Abstract
The electrocardiogram of nonhuman primates is similar to that of humans because of similar intrathoracic heart position and structure. Despite the frequent use of nonhuman primates in biologic studies, few electrocardiographic studies of Japanese monkeys (Macaca fusucata) have been reported, and no reference data are available for this species. We obtained limb-lead electrocardiograms from indoor-bred and housed ketamine-sedated Japanese macaques (48 male; 56 female; mean age, 44.3 mo; mean body weight, 4.84 kg) in the dorsal recumbency. The following quantitative data was obtained: heart rate, P wave amplitude and width, R wave amplitude, QRS duration, PR interval, QT interval, T wave height, and mean electrical axis. Corrected QT intervals were calculated by using the Bazett and Fridericia formulae. Measurements were evaluated according to sex and age. The duration of the QRS complex showed moderate correlation with age in male monkeys. All parameters, except heart rate, were similar to previous reports from Japanese, cynomolgus, and other macaques. P waves, R waves and mean electrical axis did not differ significantly between humans and Japanese macaques, but the wave amplitude in macaques was half that in humans. Our electrocardiographic measurements can serve as normal reference data for sedated, young Japanese monkeys.
Abbreviations: QTcb, QT interval corrected according to the Bazett formula; QTcf, QT interval corrected according to the Fridericia formula
Because the sequence of cardiac repolarization is similar between humans and Japanese macaques,9 this species is perhaps the most suitable animal model for studying the electrocardiogram, particularly the process of repolarization.9 Although several reports address the electrocardiogram of sedated monkeys, the number of animals evaluated in these previous studies is insufficient to establish reference values for electrocardiographic parameters.1,7,9,15-17,21 In comparison, several studies have used telemetry and Holter monitoring in conscious monkeys, thus eliminating the influence of drugs on the electrocardiogram.3,6,8
Electrocardiographic analysis of nonhuman primates is important during the evaluation of drug safety,21 because drug-associated prolongation of the QT interval is of particular clinical significance.2,3,5,8,21 QT interval prolongation can result from effects on various cardiac ion channels, including inhibition of the rapid-activating delayed rectifier K+ current or the slow-activating delayed rectifier K+ current and decreased inactivation or increased activation of Na+ or Ca2+ currents.3 In addition, the relationship between prolongation of the QT interval and torsades de pointes, a fatal arrhythmia, is important to clarify.20,21 Therefore, the International Conference on Harmonization S7B has recommended the development of animal models for nonclinical assessment of the potential of test substances to delay ventricular repolarization.5 The International Conference on Harmonization also supports the use of sedation for animal studies. The purpose of the current study was to establish reference electrocardiographic values for healthy, growing Japanese macaques under ketamine sedation to support their use as animal models for drug safety testing and other applications.
Materials and Methods
This project was approved by the Ethics Review Board of the Nihon University School of Medicine (no. 070060).
Animals.
The study population comprised 104 healthy Japanese macaques (Macaca fusucata; 48 male [weight, 4.84 ± 1.54 kg; age, 42.1 ± 10.1 mo]; 56 female [weight, 4.84 ± 1.31 kg; age, 46.2 ± 11.6 mo]). The monkeys were individually housed in indoor cages (85 × 85 × 80 cm) in a climate-controlled facility with an ambient temperature of 22 to 26 °C and managed by the Third Animal Center of Nihon University. Humidity was not controlled, but the air in each room was changed 9 times hourly by using an air conditioning and filter system. Macaques had ad libitum access to water and were supplied with commercial food (Certified Primate Diet 5048, PMI Feeds, Richmond, IN) 3 times each day. All animals at the center are allowed 90 min once a week in a recreational area. In addition, macaques underwent evaluation of their physical condition, CBC, serum chemistry panel, parasite status (for amebic dysentery and Scolecida eggs), and viral antibody status (B virus, SIV, filovirus, and simian T lymphotropic virus) are performed twice each year.
