Abstract
Recent studies have suggested that epigenetic modulation with chromatin-modifying agents can induce stemness and dedifferentiation and increase developmental plasticity. For instance, valproic acid (VPA), a histone deacetylase (HDAC) inhibitor, has been shown to promote self-renewal/expansion of hematopoietic stem cells and facilitate the generation of induced pluripotent stem cells (iPSCs). Previously, we observed that downregulation of embryonic renal stem/progenitor genes in the adult kidney was associated, at least in part, with epigenetic silencing. Therefore, we hypothesized that VPA may alter the expression of these genes and reprogram mature human adult kidney epithelial cells (hKEpCs) to a stem/progenitor-like state.
Here, using quantitative RT-PCR and flow cytometry [fluorescence-activated cell sorting (FACS)] analysis, we show in VPA-treated primary cultures of human adult and fetal kidney significant reinduction of the renal stem/progenitor markers SIX2, OSR1, SALL1, NCAM, and PSA-NCAM. Robust SIX2 mRNA re-expression was confirmed at the protein level by western blot and was associated with epigenetic changes of the histones at multiple sites of the SIX2 promoter leading to gene activation, significantly increased acetylation of histones H4, and methylation of lysine 4 on H3. Furthermore, we could demonstrate synergistic effects of VPA and Wnt antagonists on SIX2 and also OSR1 reinduction. Nevertheless, VPA resulted in upregulation of E-CADHERIN and reduction in VIMENTIN, preventing the skewing of hKEpCs towards a more replicative mesenchymal state required for clonogenic expansion and acquisition of stem cell characters, altogether inducing cell senescence at early passages. These results demonstrating that chromatin-modifying agents prevent dedifferentiation of hKEpCs have important clinical implications as they may limit ex-vivo self-renewal/expansion and possibly the in vivo renal regenerative capacity initiated by dedifferentiation.
Introduction
Complicated developmental processes such as nephrogenesis require a series of precise and coordinated changes in cellular identity to ensure nephron formation. Epigenetic mechanisms help coordinate changes in gene expression that accompany the transition from embryonic stem cells to terminally differentiated kidney cells. Hence, the molecular process that governs nephrogenesis in fetal life involves the interplay between lineage-specific transcription factors and a series of epigenetic modifications (including DNA methylation and histone tail modifications, such as acetylation/methylation) (Harari-Steinberg et al., 2011; Pleniceanu et al., 2010). Specifically, lineage-specific renal genes or renal progenitor genes (SIX2, OSR1, SALL1, WT1, and PAX2) specify early mesoderm to become specialized kidney and nephron epithelia through a process of mesenchymal-to-epithelial transition (MET). Of all gene markers, the expression of the transcription factor SIX2 is required for maintenance of the renal stem/progenitor cell population during development (Kobayashi et al., 2008). In addition, OSR1 expression demarcates a multipotent population of intermediate mesoderm that gives rise to kidney (Mugford et al., 2008). Interestingly, during ischemia–reperfusion renal injury and consequent regenerative response, there seems to be re-expression of renal developmental genes and pathways (Abbate et al., 1999; Dekel et al., 2003; Dekel et al., 2006b), although to a limited extent (Hopkins et al., 2009). It has been suggested that lack of robust SIX2 reactivation precludes a complete regenerative response (Hopkins et al., 2009).
Recent studies have indicated that epigenetic marks reversed by chromatin-modifying compounds such as the histone deacetylase (HDAC) inhibitors, valproic acid (VPA), and trichostatin A (TSA) may effect cell plasticity and identity (Bartels et al., 2010; Karantzali et al., 2008). Accordingly, epigenetic modulation has been suggested to have an impact on the developmental plasticity of several adult neural cell types (e.g., adult neurospheres and oligodendrocyte precursor cells) via activation of stemness-related lineage markers (Ruau et al., 2008; Zini et al., 2012). In addition, VPA has been shown to stimulate the self-renewal and expansion of normal hematopoietic stem cells (Debeb et al., 2012). Moreover, HDAC inhibitors, in particular VPA, enable efficient reprogramming of adult somatic cells into pluripotent stem cells (Huangfu et al., 2008). In cancer cells, the clonogenic capacity of CD34 acute myelogenous leukemia progenitor cells was improved and dedifferentiation of human breast tumor cells was stimulated with VPA treatment (Debeb et al., 2012). In zebrafish kidney, organogenesis inhibition of HDAC was shown to expand the renal progenitor cell population (de Groh et al., 2010).
These studies and our observation that embryonic renal progenitor gene downregulation in the adult human kidney was associated, at least in part, with epigenetic silencing (Metsuyanim et al., 2008), led us to hypothesize that epigenetic modulation of adult human kidney epithelial cells (hKEpC) with VPA or TSA/5-aza-2′-deoxycytidine (5-AzaC) treatment alter the expression of these genes to enhance stemness and induce dedifferentiation skewing cells toward a stem/progenitor cell-like phenotype.
Using primary hKEpC derived from surgical nephrectomies, we found that treatment with HDAC inhibitors re-expressed SIX22 and OSR1 but abrogated stemness and clonogenic capacity/expansion of hKEpC, most likely by prevention of epithelial-mesenchymal transition (EMT) and dedifferentiation. On the contrary, they may promote epithelial differentiation. These results may impact renal regenerative therapies using adult cells to generate and expand stem/progenitor cells for therapeutic applications and those aimed to induce regeneration by in vivo administration of small molecules since the renal regenerative response is initiated by dedifferentiation of surviving cells to assume stem cell character and re-dif to healthy epithelia timing of small-molecule therapeutic application is likely to be crucial.
Materials and Methods
Tissue samples
Human tissues samples were collected according to the Helsinki requirements. Human fetal kidneys were collected from elective abortions at fetal gestational ages that ranged from 15 to 19 weeks at Asaf Horofeh Medical Center. Normal human adult kidneys samples were retrieved from borders of renal cell carcinoma (RCC) tumors from partial nephrectomy patients, from both Sheba Medical Center and Wolfson hospital.
Establishment of primary cultures from human kidney tissues
Collected tissues were minced in Hanks' balanced salt solution (HBSS), soaked in Iscove's modifed Dulbecco medium (IMDM; Invitrogen) supplemented with 0.1% collagenase II (Invitrogen). The digested tissue was then gradually forced through 100-μm, 70-μm, and 50-μm cell strainers to achieve a single-cell suspension and cultured in growth medium supplemented with 10% fetal bovine serum (FBS), 1% L-glutamine, 1% penicillin-streptomycin, and the following growth factors: 50 ng/mL of basic fibroblast growth factor (bFGF), 50 ng/mL of epidermal growth factor (EGF), and 5 ng/mL of stem cell factor (SCF) (R&D Systems).
Cell treatment
Cells were treated for 24 h with growth medium supplemented with 1, 2, or 4 mM VPA (Sigma) or with H2O for the control sample. Otherwise, cells were treated for 24 h with growth medium supplemented with the combination of 75 μM TSA (Sigma) and 250 μM 5-AzaC (Sigma) or with 100% ethanol and acetic acid (acetic acid:H2O 1:1) for the control sample. In some experiments, we used Wnt pathway inhibitors in conjunction with VPA as follows: Cells were treated for 72 h with growth medium supplemented with 3 μg/mL Dickkopf-related protein 1 (DKK1; R&D Systems) or with 7 μg/mL Secreted frizzled-related protein 1 (sFRP1; R&D Systems). At 24 h before harvesting, 4 mM VPA was added to the cell culture.
