Abstract
Object
The median survival duration for patients with glioblastoma is approximately 12 months. Maximizing quality of life (QOL) for patients with glioblastoma is a priority. An important, yet understudied, QOL component is functional independence. The aims of this study were to evaluate functional outcomes over time for patients with glioblastoma, as well as identify factors associated with prolonged functional independence.
Methods
All patients who underwent first-time resection of either a primary (de novo) or secondary (prior lower grade glioma) glioblastoma at a single institution from 1996 to 2006 were retrospectively reviewed. Patients with a Karnofsky Performance Scale (KPS) score ≥ 80 were included. Kaplan-Meier, log-rank, and multivariate proportional hazards regression analyses were used to identify associations (p < 0.05) with functional independence (KPS score ≥ 60) following glioblastoma resection.
Results
The median follow-up duration time was 10 months (interquartile range [IQR] 5.6–17.0 months). A patient’s preoperative (p = 0.02) and immediate postoperative (within 2 months) functional status was associated with prolonged survival (p < 0.0001). Of the 544 patients in this series, 302 (56%) lost their functional independence at a median of 10 months (IQR 6–16 months). Factors independently associated with prolonged functional independence were: preoperative KPS score ≥ 90 (p = 0.004), preoperative seizures (p = 0.002), primary glioblastoma (p < 0.0001), gross-total resection (p < 0.0001), and temozolomide chemotherapy (p < 0.0001). Factors independently associated with decreased functional independence were: older age (p < 0.0001), coexistent coronary artery disease (p = 0.009), and incurring a new postoperative motor deficit (p = 0.009). Furthermore, a decline in functional status was independently associated with tumor recurrence (p = 0.01).
Conclusions
The identification and consideration of these factors associated with prolonged functional outcome (preoperative KPS score ≥ 90, seizures, primary glioblastoma, gross-total resection, temozolomide) and decreased functional outcome (older age, coronary artery disease, new postoperative motor deficit) may help guide treatment strategies aimed at improving QOL for patients with glioblastoma.
Keywords: functional outcome, glioblastoma, Karnofsky Performance Scale, quality of life, temozolomide, recurrence
Glioblastoma (WHO Grade IV) is the most common malignant type of primary CNS tumor in adults.17 These tumors are characterized by their propensity to invade and infiltrate surrounding normal brain parenchyma, making curative resection unlikely14 Patients with these devastating tumors have a median survival of approximately 12 months.7,15,17 Despite advances in medical and surgical therapy, this median survival rate has not substantially improved over the past decade.40 This poor survival rate places an emphasis on understanding the effects of surgery on QOL for patients harboring glioblastomas.
Whereas most clinical studies attempt to find factors associated with prolonged survival, QOL remains understudied. A key component of QOL, regardless of disease, is functional independence.5,16,20,38 Functional independence is defined as the ability to conduct normal activity with the ability to attend to most personal needs.5,16,20,38 A successful treatment would therefore prolong functional independence. Studies on functional independence for patients with glioblastoma, however, are few and limited.8,37,41,42
The aims of this study were to evaluate the functional outcomes of patients over time after glioblastoma resection, as well as identify factors associated with prolonged functional independence. An understanding of the functional outcomes, as compared with survival, may arguably be more important to some patients. Some patients may argue that prolonged functional independence is more important than prolonged survival with functional impairment. Furthermore, the ability to identify clinical factors associated with prolonged functional independence may allow clinicians to risk-stratify patients, as well as guide treatment strategies.
Methods
Patient Population and Recorded Variables
The clinical, operative, and hospital course records of all patients undergoing resection of a supratentorial glioblastoma at a single academic tertiary-care institution between 1996 and 2006 were retrospectively reviewed. The information collected from neurosurgery and neurooncology clinical notes included patient demographics, comorbidities, presenting symptoms, neuroimaging results, neurological function, and adjuvant therapy. For patients with prior tumors, the presenting characteristics were documented based on the symptoms present immediately prior to surgery for their glioblastoma.
The KPS index was used to classify patients’ functional independence.20,38 The KPS scores were assigned by 2 clinicians, blinded to patient outcomes, for each clinical visit. According to this scoring scheme, patients with a KPS score ≥ 60 were considered functionally independent, because they did not require considerable assistance and medical care.20,38 Recursive partitioning analysis was also assigned to the patients.15 The MR imaging characteristics that were recorded included the lesion’s size (largest diameter based on FLAIR imaging), specific lobe involvement, and presence of a hemorrhagic component, all confirmed by an independent neuroradiologist.
In general, the aim of surgery was to achieve GTR of the tumor when possible. Subtotal resection was performed primarily when the tumor involved eloquent brain as confirmed by intraoperative mapping and/or monitoring (awake/speech language mapping, direct cortical motor stimulation, and motor evoked or somatosensory evoked potentials). Motor and somatosensory evoked potentials were routinely used in the majority of cases, whereas surgical navigation (CT and/or MR imaging wand) was used in all cases after 2001. The use of motor mapping or electrocorticography largely depended upon the preference of the surgeon. Motor or speech mapping was primarily used when the tumor was near the speech or motor cortex, respectively. Extent of resection was retrospectively classified from MR imaging reports obtained < 48 hours after resection as either GTR or STR by an independent neuroradiologist blinded to patient outcomes. Subtotal resection and GTR were defined as those tumors with residual and no residual enhancement, respectively, achieved by comparing pre- and postoperative MR images. Notably, the extent of resection was not based on the surgeon’s intraoperative impressions, because this method would be more subjective and prone to bias. Additionally, the degree of resection was not uniformly recorded and therefore could not be analyzed. Perioperative death was defined as death within 30 days of surgery.
The use of Gliadel wafer therapy (Eisai Inc.) was determined by both the surgeon as well as the patient. Gliadel wafers were typically not implanted when tumors were multifocal, extended across the corpus callosum, required large opening of the ventricle, or believed to be subtotally resected during the operation. Likewise, the particular use of adjuvant radiation and chemotherapy was determined by the surgeon, radiation oncologist, medical oncologist, and the patients themselves. Survival data were obtained from the social security index database (http://www.ssdi.rootsweb.ancestry.com).