Electrocardiography.
Monkeys were sedated in their cages by injection of ketamine hydrochloride (10 mg/kg IM; Ketalar, Sankyo, Tokyo, Japan), and evaluations were performed after adequate sedation was achieved. We used a diagnostic electrocardiograph (Cardisuny D300, Fukuda M-E, Tokyo, Japan) to record 6-lead electrocardiograms (50 mm/s, 1 mV = 10 mm). Macaques were restrained in dorsal recumbency on the examination table. Limbs electrodes were attached to the caudal surface of the elbows and over the stifles by using alligator clips. Filters were used to eliminate electrical interference, tremor artifact, and so forth. Lead II electrocardiogram parameters were evaluated in terms of sex, age, and weight of the macaques. The following parameters were measured automatically by the recording device and were analyzed: heart rate, P wave (amplitude, time), R wave (amplitude), QRS duration, mean electrical axis, PR interval, QT interval, corrected QT interval (according to the Bazett formula12 [QTcb] and the Fridericia formula12 [QTcf]), and T wave (amplitude).
Statistical analysis.
The Spearman rank correlation coefficient was used to assess dependence. The presence of significant differences was assessed by using the Mann–Whitney U test. Differences were considered statistically significant at a P value of less than 0.05.
Results
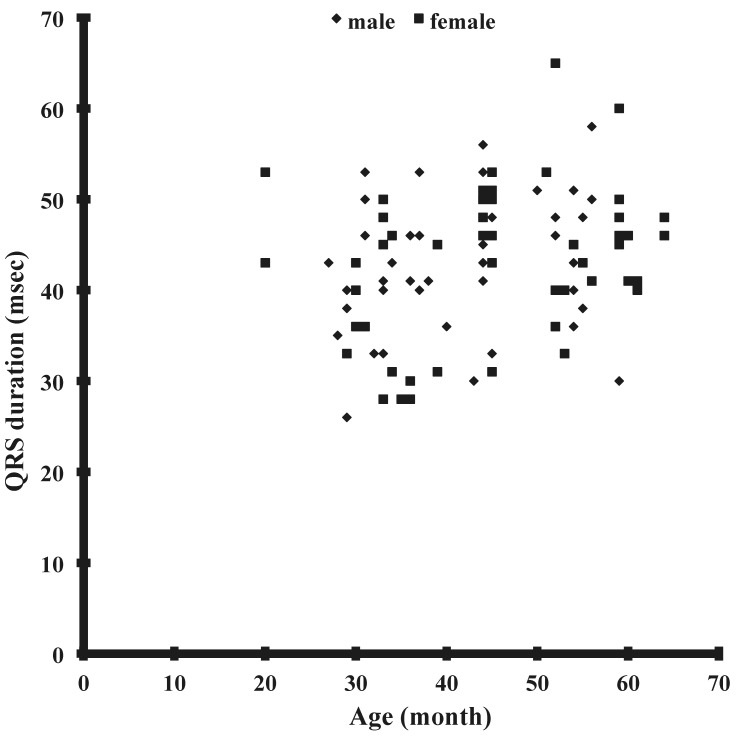
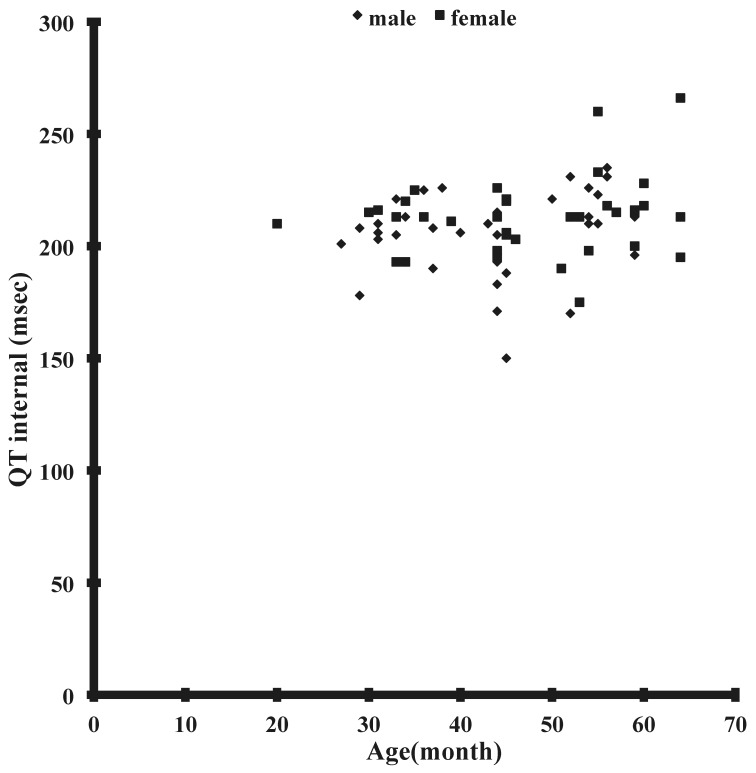
