Abstract
A definite diagnosis of Alzheimer disease (AD) can only be made at autopsy. Even at expert research centers, diagnostic accuracy is relatively low. We conducted this study to examine the accuracy of clinical diagnosis of AD and present a list of clinical and neuropsychological findings that could render the clinical diagnosis difficult. Using the National Alzheimer’s Coordinating Center database, the records of 533 patients who had been diagnosed clinically with AD and later underwent autopsy, were reviewed retrospectively. Since the pathologic results of 119 subjects did not meet the criteria for definite AD, we labeled them as Alzheimer “mimics”. The neuropathological diagnoses of Alzheimer mimics consisted of dementia with Lewy body (n=35, 29%), insufficient AD (n=22, 18%), vascular disease (n=15, 13%), frontotemporal lobar degeneration (n=14, 12%) and hippocampal sclerosis (n=10, 8%). History of pacemaker insertion (10.92% vs. 4.11%, p=0.005), congestive heart failure (13.45% vs. 6.04% p=0.007), hypertension (56.30% vs. 47.83%, p=0.037) and resting tremor (14.29% vs. 10.87%, p=0.170) was more prevalent in Alzheimer mimics. Clinical Dementia Rating score and frequency of Neuropsychiatric Inventory Questionnaire items reflecting delusions, agitation, depression and motor disturbance were more severe in confirmed AD. In addition to Mini-Mental State Examination (16.97±8.29 vs. 12.74±15.26, p<0.001), Logical Memory, Animal Fluency, Boston Naming Test and Digit Span scores showed more severe impairment in confirmed AD. Continuing systematic comparisons of the current criteria for the clinical and pathological dementia diagnoses are essential to clinical practice and research, and may also lead to further improvement of the diagnostic procedure.
Keywords: Alzheimer’s disease, diagnosis, pathology, dementia with Lewy bodies
INTRODUCTION
In addition to early detection of Alzheimer’s disease (AD), accurate distinction among dementia subtypes is important for patient care and pharmacological treatment. Accurate clinical diagnosis of dementia, especially AD, is becoming increasingly relevant as new treatment possibilities for neurodegenerative disorders become available. However, the confirmative identification of dementia subtypes relies on the neuropathological examination. The clinical diagnosis remains an estimation of the underlying neuropathology until the definite diagnosis is established upon autopsy. Historically, studies have indicated that clinical diagnoses of AD are often inaccurate in comparison to neuropathologic results [1–7]. A limitation of many of these reports is that they did not use standardized clinical criteria for the diagnosis of AD. Other studies did not use standardized neuropathologic criteria for autopsy examination. Some of these studies also reported small numbers of autopsies. Diagnostic accuracy rates in all of these studies were below 90%.
In an attempt to standardize the neuropathologic definition of AD, specific criteria for the diagnosis of definite, probable and possible AD were introduced by the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) in 1984 [8]. Standardized pathologic criteria based on specific age-related numbers of senile plaques and, to a limited degree, neurofibrillary tangles in the neocortex on neuropathologic examination were also introduced by Khachaturian in 1985 [9]. These Khachaturian criteria have been widely accepted, even though they were originally proposed as provisional. They provided some measure of standardization in the pathologic diagnosis of AD. Despite these methodology advances, a pattern of misdiagnosis in the clinical recognition of AD persists. The neuropathologic correlation studies using even the NINCDS-ADRDA criteria reveal diagnostic accuracy rates of 68% to 88% with clinical diagnosis [10–14]. To our knowledge, the study showing the best accuracy rate of clinical diagnosis in AD was performed by Morris et al. in 1988 [15]. Of 26 subjects clinically diagnosed as having AD who were examined at autopsy, all 26 met the pathologic criteria for AD. The 100% accuracy rate may be due in part to the use of criteria that were similar to, but somewhat more strict than, the NINCDS-ADRDA criteria. There also is a sample size issue. Once this research group had larger numbers of autopsies, their accuracy rate fell to 93% [16].
Ongoing systematic comparisons of the currently used criteria for the clinical and pathological dementia diagnoses provide essential feedback to clinicians and may hopefully also lead to further improvement of diagnostic procedures. The aim of this study was to investigate the concordance between clinical dementia diagnosis and neuropathological findings in a recent multicenter dementia study setting. We examined the accuracy of clinical diagnosis of AD and present here a list of clinical and neuropsychological findings that may confound the clinical diagnosis of AD.
MATERIALS AND METHODS
The National Alzheimer’s Coordinating Center (NACC, U01 AG016976) is responsible for developing and maintaining a database of patient information collected from the Alzheimer disease centers (ADCs) funded by the National Institute on Aging (NIA) [17]. The purpose of the NACC database is to account for the number and types of patients seen by the ADCs, and to use and analyze the data contained in the database for AD research. The NACC website (http://www.alz.washington.edu/) was developed to provide an efficient and secure system that allows access for data submission and retrieval. Each ADC has its own Institutional Review Board clearances for data collection.
The NACC database consists of a Uniform Data Set (UDS) [18, 19], which includes clinical and neuropsychological information at initial and follow-up visits, and a NeuroPathology Data Set (NPDS) [16]. NPDS contains data on pathological diagnoses which were performed based on the neuropathological criteria’s established by NIA/Reagan Institute neuropathological criteria [20], the Consortium to Establish a Registry of Alzheimer’s Disease (CERAD) [21] and the ADRDA/Khachaturian [9] for AD, with consideration of the Braak and Braak Neurofibrillary stage and the score of neuritic plaques [22, 23], the criteria modified from McKeith et al. for Dementia with Lewy Body (DLB) [24, 25], and the recent consensus criteria from Mackenzie et al. for FrontoTemporal Lobar Degeneration (FTLD) [26]. Histology and immunohistochemistry were also undertaken using a standard protocol. Histological stains included: hematoxylin and eosin, Luxol-fast blue Nissl, and a modified Bielschowsky silver impregnation. Immunohistochemistry was performed using anti-beta-amyloid (10D5), tau (PHF1), synuclein (LB509), and TDP-43 (ProteinTech) antibodies. Finally, the primary and contributing pathologic diagnoses were reported by the pathologists with their best judgment and the primary diagnosis was used in this study analysis (Table 1). The following information from the UDS was analyzed: demographic features including age, gender, education in years and family history of dementia; health history such as cardiovascular disease and cerebrovascular disease, and medical conditions including hypertension, diabetes and hypercholesterolemia; frequency of Neuropsychiatric Inventory Questionnaire (NPI-Q) items [27] and Unified Parkinson’s Disease Rating Scale (UPDRS)-motor scores [28]. Also, performance on the Mini-Mental State Examination (MMSE) [29], Clinical Dementia Rating (CDR) [30], and neuropsychological assessment test scores including Logical Memory [31], Digit Span [31], Category Fluency [32], Trail Making Test [33], Wechsler Adult Intelligence Scale Revised (WAIS-R) Digit Symbol [34], and Boston Naming Test (BNT) [35] were compared.