Patient Selection
Patients with a KPS score ≥ 80 and who underwent a first-time resection of a primary (de novo) or a secondary glioblastoma (prior diagnosis of a lower grade glioma) were included in the analysis. Patients with a KPS score ≥ 80 were included in this study because these patients are highly functional and this cutoff point has been found to be significantly associated with survival in several studies.9,13,25,28 These patients were subsequently followed up until they lost their functional independence (KPS score < 60). According to the KPS, a score < 60 correlates with a loss of functional independence.20,38 Furthermore, the classification of primary glioblastoma was based on the WHO classification22 and was determined by our senior neuropathology staff. The identification of secondary glioblastoma was determined by our senior neuropathology staff who were blinded to patient outcomes. The criteria for pathologically identifying secondary glioblastoma was loosely based on the WHO classification scheme, including cellular pleomorphism, mitoses, endothelial proliferation, pseudopalisading, and necrosis. The designation of primary versus secondary glioblastoma was based on clinical criteria because genomic data were not routinely obtained at our institution. Primary glioblastoma were those that arose de novo, whereas secondary glioblastoma presumably arose in patients with a prior history of a lower grade glioma. Patients with repeat glioblastoma resections, as well as those with infratentorial glioblastomas or who underwent needle biopsy procedures, were excluded from the analysis. Furthermore, patients without sufficient clinical information to assign a KPS score were also excluded. These criteria were used to create a more uniform patient population with functionally independent patients to help understand the effects of resection on functional outcomes for patients with glioblastoma.
Statistical Analysis
All analyses were performed using the statistical program JMP version 7 (SAS Institute) unless otherwise noted. Summary data were presented as means ± SDs for parametric data and as median (IQR) for nonparametric data. The Kappa statistic was used to assess interobserver reliability for assigning KPS score.2 Percentages were compared using the Fisher exact test. For intergroup comparison, the Student t-test was used for parametric data and the Mann-Whitney U-test for nonparametric data. The Kaplan-Meier method was used to plot functional independence for patients with glioblastoma as a function of time, and the log-rank analysis was used to compare Kaplan-Meier plots (GraphPad Prism 5). Multivariate proportional hazards regression analysis was used to identify factors associated with functional independence for patients with glioblastoma. In this analysis, all variables associated with functional independence in the univariate analysis (p < 0.10) were included in a stepwise multivariate logistical regression model. Probability values < 0.05 in these analyses were considered statistically significant. The percentage of functional independence duration was determined by dividing the functionally independent time by survival time following resection.
Results
Preoperative Characteristics
The patient information is summarized in Table 1. A total of 844 patients underwent resection of a glioblastoma during the reviewed period. Of these 844 patients, 544 patients met the inclusion/exclusion criteria, and 333 (61%) of the patients were male. The average patient age was 52 ± 16 years at the time of surgery. Sixty-three (12%), 198 (36%), and 282 (52%) patients had a KPS score of 100, 90, and 80, respectively. Ninety-six of the patients (18%) had an RPA Class of III, whereas 448 (82%) had an RPA Class of IV. Ninety-four patients (17%) presented with seizures, 141 (26%) with headaches, 32 (6%) with nausea and vomiting, 122 (22%) with motor deficits, 96 (18%) with speech or language difficulty, 80 (15%) with visual deficits, and 28 (5%) with sensory deficits. The average size of the tumor was 4.2 ± 1.6 cm, and 259 (48%) involved the frontal lobe, 114 (21%) the parietal lobe, 138 (25%) the temporal lobe, and 33 (6%) the occipital lobe. The tumor primarily involved the cortex in 156 patients (29%). The tumor was a primary and secondary glioblastoma in 328 (60%) and 216 (40%) patients, respectively. Among patients with secondary glioblastoma, 142 (66%) had received prior radiation therapy, 19 (9%) prior temozolomide chemotherapy, and 12 (6%) a prior nontemozolomide chemotherapeutic agent (cisplatin, BCNU, and others). In addition, 21 patients (10%) had more than 1 recurrence of a nonglioblastoma glioma.
TABLE 1.
Preoperative characteristics of 544 patients with glioblastoma
| Preoperative Characteristics | Value (%) |
|---|---|
| male | 333 (61) |
| female | 211 (39) |
| mean age (yrs) ± SD | 52 ±16 |
| KPS score | |
| 100 | 63 (12) |
| 90 | 198 (36) |
| 80 | 282 (52) |
| RPA class | |
| III | 96(18) |
| IV | 448 (82) |
| preop Sx | |
| seizures | 94 (17) |
| headaches | 141 (26) |
| nausea/vomiting | 32(6) |
| motor deficit | 122 (22) |
| sensory deficit | 28(5) |
| language deficit | 96(18) |
| visual deficit | 80(15) |
| gait deficit | 26(5) |
| confusion/memory loss | 17(3) |
| urinary incontinence | 2 (0.4) |
| tumor | |
| mean size (cm) ± SD | 4.2 ±1.6 |
| frontal | 259 (48) |
| temporal | 138 (25) |
| parietal | 114(21) |
| occipital | 33(6) |
| hemorrhagic | 41 (8) |
| cortical | 156 (29) |
| primary resection | 328 (60) |
| secondary resection | 216 (40) |
Perioperative and Postoperative Outcomes
Gross-total resection was achieved in 249 patients (46%; Table 2). There were 5 cases of perioperative death (1%). Thirty-five (6%), 5 (1%), and 25 (5%) patients incurred a new or increased motor, visual, and language deficit, respectively. Two hundred and three patients (37%) had Gliadel wafers placed at the time of surgery, while 159 (29%), 39 (7%), 24 (4%), and 23 (4%) underwent temozolomide, BCNU, CCNU, and cisplatin chemotherapy, respectively. Ninety-five patients (17%) underwent some other type of chemotherapy including methotrexate, procarbazine, and vincristine. Of the 159 patients who underwent temozolomide therapy, 89 (56%) received temozolomide according to the protocol used in the study of Stupp et al.39 Three hundred ninety-six patients (73%) underwent radiotherapy, with a median dose of 6000 cGy (IQR 5940–6000 cGy).
TABLE 2.