The results obtained from lead II of the electrocardiogram (Figure 1) are summarized in Table 1. The mean values of all parameters did not differ between male and female Japanese macaques. The QRS duration of Japanese macaques showed modest age-associated prolongation in male monkeys, but no additional correlations were seen between any other parameters and age or weight (Figure 2). In particular, no correlation was noted between age and QT interval (Figure 3), QTcb, or QTcf. None of the monkeys had any arrhythmia.
Figure 1.
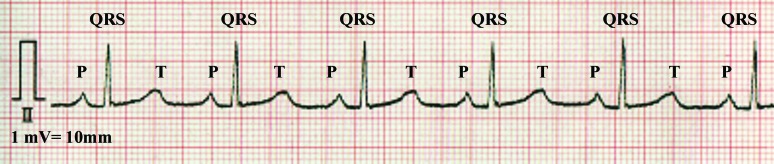
An electrocardiogram obtained from a sedated Japanese macaque. Lead II was recorded at 50 mm/s and 1 mV = 10 mm.
Table 1.
Lead II ECG data (mean ± 1 SD) and range for intervals, wave amplitude, and heart rate from 104 clinically normal Japanese macaques
|
Macaca fusucata |
|||
| male | female | Macaca fascicularis | |
| Heart rate (bpm) | 157 ± 19 | 159 ± 21 | 168 ± 22 |
| QRS axis (°) | 66 ± 22 | 75 ± 18 | 55 ± 41 |
| QRS width (msec) | 44 ± 7 | 43 ± 8 | 36 ± 6 |
| P width (msec) | 37 ± 18 | 41 ± 18 | 50 ± 7 |
| PR interval (msec) | 84 ± 13 | 80 ± 11 | 89 ± 11 |
| QT interval (msec) | 205 ± 18 | 213 ± 17 | 202 ± 30 |
| QTcb interval (msec) | 329 ± 20 | 339 ± 19 | 209 ± 30 |
| QTcf interval (msec) | 281 ± 19 | 290 ± 19 | 282 ± 32 |
| P wave amplitude (mV) | 0.11 ± 0.88 | 0.13 ± 0.07 | 0.11 ± 0.02 |
| R wave amplitude (mV) | 1.12 ± 0.42 | 1.25 ± 0.38 | 0.69 ± 0.23 |
| T wave amplitude (mV) | 0.14 ± 0.11 | 0.12 ± 0.33 | 0.19 ± 0.10 |
QTcb, QT interval as corrected by using the formula of Bazett; QTcf, QT interval corrected by using the formula of Fridricia.
Data for reference range for M. fascicularis were obtained from reference 21, except for T wave amplitude (reference 1).
Figure 2.