Table 1.
Primary and contributing pathologic diagnoses of 533 patients with clinical AD
| Primary | Contributing | Number(%) | ||
|---|---|---|---|---|
| AD | 414 | |||
|
| ||||
| AD | only | 210 (50.72%) | ||
| AD | DLB | 68 (16.43%) | ||
| AD | DLB | Vascular disease | 10 (2.42%) | |
| AD | DLB | Vascular disease | HS | 3 (0.72%) |
| AD | DLB | Vascular disease | Others | 2 (0.48%) |
| AD | DLB | HS | 5 (1.21%) | |
| AD | DLB | Others | 8 (1.93%) | |
| AD | Vascular disease | 70 (16.91%) | ||
| AD | Vascular disease | HS | 4 (0.97%) | |
| AD | Vascular disease | Others | 3 (0.72%) | |
| AD | FTLD | 2 (0.48%) | ||
| AD | HS | 10 (2.42%) | ||
| AD | HS | Others | 4 (0.97%) | |
| AD | Others | 15 (3.62%) | ||
| DLB | 35 | |||
|
| ||||
| DBL | only | 3 (8.57%) | ||
| DLB | Insufficient AD | 6 (17.14%) | ||
| DLB | Insufficient AD | Vascular disease | 1 (2.86%) | |
| DLB | Insufficient AD | Others | 2 (5.71%) | |
| DLB | AD | 16 (45.71%) | ||
| DLB | AD | Vascular disease | 1 (2.86%) | |
| DLB | AD | Vascular disease | HS | 1 (2.86%) |
| DLB | AD | HS | 2 (5.71%) | |
| DLB | AD | Others | 1 (2.86%) | |
| DLB | Vascular disease | 1 (2.86%) | ||
| DLB | HS | 1 (2.86%) | ||
| Insufficient AD | 22 | |||
|
| ||||
| Insufficient AD | only | 13 (59.09%) | ||
| Insufficient AD | DLB | 3 (13.64%) | ||
| Insufficient AD | Vascular disease | 4 (18.18%) | ||
| Insufficient AD | Vascular disease | HS | 1 (4.55%) | |
| Insufficient AD | Others | 1 (4.55%) | ||
| Vascular disease | 15 | |||
|
| ||||
| Vascular disease | only | 5 (33.33%) | ||
| Vascular disease | Insufficient AD | 1 (6.67%) | ||
| Vascular disease | Insufficient AD | Others | 1 (6.67%) | |
| Vascular disease | AD | 6 (40.00%) | ||
| Vascular disease | AD | DLB | 1 (6.67%) | |
| FTLD | 14 | |||
|
| ||||
| FTLD | only | 5 (35.71%) | ||
| FTLD | Insufficient AD | 3 (21.43%) | ||
| FTLD | AD | DLB | HS | 1 (7.14%) |
| FTLD | Vascular disease | 1 (7.14%) | ||
| FTLD | Vascular disease | Others | 1 (7.14%) | |
| FTLD | HS | 2 (14.29%) | ||
| FTLD | Others | 1 (7.14%) | ||
| HS | 10 | |||
|
| ||||
| HS | only | 3 (30.00%) | ||
| HS | Insufficient AD | DLB | 1 (10.00%) | |
| HS | Insufficient AD | Others | 1 (10.00%) | |
| HS | AD | 1 (10.00%) | ||
| HS | DLB | Others | 1 (10.00%) | |
| HS | Others | 3 (30.00%) | ||
Values are presented as number and percentage.
Among 119 Alzheimer mimics, main 5 subtypes of DLB, vascular disease, insufficient AD, FTLD, and HS are showed, in addition to pathologically confirmed AD, in this table.
AD, Alzheimer’s Disesae; DLB, dementia with Lewy body; HS, hippocampal sclerosis; FTLD, fontotemporal lobar degeneration.
The records in the NACC database for 533 participants diagnosed clinically with AD at their last ADC visit before autopsy during the period from December 19, 2005 until July 7, 2010 were reviewed retrospectively. The mean duration between the last UDS visit and autopsy was 10.42±7.97 (range, 0.3~40.6) months. Among them, 440 were probable AD and 93 were possible AD. 119 subjects whose pathologic results did not meet the criteria for primary pathologic AD (defined as meeting AD criteria according to the ADRDA/Khachaturian, CERAD, and NIA/Reagan criteria) were labeled as Alzheimer mimics. Their clinical diagnoses were 89 probable AD and 30 possible AD. We compared the clinical and neuropsychological differences between these 119 Alzheimer mimics (22.33%) and 414 (77.67%) individuals with pathologically proven AD. Comparison between confirmed AD and Alzheimer mimics was performed using Student t-test and Fisher’s exact test. Comparison among dementia subtypes such as confirmed AD, DLB, insufficient AD, vascular disease, FTLD and hippocampal sclerosis (HS) were performed using Kruskall-Wallis test and the Scheffe method after analysis of covariance (ANCOVA). All analyses were adjusted for age. Computerized statistical analysis was performed using SPSS (version 15.0) for Windows (SPSS, Chicago, IL, USA). For statistical significance, the p-value was set at 0.05 for all analyses.
RESULTS
1. Neuropathological diagnoses
Among the 119 subjects who did not conform to the criteria for primary pathologic AD, the neuropathological diagnoses that mimicked AD were DLB (n=35, 29%), insufficient AD (n=22, 18%), vascular disease (n=15, 13%), FTLD (n=14, 12%) and HS (n=10, 8%) in this order (Fig. 1). The diagnosis of insufficient AD is used for NIA/Reagan low likelihood, or cases which were not classified by NIA/Reagan criteria. Eight patients did not show any specific abnormal pathologic manifestations (7%). Other pathologic results (n=15, 13%) consisted of tangle predominant senile dementia (n=5), tau-negative (ubiquitin-positive) frontotemporal dementia (n=3), progressive supranuclear palsy (PSP, n=2), diffuse grain disease (n=2), amyloid angiopathy (n=1), corticobasal degeneration (CBD, n=1) and Rosenthal fiber encephalopathy (n=1).
Fig. 1. Pathological diagnoses of 119 Alzheimer mimics.