Perioperative and postoperative characteristics of 544 patients with glioblastoma
| Characteristic | Value (%) |
|---|---|
| GTR | 249 (46) |
| STR | 295 (54) |
| periop death | 5(1) |
| postop motor deficit | 35(6) |
| postop visual deficit | 5(1) |
| postop language deficit | 25(5) |
| adjuvant therapy | |
| Gliadel | 203 (37) |
| temozolomide | 159 (29) |
| BCNU | 39(7) |
| CCNU | 24(4) |
| cisplatin | 23(4) |
| other chemotherapy | 95 (17) |
| radiation therapy | 396 (73) |
| median radiation dose (cGy) | 6000 (IQR 5940–6000) |
| median follow-up duration in mos | 10 (IQR6–16) |
| loss of functional independence | 302 (56) |
| median time to loss of function in mos | 10 (IQR 4–19) |
Interobserver Reliability
The Kappa statistic was used to assess interobserver reliability for assigning a KPS score.2 The Kappa value between the 2 clinicians was 0.94 (95% CI 0.90–0.98). This value is associated with near-perfect agreement between the 2 clinicians in assigning KPS scores.2
Functional Outcome Over Time
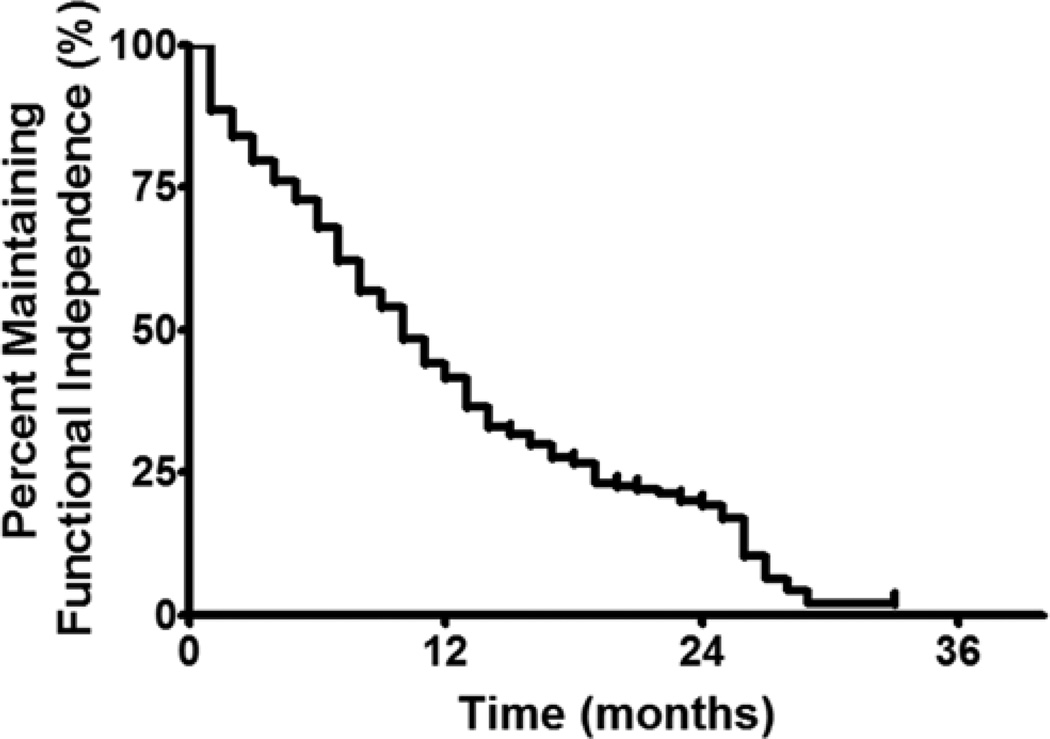
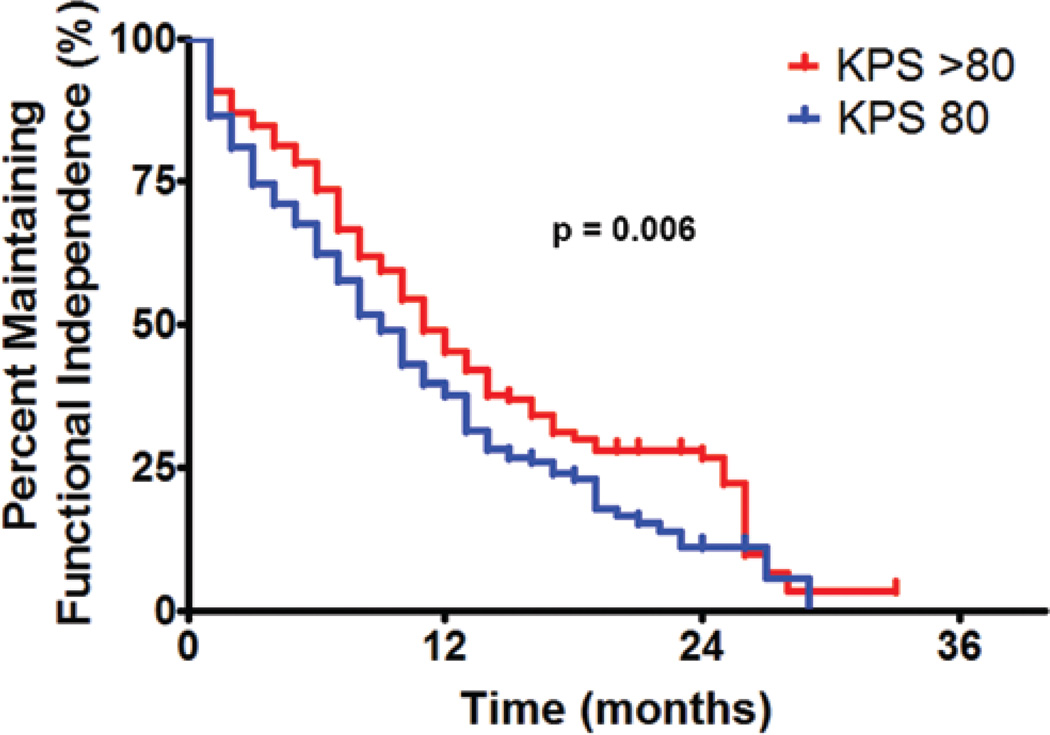
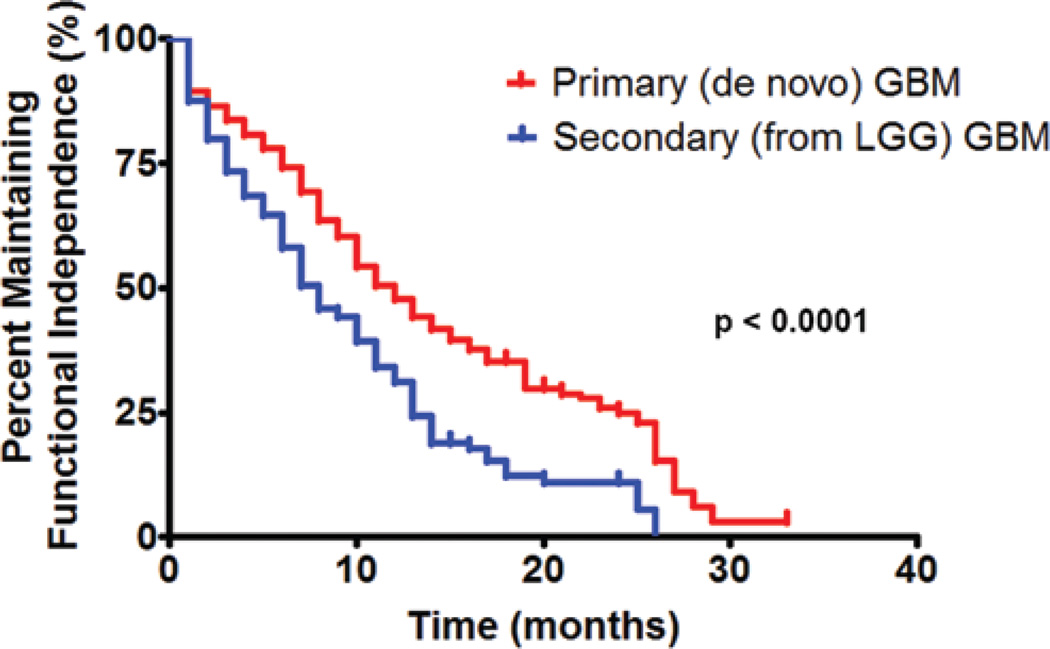
At a median follow-up time of 10 months (IQR 5.6–17.0 months), 302 patients (56%) lost functional independence (KPS score < 60; Fig. 1). In a subgroup analysis, patients with a preoperative KPS score > 80 were able to remain functionally independent for a median duration of 11 months, as compared with 9 months for patients with a KPS score of 80 (p = 0.006). The 6-, 12-, and 18-month functional independence rates for patients with a preoperative KPS score > 80 were 74%, 45%, and 30%, respectively. Patients with a preoperative KPS score of 80 had 6-, 12-, and 18-month functional independence rates of 62%, 38%, and 23%, respectively (Fig. 2). Furthermore, patients with primary glioblastoma had a median functional independence duration of 12 months as compared with 8 months for patients with secondary glioblastoma (p < 0.0001). The 6-, 12-, and 18-month functional independence rates for patients with primary glioblastoma were 74%, 48%, and 35%, respectively. In patients with secondary glioblastoma, the 6-, 12-, and 18-month functional independence rates were 58%, 31%, and 12%, respectively (Fig. 3). In regards to temozolomide chemotherapy, the median functional independence duration was 14 months for patients who received temozolomide as compared with 8 months for patients who did not receive temozolomide (p = 0.0001).
Fig 1.
Kaplan-Meier plot of maintaining functional independence for all patients with glioblastoma in this study. The median time for maintaining functional independence was 10 months, whereas the 6-, 12-, and 18-month functional independence rates were 68%, 41%, and 27%, respectively.
Fig. 2.