Flow cytometry
Cells were detached from cultures plated with nonenzymatic cell dissociation solution (Sigma-Aldrich). Cells (1×105 in each reaction) were suspended in 50 μL of FACS buffer, 0.5% bovine serum albumin (BSA), and 0.02% sodium azide in phosphate-buffered saline (PBS; Sigma-Aldrich and Invitrogen, respectively)] and blocked with FcR Blocking Reagent (MiltenyiBiotec) and human serum (1:1) for 15 min. Cells were then incubated for 45 min with the following primary antibodies: NCAM1-PE (eBioscience); CD133-APC, PSA-NCAM-PE (MiltenyiBiotec); or a matching isotype control. Cell labeling was detected using FACSCalibur (BD Biosciences). Flow cytometry results were analyzed using FlowJo analysis software. Viable cells were gated by both their forward scatter (FSC)/side scatter (SSC) profile and 7-amino actinomycin D (7AAD) (eBioscience) exclusion.
Quantitative reverse transcription-PCR
Total RNA from kidney tissue cultured cells was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's protocols. cDNA was synthesized using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, CA) on total RNA. Quantitative RT-PCR was performed using an ABI7900HT sequence detection system (Perkin-Elmer/Applied Biosystems) in the presence of SYBR Green (SYBR Green PCR kit, Qiagen). Sequences of the specific primers used for PCR are: SIX2, 5′-CCAAGGAA AGGGAGAACAACG-3′ and 5′-GCTGGATGATGAGTGGT CTGG-3′; OSR1, 5′-TGT ATG GTT TCA GCG CGT TG - 3′ and 5′-GGG TTG AAT GAC ATG AGG GAA-3′; WT1 5′-GCTGTCCCACTTACAGATGCA-3′ and 5′-TCAAAGCG CCAGCTGGAGTTT-3′; PAX2 5′-CCCAGCGTCTCTTCCA TCA-3′ and 5′-GGCGTTGGGTGGAAAGG-3′; SALL1, 5′-CA ATCTTAAGGTACACATGGGCAC-3′ and 5′-TGCCTCCTA GAAATGTCATGGG-3′; E-CADHERIN, 5′-AGTGCCAACT GGACCATTCA-3′ and 5′-TCTTTGACCACCGCTCTCCT -3′; VIMENTIN, 5′-ACA CCC TGC AAT CTT TCA GAC A-′ and 5′-GAT TCC ACT TTG CGT TCA AGG T -3′; OCT4, 5′-GAG AAC CGA GTG AGA GGC AAC C-3′ and 5′-CAT AGT CGC TGC TTG ATC GCT TG -3′; NANOG, 5′-AATACCTCAGCC TCCAGCAGATG -3′ and 5′-TGCGTCACACCATTGCTAT TC TTC-3′; KLF4, 5′-ACCAGGCACTACCGTAAACACA-3′ and 5′-GGTCCGACCTGGAAAATGCT-3′. Each analysis reaction was performed in duplicates or triplicates. GAPDH was used as an endogenous control throughout all experimental analyses. Analysis was performed using the −ΔΔCt method, which determines fold changes in gene expression relative to a comparator sample.
Chromatin immunoprecipitation assay
A total of 10×106 hKEpC were grown. Untreated cells (control) or cells treated with VPA (4 mM) were cross-linked with 1% formaldehyde for 10 min at room temperature in culture medium. The cells were washed and harvested in cold PBS containing protease inhibitors (cOmplete Mini, Roche Applied Science) and Pepstatin (Sigma). The cells were then washed with Buffer B [20 mM HEPES (pH 7.6), 0.25% Triton-X, 10 mM EDTA, 0.5 mM EGTA] and Buffer C [50 mM HEPES (pH 7.6), 150 mM NaCl, 1 mM EDTA, 0.5 mM EGTA], resuspended in 300 μL of sodium dodecyl sulfate (SDS) lysis buffer [1% SDS, 10 mM EDTA, and 50 mM Tris–HCl (pH 8.1)], and incubated on ice for 10 min. Lysates were sonicated with 8×10-sec bursts, and debris was removed by centrifugation for 10 min at 1000×g, at 40°C.
Supernatants were diluted 10-fold in chromatin immunoprecipitation (ChIP) dilution buffer [0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl (pH 8.1), and 167 mM NaCl] and precleared by incubating with 40 μL of protein A+salmon sperm beads (Upstate Biotechnology) for 30 min at 40°C. Beads were pelleted for 1 min at 1000×g at 40°C. A total of 10 μL of the supernatant was saved as input, and the rest was divided into equal aliquots and incubated by rocking with either control antibody [immunoglobulin G (IgG)] or with specific antibodies, including Trimethyl-Histone 3K27 antibody (Upstate Biotechnology), Trimethyl-Histone 3K4 antibody (Upstate or abcam), or anti-acetylated histone H4 (Upstate Biotechnology) overnight at 40°C. A total of 60 μL of protein A+salmon sperm beads was added, and the samples were rocked for 2 h at 40°C. The complexes on the beads were washed for 5 min at 40°C with the following buffers: low salt [0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris–HCl (pH 8.1) and 150 mM NaCl], high salt [0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris–HCl (pH 8.1), 500 mM NaCl], LiCl wash [0.25 M LiCl, 1% Nonidet P-40, 1% deoxycholate, 1 mM EDTA and 10 mM Tris–HCl (pH 8.1)], and twice with TE buffer [10 mM Tris–HCl and 1 mM EDTA (pH 8.0)].
Immune complexes were eluted twice with 200 μL of elution buffer [1% SDS and 0.1 M NaHCO3) for 15 min at room temperature. The samples were de-crosslinked by adding 16 μL of 5 M NaCl, 8 μL of 0.5 M EDTA (pH 6.5), and 16 μL of 1 M Tris–HCl (pH 8.1) and incubated overnight at 65°C. Proteinase K (10 mg/mL) and the samples were incubated for additional 2 h at 55°C.
Immunoprecipitated DNA was recovered by phenol/chloroform extraction and ethanol precipitation and was analyzed by quantitative RT-PCR. Primer sets of SIX and GAPDH promoters were as follows: SIX2 CpG1157, 5′-AAG CAAAAAACAGGACCCCC-3′ and 5′-AACGGAGGCAAG ATTCCCA-3′; SIX2 CpG −955, 5′-GAAGCCCCACCACCGT CCTAGA-3′ and 5′-ACTTAACCCCACGGGTCCCACA-3′; SIX2 CpG −731, 5′-GAAGTCGATTCTCCGGCGT-3′ and 5′-CCCACCCCCATCCTAGAA AC-3′; SIX2 CpG −541, 5′-GGGTAGAATGTGCCCGGTGAACAGA-3′ and 5′-AGGGAAGGCGGAGACCGTTTAAGG-3′; SIX2 CpG −412, 5′-GCTGCCCAAACTTTCTTCCCCTG-3′ and 5′-CGAAAAG GGGTGGATCGAGGTG-3′; SIX2 CpG −92, 5′-CGAGGCT CGGGTTACCAGT-3′ and 5′-CCCTGATTGGTCCGGTTA TCT-3′.