Correlation between age and QRS duration in male (r = 0.25, n = 47) and female (r = 0.16, n = 56) Japanese macaques.
Figure 3.
Correlation between age (in months) and QT interval.
Discussion
Although the electrocardiogram of Japanese macaques has been evaluated previously, the studies involved fewer subjects than we used in the current study, and experimental methods and breeding conditions differ among the various studies; these previous efforts thus yielded insufficient data to derive reference electrocardiographic values for this species.11,17 Our current study is the first large-scale electrocardiographic evaluation of indoor-bred, young Japanese macaques. We accordingly have determined reference electrocardiographic values for sedated Japanese macaque for future use in pharmacologic and other studies.
The use of ketamine sedation in nonhuman primates is safe and produces little effect on the electrocardiogram or heart rate. The electrocardiogram of ketamine-sedated nonhuman primates correlates closely with that of nonsedated animals.1,21
We evaluated the correlation between various electrocardiographic parameters and age or weight. Our results demonstrated a weak positive correlation between age and QRS duration in male macaques (Figure 2). This prolongation of the QRS complex probably is due to thickening of the myocardium with age and growth, thus increasing the conduction time.
The heart rates we noted (males, 157 ± 19 bpm; females, 159 ± 21 bpm) were similar to those of Japanese macaques sedated with secobarbital.9 The heart rates in our study were, however, higher than those of unanesthetized Japanese monkeys.16 Furthermore, the heart rate of Japanese monkeys in our study was lower than the heart rate of Macaca fascicularis, Saimiri sciureus, Macaca arctoides, Macaca mulatta, Macaca radiata, Macaca nemestrina, Cercopithecus aethiops, Erythrocebus patas, and Cebus apella that had been sedated with ketamine.7,14,21 Excitement of animals due to handling by the researcher might cause the differences between our results and previous findings.
The mean electrical axis of the electrocardiogram in humans demonstrates right-axis deviation soon after birth and then tends toward the leftward deviation that is first observed at 1 to 3 mo of age.4 In comparison, 12% of our monkeys showed right-axis deviation, and the mean electrical axis did not correlate with age. However, most previous records of electrocardiograms from nonhuman primates were obtained as animals were sitting in a restraint chair. Electrocardiograms obtained from subjects whose body positions during examination differed should not be compared, and position-specific reference ranges are required.21 In our present study, we recorded electrocardiograms from macaques in dorsal recumbency, similar to that during electrocardiography in humans.4,18
Although the QRS duration of our Japanese macaques did not differ from those of other monkey species,1,7,9,17 the interval was half the reference value for QRS duration in humans.18 We suspect that the myocardium is thinner in macaques than humans, accounting for the observed difference.
The amplitude of the P wave of Japanese monkeys in our study was lower than that of other species.1,7 Although Japanese macaques have been known to show a prominent P wave, similar to P pulmonale, we did not note this characteristic in our current study.8,16 The duration of the P wave in our study was shorter than that previously reported for Japanese macaques,9 perhaps reflecting the small size and young age of our monkeys.