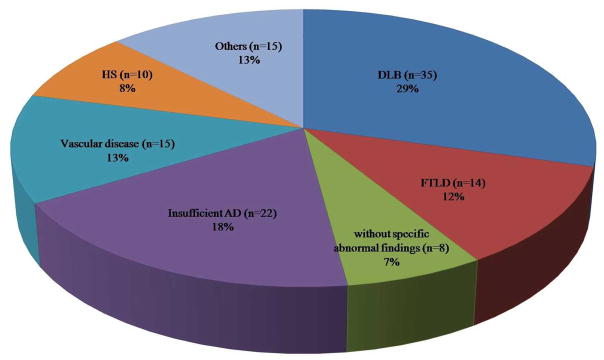
DLB, dementia with Lewy body; AD, Alzheimer’s Disease; FTLD, frontotemporal lobar degeneration; HS, hippocampal sclerosis.
2. Clinical and neuropsychological differences
The mean age(±standard deviation) of Alzheimer mimic participants was 81.58±9.29, significantly older than the mean of 78.54±10.17 for subjects with confirmed AD (t=3.038, p=0.001). Therefore, age was adjusted in other analyses. Although Alzheimer mimics were older, patients with confirmed AD showed lower MMSE and more severe CDR scores. Mean MMSE scores were 12.74±15.26 for patients with confirmed AD and 16.97±8.29 for Alzheimer mimics (t=6.124, p<0.001). Mean CDR scores of subjects with confirmed AD were 2.31 (range, 0.5~3), and those of subjects with Alzheimer mimics were 1.91 (range, 0.5~3) (t=4.629, p<0.001). Patients with confirmed AD were more likely to have mothers who had dementia (32.37% vs. 19.33%, χ2=9.669, p=0.001). A history, or evidence, of cardiovascular diseases, especially pacemaker insertion (10.92% vs. 4.11%, χ2=8.687, p=0.005), congestive heart failure (CHF, 13.45% vs. 6.04%, χ2=7.605, p=0.007) and hypertension (56.30% vs. 47.83%, χ2=3.563, p=0.037) was more prevalent in Alzheimer mimics. Other neurological conditions such as seizure and traumatic brain injury were found more prevalently in participants with confirmed AD (21.98% vs. 14.29%, χ2=3.381, p=0.041). Comparisons among dementia subtypes revealed that subjects with vascular disease were the oldest (88.12±8.70). Also, the CDR scores of subjects with insufficient AD (mean score 1.73, range 1~3) were less severe compared with those of subjects with confirmed AD. A history of cerebrovascular and cardiovascular diseases/evidence such as pacemaker insertion and CHF was dominant in vascular disease (Table 2).
Table 2.
Clinical characteristics of pathologically confirmed AD and 5 subtypes of Alzheimer mimics
| Confirmed AD (n=414 | Alzheimer mimics (n=119) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| DLB (n=35) | Insufficient AD (n=22) | Vascular D. (n=15) | FTLD (n=14) | HS (n=10) | p value† | |||
| Age (yrs) | 78.54±10.17 | 81.58±9.29* | 78.30±8.01 | 80.76±8.93 | 88.12±8.70‡ | 79.65±9.71 | 80.19±8.10 | 0.008 |
| Gender (M:F) | 239:175 | 69:50 | 22:13 | 13:09 | 5:10 | 10:04 | 5:05 | 0.374 |
| Education (yrs) | 15.09±5.29 | 15.99±11.47 | 17.51±14.63 | 13.68±3.51 | 14.33±3.22 | 15.07±4.12 | 13.50±5.06 | 0.4 |
| FHx | ||||||||
| Mother | 32.37%(134) | 19.33%(23)* | 20.00%(7) | 13.64%(3) | 6.67%(1) | 21.43%(3) | 20.00%(2) | 0.034 |
| Father | 15.94%(66) | 11.76%(14) | 20.00%(7) | 9.09%(2) | 6.67%(1) | 0.00%(0) | 10.00%(1) | 0.405 |
| Siblings | 1.45%(6) | 3.36%(4) | 2.86%(1) | 4.55%(1) | 6.67%(1) | 0.00% | 0.00% | 0.557 |
| MMSE | 12.74±15.26 | 16.97±8.29* | 13.81±9.24 | 19.82±8.47 | 21.23±5.18 | 10.83±9.55 | 17.78±5.72 | <0.001 |
| CDR | 2.31 (0.5~3) | 1.91 (0.5~3)* | 2.01 (0.5~3) | 1.73 (1~3)‡ | 1.69 (1~3) | 2.64 (1~3) | 1.78 (1~3) | <0.001 |
| HIS | 1.16±1.74 | 1.13±1.31 | 1.37±1.92 | 0.93±1.03 | 1.25±1.14 | 1.15±0.99 | 0.63±0.52 | 0.781 |
| Cardiovascular disease | 34.06%(141) | 40.34%(48) | 25.71%(9) | 50.00%(11) | 40.00%(6)* | 57.14%(8) | 40.00%(4) | 0.132 |
| Heart attack/cardiac arrest | 8.45%(35) | 9.24%(11) | 8.57%(3) | 13.64%(3) | 0.00%(0)* | 7.14%(1) | 10.00%(1) | 0.864 |
| Atrial fibrillation | 12.80%(53) | 14.29%(17) | 11.43%(4) | 4.55%(1) | 26.67%(4) | 28.57%(4) | 10.00%(1) | 0.156 |
| Angioplasty/endarterectomy/stent | 6.28%(26) | 8.40%(10) | 5.71%(2) | 4.55%(1) | 6.67%(1) | 0.00%(0) | 20.00%(2) | 0.372 |
| Cardiac bypass procedure | 5.07%(414) | 5.26%(6) | 5.71%(2) | 4.55%(1) | 0.00%(0) | 0.00%(0) | 20.00%(2) | 0.202 |
| Pacemaker | 4.11%(17) | 10.92%(13)* | 2.86%(1) | 9.09%(2) | 26.67%(4)‡ | 7.14%(1) | 10.00%(1) | 0.008 |
| CHF | 6.04%(25) | 13.45%(16)* | 8.57%(3) | 13.64%(3) | 26.67%(4)‡ | 14.29%(2) | 20.00%(2) | 0.015 |
| Cerebrovascular disease | 16.67%(69) | 19.33%(23) | 17.14%(6) | 18.18%(4) | 53.33%(8)‡ | 28.57%(4) | 0.00%(0) | 0.007 |