Methylation depletion immunoprecipitation (MeDIP)
Sonicated genomic DNA (10 μg, 100–400 bp in length) was denatured, incubated overnight at 4°C with 10 μg/μL of anti-methyl cytosine antibody (Diagenode, Belgium), and subsequently with 60 μL of Protein A fast flow beads (Upstate Biotechnologies) for 2 h at 4°C. The beads were washed and incubated with digestion buffer [50 mM Tris-HCl (pH 8.0), 10 mM EDTA, 0.5% SDS] and proteinase K for 3 h, and the DNA was extracted by phenol-chloroform and ethanol precipitation. The samples were tested by quantitative RT-PCR with the primers indicated above and D4Z4 for positive control: 5′-TCGCTCTGGTCTTCTACGTGG-3′ and 5′-AGTC TTGAGTGTGCCAGGCC-3′. The sonicated DNA served as input.
Western blot
hKEpC, human fetal kidney cells (hFKC) (treated and untreated) and melanoma cells were harvested with trypsin/EDTA. Cell extracts were prepared with lysis buffer [50 mM Tris-HCl, 150 mM NaCl, 0.1 mM EDTA, 0.5 mM dithiothreitol (DTT), 1.5 mM MgCl, 0.5% Triton-X] and their concentrations were detected by BCA Protein Assay kit (Thermo Scientific). A 100-μg amount of total proteins was heated at 95°C for 5 min with Laemmli sample buffer (Bio-Rad) and then were loaded on a SDS 10% polyacrylamide gel. After electrophoresis, proteins were transferred to nitrocellulose membrane that was blocked with 5% nonfat dry milk overnight at 4°C. The membrane was reacted with the selected antibodies—SIX2 (Affinity Bioreagents), WT1 (Santa Cruz), and goat anti-rabbit IgG (Jackson). The membrane was reacted with ECL Substrate (Thermo Scientific) and was exposed to medical X-ray film (Fuji).
Immunofluorescence staining
hKEpC and hFKC (treated and untreated, a human epithelial cell line and fibroblast cell line) were fixed with acetone at −20°C for 7 min and washed with PBS. Cells were blocked with 7% human serum in PBS for 15 min followed by incubation with β-catenin antibodies (Chemicon International) for 60 min. Cells were washed and then incubated with Cy-3-conjugated anti-mouse IgG secondary antibody (1:200) (Jackson) for 30 min. For nuclear staining, cells were washed with PBS containing Hoechst (Dako). The presence of β-catenin was examined under a Zeiss confocal fluorescence microscope.
Chick chorioallantoic membrane assay
Fertile eggs were incubated (blunt side facing down), and on incubation day (ID) three eggs were placed upside down and a small hole was punctured and sealed on the blunt side. On ID 8 or 9, the blunt side of the egg was cut open to unravel the chick chorioallantoic membrane (CAM). A small plastic ring was placed on a large CAM blood vessel, and hKEpC, treated and untreated with VPA, were suspended in 20 μL of Matrigel (Sigma) (1:1 by volume) and then slowly pipetted into the plastic ring. The egg was sealed and incubated for 7 more days. On ID15 or 16, the grafted cells were harvested, fixed using 4% paraformaldehyde (PFA), and embedded in paraffin.
Hematoxylin &Eosin staining
Sections (6 μm) of paraffin-embedded of cells grafted on chick CAM were mounted on polylysine-coated glass and deparaffinized by toluene and descending concentrations of ethanol (100%, 95%, 70%, and 50%). The sections were incubated in Mayer's Hematoxylin (Sigma) and washed with 95% alcohol and double-distilled water (DDW). Sections were then incubated with eosin-phloxine B solution and washed again with 95% alcohol and DDW. Sections were dehydrated in ascending concentrations of ethanol (70%, 95%, and 100%) and xylene and sealed with Entellan (Merck Millipore).
Statistical analysis
Results are expressed as the mean values ± standard deviation (SD). Statistical differences of data for two groups were compared by Student's t-test. Where indicated, a t-test was performed after logarithmic transformation to achieve normality. For all statistical analyses, the level of significance was set as p<0.05.
Results
VPA treatment reactivates renal progenitor genes
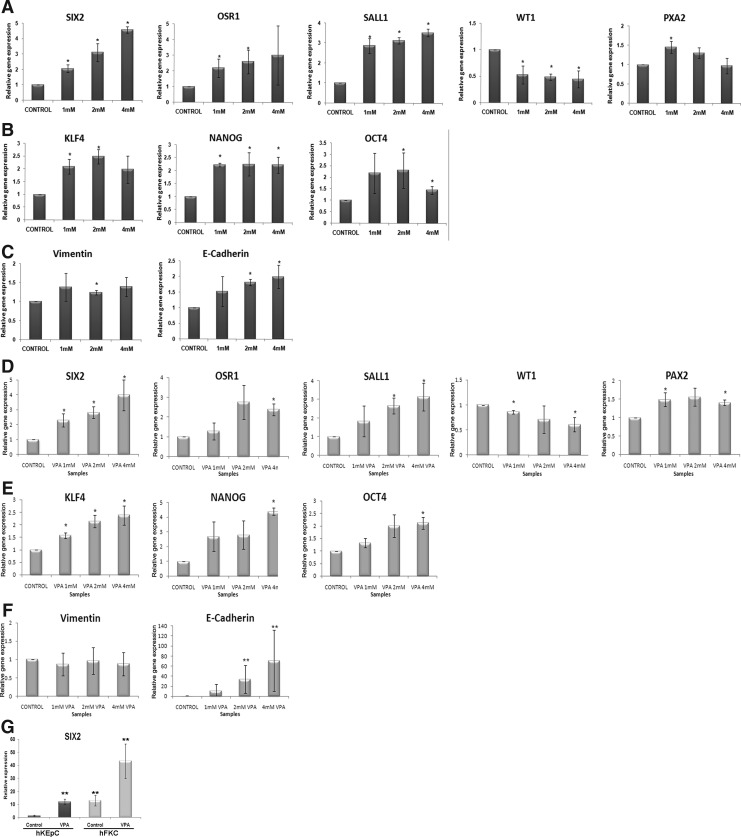
On the basis of previous evidence of epigenetic silencing of renal progenitor genes in adult human kidney (Metsuyanim et al., 2008), we hypothesized that the epigenetically repressed genes could be reactivated by treatment with VPA on hKEpC and hFKC. VPA has recently been shown to be extremely efficient in improving the reprogramming of mouse and human somatic cells (Huangfu et al., 2008). Changes in renal progenitor gene levels were assessed initially in hKEpC following a 24-h VPA exposure. VPA led to consistent and significant SIX2 activation among independent samples, demonstrating dose-dependent reinduction of SIX2 (Fig. 1A). Similarly, both OSR1 and SALL1 were upregulated in a dose-dependent manner, whereas PAX2 levels were mildly elevated and WT1 levels reduced (Fig. 1A). Because activation of pluripotency genes (alone or in concert) has been shown to induce in vitro stemness characteristics (Liu et al., 2009; Tsai and Hung, 2012), we analyzed the expression profile of these genes in VPA-treated hKEpC alongside the renal progenitor genes. Analysis of the pluripotency genes showed NANOG and KLF4 to be significantly upregulated in all VPA concentrations, and OCT4 particularly in 2 mM VPA (Fig. 1B). Because kidney development progresses through MET of the early mesenchyme (VIMENTIN+) to epithelial progenitors (E-CADHERIN+) (Dressler, 2006), we analyzed whether the reinduction of SIX2 and OSR1 and pluripotency genes recapitulates early renal development and is associated with VIMENTIN upregulation. Analysis of EMT-related genes demonstrated VIMENTIN levels to be mostly unchanged, whereas E-CADHERIN levels were significantly elevated (2 mM and 4 mM VPA) (Fig. 1C).
