 126 ± 4 mg/dL; standard chow, 74 ± 7 mg/dL; t6 = 6.82, P < 0.001). Lipid particle-size analyses—performed only in female monkeys at the 12-wk point—indicated no difference between those fed standard chow compared with the Western diet. Neither LDL particle size (standard chow, 11.28 ± 0.07 nm/L; Western diet, 11.33 ± 0.09 nm/L; t9 = 0.36, nonsignificant) or HDL particle size (standard chow, 6.08 ± 0.18 nm/L; Western diet, 5.74 ± 0.04 nm/L; t9 = 2.01, P = 0.08) showed a significant difference. In addition, triglyceride, insulin, and fructosamine levels did not differ by diet in either male or female monkeys. Over the 12-wk evaluation, glucose went down 21% in male monkeys maintained on standard chow and increased 7% in those switched to the Western diet; glucose did not vary in female monkeys.
Given the potential effects of age on the various analyates, Table 2 shows the correlations between age at baseline and the percentage change from baseline to 12-wk for each measurement. For female monkeys, significant correlations were identified between age and levels of LDL cholesterol (r = –0.56, P <0.05) and fructosamine (r = 0.58, P < 0.05). In light of these findings, analyses in Table 1 were repeated by using ANCOVA with age as a covariate. The only notable change in overall results was that triglycerides were significantly decreased in female monkeys switched to the Western diet (–20.5% compared with 13.8% for females maintained on chow, F1,8 = 7.24, P < 0.05). All other effects described in Table 1 were maintained.
Table 2.
Correlations between age and percentage change from baseline to 12 wk after diet change
| Male monkeys (n = 8) | Female monkeys (n = 11) | |
| Body weight | −0.57 | 0.00 |
| Waist circumference | −0.44 | 0.02 |
| Body mass index | −0.34 | −0.03 |
| Total plasma cholesterol | 0.26 | −0.47 |
| HDL cholesterol | 0.10 | −0.38 |
| LDL cholesterol | 0.27 | −0.56a |
| Triglycerides | 0.10 | −0.26 |
| Glucose | −0.23 | 0.26 |
| Insulin | 0.19 | 0.41 |
| Fructosamine | −0.32 | 0.58a |
Significant (P < 0.05) Pearson correlation (r) between age at baseline and percentage change from baseline to 12 wk after diet change.
Isoflavone concentrations were higher in male monkeys eating chow than in those eating the Western diet. At the 6-wk point, daidzein was 69.3 ± 2.7 nM in males fed chow compared with 11.1 ± 3.8 nM in those fed the Western diet (t6 = 4.90, P < 0.01). The same was true for equol (129.7 ± 5.9 nM compared with 5.5 ± 1.3 nM; t6 = 7.48, P < 0.001). For genistein levels, the difference approached significance (16.5 ± 3.4 compared with 0.3 ± 0.2; t6 = 2.32, P = 0.059).
Discussion
This study indicates that African green monkeys given a Western diet readily ate the diet and showed no remarkable changes in body weight, abdominal obesity, or fecal quality, even after 12 wk, but demonstrated an increase in plasma cholesterol levels and other adverse health effects. Plasma isoflavone concentrations were significantly higher in animals fed monkey chow, a diet high in soy, compared with those fed the more Western diet, which contained primarily animal-source protein.
In general, total plasma cholesterol and HDL cholesterol tend to covary in green monkeys.15 Therefore, the observation that both total plasma cholesterol and HDL cholesterol were higher in African green monkeys fed the Western diet is not necessarily surprising, but this characteristic should not distract from the overall result that feeding this Western diet led to an increase in plasma cholesterol concentrations.
Despite the absence of changes in overall body weight or waist circumference, changes in lean mass, fat mass, or bone mass may have occurred. Because neither dual-energy X-ray absorptiometry nor equivalent measures were performed, our study cannot address this issue. Future studies on the effect of this Western-type diet would benefit from these additional measures.