| Other neurologic conditions | 21.98%(91) | 14.29%(17)* | 17.14%(6) | 22.73%(5) | 6.67%(1) | 14.29%(2) | 10.00%(1) | 0.617 |
| Resting tremor | 10.87%(45) | 14.29%(17) | 28.57%(10) | 4.55%(1) | 0.00%(0) | 0.00%(0) | 40.00%(4) | <0.001 |
| UPDRS score | 0.29±1.04 | 0.64±1.84* | 1.34±2.67‡ | 0.19±0.87 | 0.00±0.00 | 0.00±0.00 | 1.90±2.69‡ | <0.001 |
| Medical/metabolic conditions | ||||||||
| HTN | 47.83%(198) | 56.30%(67)* | 54.29%(19) | 50.00%(11) | 40.00%(6) | 78.57%(11) | 40.00%(4) | 0.323 |
| Hypercholesterolemia | 39.13%(162) | 42.86%(51) | 37.14%(13) | 40.91%(9) | 26.67%(4) | 42.86%(6) | 50.00%(5) | 0.891 |
| Diabetes | 10.63%(44) | 10.08%(12) | 5.71%(2) | 4.55%(1) | 0.00%(0) | 14.29%(2) | 20.00%(2) | 0.42 |
| B12 deficiency | 6.76%(28) | 8.40%(10) | 11.43%(4) | 4.55%(1) | 6.67%(1) | 0.00%(0) | 0.00%(0) | 0.673 |
| Thyroid disease | 17.63%(73) | 15.13%(18) | 8.57%(3) | 13.64%(3) | 26.67%(4) | 14.29%(2) | 20.00%(2) | 0.569 |
| Urinary incontinence | 58.94%(244) | 56.30%(67) | 60.00%(21) | 50.00%(11) | 46.67%(7) | 78.57%(11) | 60.00%(6) | 0.657 |
| Depression | 50.48%(209) | 49.58%(59) | 57.14%(20) | 59.09%(13) | 26.67%(4) | 50.00%(7) | 40.00%(4) | 0.074 |
| Alcohol | 8.21%(34) | 10.08%(12) | 14.29%(5) | 9.09%(2) | 6.67%(1) | 14.29%(2) | 0.00%(0) | 0.712 |
| Smoking | 2.90%(12) | 1.68%(2) | 0.00%(0) | 4.55%(1) | 6.67%(1) | 0.00%(0) | 0.00%(0) | 0.644 |
| Psychiatric disorders | 7.25%(30) | 3.36%(4) | 8.57%(3) | 0.00%(0) | 6.67%(1) | 0.00%(0) | 0.00%(0) | 0.555 |
Values are presented as mean±standard deviation (range), number and percentage.
Significant difference between confirmed AD and Alzheimer mimics by Student t-test.
Comparisons among dementia subtypes such as confirmed AD, DLB, insufficient AD, vascular disease, FTLD, and HS by analysis of covariances
Significant difference after multiple comparisons by Scheffee method, compared to confirmed AD.
Age was adjusted for the analyses.
AD, Alzheimer’s Disesae; DLB, dementia with Lewy body; FTLD, fontotemporal lobar degeneration; HS, hippocampal sclerosis; FHx, family history; MMSE, mini-mental state examinatin; CDR, clinical dementia rating; HIS, Hachinski ischemic score; CHF, congestive heart failure; UPDRS, unified Parkinson’s disease rating scale; HTN, hypertension.
Compared with Alzheimer mimics, participants with confirmed AD had a higher prevalence of delusions (27.56% vs. 15.31%, χ2=6.140, p=0.008), agitation or aggression (47.16% vs. 31.63%, χ2=7.492, p=0.004), depression or dysphoria (38.35% vs. 24.49%, χ2=6.433, p=0.007) and motor disturbance (32.10% vs. 18.37%, χ2=6.992, p=0.005) on the NPI-Q.
The mean UPDRS score of resting tremor was 0.29±1.04 in subjects with confirmed AD and 0.64±1.84 in Alzheimer mimics (t=1.992, p=0.004). Results of ANCOVA revealed that the scores of resting tremor (F=8.353, p<0.001), rigidity (F=4.109, p=0.001), and bradykinesia (F=2.433, p=0.034) were different among the 6 dementia subtypes such as confirmed AD, DLB, insufficient AD, vascular disease, FTLD and HS. In particular, patients with DLB showed higher resting tremor score of 1.34±2.67 compared with those with confirmed AD at the post hoc analysis (Table 2). In addition to resting tremor, rigidity and bradykinesia were more severe in patients with DLB. The mean rigidity score was 3.51±5.45 for confirmed AD patients and 6.03±6.34 for DLB patients, and that of bradykinesia was 1.12±1.31 for confirmed AD patients and 1.60±1.50 for DLB patients. Similar results were observed for the frequency of occurrence of these conditions, although not significant. Resting tremor was 10.87% in confirmed AD and 14.29% in Alzheimer mimics including DLB (28.75%). Rigidity and bradykinesia were 45.65% and 55.80% in confirmed AD, and 68.57% and 68.57% in DLB.
As with the CDR and NPI-Q results, neuropsychological test scores showed more severe impairment in patients with confirmed AD (Table 3). These participants showed more severe logical memory decline, compared with those with DLB, insufficient AD and vascular disease.
Table 3.
Neuropsychological findings of confirmed AD and 5 subtypes of Alzheimer mimics at the last examination.