FIG. 1.
Valproic acid (VPA) treatment reactivates renal progenitor genes. qRT-PCR analysis of hKEpC (A–C) and hFKC (D–F) subjected to VPA (24 h). Shown are renal progenitor (A, D), pluripotency/self renewal (B, E), and mesenchymal-to-epithelial transition (MET) (C, F) genes. At least three independent samples of human kidneys were used. The values shown were calculated with respect to nontreated control-hFKC/hKEpC (therefore equal 1). Data were calculated as average±standard deviation (SD). (*) p<0.05; (**) p<0.05 after logarithmic transformation versus untreated controls. (G) Comparison of SIX2 expression levels between hFKC and hKEpC before and after VPA treatment. Data were calculated as average±SD. (*) p<0.05; (**) p<0.005.
We next evaluated hFKC derived from mid-gestation kidneys. At this stage of nephrogenesis, there are opposing factors that govern the effects of treatment; on the one hand, the relative proportion of early progenitor cells is rather low compared to more differentiated epithelial cell types. On the other hand, renal progenitor genes are still expected to be more abundantly expressed and less methylated or deacylated and thus less influenced (Metsuyanim et al., 2008). Almost the same findings were noted in hFKC exposed to VPA. The most prominent induction was noted in SIX2 levels (four-fold) and to a lesser extent in OSR1 and SALL1, with dose-dependent activation, concomitant with reduction of WT1 and mild elevation in PAX2 levels (Fig. 1D). Furthermore, levels of all pluripotency genes, NANOG, KLF4, and OCT4 (two- to five-fold increase at 4 mM VPA) and also E-CADHERIN, were found to be significantly elevated along with the renal progenitor genes (Figs. 1E, F). Importantly, to add a quantitative perspective to the effects of VPA on SIX2 reinduction in adult cells, we compared SIX2 levels in hKEpC and hFKC. We found that SIX2 levels in hKEpC following VPA treatment reached those observed under control basal conditions in developing kidney cells (Fig. 1G).
Effects of TSA/5-AzaC treatment
To further analyze the effects of additional chromatin-modifying agents on kidney cells, we tested the combination of the demethylating agent 5-AzaC and the HDAC inhibitor TSA. hKEpC were initially cultured in the presence or absence of TSA/5-AzaC for 24 h. We analyzed changes in gene expression in at least three independent samples of human adult kidneys after a 24-h TSA/5-AzaC treatment by quantitative RT-PCR. Gene expression profiles were similar to those observed following VPA treatment in both hKEpC and hFKC (Fig. S1) (Supplementary Data are available at www.liebertpub.com/cell/.) However, VPA treatment induced significant induction in more genes (e.g., SALL1), and changes were more constant across human kidney samples, favoring the use of VPA for further experimentation.
VPA treatment induces changes at the protein level
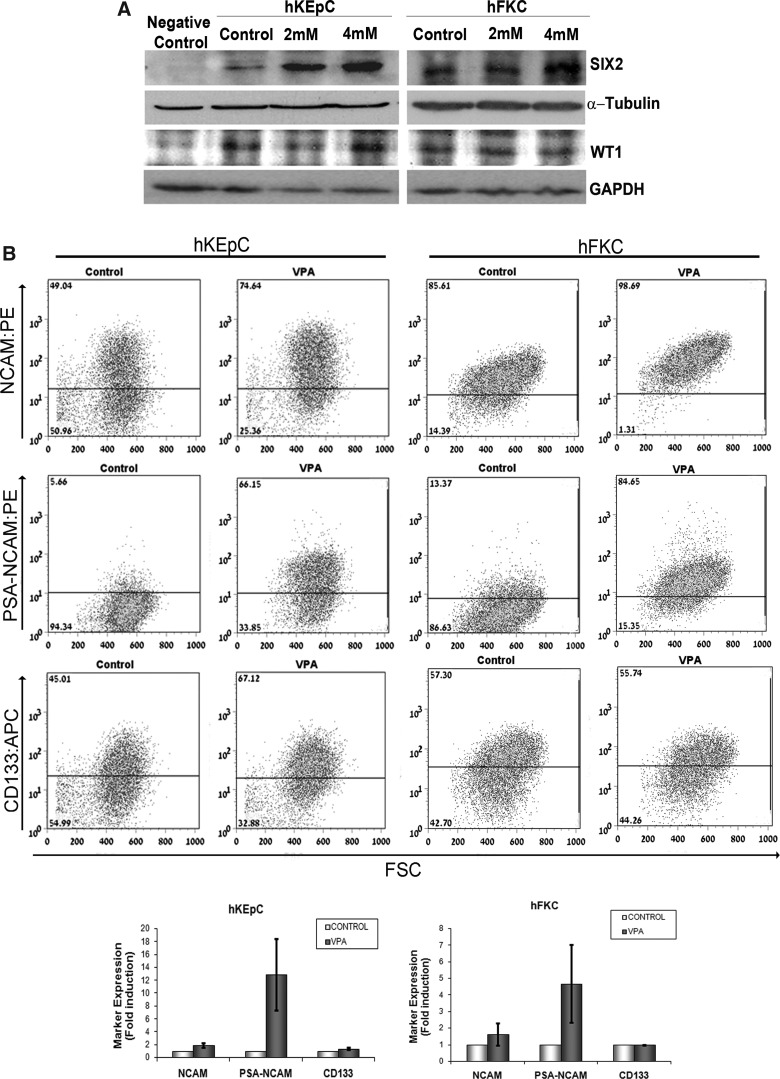
We analyzed changes at the protein level following exposure to 2 mM and 4 mM VPA. For western blot analysis, we focused on the SIX2 protein because it was most substantially induced and it has a major role in specifying the renal stem cell population in the metanephric mesenchyme (MM) (Kobayashi et al., 2008). An additional renal progenitor gene, WT1, which was not induced at the gene level, was analyzed concomitantly. Western blots performed on both human fetal and adult kidney cells 24 h after VPA exposure clearly demonstrated an increment in SIX2 protein levels in both types of cells (4 mM) and no changes in WT1, both following their gene expression pattern (Fig. 2A).
FIG. 2.
Valproic acid (VPA) treatment induces changes at the protein level. (A) Western blot analysis for SIX2 and WT1 in VPA-treated hKEpC and hFKC, showing elevated SIX2 but not WT1 protein levels in both cell types following treatment. Melanoma cells were used as negative control and α-tubulin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used for loading control. (B) Flow cytometric analyses of embryonic renal progenitor surface markers [neural cell adhesion molecule (NCAM), polysialylated (PSA)-NCAM] and a marker of differentiated renal epithelia in culture (CD133), in hKEpC and hFKC following a 24-h treatment with 4 mM VPA. At least three independent samples of human kidneys were used. Shown are representative fluorescence-activated cell sorting (FACS) analyses and fold induction in expression levels demonstrating significant elevation of NCAM and PSA-NCAM. FSC, forward scatter; APC, Allophycocyanin; PE, Phycoerythrin.