Graph of maintaining functional independence for patients with glioblastoma comparing those patients with a preoperative KPS score > 80 versus 80. The median time for maintaining functional independence for patients with a preoperative KPS score > 80 was 11 months, whereas the 6-, 12-, and 18-month functional independence rates were 74%, 45%, and 30%, respectively. The median time for maintaining functional independence for patients with a preoperative KPS score of 80 was 9 months, whereas the 6-, 12-, and 18-month functional independence rates were 62%, 38%, and 23%, respectively. These groups were significantly different (p = 0.006).
Fig. 3.
Graph of maintaining functional independence in patients with a primary (de novo) versus secondary (from lower grade glioma [LGG]) glioblastoma resection. The median duration for maintaining functional independence for patients with primary glioblastoma was 12 months, whereas the 6-, 12-, and 18-month functional independence rates were 74%, 48%, and 35%, respectively. The median time for maintaining functional independence for patients with secondary glioblastoma was 8 months, whereas the 6-, 12-, and 18-month functional independence rates were 58%, 31%, and 12%, respectively. These groups were statistically different (p < 0.0001).
The median survival duration for patients in this study was 11.6 months (IQR 6.5–21.7 months). Patients in this study remained functionally independent for a median of 10 months (IQR 6–16 months). For all patients, this corresponded to a median percentage functional independence duration after surgery of 65% (IQR 39–89%).
Functional Status and Survival
Among the patients in this study, improved preoperative functional status was associated with prolonged survival (RR 0.987, 95% CI 0.975–0.998; p = 0.02); patients with KPS scores ≥ 90 had the greatest statistical association with survival (RR 0.796. 95% CI 0.674–0.939; p = 0.008). Importantly, higher immediate postoperative (within 1 month) KPS score was also significantly associated with prolonged survival (RR 0.968, 95% CI 0.962–0.974; p < 0.0001); patients with KPS scores ≥ 90 had the greatest statistical association with survival (RR 0.628, 95% CI 0.528–0.747; p < 0.0001). Interestingly, immediate postoperative KPS score had a stronger association with prolonged survival as compared with preoperative functional status.
Factors Associated With Maintaining Functional Independence
Univariate Analysis
In the univariate analysis, the factors that were associated with maintaining prolonged functional independence were: preoperative KPS score ≥ 90, presence of preoperative seizures or headaches, patients with primary glioblastoma, those with GTR of their glioblastoma, and those who received Gliadel, temozolomide, or radiation adjuvant therapy. Additionally, the factors that were associated with a decreased ability to maintain functional independence in the univariate analysis were: increasing age at the time of surgery, presence of specific comorbidities (CAD, chronic obstructive pulmonary disorder, or hypertension), preoperative motor deficits or language deficits, and those who incurred a new postoperative motor or language deficit. No other clinical, imaging, or pathological variables were found to be associated with functional independence, including postoperative visual and sensory deficits.