| Confirmed AD (n=414) | Alzheimer mimics (n=119) | p value* | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| DLB (n=35) | Insufficient AD (n=22) | Vascular D. (n=15) | FTLD (n=14) | HS (n=10) | p value† | ||||
| MMSE | 12.74±15.26 | 16.97±8.29 | 0.02 | 13.81±9.24 | 19.82±8.47 | 21.23±5.18 | 10.83±9.55 | 17.78±5.72 | <0.001 |
| Logical Memory IA-Immediate | 2.26±2.84 | 5.11±4.41 | <0.001 | 4.86±4.70‡ | 6.27±4.15‡ | 6.55±4.74‡ | 4.50±1.73 | 3.13±2.36 | <0.001 |
| Logical Memory IIA-Delayed | 1.04±2.15 | 2.86±4.03 | <0.001 | 3.19±4.20‡ | 4.73±4.63‡ | 3.00±2.94 | 0.75±0.96 | 1.13±1.55 | <0.001 |
| Digit Span Forward | 5.13±1.89 | 5.96±1.36 | 0.002 | 5.43±1.89 | 6.42±1.24 | 6.38±0.96 | 5.80±0.84 | 6.13±1.25 | 0.016 |
| Digit Span Backward | 2.84±1.54 | 3.47±1.37 | 0.01 | 2.80±1.19 | 3.58±1.08 | 4.08±1.04‡ | 3.00±0.71 | 3.501.93 | 0.041 |
| Category Fluency | |||||||||
| Animals | 6.43±4.84 | 8.65±4.67 | 0.001 | 8.08±4.99 | 9.00±5.13 | 9.38±4.77 | 7.00±3.39 | 7.75±3.15 | 0.075 |
| Vegetables | 3.77±3.33 | 5.51±3.27 | <0.001 | 4.87±3.65 | 6.30±2.75 | 5.62±3.82 | 5.40±2.51 | 5.50±3.16 | 0.019 |
| Trail Making Test (sec) | |||||||||
| Part A | 97.54±44.96 | 96.98±42.44 | 0.954 | 124.95±80.82 | 80.73±42.08 | 91.73±51.08 | 61.50±23.10 | 100.63±41.66 | 0.036 |
| Part B | 244.29±80.82 | 237.32±80.10 | 0.628 | 300.00±0.00 | 198.88±81.55 | 200.33±90.89 | 216.25±97.88 | 237.50±109.59 | 0.074 |
| WAIS-Digit Symbol | 18.94±17.12 | 19.56±12.28 | 0.827 | 9.50±7.113 | 22.45±15.43 | 20.00±11.94 | 30.75±8.06 | 17.86±7.27 | 0.04 |
| BNT | 14.88±8.65 | 18.96±7.33 | <0.001 | 18.22±7.70 | 20.27±7.03 | 16.15±8.20 | 19.33±6.19 | 20.63±5.45 | 0.067 |
Values are presented as mean±standard deviation.
Comparisons between confirmed AD and Alzheimer mimics by Student t-test.
Comparisons among dementia subtypes such as confirmed AD, DLB, insufficient AD, vascular disease, FTLD, and HS by analysis of covariances
Significant difference after multiple comparisons by Scheffee method, compared to confirmed AD.
Age was adjusted for the analyses.
AD, Alzheimer’s Disesae; DLB, dementia with Lewy body; FTLD, fontotemporal lobar degeneration; HS, hippocampal sclerosis; MMSE, mini-mental state examination; WAIS, Wechsler Adult Intelligence Scale; BNT, Boston naming test.
3. Follow-up of Neuropsychological findings
The mean follow-up duration of neuropsychological examinations was 19.19±8.79 months for confirmed AD patients (n=206) and 19.46±9.31 for Alzheimer mimics (n=64, t=−0.211, p=0.833).
During the follow-up assessments of neuropsychological examinations, patients with confirmed AD showed more severe changes in the scores of BNT (−4.52±5.01, t=−3.086, p=0.001) and Digit Span Forward (−0.73±1.55, t=−2.114, p=0.023) and Backward (−0.81±1.24, t=−2.772, p=0.004) examinations. Alzheimer mimics had smaller changes in BNT (−1.78±4.14) and Digit Span Forward (−0.18±1.22) and Backward (−0.18±1.32) (Fig. 2).
Fig. 2. Comparisons of follow-up neuropsychological findings between the first and the last examinations.
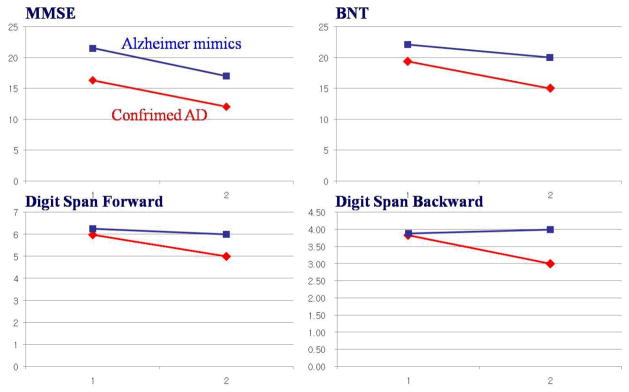
Similar to those of MMSE (−4.38±5.04 for Alzheimer mimics and −4.80±4.94 for confirmed AD, p=0.287), the changes in other neuropsychological findings were not different. The only differences were of BNT (−1.78±4.14 vs. −4.52±5.01, p=0.001) and Digit Span Forward (−0.18±1.22 vs. −0.73±1.55, p=0.023) and Backward (−0.18±1.32 vs. −0.81±1.24, p=0.004) examinations.
AD, Alzheimer’s Disesae; MMSE, mini-mental state examination; BNT, Boston naming test.
Post-hoc analysis revealed that changes in BNT and Digit Span Forward were less severe with insufficient AD and vascular disease compared with confirmed AD (Table 4). Like the MMSE results (−4.38±5.04 for Alzheimer mimics and −4.80±4.94 for confirmed AD patients, t=−0.563, p=0.287), changes in other neuropsychological findings were not different between groups.
Table 4.
Follow-up results of neuropsychological findings among dementia subtypes including confirmed AD
| Confirmed AD (n=206) | DLB (n=11) | Insufficient AD (n=11) | Vascular D. (n=10) | FTLD (n=7) | HS (n=6) | p value* | |
|---|---|---|---|---|---|---|---|
| Δ MMSE | −4.80±4.94 | −4.18±5.19 | −3.09±4.18 | −2.90±3.28 | −7.43±7.04 | −6.50±6.28 | 0.542 |
| Δ Logical Memory IA-Immediate | −1.68±2.67 | −0.71±5.12 | −0.71±1.80 | −0.33±4.36 | −1.00±0.00 | −3.00±2.45 | 0.691 |
| Δ Digit Span Forward | −0.73±1.55 | 0.17±2.14 | 0.43±0.98† | 0.10±0.32† | −1.50±0.71 | −1.20±1.26 | 0.044 |
| Δ Digit Span Backward | −0.81±1.24 | 0.17±2.14 | −0.57±0.98 | −0.10±0.99† | −1.50±0.71 | −0.25±0.50 | 0.228 |
| Δ Category Fluency | |||||||
| Δ Animals | −3.07±3.50 | −3.13±3.72 | −4.00±4.90 | −1.40±2.67 | −4.00±0.00 | −2.25±2.50 | 0.718 |
| Δ Vegetables | −2.23±2.61 | −2.75±2.49 | −3.33±3.01 | −1.70±2.79 | −0.50±4.95 | −2.50±0.58 | 0.729 |
| Δ Trail Making Test (sec) | |||||||
| Δ Part A | 21.81±34.09 | 36.83±18.16 | 33.17±42.05 | 13.56±39.78 | 53 | 56.75±58.77 | |
| Δ Part B | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0 | 0 | |
| Δ WAIS-Digit Symbol | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | ||
| Δ Logical Memory IIA-Delayed | −0.63±2.44 | −0.57±2.99 | 2.00±3.83† | −1.89±3.06 | 0 | −0.50±1.29 | 0.183 |
| Δ BNT | −4.52±5.01 | −2.75±3.58 | −0.67±1.86† | −1.60±5.27† | −1.67±8.33 | −4.50±5.69 | 0.048 |
Values are presented as mean±standard deviation, and differences between the last and first examinations.