Because certain surface markers characterize renal progenitor cells (Dekel et al., 2006; Metsuyanim et al., 2009; Pode-Shakked et al., 2008), we next performed fluorescence-activated cell sorting (FACS) analysis of human adult and fetal kidney cells so as to determine whether VPA exposure can also alter their expression levels. Neural cell adhesion molecule (NCAM) and its embryonic form, polysialylated (PSA)-NCAM, which are both expressed on nephron progenitor cells during early kidney development (Bard et al., 2001), showed significant upregulation in expression levels, with dramatic elevation (5- to 10-fold) in PSA-NCAM expression, especially in the adult kidney (Fig. 2B). Concomitantly, CD133, a marker of differentiated renal epithelia in culture (Metsuyanim et al., 2009), was not significantly changed upon VPA treatment (Fig. 2B).
Epigenetic modification of the SIX2 promoter
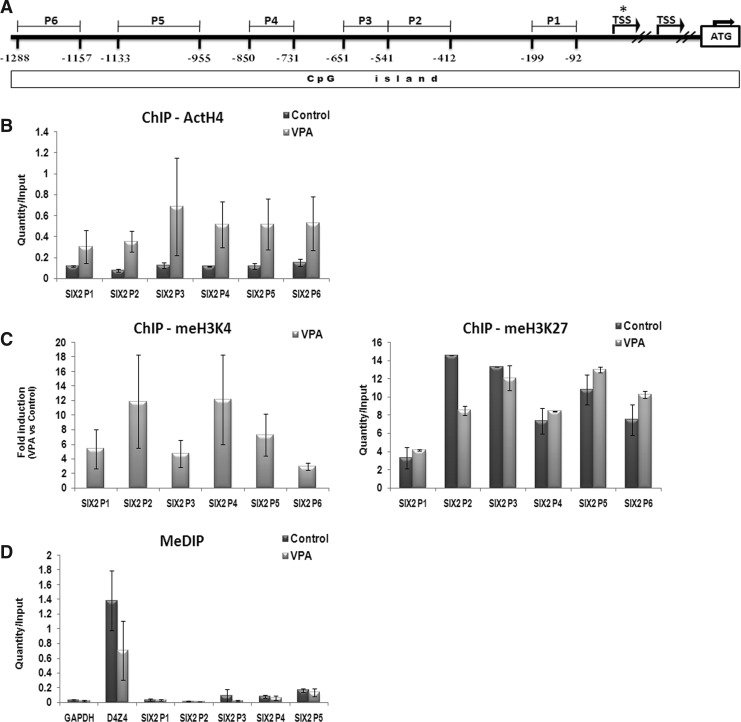
To test whether HDAC inhibition following VPA treatment functions in part through chromatin remodeling, ChIP was used to examine the posttranscriptionally modified state of the histones at the SIX2 promoter. Recent evidence has indicated that unique patterns of histone modifications are responsible for transcriptional and lineage control (Bernstein et al., 2007). In particular, methylation of lysine 27 on histone subunit H3 (H3K27me) by the Polycomb complex is associated with transcriptional repression, whereas methylation of lysine 4 on H3 (H3K4me) is associated with gene activation. More generally, lysine acetylation of histones, such as acetylated H4 (ActH4), is also associated with transcriptional activation (Kouzarides, 2007). Therefore, we examined multiple loci at the SIX2 promoter region for such changes by ChIP analysis. The promoter sites examined were designed at consecutive distances from the 5′ transcription start site (TSS) (from ∼90 bp upstream to ∼1300 bp) (Fig. 3A).
FIG. 3.
Epigenetic modification of the SIX2 promoter. (A) Scheme of the CpG island region in the SIX2 promoter. Marked sections refer to the tested region upstream to the starred transcriptional start site (TSS) (the unstarred TSS is an alternative one). (B) Chromatin immunoprecipitation (ChIP) assays. Comparison of histone modifications between valproic acid (VPA)-treated and untreated hKEpC. DNA was immunoprecipitated using antibodies directed against ActH4 (B), meH3K4 (C), meH3K27 and was analyzed by qRT-PCR using primers from different regions of the SIX2 promoter. To evaluate the level of histone acetylation and methylation, the ratio of PCR products from immunoprecipitated DNA versus input DNA was calculated. ChIP assays results for meH3K4 are presented as fold induction after VPA treatment. (D) Methylation state of the SIX2 promoter was analyzed by methylation depletion immunoprecipitation (MeDIP); GAPDH was used as negative control and D4Z4 as positive control. To evaluate the level of DNA methylation, the ratio of PCR products from immunoprecipitated DNA vs. input DNA was calculated.
In addition to the chromatin state, we determined DNA methylation profiles at the SIX2 promoter regions so as to detect epigenetic modifications at the DNA level. We observed that treatment with 4 mM VPA significantly increased ActH4 in multiple SIX2 promoter regions (upstream to the TSS −92, −412, −541, −731, −955, and −1157) (Fig. 3B) accompanied with significant increases in H3K4me but no change in H3K27me levels (Fig. 3C). In addition, MeDIP revealed DNA hypomethylation at the SIX2 promoter loci regardless of VPA treatment (Fig. 3D). Although the observed histone acetylation is directly modulated by HDAC inhibitor treatment, changes in methylation likely result from secondary effects (Klose and Zhang, 2007). These results indicated that reactivation of SIX2 is linked to epigenetic modification of histones at its promoter region leading to gene induction.
Wnt pathway modulation synergizes with VPA to further induce SIX2/OSR1
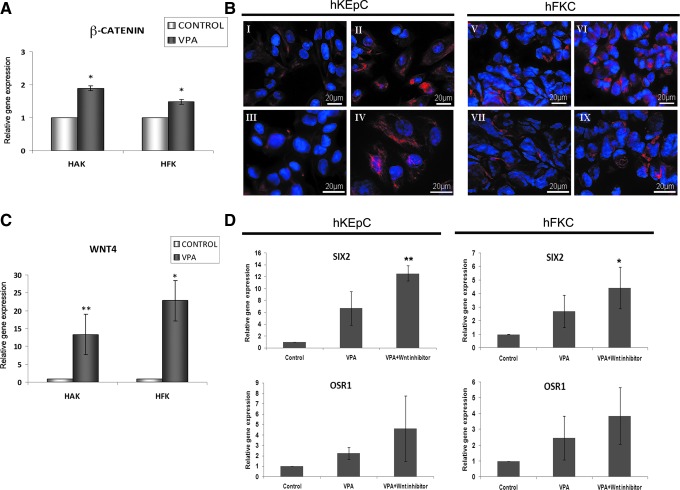
It has been suggested that in addition to its major role as an HDAC inhibitor, VPA can inhibit GSK3β enabling the activation of the canonical Wnt pathway (Doble and Woodgett, 2003; Zhurinsky et al., 2000). Indeed, quantitative RT-PCR analysis showed an elevation in β-CATENIN mRNA levels in VPA-treated human kidney cells (Fig. 4A), and immunofluorescence disclosed cyoplasmic and nuclear accumulation of β-CATENIN compared to untreated cells (Fig. 4B). In addition, we found strong upregulation of WNT4 mRNA (Fig. 4C), shown to induce epithelial differentiation in the early renal progenitor population by opposing SIX2 effects (Kispert et al., 1998; Nishinakamura, 2008). To evaluate the contribution of VPA-induced Wnt activation on renal progenitor gene expression, we added Wnt inhibitors (sFRP1 or DKK1) to VPA and determined their effect. Indeed, application of a combination of VPA and Wnt pathway antagonists resulted in enhanced reinduction of SIX2/OSR1 compared to VPA alone in both hKEpC and hFKC (Fig. 4D).