Multivariate Analysis
In stepwise multivariate analysis, the factors significantly associated with prolonged functional independence were: preoperative KPS score ≥ 90 (OR 0.721, 95% CI 0.581–0.898; p = 0.004), preoperative seizures (OR 0.606, 95% CI 0.431–0.832; p = 0.002), primary glioblastoma (OR 0.588, 95% CI 0.467–0.742; p < 0.0001), GTR (OR 0.579, 95% CI 0.467–0.718; p <0.0001), and temozolomide chemotherapy (OR 0.622, 95% CI 0.487–0.789; p < 0.0001; Table 3). The factors that were associated with decreased ability to maintain functional independence were: increasing age (OR 1.020, 95% CI 1.012–1.028; p < 0.0001), CAD (OR 2.898, 95% CI 1.348–5.487; p = 0.009), and new postoperative motor deficit (OR 1.677, 95% CI 1.133–2.400; p = 0.01). Of note, patients who were older than 50 at the time of surgery had the greatest statistical association with decreased functional independence (OR 2.658, 95% CI 1.316–4.744; p = 0.009). Interestingly, preoperative language deficit was not statistically associated with maintaining functional independence (p = 0.27).
TABLE 3.
Multivariate associations with maintaining functional independence (KPS score ≥ 60) for patients with glioblastoma
| Variable | OR (95% CI) | p Value |
|---|---|---|
| positive associations w/ prolonged functional independence | ||
| preop KPS score ≥90 | 0.721 (0.581–0.898) | 0.004 |
| preop seizures | 0.606 (0.431–0.832) | 0.002 |
| primary glioblastoma | 0.588 (0.467–0.742) | <0.0001 |
| GTR | 0.579 (0.467–0.718) | <0.0001 |
| temozolomide chemotherapy | 0.622 (0.487–0.789) | <0.0001 |
| negative associations w/ prolonged functional independence | ||
| older age | 1.020(1.012–1.028) | <0.0001 |
| CAD | 2.898(1.348–5.487) | 0.009 |
| new postop motor deficit | 1.677(1.133–2.400) | 0.01 |
In a subgroup analysis, after stratifying patients by preoperative KPS score, patients with primary glioblastoma had a significantly longer period of prolonged functional independence as compared with patients with secondary glioblastoma. Among patients with a preoperative KPS score ≥ 90, patients with primary glioblastoma had a median functional independence duration of 14 months as compared with 8 months for patients with secondary glioblastoma (p = 0.0001). Among patients with a preoperative KPS score of 80, patients with primary glioblastoma had a median functional independence duration of 10 months as compared with 7 months for patients with secondary glioblastoma (p = 0.009). Additionally, after controlling for factors known to be associated with seizures (temporal lobe involvement, cortical location, and tumor size), seizures remained significantly associated with prolonged functional independence (RR 0.530, 95% CI 0.362–0.751; p = 0.0002). Furthermore, even after controlling for medications associated with CAD (statins, beta-blockers), as well as other related health conditions (hypertension, diabetes), CAD remained associated with decreased functional independence (RR 2.203, 95% CI 0.992–4.445; p = 0.05). Based on neurosurgery and neurooncology clinical notes, patients with CAD did not suffer any cardiac problems after the perioperative period. The presence of CAD also did not limit any therapeutic intervention. Finally, to determine if there was an association between extent of resection and postoperative motor deficit, a Fisher exact analysis was performed. There was no statistical association between extent of resection and developing a postoperative motor deficit (p = 0.28).
Loss of Functional Independence and Tumor Recurrence
Three hundred and two patients (56%) lost their functional independence at last follow-up. This loss of functional independence corresponded with tumor recurrence in 256 patients (85%). Patients who experienced a decrease in functional ability, as compared with those who maintained their functional status, were more likely to have tumor recurrence (p = 0.002). In multivariate pro- portional hazards regression analysis, loss of functional independence was independently associated with tumor progression (RR 1.468, 95% CI 1.060–2.024; p = 0.01). This association was independent of patient age at surgery, preoperative KPS score, tumor size, extent of resection, Gliadel wafer therapy, and temozolomide chemotherapy.
Discussion
In this series of 544 patients with glioblastoma and a preoperative KPS score ≥ 80, 302 patients (56%) lost their functional independence (KPS score < 60) at a median duration of 10 months (IQR 6–16 months). The median survival for patients in this study was 11.6 months (IQR 6.5–21.7 months). These data indicate that patients in this study had a median percentage functional independence duration after surgery of 65% (IQR 39–89%). Both preoperative and immediately postoperative functional status was associated with prolonged survival; a patient’s immediate postoperative functional status was most significantly associated with survival. The factors that were independently associated with prolonged functional independence were: a preoperative KPS score ≥ 90, preoperative seizures, primary glioblastoma resection, GTR, and temozolomide chemotherapy. The factors that were independently associated with decreased functional independence were: older age at the time of surgery, coexistent CAD, and incurring a new postoperative motor deficit. A decline in functional status was independently associated with tumor recurrence.
Functional Outcomes for Patients With Glioblastoma
It is well known that patients harboring glioblastoma have a poor survival prognosis.7,15,17 Most of the current clinical studies aim to optimize survival, even though median survival has shown little improvement over the past 10–15 years.40 This poor survival rate has led many patients and clinicians to question whether aggressive treatment is even warranted. Many patients, and even clinicians, would argue that prolonged functional independence is more important than prolonged survival with functional impairment. This argument stresses the need to understand the factors associated with functional outcomes, because an understanding may not only risk-stratify patients and guide clinical management, but also supply useful information regarding patients’ functional prognosis. In fact, the present study illustrates the importance that both preoperative and immediately postoperative functional status have on prolonging survival for patients with glioblastoma.
Prior clinical studies on the ability of patients to retain functional independence following glioblastoma resection are few and limited.6,8,37,41 Bussière et al.8 studied 143 patients with malignant gliomas, which included glioblastoma, anaplastic astrocytomas, and anaplastic oligodendrogliomas. These investigators found that functional outcome in this heterogeneous population was related to age, preoperative KPS score, and patients who received nonspecific multimodal treatments (surgery, radiation, and chemotherapy).8 Using a KPS-incorporated independent living score, Recht et al.37 also reported a similar positive correlation between aggressive treatment (surgery and adjuvant therapy) and functional outcomes in 75 patients with WHO Grade III and IV intracranial tumors. Furthermore, Taphoorn and colleagues41 compared the effect of radiotherapy alone versus radiotherapy and temozolomide on QOL for patients with glioblastoma in a multicenter clinical trial. They found no significant QOL differences between the 2 cohorts, and concluded that temozolomide significantly improved survival without affecting functional outcomes.41 Finally, Brown et al.6 found that GTR was associated with an improved QOL for patients with high-grade gliomas (WHO Grade III and IV). These studies were limited by relatively small patient sample sizes, heterogeneous pathologies, and analysis of only those variables previously shown to be associated with survival (age, KPS score, temozolomide chemotherapy, and extent of resection).6,8,37,41 Therefore, the effects of functional outcome over time, as well as the factors associated with functional independence, remain poorly understood.