Comparisons among dementia subtypes such as confirmed AD, DLB, insufficient AD, vascular disease, FTLD, and HS by analysis of covariances.
Significant difference after multiple comparisons by Scheffee method, compared to confirmed AD.
Age was adjusted for the analyses.
AD, Alzheimer’s Disesae; DLB, dementia with Lewy body; FTLD, fontotemporal lobar degeneration; HS, hippocampal sclerosis; MMSE, mini-mental state examination; WAIS, Wechsler Adult Intelligence Scale; BNT, Boston naming test.
DISCUSSION
In this study, we compared the clinical and neuropsychological characteristics of different dementia subtypes based on the reports of neuropathological examinations on a large number of individuals with clinically diagnosed AD. The pathological confirmation of AD in patients with a clinical diagnosis recorded in the NACC database was found for 77.67%. DLB was the disorder most commonly misdiagnosed as AD. Comorbidities such as hypertension, CHF and resting tremor were more common in Alzheimer mimics than in those with neuropathological AD. Dementia severity, behavioral symptoms and cognitive impairments were more severe in confirmed AD patients, although the average age of Alzheimer mimics was older at clinical examination. Ageing is known to be associated with an increasing number of clinical and neuropathological diagnoses [36].
Like a previous study [1], this study showed that the most common clinical errors involved misdiagnosis of dementias due to DLB and cerebrovascular disease. Pathologically insufficient AD was also diagnosed clinically as AD dementia (4.13%) in the present study database. Although the persons had an AD clinical phenotype resulting in the clinical diagnosis, the AD pathology was “insufficient” to warrant a neuropathological diagnosis of AD according to all three of the criteria used. The pathology apparently was sufficient to cause the phenotype, so maybe it is the neuropathologic criteria that are inadequate here. The diagnosis of insufficient AD in this database is used for NIA/Reagan low likelihood, or cases which are not classified by NIA/Reagan criteria. This category has been added for normal controls or subjects with mild cognitive impairment or early dementia who have low level of AD pathology such as Braak stage III or IV and moderate or frequent plaques. The high likelihood category of NIA/Reagan for AD, which requires the presence of tangles in the neocortex, is highly specific, but insufficiently sensitive to AD. Subjects with the plaque-predominant form of AD and up to 50% of cases with mild stage of AD at death have in fact moderate to frequent plaques, but no tangles in the neocortex. These subjects, although having “insufficient AD” according to the NIA/Reagan criteria, usually fulfill the less demanding the Khachaturian and CERAD criteria. An exact distinction between prodromal AD and AD dementia is important and should be emphasized in further research and treatment planning.
This study identified some clinical characteristics of Alzheimer mimics. First, a history of cerebrovascular and cardiovascular diseases such as pacemaker insertion and CHF was prevalent in Alzheimer mimics. However, the mean Hachinski ischemic score [37] was not different between patients with confirmed AD (1.16±1.74) and Alzheimer mimics (1.13±1.31), including mimics with vascular disease (1.25±1.14). For the distinction of pure vascular diseases, comparison with neuropsychological findings is essentially needed. The occurrence of extrapyramidal signs in patients with AD was also not uncommon. In addition to resting tremor, rigidity and bradykinesia were common with DLB in this study. Among patients with AD, estimates of extrapyramidal signs range from 6% to 92% [38–41]. This frequency increases with increasing severity of the illness [38, 39, 42, 43]. However, some signs were found to be much more common than others. Rigidity and hypokinesia were the most commonly reported signs, with frequencies up to 67% and 78%, respectively [41]; the presence of resting tremor were found to be much less common, from 0% to 16% [38, 41, 44, 45]. Therefore, the presence of certain parkinsonian signs, especially resting tremor, in patients with suspected AD should alert the clinician to the possibility of an alternative diagnosis. However, some patients with resting tremor, labeled as having DLB, may have actually had Parkinson disease-dementia during life, since resting tremor is common in patients with Parkinson disease-dementia and this condition is often indistinguishable from DLB at autopsy. Although HS also showed resting tremor with the frequency and severity similar to that of DLB, the rigidity and bradykinesia found in patients with HS was similar to those found in patients with confirmed AD, not DLB. Only focal neurologic findings are identified as an exclusionary criterion in the NINCDS-ADRDA criteria. However, McDaniel et al. have also reported the presence of parkinsonian features as well as focal neurologic findings as risk factors for misdiagnosis of AD [46].
There are several consecutive autopsy studies or investigations on comparable populations of dementia patients neuropathologically examined upon death. However, only a few of these studies include a reasonably large number of patients and present detailed information on dementia subtypes. Additionally, from a neuropathological point of view, it is most likely that the presence of LB was underreported, as staining with antibodies against ubiquitin or α-synuclein was not a routine procedure during most years of previous studies. LB might be difficult to detect without either of these stains [47, 48], at least when the presence of LB is sparse. Moreover, concomitant LBs may have contributed to the dementia disorder. When we consider the concomitance of LB in pathologic AD patients (96 cases), the presence of LB would be increased to above 24.58%. Many DLB patients, although not fulfilling the NIA/Reagan criteria for AD, might have a number of senile neuritic plaques sufficient to meet the Khachaturian or CERAD criteria for AD, and the 2005 McKeith criteria provide a probability that the cognitive deficit is linked to the Lewy pathology. This database was designed to check one of brainstem predominant, transitional limbic or diffuse neocortical type in the “Lewy Body Pathology” section. Pathologic characterization of Lewy body pathology is to be performed independently of Alzheimer-related pathology for this neuropathologic database. However, to answer the other question in the “Lewy Body Pathology” section, the likelihood that DLB clinical syndrome was due to DLB pathology, Alzheimer pathology as recorded in the previous “Alzheimer Type Pathology” section is used by comparison with the NIA-Reagan (Braak stage) likelihood, adapted from the 2005 guidelines. Finally, the primary diagnosis by the pathologists with their best judgment was used in this analysis. Among 35 primary DLB patients included in this study, there were 21 patients who had AD pathologies as the contributing diagnosis. Likewise, the present study did not address limitations regarding mixed pathological findings. Besides LB, the diagnoses of AD+vascular dementia (VaD) have, more importantly, remained constant over the years [49–51]. In previous studies, only the patients with Alzheimer disease and vascular pathology of such degree that both were likely to have caused or contributed to the dementia were classified as AD+VaD, while those with significant Alzheimer pathology and a minor vascular component, such as a single minor infarction, were classified as AD [49–51]. We did not consider AD+VaD. However, the subjects included in this study could be classified as patients with primary AD. Another limitation is that we could not rule out the possibility that CBD and PSP were included as FTLD diagnoses, considering the similarities of these two diseases to the frontotemporal dementia variants [52], although 2 cases of PSP and one case of CBD (n=1) were distinguished in this database. This is in accordance with the recently published consensus statement on FTLD [53], FTLD today being a commonly used umbrella term for the group.