FIG. 4.
Wnt pathway modulation synergizes with valproic acid (VPA) to further induce SIX2/OSR1. (A) qRT-PCR analysis of β-CATENIN in hKEpC and hFKC subjected to VPA. (B) β-CATENIN immunofluorescence in hKEpC and hFKC after treatment with VPA for 24 h; β-CATENIN expression in hKEpC or hFKC before (I, III and V, VII) and after (II, IV and VI, IX) treatment, respectively, is shown in low (63×) and high magnification (100×), showing strong and extensive expression in VPA-treated cells, as well as nuclear localization (IV). Bar, 20 μM. (C) qRT-PCR analysis of WNT4 in hKEpC and hFKC subjected to VPA. (D) qRT-PCR analysis of SIX2 and OSR1 following the addition of Wnt pathway antagonist to untreated and VPA-treated hKEpC and hFKC. In sections A, C, and D, the values for untreated control hFKC/hKEpC were used to normalize (therefore equal 1) and all other values were calculated with respect to them. Data were calculated as average±standard deviation (SD). (*) p<0.05; (**) p<0.005. Color images available online at www.liebertpub.com/cell
VPA-reactivated embryonic genes do not confer stemness to hKEpC
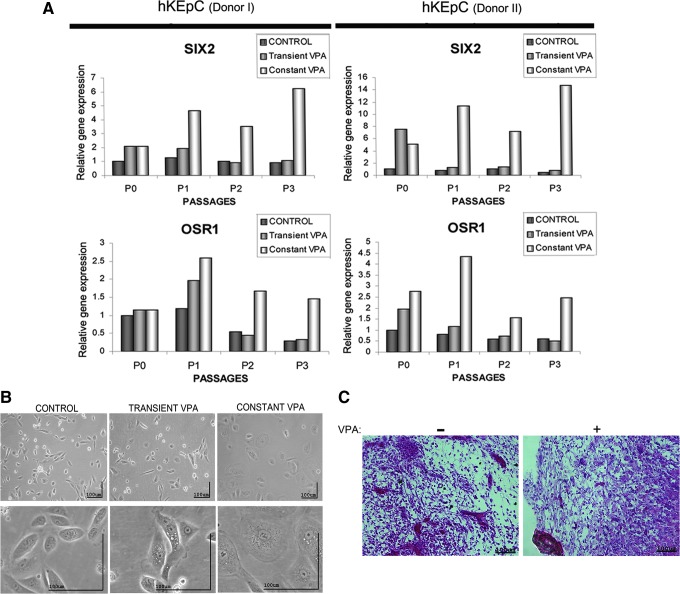
Having observed that VPA can lead to significant reactivation of SIX2/OSR1 along with stemness-related genes, we interrogated whether these changes can confer growth advantage or stemness traits. We first employed 24-h exposure to 4 mM VPA. OSR1/SIX2 gene activity in cells transiently (24 h) exposed to VPA was reinduced in P0 cells, but rapidly downregulated and returned to baseline levels in later passages. At the culture level, no differences were noted between transiently VPA-treated and control untreated hKEpC, both appearing as spindle shaped, with similar proliferation rates and clonogenic capacities (clonogenic efficency of 2–5% in cells obtained from three different kidney donors). We then employed a second protocol of continuous exposure to VPA. In contrast to transient administration, constant VPA treatment resulted in continuous reinduction of both SIX2 and OSR1, where SIX2 levels were further upregulated in P3 cells (Fig. 5A). In parallel, large epithelial-like cell morphology, reduced proliferation, and complete lack of clonogenicty were noted within 21 days; expansion was terminated at P3 (Fig. 5B).
FIG. 5.
Valproic acid (VPA) treatment can maintain SIX2 and OSR1 reinduction but leads to cellular senescence and decreased proliferation. (A) Temporal expression patterns of SIX2 and OSR1 mRNA in hKEpC undergoing culture passages and subjected to constant VPA exposure (const VPA), 24-h exposure to 4 mM VPA and control samples. Total RNA was isolated from the hKEpC in P1–P3, and transcript levels of SIX2 and OSR1 were analyzed by qRT-PCR. The values for untreated control-hKEpC in P1 were used as reference (therefore equal 1) and all other values were calculated with respect to them. Shown are two examples of independent samples. (B) Large epithelial-like cell morphology in VPA constant-treated cells compared to fibroblastic appearance in transient VPA exposure and control group. (C) VPA-treated hKEpC did not show any advantage when grafted onto the chick embryo CAM. Both treated and untreated cells failed to generate tubular structures. Hematoxylin & Eosin (H&E) staining. Magnification, 40×. Color images available online at www.liebertpub.com/cell
To corroborate in vivo data, we determined whether VPA-treated hKEpC and reactivation of embryonal genes would prove superior in nephrogenic potential compared to control hKEpC upon grafting onto the CAM of the chick embryo. This model has been used by our group to study kidney-forming potency of various cell types, including cells grown as nephrospheres (Buzhor et al., 2011; Noiman et al., 2011). We tested the potency of hKEpC expanded in adhesion culture and subjected to VPA treatment in this model and found that VPA did not confer any advantage in kidney generation (Fig. 5C), failing to generate renal tubular structures (0/5 grafts).
Discussion
We show that VPA or TSA/5-AzaC treatment induced robust and prompt re-expression of a specific set of developmental renal stem/progenitor genes SIX2 and OSR1 in human kidney cells. VPA was superior to TSA/5-AzaC because it induced a wider array of progenitor genes (including reactivation of SALL1) as well as enhancing the expression of the cell-surface markers NCAM and PSA-NCAM, all associated with a renal developmental stem/progenitor cell phenotype (Bard et al., 2001; Kobayashi et al., 2008; Metsuyanim et al., 2009; Mugford et al., 2008; Nishinakamura, 2008; Pode-Shakked et al., 2008). In addition, concomitant induction of pluripotency/self-renewal genes (such as Oct4) was observed. Both human adult and fetal kidney cells (from mid-gestation) behaved similarly. We examined SIX2 more closely and showed that in response to VPA multiple sites in its promoter region are epigenetically modified at the histone level in a way that favors transcriptional activation and that this treatment reinduces protein expression. These results favor the idea that VPA increases SIX2, and likely the other stem/progenitor genes, through its capacity to modify chromatin (mostly histone acetylation) in a dose-dependent manner. Importantly, VPA has been shown also to inhibit GSK3β in various cell types, enabling transcriptional activation of canonical Wnt signaling (Doble and Woodgett, 2003; Zhurinsky et al., 2000). Indeed, Wnt-related molecules such as the Wnt ligand WNT4 were dramatically upregulated following VPA exposure. Wnt4 and canonical Wnt signaling were recently shown to oppose the expression and function of SIX2 in early renal progenitors (Kispert et al., 1998; Kuure et al., 2007). Along these lines, once SIX2 was reactivated by VPA, we could show that concomitant treatment with Wnt antagonists (DKK1, sFRP1) could further elevate SIX2 levels in adult kidney cells to levels observed in developing human kidney cells.