In light of the results of this study, we plan to test the long-term effects of this Western diet (LabDiet 5L3K) in a larger cohort of animals from our breeding colony of African green monkeys. Given the palatability of this diet, the lack of adverse effects, and the expected changes in plasma lipid and isoflavone concentrations, using this diet in these nonhuman primates may be appropriate for mimicking the nutritional context of humans. Although the Western-type diet we used may not be ideal for maximizing the health of captive nonhuman primates, it likely will be valuable for studying the effects of diet on a wide-range of biomedically relevant traits.
Nonhuman primates play a crucial role in the translation between basic and clinical science. Although rodents are an increasingly important tool for biomedical research, there are numerous instances in which nonhuman primates provide greater generalizability to humans. In particular, nonhuman primates have been shown to be excellent models for reproductive function, cancer, obesity, diabetes, cardiovascular disease, aging, cognition, and many other areas of interest.16
Nutritional context often plays a key role in many of these areas of inquiry. Relying on commercial diets that are high in soy isoflavones can constitute an often unrecognized experimental confound.18 For example, isoflavones bind to estrogen receptors and can have a profound effect on reproductive function. Feeding female monkeys diets rich in soy isoflavones results in plasma concentrations of genistein, daidzein, and equol that are several orders of magnitude higher than endogenous estrogen concentrations and which therefore are quite dissimilar from plasma concentrations in women.5 Feeding of soy-heavy compared with soy-free diets can alter the progression of menopause.2
In conclusion, we here demonstrated that a Western-type diet (LabDiet 5L3K) is suitable for use in African green monkeys and potentially for other Old World primate species. The use of more Western diets, in particular those devoid of soy isoflavones, will help expand the generalizability of biomedical research using these important translational models.
Acknowledgments
Support for this research was provided in part by NIH grant RR019963/OD010965 (PI: Jay R Kaplan) and funding provided by the Veterans Administration. We thank Tara Chavanne and Cameron (Kirkus) Sousa for technical effort during the study. We also thank Drs Susan Appt and Kylie Kavanagh for consultation regarding creation of the Western diet and study design and thank Carrie Schultz (LabDiet) for the formulation of the diet. We appreciate the effort of the following persons and laboratories for running assays: Dr Adrian Franke's laboratory (Research Corporation of the University of Hawaii) for isoflavone assays; Dr Lawrence Rudel's laboratory (Wake Forest) for lipid particle-size assays; and Maryanne Post (Wake Forest Primate Center) for running lipid and glycemic assays.
References
- 1.Adams MR, Golden DL, Williams JK, Franke AA, Register TC, Kaplan JR. 2005. Soy protein containing isoflavones reduces the size of atherosclerotic plaques without affecting coronary artery reactivity in adult male monkeys. J Nutr 135:2852–2856 [DOI] [PubMed] [Google Scholar]
- 2.Appt SE, Chen H, Goode AK, Hoyer PB, Clarkson TB, Adams MR, Wilson ME, Franke AA, Kaplan JR. 2010. The effect of diet and cardiovascular risk on ovarian aging in cynomolgus monkeys (Macaca fascicularis). Menopause 17:741–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi J, Li C, McDonald TJ, Comuzzie A, Mattern V, Nathanielsz PW. 2011. Emergence of insulin resistance in juvenile baboon offspring of mothers exposed to moderate maternal nutrient reduction. Am J Physiol Regul Integr Comp Physiol 301:R757–R762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarkson TB, Anthony MS, Morgan TM. 2001. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab 86:41–47 [DOI] [PubMed] [Google Scholar]
- 5.Clarkson TB, Appt SE.2004. Cardiovascular effects of dietary soy. In: Watson RR, Preedy VR. Nutrition and heart disease. Boca Raton (FL): CRC Press.