A recently published clinicopathological study reported high concordance between clinical diagnosis and ultimate pathological diagnoses [54]. FTLD was identified with 100% sensitivity and 97% specificity and AD with 97% sensitivity and 100% specificity. Although it deals mainly with the differentiation between the cortical dementias of AD and FTLD-related syndromes, the study could increase the accuracy of pathologic diagnosis through the clinical differentiation with attention being given to (i) the evolution and course of illness; (ii) the relative salience of cognitive, behavioral and physical symptoms and signs; (iii) the pattern of cognitive deficits; and (iv) the degree of selectivity of those deficits. However, our study could not evaluate the exact accuracy of pathologic diagnosis, because we included just patients with clinically diagnosed AD, instead of all causes of dementia. Moreover, Alzheimer mimics, the new entity used in this study, who included all causes of dementia except for AD are heterogeneous pathologically. In the present study, a different pathology was responsible for the AD phenotype. Precise clinical differentiation including the course of illness and the pattern of cognitive deficits and the larger number of individual subgroups of Alzheimer mimics also will be needed. This discordance like Alzheimer mimics might be, even partly, related to the low clinical threshold of AD diagnosis, with no corresponding precision on etiology. Especially, the possible AD category could actually include more cases with many causes different from AD pathologically. In the present study, the pathological confirmation of AD was 79.77% (351/440) in patients with clinically probable AD, but 68.82% (64/93) in possible AD.
Continuing systematic comparisons of the current criteria for the clinical and pathological dementia diagnoses are essential to clinical practice and research, and may also lead to further improvement of the diagnostic procedure. Also for early detection of preclinical AD, prodromal AD and AD dementia, which would be focuses in the current research, both clinical and pathological diagnostic criteria would continuously be reviewed and revised further. Comparing these criteria and subdividing the coexistence of pathological findings could strengthen our study results. Additionally, more serial clinical findings in patients with pathologically normal and insufficient AD will also be needed.
Acknowledgments
The NACC database is funded by a National Institute on Aging (NIA) Grant U01 AG016976. This study was supported in part by a grant from the Korea Healthcare Technology R&D project, Ministry of Health & Welfare, Republic of Korea (A0102065).
Footnotes
We have no conflicts of interest.
References
- 1.Klatka LA, Schiffer RB, Powers JM, Kazee AM. Incorrect diagnosis of Alzheimer’s disease. A clinicopathologic study. Arch Neurol. 1996;53:35–42. doi: 10.1001/archneur.1996.00550010045015. [DOI] [PubMed] [Google Scholar]
- 2.Neary D, Snowden JS, Bowen DM, Sims NR, Mann DM, Benton JS, Northen B, Yates PO, Davison AN. Neuropsychological syndromes in presenile dementia due to cerebral atrophy. J Neurol Neurosurg Psychiatry. 1986;49:163–174. doi: 10.1136/jnnp.49.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sulkava R, Haltia M, Paetau A, Wikstrom J, Palo J. Accuracy of clinical diagnosis in primary degenerative dementia: correlation with neuropathological findings. J Neurol Neurosurg Psychiatry. 1983;46:9–13. doi: 10.1136/jnnp.46.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molsa PK, Paljarvi L, Rinne JO, Rinne UK, Sako E. Validity of clinical diagnosis in dementia: a prospective clinicopathological study. J Neurol Neurosurg Psychiatry. 1985;48:1085–1090. doi: 10.1136/jnnp.48.11.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wade JP, Mirsen TR, Hachinski VC, Fisman M, Lau C, Merskey H. The clinical diagnosis of Alzheimer’s disease. Arch Neurol. 1987;44:24–29. doi: 10.1001/archneur.1987.00520130016010. [DOI] [PubMed] [Google Scholar]
- 6.Becker JT, Boller F, Lopez OL, Saxton J, McGonigle KL. The natural history of Alzheimer’s disease. Description of study cohort and accuracy of diagnosis. Arch Neurol. 1994;51:585–594. doi: 10.1001/archneur.1994.00540180063015. [DOI] [PubMed] [Google Scholar]
- 7.Joachim CL, Morris JH, Selkoe DJ. Clinically diagnosed Alzheimer’s disease: autopsy results in 150 cases. Ann Neurol. 1988;24:50–56. doi: 10.1002/ana.410240110. [DOI] [PubMed] [Google Scholar]
- 8.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 9.Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 10.Risse SC, Raskind MA, Nochlin D, Sumi SM, Lampe TH, Bird TD, Cubberley L, Peskind ER. Neuropathological findings in patients with clinical diagnoses of probable Alzheimer’s disease. Am J Psychiatry. 1990;147:168–172. doi: 10.1176/ajp.147.2.168. [DOI] [PubMed] [Google Scholar]
- 11.Boller F, Lopez OL, Moossy J. Diagnosis of dementia: clinicopathologic correlations. Neurology. 1989;39:76–79. doi: 10.1212/wnl.39.1.76. [DOI] [PubMed] [Google Scholar]
- 12.Gilleard CJ, Kellett JM, Coles JA, Millard PH, Honavar M, Lantos PL. The St. George’s dementia bed investigation study: a comparison of clinical and pathological diagnosis. Acta Psychiatr Scand. 1992;85:264–269. doi: 10.1111/j.1600-0447.1992.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 13.Burns A, Luthert P, Levy R, Jacoby R, Lantos P. Accuracy of clinical diagnosis of Alzheimer’s disease. BMJ. 1990;301:1026. doi: 10.1136/bmj.301.6759.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tierney MC, Fisher RH, Lewis AJ, Zorzitto ML, Snow WG, Reid DW, Nieuwstraten P. The NINCDS-ADRDA Work Group criteria for the clinical diagnosis of probable Alzheimer’s disease: a clinicopathologic study of 57 cases. Neurology. 1988;38:359–364. doi: 10.1212/wnl.38.3.359. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC, McKeel DW, Jr, Fulling K, Torack RM, Berg L. Validation of clinical diagnostic criteria for Alzheimer’s disease. Ann Neurol. 1988;24:17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- 16.Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1988;55:326–35. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 17.Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME, Kukull WA NIA-Alzheimer’s Disease Centers. The National Alzheimer’s Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18:270–277. [PubMed] [Google Scholar]