Factors Associated With Prolonged Functional Independence
Among patients who were clearly functionally independent (KPS score ≥ 80), patients with a preoperative KPS score of 100 or 90 maintained their functional independence significantly longer than patients with a preoperative KPS score of 80. Interestingly, preoperative language deficit was not significantly associated with maintaining functional independence (p = 0.27).24,25,28 This study therefore supports the notion that more functional patients with glioblastoma not only have prolonged survival,24,25,28 but may also have prolonged functional ability.
Patients with preoperative seizures had a 40% increase in functional independence duration as compared with patients who did not have seizures. The presence of seizures is more common in low-grade gliomas,27,35,43 which has been attributed to their slow growth.4 Seizures have been associated with prolonged survival for patients with malignant glioma in some studies,34 but not others.15 The presence of seizures may therefore indicate a slower growing or less virulent glioblastoma, which allows these patients with seizures to have prolonged functional independence.
Patients who had primary (de novo) glioblastoma had an approximate 4-month increase in the median functional independence duration as compared with patients with secondary (prior lower grade glioma) glioblastoma. These subtypes of glioblastoma constitute distinct disease entities that evolve through different genetic pathways and affect patients at different ages.10,23,45 Previous studies have found no association between primary versus secondary glioblastoma and survival.1,18 This present study supports the notion that while patients with primary and secondary glioblastoma may not have different survival times, they may have different long-term functional outcomes.
The effects of extensive resection on clinical outcomes for patients with glioblastoma have been primarily limited to survival studies. Recent studies have found that GTR of malignant gliomas is correlated with prolonged survival.3,12,21,31 In fact, some studies have shown that GTR leads to an approximate 40% increase in median survival as compared with STR.31 Likewise, we found that GTR results in an approximate 45% increase in prolonged functional independence. However, this procedure should not be performed at the cost of causing a new postoperative deficit, which has been shown to be negatively associated with prolonged functional independence in this study.
Temozolomide in this study was associated with an approximate 40% increase (or approximately 6 months) in prolonged functional independence duration. Stupp et al.39 found that the addition of temozolomide to concurrent radiation therapy resulted in a significant increase in median survival time (from 12.1 to 14.6 months) in adult patients with newly diagnosed malignant astrocytomas. Other studies have found that the use of temozolomide increases survival, without the cost of impacting QOL.37,41 Although it was difficult to ascertain all of the patients’ therapeutic regimens and courses, this study supports the finding that temozolomide increases functional outcomes. The effects of other types of chemotherapy were not shown to be statistically significant in this analysis.
Factors Associated With Decreased Functional Independence
Older patients were less likely to retain prolonged functional independence in this study. In fact, patients who were older than 50 at the time of surgery were 3 times more likely to lose functional independence as compared with younger patients. This finding is not entirely surprising given that older age has been associated with poorer prognosis in terms of survival and tolerance to treatment as compared with younger patients with glioblastoma.29 This decreased ability to remain functionally independent may be a result of an age-related decrease in ability to withstand neurological insults caused by the tumor, surgery, and/or adjuvant therapy.24,25,28,29
Patients with a diagnosis of CAD were also approximately 3 times less likely to maintain their functional independence as compared with patients without CAD. This loss of functional independence for patients with CAD occurred over time; no patients were found to have a myocardial infarct or known stroke. An association between CAD and survival for patients with glioblastoma has not been previously documented. As with older age, patients with CAD may have less tolerance to neurological insults. The finding that CAD may predispose patients to poorer outcomes indicates a need to make sure patients are receiving optimal cardiac medications after surgery for malignant astrocytomas, including beta-blockers, statins, and aspirin. However, this finding may also be just a statistical correlation because patients with CAD did not suffer any cardiac problems after the perioperative period, and their comorbidity did not limit any therapeutic intervention.
The development of a new postoperative motor deficit has also been associated with decreased functional independence in this study. Even though GTR of these tumors has been associated with prolonged survival and delayed recurrence,3,12,21,31 care should be taken to avoid causing new motor deficits to preserve one’s functional status.5,6 Patients who develop new motor deficits have also been demonstrated to have poorer survival in previous studies.32 Interestingly, the development of postoperative language deficits was associated with functional independence in univariate but not multivariate analysis. A possible explanation is that patients who develop language deficits might not be as functionally limited as patients with motor deficits. Their language deficits may be minor (such as paraphasic errors) or may improve following the perioperative period. Nonetheless, because patients with new deficits may have a worse prognosis, the use of adjunctive surgical modalities including surgical navigation, motor mapping, and motor evoked potentials to guide resection should be considered.
Tumor Recurrence and Functional Decline
In this study, functional decline was significantly associated with tumor progression. The exact mechanism of this finding is unknown, but it appears intuitive that lesions that recur will cause clinical decline. Therefore, patients who functionally decline must be investigated for tumor recurrence.
Study Strengths and Limitations
The overwhelming majority of clinical studies on glioblastoma have focused on factors associated with survival and recurrence, which have changed minimally despite advances in medical and surgical therapy.11,19,30,36,44,46 An understanding of these factors is important for several reasons. First, QOL and the ability to resume activities of daily living are important for patients with glioblastoma, and may be as important as prolonged survival in many instances. Second, the effects of surgical therapy on functional outcome for patients with glioblastoma remain poorly understood. The ability to risk-stratify patients at risk for poor outcomes may help guide clinical decision making. Finally, developing strategies to prolong functional independence may provide a better approach to improving outcome for patients with glioblastoma.