Despite the difference in body positions of the Japanese macaques evaluated in the current and previous reports, R wave did not differ between the studies.9 In nonhuman primates, the PR interval varies mainly in relation to heart rate. We did not note prolongation of the PR interval in our study. In addition, our results were similar to those previously reported for Japanese monkeys.9,17
The QT interval in our current study was consistent regardless of the age or sex of the macaque evaluated. Although the QT interval in our monkeys was similar to those in other monkey species, it was longer than that reported in a previous study of unanesthetized Japanese monkeys16 and shorter than that reported for anesthetized monkeys.9 These difference could be due to differences associated with anesthesia, sedation, physical restraint and excitement, all of which can affect heart rate and consequently QT interval. A previous study in female cynomolgus monkeys reported a significant age-related difference in QT interval.11 Another study postulated that fluctuations in sympathetic nervous activity are responsible for the shortened QT interval in cynomolgus monkeys.19
We used the Bazett and Fridericia correction formulae to calculate the corrected QT intervals in the present study. However, QTcb in our study was shorter than that previously reported for Japanese monkeys9 and longer than that in cynomolgus macaques, with QTcf showing almost the same tendency.5,19 There are several formulae for correction of the QT interval, with the choice of formula depending on the species and age of the subjects.2,12,19 For example, the Hodge formula, which does not correlate with heart rate, is considered the most useful formula for adult humans,13 whereas the Fridericia and Framingham formula is reportedly most useful in the evaluation of long QT syndrome in children.2 The Bazett formula is considered useful in the assessment of rhesus monkeys and dogs.8,12
No differences in T wave amplitude were noted between the Japanese monkeys in our current study and previous reports of this and other species.1,7,9,17 One previous report mentioned that, regardless of species of nonhuman primate, the T wave can be negative in a few animals;17 approximately 6% of our monkeys had a negative T wave in lead II. In a previous report, a negative T wave was present in approximately 10% of lead II, III, and aVF electrocardiograms.16 Although a negative T wave usually indicates abnormal repolarization, it has been reported in rhesus monkeys after anesthetic administration.16 In some humans, the T wave becomes negative with fear and anxiety.22 Negative T waves in monkeys have also been known to return to normal several months later.18
The form of the electrocardiogram of our monkeys was similar to that of dogs, although the R wave potential was lower. Conversely, the R wave of Japanese monkeys was higher than that in previous reports on cynomolgus monkeys, whereas the PR interval of our monkeys was very similar to that of cynomolgus monkeys (Table 1).
In the current study, we obtained reference values for electrocardiography in Japanese macaques. There was also no significant difference between humans and macaques in terms of the amplitudes of the P and R waves. The PR, QRS, and QT intervals and the P wave amplitude in our macaques were about half those in humans; these differences likely reflect differences in body size.9,18
Because they are less aggressive and therefore easier to handle, Japanese macaques often are considered to be a better choice than are cynomolgus monkeys.10 However, cynomolgus macaques currently are the most commonly used nonhuman primate for electrocardiographic studies. Given the electrocardiographic similarity between Japanese macaques, cynomolgus monkeys, and humans, we find that Japanese macaques have the potential to serve as nonhuman primate models in future studies.
Acknowledgments
We thank Shunsaku Iwaki and Satoko Ogiwara for help and encouragement. This work was partially supported by a Nihon University Individual Research Grant to H Koie (2006).
References
- 1.Atkins CE, Dickie BC. 1986. Electrocardiogram of the clinically normal ketamine-sedated Macaca fascicularis. Am J Vet Res 47:455–457 [PubMed] [Google Scholar]