- 18.Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA NIA Alzheimer’s Disease Centers. The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–58. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 19.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 20.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Mirra SS, Hart MN, Terry RD. Making the diagnosis of Alzheimer’s disease. A primer for practicing pathologists. Arch Pathol Lab Med. 1993;117:132–144. [PubMed] [Google Scholar]
- 22.Nagy Z, Yilmazer-Hanke DM, Braak H, Braak E, Schultz C, Hanke J. Assessment of the pathological stages of Alzheimer’s disease in thin paraffin sections: a comparative study. Dement Geriatr Cogn Disord. 1998;9:140–144. doi: 10.1159/000017038. [DOI] [PubMed] [Google Scholar]
- 23.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 24.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 25.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 26.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, 3rd, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM Consortium for Frontotemporal Lobar Degeneration. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 28.Fahn S, Elton RL . UPDRS Development Committee. The Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Macmillan Healthcare Information; Florham Park, NJ: 1987. pp. 153–163. [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D, Stone CP. Manual: Wechsler Memory Scale. Psychological Corporation; NewYork: 1973. [Google Scholar]
- 32.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 33.Armitage SG. An analysis of certain psychological tests used in the evaluation of brain injury. Psych Mono. 1946;60:1–48. [Google Scholar]
- 34.Wechsler D. Manual: Wechsler Adult Intelligence Scale. Psychological Corporation; NewYork: 1955. [Google Scholar]
- 35.Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. Lee & Ferbiger; Philadelphia: 1983. [Google Scholar]
- 36.Gay BE, Taylor KI, Hohl U, Tolnay M, Staehelin HB. The validity of clinical diagnoses of dementia in a group of consecutively autopsied memory clinic patients. J Nutr Health Aging. 2008;12:132–137. doi: 10.1007/BF02982566. [DOI] [PubMed] [Google Scholar]
- 37.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 38.Burns A, Jacoby R, Levy R. Neurological signs in Alzheimer’s disease. Age Ageing. 1991;20:45–51. doi: 10.1093/ageing/20.1.45. [DOI] [PubMed] [Google Scholar]
- 39.Chen JY, Stern Y, Sano M, Mayeux R. Cumulative risks of developing extrapyramidal signs, psychosis, or myoclonus in the course of Alzheimer’s disease. Arch Neurol. 1991;48:1141–1143. doi: 10.1001/archneur.1991.00530230049020. [DOI] [PubMed] [Google Scholar]
- 40.Corey-Bloom J, Galasko D, Hofstetter CR, Jackson JE, Thal LJ. Clinical features distinguishing large cohorts with possible AD, probable AD, and mixed dementia. J Am Geriatr Soc. 1993;41:31–37. doi: 10.1111/j.1532-5415.1993.tb05944.x. [DOI] [PubMed] [Google Scholar]
- 41.Molsa PK, Marttila RJ, Rinne UK. Extrapyramidal signs in Alzheimer’s disease. Neurology. 1984;34:1114–1116. doi: 10.1212/wnl.34.8.1114. [DOI] [PubMed] [Google Scholar]
- 42.Stern Y, Mayeux R, Sano M, Hauser WA, Bush T. Predictors of disease course in patients with probable Alzheimer’s disease. Neurology. 1987;37:1649–1653. doi: 10.1212/wnl.37.10.1649. [DOI] [PubMed] [Google Scholar]
- 43.Mayeux R, Stern Y, Spanton S. Heterogeneity in dementia of the Alzheimer type: evidence of subgroups. Neurology. 1985;35:453–461. doi: 10.1212/wnl.35.4.453. [DOI] [PubMed] [Google Scholar]
- 44.Ditter SM, Mirra SS. Neuropathologic and clinical features of Parkinson’s disease in Alzheimer’s disease patients. Neurology. 1987;37:754–760. doi: 10.1212/wnl.37.5.754. [DOI] [PubMed] [Google Scholar]
- 45.Galasko D, Kwo-on-Yuen PF, Klauber MR, Thal LJ. Neurological findings in Alzheimer’s disease and normal aging. Arch Neurol. 1990;47:625–627. doi: 10.1001/archneur.1990.00530060033012. [DOI] [PubMed] [Google Scholar]
- 46.McDaniel LD, Lukovits T, McDaniel KD. Alzheimer’s disease: the problem of incorrect clinical diagnosis. J Geriatr Psychiatry Neurol. 1993;6:230–234. doi: 10.1177/089198879300600409. [DOI] [PubMed] [Google Scholar]
- 47.Lennox G, Lowe J, Morrell K, Landon M, Mayer RJ. Anti-ubiquitin immunocytochemistry is more sensitive than conventional techniques in the detection of diffuse Lewy body disease. J Neurol Neurosurg Psychiatry. 1989;52:67–71. doi: 10.1136/jnnp.52.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 49.Jellinger KA. Clinicopathological analysis of dementia disorders in the elderly-an update. J Alzheimers Dis. 2006;9:61–70. doi: 10.3233/jad-2006-9s308. [DOI] [PubMed] [Google Scholar]
- 50.Knopman DS, Parisi JE, Boeve BF, Cha RH, Apaydin H, Salviati A, Edland SD, Rocca WA. Vascular dementia in a population-based autopsy study. Arch Neurol. 2003;60:569–575. doi: 10.1001/archneur.60.4.569. [DOI] [PubMed] [Google Scholar]
- 51.Brunnstrom H, Englund E. Clinicopathological concordance in dementia diagnostics. Am J Geriatr Psychiatry. 2009;17:664–670. doi: 10.1097/jgp.0b013e3181a6516e. [DOI] [PubMed] [Google Scholar]
- 52.Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, Parisi JE, Dickson DW. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66:41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- 53.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, 3rd, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM Consortium for Frontotemporal Lobar Degeneration. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snowden JS, Thompson JC, Stopford CL, Richardson AMT, Gerhard A, Neary D, Mann DMA. The clinical diagnosis of early-onset dementias: diagnostic accuracy and clinicopathological relationships. Brain. 2011;134:2478–2492. doi: 10.1093/brain/awr189. [DOI] [PubMed] [Google Scholar]