This study, however, has some limitations. This study was designed to evaluate functional outcomes based on the well-accepted KPS score.20,38 This index allows for the retrospective classification of a patient’s functional status at different time points, making it ideal for this study.20,38 The use of questionnaires, on the other hand, may be flawed by poor patient response, as well as being limited to a small study population because patients with glioblastoma have poor survival times. Better data, however, could be obtained by prospectively following patients after glioblastoma resection, as well as using scales that could be determined prospectively, including the 36-Item Short Form Health Survey33 and Functional Independence Measure scores.26 These scoring systems may also better reflect a patient’s perception of his or her QOL. Second, this study follows patients who presented with a KPS score ≥ 80 and follows these patients until they lose their functional independence (KPS score < 60). Therefore, the results of these studies do not necessarily apply to patients who present with lower functional ability prior to surgery. Additionally, many of the patients in this study received different types and courses of chemotherapy and radiation therapy, which limits the efficacy of comparing different treatment regimens. Interestingly, only 73% of the patients in this study received postoperative radiation therapy. The remaining 27% of patients appear to not have received radiation therapy, but the chance that some of these patients may have received radiation therapy at another institution that was not documented could not be excluded. Another potential limitation is the identification of patients with secondary glioblastoma. The identification of patients with secondary glioblastoma does not necessarily fit the WHO classification scheme because treatment effects may hinder accurate diagnoses. These patients may actually have lower grade gliomas, and thus better outcomes, but this was not the case in this study. Lastly, this study is inherently limited by its retrospective design, and therefore it is not appropriate to infer direct causal relationships. However, we tried to create a uniform patient population by utilizing strict inclusion criteria, therefore the results of this study may not apply to all patients with glioblastoma. We included only functional patients (KPS score ≥ 80) who underwent primary resection of their glioblastoma, and excluded variants that may mask the effects of resection and functional outcome including infratentorial tumors, repeat glioblastoma resections, and patients undergoing biopsy procedures. In addition, we used multivariate analyses to control for confounding variables for patients with glioblastoma. Given these statistical controls and a relatively precise outcome measure, we believe our findings offer useful insights into the management of patients with glioblastoma. However, prospective studies are needed to provide better data to guide clinical decision making.
Conclusions
Survival for patients with glioblastoma remains dismal. This poor survival places an emphasis on understanding the effects that surgery has on QOL and, more specifically, functional independence for patients with glioblastoma. An understanding of the functional outcome over time and the factors associated with prolonged functional independence may therefore provide insight into developing effective treatment strategies aimed at improving patients’ QOL.
Abbreviations used in this paper
- BCNU
l,3-bis-(2-chloroethyl)-l-nitrosourea
- CAD
coronary artery disease
- CCNU
l-(2-chloroethyl)-3-cyclohexyl-nitrosourea
- GTR
gross-total resection
- IQR
interquartile range
- KPS
Karnofsky Performance Scale
- QOL
quality of life
- RPA
recursive partitioning analysis
- RR
relative risk
- STR
subtotal resection
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Chaichana. Acquisition of data: Chaichana, Halthore, Parker. Analysis and interpretation of data: Chaichana, Olivi, Weingart, Brem. Drafting the article: Chaichana. Critically revising the article: Quinones-Hinojosa, Chaichana, Weingart. Reviewed final version of the manuscript and approved it for submission: all authors. Statistical analysis: Chaichana, Halthore. Administrative/technical/material support: Quinones-Hinojosa, Brem. Study supervision: Chaichana.
References
- 1.al-Sarraj S, Bridges LR. p53 immunoreactivity in astrocytomas and its relationship to survival. Br J Neurosurg. 1995;9:143–149. doi: 10.1080/02688699550041476. [DOI] [PubMed] [Google Scholar]
- 2.Altman DG. Practical Statistics for Medical Research. New York: Chapman & Hall/CRC; 1991. [Google Scholar]
- 3.Barker FG, II, Prados MD, Chang SM, Gutin PH, Lamborn KR, Larson DA, et al. Radiation response and survival time in patients with glioblastoma multiforme. J Neurosurg. 1996;84:442–448. doi: 10.3171/jns.1996.84.3.0442. [DOI] [PubMed] [Google Scholar]
- 4.Boarini DJ, Beck DW, VanGilder JC. Postoperative prophylactic anticonvulsant therapy in cerebral gliomas. Neurosurgery. 1985;16:290–292. doi: 10.1227/00006123-198503000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Brown PD, Ballman KV, Rummans TA, Maurer MJ, Sloan JA, Boeve BF, et al. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neurooncol. 2006;76:283–291. doi: 10.1007/s11060-005-7020-9. [DOI] [PubMed] [Google Scholar]
- 6.Brown PD, Maurer MJ, Rummans TA, Pollock BE, Ballman KV, Sloan JA, et al. A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: the impact of the extent of resection on quality of life and survival. Neurosurgery. 2005;57:495–504. doi: 10.1227/01.neu.0000170562.25335.c7. [DOI] [PubMed] [Google Scholar]
- 7.Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003;30(6 Suppl 19):10–14. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Bussiére M, Hopman W, Day A, Pombo AP, Neves T, Espinosa F. Indicators of functional status for primary malignant brain tumour patients. Can J Neurol Sci. 2005;32:50–56. doi: 10.1017/s0317167100016875. [DOI] [PubMed] [Google Scholar]
- 9.Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol. 2007;25:2601–2606. doi: 10.1200/JCO.2006.08.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter AN, Cole CL, Playle AG, Ramsay EJ, Shervington AA. GPR26: a marker for primary glioblastoma? Mol Cell Probes. 22:133–137. doi: 10.1016/j.mcp.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Chang EF, Potts MB, Keles GE, Lamborn KR, Chang SM, Barbaro NM, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108:227–235. doi: 10.3171/JNS/2008/108/2/0227. [DOI] [PubMed] [Google Scholar]
- 12.Chang SM, Parney IF, Huang W, Anderson FA, Jr, Asher AL, Bernstein M, et al. Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293:557–564. doi: 10.1001/jama.293.5.557. [DOI] [PubMed] [Google Scholar]