- 2.Benatar A, Decraene T. 2001. Comparison of formulae for heart rate correction of QT interval in exercise ECGs from healthy children. Heart 86:199–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaves AA, Keller WJ, Sullivan SO, Fitzgerald LE, McPherson HE, Goykhman D, Ward PD, Hoe CM, Mixon L, Briscoe RJ. 2006. Cardiovascular monkey telemetry: sensitivity to detect QT interval prolongation. J Pharmacol Toxicol Methods 54:150–158 [DOI] [PubMed] [Google Scholar]
- 4.Dickinson DF. 2005. The normal ECG in childhood and adolescence. Heart 91:1626–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food and Drug Administration, HHS 2005. International Conference on Harmonization; guidance on S7B nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals; availability. Notice. Fed Regist 70:61133–61134. [PubMed] [Google Scholar]
- 6.Gauvin DV, Tilley LP, Smith FW, Jr, Baird TJ. 2006. Electrocardiogram, hemodynamics, and core body temperatures of the normal freely moving cynomolgus monkey by remote radiotelemetry. J Pharmacol Toxicol Methods 53:140–151 [DOI] [PubMed] [Google Scholar]
- 7.Gonder JC, Gard EA, Lott NE. 1980. Electrocardiograms of 9 species of nonhuman primates sedated with ketamine. Am J Vet Res 41:972–975 [PubMed] [Google Scholar]
- 8.Hassimoto M, Harada T, Kaga N, Murano H, Obata M. 2002. Accurate evaluation of QT interval in conscious rhesus monkeys (Macaca mulatta) by use of Holter ECG. J Electrocardiol 35:333–342 [DOI] [PubMed] [Google Scholar]
- 9.Imanishi S, Arita M, Aomine M, Kiyosue T. 1983. Electrocardiogram and His bundle electrogram of Japanese monkeys (Macaca fuscata). Jikken Dobutsu 32:167–173 [DOI] [PubMed] [Google Scholar]
- 10.Isa T, Yamane I, Hamai M, Inagaki H. 2009. Japanese macaques as laboratory animals. Exp Anim 58:451–457 [DOI] [PubMed] [Google Scholar]
- 11.Ishizaka T, Yoshimatu Y, Ozawa M, Kimotsuki T, Takasaki W, Manabe S, Yasuda M. 2009. Age-related differences of QT interval and autonomic nervous system activity in female cynomolgus monkeys. J Pharmacol Toxicol Methods 60:288–295 [DOI] [PubMed] [Google Scholar]
- 12.Koyama H, Yoshii H, Yabu H, Kumada H, Fukuda K, Mitani S, Rousselot JF, Hirose H, Uchino T. 2004. Evaluation of QT interval prolongation in dogs with heart failure. J Vet Med Sci 66:1107–1111 [DOI] [PubMed] [Google Scholar]
- 13.Luo S, Michler K, Johnston P, Macfarlane PW. 2004. A comparison of commonly used QT correction formulae: the effect of heart rate on the QTc of normal ECGs. J Electrocardiol 37 Suppl:81–90 [DOI] [PubMed] [Google Scholar]
- 14.Malhotra V, Pick R, Pick A, Glick G. 1975. Electrocardiographic studies in stumptail macaque (Macaca arctoides). J Electrocardiol 8:247–251 [DOI] [PubMed] [Google Scholar]
- 15.Malinow MR. 1966. An electrocardiographic study of Macaca mulatta. Folia Primatol (Basel) 4:51–65 [DOI] [PubMed] [Google Scholar]
- 16.Malinow MR, DeLannoy CW. 1967. The electrocardiogram of Macaca fuscata. Folia Primatol (Basel) 7:284–291 [DOI] [PubMed] [Google Scholar]
- 17.Malinow MR, Hackel DB. 1966. Abnormal ventricular repolarization in Macaca mulatta. Am Heart J 71:140. [DOI] [PubMed] [Google Scholar]
- 18.Mason JW, Ramseth DJ, Chanter DO, Moon TE, Goodman DB, Mendzelevski B. 2007. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol 40: 228–234 [DOI] [PubMed] [Google Scholar]
- 19.Soloviev MV, Hamlin RL, Barrett RM, Chengelis CP, Schaefer GJ. 2006. Different species require different correction factors for the QT interval. Cardiovasc Toxicol 6:145–157 [DOI] [PubMed] [Google Scholar]
- 20.Sugiyama A. 2008. Sensitive and reliable proarrhythmia in vivo animal models for predicting drug-induced torsades de pointes in patients with remodeled hearts. Br J Phrmacol 154:1528–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor K, Gleason C. 2010. Effect of body position on limb-lead electrocardiographic findings in sedated cynomolgus macaques (Macaca fascicularis). J Am Assoc Lab Anim Sci 49:352–356 [PMC free article] [PubMed] [Google Scholar]
- 22.Wilde H. 1958. Functional electrocardiographic abnormalities. N Engl J Med 258:735–738 [DOI] [PubMed] [Google Scholar]