Regeneration in the kidney likely occurs by a pool of replicating cells that transiently dedifferentiate, resulting in cellular turnover and growth of its compartments (Humphreys et al., 2008; Pleniceanu et al., 2010). One of the striking features of the regenerative response that occurs during recovery from acute kidney injury is the reactivation of embryonic renal progenitor genes, drawing similarities between renal development and regeneration (Dekel et al., 2006; Metsuyanim et al., 2008). Therefore, therapeutics aimed at increasing levels of renal stem/progenitor genes along with increase in the proliferation and self-renewal potential of adult kidney cells may have important therapeutic consequences.
VPA has been shown to harbor a positive therapeutic effect in chronic renal models of tubulointerstitial fibrosis and lupus (Marumo et al., 2010; Pang et al., 2009; Van Beneden et al., 2011) as well as beneficial in vivo effect of VPA in an experimental model of glomerulosclerosis (Sayyed et al., 2010). In addition, the renal progenitor pool has been shown to expand in the zebrafish model by HDAC inhibition with a small molecule 4-(phenylthio)butanoic acid (PTBA) (de Groh et al., 2010). Nevertheless, we show that VPA has unexpected effects on the generation of a renal stem/progenitor phenotype, substantially decreasing stemness (both proliferation and clonogenic self-renewal potential) in human kidney cells. While treatment with chromatin-modifying agents indeed prompted re-expression of stem/progenitor genes, this did not lead to induction of a renal stem/progenitor phenotype in culture. On the contrary, VPA-treated human kidney epithelia underwent terminal differentiation, as evidenced by low VIMENTIN and high E-CADHERIN expression, and failed to undergo epithelial to mesenchymal transition (EMT). Cells featured epithelial morphology accompanied by reduced proliferation/expansion/clonogenicity, and cell senescence appeared within a few passages. In addition, VPA induction of pluripotency/self-renewal related genes did not promote “stemness” in primary hKEpC cultures, nor did it increase or preserve in vivo kidney-forming potency analyzed upon cell grafting onto the chick CAM.
In contrast to VPA-treated cells, other adult kidney cell types, such as adult renal progenitors cultured as spheres (Buzhor et al., 2011; Noiman et al., 2011), have been shown to harbor strong tubogenic potential. Altogether, the “transcriptional reprogramming” by chromatin-modifying agents induced signals of epithelial differentiation while limiting dedifferentiation required for a proliferative stem/progenitor character in human kidney epithelia.
Importantly, our results recommend the reassessment of the role of VPA and other chromatin-modifying agents in the treatment of renal disease, especially when expansion of large numbers of adult stem/progenitors in vitro is required, or in pathological conditions for which the acquisition of stem cell characters by kidney epithelia and clonogenic cell growth may lead to an enhanced regenerative response. Importantly, they might be indeed useful in the redifferentiation phase of the renal regenerative response when MET back to healthy epithelia is required. Therefore, timing of administration of VPA/chromatin-modifying agents is likely to be crucial for a therapeutic benefit.
Supplementary Material
Acknowledgments
The work is supported by the ISF grant number 910/11, Israel Ministry of Industry 'NOFAR' program, Wolfson Clore Mayer, TAU Stem Cell Research Center, Sackler School of Medicine, Tel Aviv University and the Mary Barry International Scientific Exchange Fund, Cedars-Sinai Medical Center, Los Angeles, CA (B.D). This work was performed in partial fulfillment of the requirements for a Ph.D. degree of Dorit Omer. E.N.G. is a fellow of the Talpiot Medical Leadership program of the Sheba Medical Center. Her work is supported by ISF grant number 1609/09, and the Israel Cancer Association.
Author Disclosure Statement
No competing financial interests exist.
References
- Abbate M. Brown D. Bonventre J.V. Expression of NCAM recapitulates tubulogenic development in kidneys recovering from acute ischemia. Am. J. Physiol. 1999;277:F454–463. doi: 10.1152/ajprenal.1999.277.3.F454. [DOI] [PubMed] [Google Scholar]
- Bard J.B. Gordon A. Sharp L. Sellers W.I. Early nephron formation in the developing mouse kidney. J. Anat. 2001;199:385–392. doi: 10.1046/j.1469-7580.2001.19940385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels M. Geest C.R. Bierings M. Buitenhuis M. Coffer P.J. Histone deacetylase inhibition modulates cell fate decisions during myeloid differentiation. Haematologica. 2010;95:1052–1060. doi: 10.3324/haematol.2009.008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein B.E. Meissner A. Lander E.S. The mammalian epigenome. Cell. 2007;128:669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Buzhor E. Harari-Steinberg O. Omer D. Metsuyanim S. Jacob-Hirsch J. Noiman T. Dotan Z. Goldstein R.S. Dekel B. Kidney spheroids recapitulate tubular organoids leading to enhanced tubulogenic potency of human kidney-derived cells. Tiss. Eng. Part A. 2011;17:2305–2319. doi: 10.1089/ten.TEA.2010.0595. [DOI] [PubMed] [Google Scholar]
- de Groh E.D. Swanhart L.M. Cosentino C.C. Jackson R.L. Dai W. Kitchens C.A. Day B.W. Smithgall T.E. Hukriede N.A. Inhibition of histone deacetylase expands the renal progenitor cell population. J. Am. Soc. Nephrol. 2010;21:794–802. doi: 10.1681/ASN.2009080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeb B.G. Lacerda L. Xu W. Larson R. Solley T. Atkinson R. Sulman E.P. Ueno N.T. Krishnamurthy S. Reuben J.M., et al. Histone deacetylase inhibitors stimulate dedifferentiation of human breast cancer cells through WNT/beta-catenin signaling. Stem Cells. 2012;30:2366–2377. doi: 10.1002/stem.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekel B. Biton S. Yerushalmi G.M. Altstock R.T. Mittelman L. Faletto D. Smordinski N.J. Tsarfaty I. In situ activation pattern of MET docking site following renal injury and hypertrophy. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2003;18:1493–1504. doi: 10.1093/ndt/gfg215. [DOI] [PubMed] [Google Scholar]
- Dekel B. Metsuyanim S. Schmidt-Ott K.M. Fridman E. Jacob-Hirsch J. Simon A. Pinthus J. Mor Y. Barasch J. Amariglio N., et al. Multiple imprinted and stemness genes provide a link between normal and tumor progenitor cells of the developing human kidney. Cancer Res. 2006a;66:6040–6049. doi: 10.1158/0008-5472.CAN-05-4528. [DOI] [PubMed] [Google Scholar]