- 13.Chang SM, Parney IF, McDermott M, Barker FG, II, Schmidt MH, Huang W, et al. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg. 2003;98:1175–1181. doi: 10.3171/jns.2003.98.6.1175. [DOI] [PubMed] [Google Scholar]
- 14.Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007;114:443–458. doi: 10.1007/s00401-007-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 16.De Haan R, Horn J, Limburg M, Van Der Meulen J, Bossuyt R. A comparison of five stroke scales with measures of disability, handicap, and quality of life. Stroke. 1993;24:1178–1181. doi: 10.1161/01.str.24.8.1178. [DOI] [PubMed] [Google Scholar]
- 17.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 18.Dropcho EJ, Soong SJ. The prognostic impact of prior low grade histology in patients with anaplastic gliomas: a casecontrol study. Neurology. 1996;47:684–690. doi: 10.1212/wnl.47.3.684. [DOI] [PubMed] [Google Scholar]
- 19.Hildebrand J, Lecaille C, Perennes J, Delattre JY. Epileptic seizures during follow-up of patients treated for primary brain tumors. Neurology. 2005;65:212–215. doi: 10.1212/01.wnl.0000168903.09277.8f. [DOI] [PubMed] [Google Scholar]
- 20.Hollen PJ, Gralla RJ, Kris MG, Cox C, Belani CP, Grunberg SM, et al. Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies. Psychometric assessment of the Lung Cancer Symptom Scale. Cancer. 1994;73:2087–2098. doi: 10.1002/1097-0142(19940415)73:8<2087::aid-cncr2820730813>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 21.Keles GE, Chang EF, Lamborn KR, Tihan T, Chang CJ, Chang SM, et al. Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg. 2006;105:34–40. doi: 10.3171/jns.2006.105.1.34. [DOI] [PubMed] [Google Scholar]
- 22.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–229. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- 23.Kleihues P, Ohgaki H. Primary and secondary glioblastomas: from concept to clinical diagnosis. Neuro Oncol. 1999;1:44–51. doi: 10.1093/neuonc/1.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, et al. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596–2606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 25.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6:227–235. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Law J, Fielding B, Jackson D, Turner-Stokes L. The UK FIM+FAM Extended Activities of Daily Living module: evaluation of scoring accuracy and reliability. Disabil Rehabil. 2009;31:825–830. doi: 10.1080/09638280802355049. [DOI] [PubMed] [Google Scholar]
- 27.Laws ER, Jr, Taylor WF, Clifton MB, Okazaki H. Neurosurgical management of low-grade astrocytoma of the cerebral hemispheres. J Neurosurg. 1984;61:665–673. doi: 10.3171/jns.1984.61.4.0665. [DOI] [PubMed] [Google Scholar]
- 28.Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–473. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- 29.Lopez S, Taillibert S, Idbaih A, Simon JM, Mazeron JJ. [Should elderly patients with glioblastoma be proposed to radiotherapy?] Cancer Radiother. 2008;12:827–830. doi: 10.1016/j.canrad.2008.05.003. (Fr) [DOI] [PubMed] [Google Scholar]
- 30.Maschio M, Albani F, Baruzzi A, Zarabla A, Dinapoli L, Pace A, et al. Levetiracetam therapy in patients with brain tumour and epilepsy. J Neurooncol. 2006;80:97–100. doi: 10.1007/s11060-006-9162-9. [DOI] [PubMed] [Google Scholar]
- 31.McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110:156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 32.McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65:463–470. doi: 10.1227/01.NEU.0000349763.42238.E9. [DOI] [PubMed] [Google Scholar]
- 33.McKee G. Are there meaningful longitudinal changes in health related quality of life- SF36, in cardiac rehabilitation patients? Eur J Cardiovasc Nurs. 2008;8:40–47. doi: 10.1016/j.ejcnurse.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Miller PJ, Hassanein RS, Giri PG, Kimler BF, O’Boynick P, Evans RG. Univariate and multivariate statistical analysis of high-grade gliomas: the relationship of radiation dose and other prognostic factors. Int J Radiat Oncol Biol Phys. 1990;19:275–280. doi: 10.1016/0360-3016(90)90534-q. [DOI] [PubMed] [Google Scholar]
- 35.North CA, North RB, Epstein JA, Piantadosi S, Wharam MD. Low-grade cerebral astrocytomas. Survival and quality of life after radiation therapy. Cancer. 1990;66:6–14. doi: 10.1002/1097-0142(19900701)66:1<6::aid-cncr2820660103>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 36.Perry JR, Sawka C. Add-on gabapentin for refractory seizures in patients with brain tumours. Can J Neurol Sci. 1996;23:128–131. doi: 10.1017/s0317167100038853. [DOI] [PubMed] [Google Scholar]
- 37.Recht L, Glantz M, Chamberlain M, Hsieh CC. Quantitative measurement of quality outcome in malignant glioma patients using an independent living score (ILS). Assessment of a retrospective cohort. J Neurooncol. 2003;61:127–136. doi: 10.1023/a:1022187502917. [DOI] [PubMed] [Google Scholar]
- 38.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2:187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 39.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 40.Tait MJ, Petrik V, Loosemore A, Bell BA, Papadopoulos MC. Survival of patients with glioblastoma multiforme has not improved between 1993 and 2004: analysis of 625 cases. Br J Neurosurg. 2007;21:496–500. doi: 10.1080/02688690701449251. [DOI] [PubMed] [Google Scholar]
- 41.Taphoorn MJ, Stupp R, Coens C, Osoba D, Kortmann R, van den Bent MJ, et al. Health-related quality of life in patients with glioblastoma: a randomised controlled trial. Lancet Oncol. 2005;6:937–944. doi: 10.1016/S1470-2045(05)70432-0. [DOI] [PubMed] [Google Scholar]
- 42.Taylor BV, Buckner JC, Cascino TL, O’Fallon JR, Schaefer PL, Dinapoli RP, et al. Effects of radiation and chemotherapy on cognitive function in patients with high-grade glioma. J Clin Oncol. 1998;16:2195–2201. doi: 10.1200/JCO.1998.16.6.2195. [DOI] [PubMed] [Google Scholar]
- 43.van Veelen ML, Avezaat CJ, Kros JM, van Putten W, Vecht C. Supratentorial low grade astrocytoma: prognostic factors, dedifferentiation, and the issue of early versus late surgery. J Neurol Neurosurg Psychiatry. 1998;64:581–587. doi: 10.1136/jnnp.64.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner GL, Wilms EB, Van Donselaar CA, Vecht ChJ. Levetiracetam: preliminary experience in patients with primary brain tumours. Seizure. 2003;12:585–586. doi: 10.1016/s1059-1311(03)00096-7. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe K, Tachibana O, Sata K, Yonekawa Y, Kleihues P, Ohgaki H. Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996;6:217–224. doi: 10.1111/j.1750-3639.1996.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 46.Wick W, Menn O, Meisner C, Steinbach J, Hermisson M, Tatagiba M, et al. Pharmacotherapy of epileptic seizures in glioma patients: who, when, why and how long? Onkologie. 2005;28:391–396. doi: 10.1159/000086375. [DOI] [PubMed] [Google Scholar]