- 6.Fabbrini E, Higgins PB, Magkos F, Bastarrachea RA, Voruganti VS, Comuzzie AG, Shade RE, Gastaldelli A, Horton JD, Omodei D, Patterson BW, Klein S. 2013. Metabolic response to high-carbohydrate and low-carbohydrate meals in a nonhuman primate model. Am J Physiol Endocrinol Metab 304:E444–E451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franke AA, Custer LJ, Wilkens LR, Le Marchand LL, Nomura AM, Goodman MT, Kolonel LN. 2002. Liquid chromatographic–photodiode array mass spectrometric analysis of dietary phytoestrogens from human urine and blood. J Chromatogr B Analyt Technol Biomed Life Sci 777:45–59 [DOI] [PubMed] [Google Scholar]
- 8.Garber DW, Kulkarni KR, Anantharamaiah GM. 2000. A sensitive and convenient method for lipoprotein profile analysis of individual mouse plasma samples. J Lipid Res 41:1020–1026 [PubMed] [Google Scholar]
- 9.Higgins PB, Bastarrachea RA, Lopez-Alvarenga JC, Garcia-Forey M, Proffitt JM, Voruganti VS, Tejero ME, Mattern V, Haack K, Shade RE, Cole SA, Comuzzie AG. 2010. Eight-week exposure to a high-sugar, high-fat diet results in adiposity gain and alterations in metabolic biomarkers in baboons (Papio hamadryas sp.). Cardiovasc Diabetol 9:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press.
- 11.Jasinska AJ, Lin MK, Service S, Choi OW, DeYoung J, Grujic O, Kong SY, Jung Y, Jorgensen MJ, Fairbanks LA, Turner T, Cantor RM, Wasserscheid J, Dewar K, Warren W, Wilson RK, Weinstock G, Jentsch JD, Freimer NB. 2012. A nonhuman primate system for large-scale genetic studies of complex traits. Hum Mol Genet 21:3307–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mubiru JN, Garcia-Forey M, Higgins PB, Hemmat P, Cavazos NE, Dick EJ, Jr, Owston MA, Bauer CA, Shade RE, Comuzzie AG, Rogers J. 2011. A preliminary report on the feeding of cynomolgus monkeys (Macaca fascicularis) with a high-sugar, high-fat diet for 33 weeks. J Med Primatol 40:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nechuta SJ, Caan BJ, Chen WY, Lu W, Chen Z, Kwan ML, Flatt SW, Zheng Y, Zheng W, Pierce JP, Shu XO. 2012. Soy food intake after diagnosis of breast cancer and survival: an in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am J Clin Nutr 96:123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Register TC, Cann JA, Kaplan JR, Williams JK, Adams MR, Morgan TM, Anthony MS, Blair RM, Wagner JD, Clarkson TB. 2005. Effects of soy isoflavones and conjugated equine estrogens on inflammatory markers in atherosclerotic, ovariectomized monkeys. J Clin Endocrinol Metab 90:1734–1740 [DOI] [PubMed] [Google Scholar]
- 15.Rudel LL, Reynolds JA, Bullock BC. 1981. Nutritional effects on blood lipid and HDL-cholesterol concentrations in 2 subspecies of African green monkeys (Cercopithecus aethiops). J Lipid Res 22:278–286 [PubMed] [Google Scholar]
- 16.Shively CA, Clarkson TB. 2009. The unique value of primate models in translational research. Nonhuman primate models of women's health: introduction and overview. Am J Primatol 71:715–721 [DOI] [PubMed] [Google Scholar]
- 17.Simon NG, Kaplan JR, Hu S, Register TC, Adams MR. 2004. Increased aggressive behavior and decreased affiliative behavior in adult male monkeys after long-term consumption of diets rich in soy protein and isoflavones. Horm Behav 45:278–284 [DOI] [PubMed] [Google Scholar]
- 18.Stroud FC, Appt SE, Wilson ME, Franke AA, Adams MR, Kaplan JR. 2006. Concentrations of isoflavones in macaques consuming standard laboratory monkey diet. J Am Assoc Lab Anim Sci 45:20–23 [PubMed] [Google Scholar]
- 19.Wood CE, Register TC, Anthony MS, Kock ND, Cline JM. 2004. Breast and uterine effects of soy isoflavones and conjugated equine estrogens in postmenopausal female monkeys. J Clin Endocrinol Metab 89:3462–3468 [DOI] [PubMed] [Google Scholar]