- Dekel B. Shezen E. Even-Tov-Friedman S. Katchman H. Margalit R. Nagler A. Reisner Y. Transplantation of human hematopoietic stem cells into ischemic and growing kidneys suggests a role in vasculogenesis but not tubulogenesis. Stem Cells. 2006b;24:1185–1193. doi: 10.1634/stemcells.2005-0265. [DOI] [PubMed] [Google Scholar]
- Doble B.W. Woodgett J.R. GSK-3: Tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler G.R. The cellular basis of kidney development. Ann. Rev. Cell Dev. Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- Harari-Steinberg O. Pleniceanu O. Dekel B. Selecting the optimal cell for kidney regeneration: Fetal, adult or reprogrammed stem cells. Organogenesis. 2011;7:123–134. doi: 10.4161/org.7.2.15783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C. Li J. Rae F. Little M.H. Stem cell options for kidney disease. Journal of Pathology. 2009;217:265–281. doi: 10.1002/path.2477. [DOI] [PubMed] [Google Scholar]
- Huangfu D. Maehr R. Guo W. Eijkelenboom A. Snitow M. Chen A.E. Melton D.A. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys B.D. Valerius M.T. Kobayashi A. Mugford J.W. Soeung S. Duffield J.S. McMahon A.P. Bonventre J.V. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Karantzali E. Schulz H. Hummel O. Hubner N. Hatzopoulos A. Kretsovali A. Histone deacetylase inhibition accelerates the early events of stem cell differentiation: Transcriptomic and epigenetic analysis. Genome Biol. 2008;9:R65. doi: 10.1186/gb-2008-9-4-r65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispert A. Vainio S. McMahon A.P. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125:4225–4234. doi: 10.1242/dev.125.21.4225. [DOI] [PubMed] [Google Scholar]
- Klose R.J. Zhang Y. Regulation of histone methylation by demethylimination and demethylation. Nat. Rev. Mol. Cell. Biol. 2007;8:307–318. doi: 10.1038/nrm2143. [DOI] [PubMed] [Google Scholar]
- Kobayashi A. Valerius M.T. Mugford J.W. Carroll T.J. Self M. Oliver G. McMahon A.P. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kuure S. Popsueva A. Jakobson M. Sainio K. Sariola H. Glycogen synthase kinase-3 inactivation and stabilization of beta-catenin induce nephron differentiation in isolated mouse and rat kidney mesenchymes. J. Am. Soc. Nephrol. 2007;18:1130–1139. doi: 10.1681/ASN.2006111206. [DOI] [PubMed] [Google Scholar]
- Liu T.M. Wu Y.N. Guo X.M. Hui J.H. Lee E.H. Lim B. Effects of ectopic Nanog and Oct4 overexpression on mesenchymal stem cells. Stem Cells Dev. 2009;18:1013–1022. doi: 10.1089/scd.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumo T. Hishikawa K. Yoshikawa M. Hirahashi J. Kawachi S. Fujita T. Histone deacetylase modulates the proinflammatory and -fibrotic changes in tubulointerstitial injury. Am. J. Physiol. Renal Physiol. 2010;298:F133–F141. doi: 10.1152/ajprenal.00400.2009. [DOI] [PubMed] [Google Scholar]
- Metsuyanim S. Pode-Shakked N. Schmidt-Ott K.M. Keshet G. Rechavi G. Blumental D. Dekel B. Accumulation of malignant renal stem cells is associated with epigenetic changes in normal renal progenitor genes. Stem Cells. 2008;26:1808–1817. doi: 10.1634/stemcells.2007-0322. [DOI] [PubMed] [Google Scholar]
- Metsuyanim S. Harari-Steinberg O. Buzhor E. Omer D. Pode-Shakked N. Ben-Hur H. Halperin R. Schneider D. Dekel B. Expression of stem cell markers in the human fetal kidney. PLoS One. 2009;4:e6709. doi: 10.1371/journal.pone.0006709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugford J.W. Sipila P. McMahon J.A. McMahon A.P. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol. 2008;324:88–98. doi: 10.1016/j.ydbio.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishinakamura R. Stem cells in the embryonic kidney. Kidney Int. 2008;73:913–917. doi: 10.1038/sj.ki.5002784. [DOI] [PubMed] [Google Scholar]
- Noiman T. Buzhor E. Metsuyanim S. Harari-Steinberg O. Morgenshtern C. Dekel B. Goldstein R.S. A rapid in vivo assay system for analyzing the organogenetic capacity of human kidney cells. Organogenesis. 2011;7:140–144. doi: 10.4161/org.7.2.16457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang M. Kothapally J. Mao H. Tolbert E. Ponnusamy M. Chin Y.E. Zhuang S. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am. J. Physiol. Renal Physiol. 2009;297:F996–F1005. doi: 10.1152/ajprenal.00282.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleniceanu O. Harari-Steinberg O. Dekel B. Concise review: Kidney stem/progenitor cells: Differentiate, sort out, or reprogram? Stem Cells. 2010;28:1649–1660. doi: 10.1002/stem.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pode-Shakked N. Metsuyanim S. Rom-Gross E. Mor Y. Fridman E. Goldstein I. Amariglio N. Rechavi G. Keshet G. Dekel B. Developmental Tumorigenesis: NCAM as a putative marker for the malignant renal stem/progenitor cell population. J. Cell. Mol. Med. 2008;13:1798–1808. doi: 10.1111/j.1582-4934.2008.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruau D. Ensenat-Waser R. Dinger T.C. Vallabhapurapu D.S. Rolletschek A. Hacker C. Hieronymus T. Wobus A.M. Muller A.M. Zenke M. Pluripotency associated genes are reactivated by chromatin-modifying agents in neurosphere cells. Stem Cells. 2008;26:920–926. doi: 10.1634/stemcells.2007-0649. [DOI] [PubMed] [Google Scholar]
- Sayyed S.G. Gaikwad A.B. Lichtnekert J. Kulkarni O. Eulberg D. Klussmann S. Tikoo K. Anders H.J. Progressive glomerulosclerosis in type 2 diabetes is associated with renal histone H3K9 and H3K23 acetylation, H3K4 dimethylation and phosphorylation at serine 10. Nephrol. Dialysis Transplant. 2010;25:1811–1817. doi: 10.1093/ndt/gfp730. [DOI] [PubMed] [Google Scholar]
- Tsai C.C. Hung S.C. Functional roles of pluripotency transcription factors in mesenchymal stem cells. Cell Cycle. 2012;11:3711–3712. doi: 10.4161/cc.22048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beneden K. Geers C. Pauwels M. Mannaerts I. Verbeelen D. van Grunsven L.A. Van den Branden C. Valproic acid attenuates proteinuria and kidney injury. J. Am. Soc. Nephrol. 2011;22:1863–1875. doi: 10.1681/ASN.2010111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhurinsky J. Shtutman M. Ben-Ze'ev A. Plakoglobin and beta-catenin: protein interactions, regulation and biological roles. J. Cell Sci. 2000;113:3127–3139. doi: 10.1242/jcs.113.18.3127. [DOI] [PubMed] [Google Scholar]
- Zini R. Norfo R. Ferrari F. Bianchi E. Salati S. Pennucci V. Sacchi G. Carboni C. Ceccherelli G.B. Tagliafico E., et al. Valproic acid triggers erythro/megakaryocyte lineage decision through induction of GFI1B and MLLT3 expression. Exp. Hematol. 2012;40:1043–1054. doi: 10.1016/j.exphem.2012.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.