Abstract
The experience of pain can be significantly influenced by expectancy (predictive cues). This ability to modulate pain has the potential to affect therapeutic analgesia substantially and constitutes a foundation for non-pharmacological pain relief. In this study, we investigated 1) brain regions involved in visual cue modulation of pain during anticipation of pain, pain administration, and pain rating; and 2) the association between pre-test resting-state functional connectivity and the magnitude of cue effects on pain ratings. We found that after cue conditioning, visual cues can significantly modulate subjective pain ratings. fMRI results suggested that brain regions pertaining to the frontoparietal network (prefrontal and parietal cortex) and a pain/emotion modulatory region (rostral anterior cingulate cortex, rACC) are involved in cue modulation during both pain anticipation and administration stage. Most interestingly, we found that pre-test resting state functional connectivity between the frontoparietal network (as identified by independent component analysis) and the rACC/MPFC was positively associated with cue effects on pain rating changes. We believe that these finding will shed new light on our understanding of variable cue/expectancy effects across individuals and how the intrinsic connectivity of the brain may influence expectancy induced modulation of pain.
Keywords: fMRI, expectancy, cue effect, resting state, independent component analysis, functional connectivity, placebo analgesia
Introduction
The experience of pain can be significantly influenced by our expectations [1; 23; 30; 42]. Previous studies have demonstrated that positive expectancy (anticipating low intensity pain) can significantly reduce pain, whereas negative expectancy (anticipating high intensity pain) can significantly increase the painful experience. Paired with expectancy-induced changes in subjective pain ratings, there are widespread changes in the brain during the anticipation and experience of painful stimuli. Nevertheless, the capacity to modulate pain in response to expectancy varies substantially across individuals [54; 61], which may reflect a crucial difference in the ability to recruit endogenous analgesia and protect our bodies from long-term exposure to pain. Despite the large impact of expectancy cues on pain modulation, the underlying mechanism remains unclear.
Previous investigators have hypothesized that two specific brain networks may be crucial for the modulatory effect of expectancy cues [1]: 1) the frontoparietal control network involved in attention and cognitive control [11] and 2) the limbic network involved in emotion modulation [38]. However, the role of these two networks for cognitive modulation of pain, and its relation to the individual variability of cue effects, is still unknown.
In recent years, the spontaneous low-frequency fluctuations in brain activity, observed during rest, have drawn the attention of neuroimaging investigators [6; 19; 44]. Investigators believe that the slow-frequency fluctuations may provide information about the intrinsic functional organization of the brain. Accumulating evidence suggests that resting state functional connectivity may be relevant for the understanding of human cognition and behavior [29; 51; 56; 57; 59]. For example, it has been shown that resting-state scans can be used to predict inhibition responses [51], reading competency [29] and memory performance [56; 57] across individuals. These results indicate that functional synchrony within a specific brain network during rest may influence subsequent behavioral responses that engage regions within that same network.
Thus, using a modified cue model [1; 23; 30; 40; 47] in a relatively large cohort of subjects (n=46), we combined task-related fMRI data with pre-test resting state fMRI data to investigate 1) the brain networks involved in cue modulation of pain and 2) how pre-test intrinsic resting state functional connectivity may influence subsequent pain modulation. Specifically, we first investigated the brain activations during task-related cue modulation using a paradigm including periods pain anticipation, pain application, and subjective pain ratings. Then, we explored the association between the pre-test resting state functional connectivity and the amplitude of the cue effects, using a data driven data analysis: independent component analysis (ICA).
Based on previous ICA analyses, three networks were identified as networks of interest in the present study. These networks, the sensory-motor, executive-control and right frontoparietal control network [2; 7; 15; 18; 49], have reliably been observed in previous ICA studies. These three particular networks were chosen because of their association with perception–somesthesis–pain [49], and in particular, the frontoparietal network was chosen because it has been implicated in cue modulation effects [1].
Experimental procedures
Subjects
Forty-eight right-handed healthy volunteers (29 female), aged 21–33 years (mean 26.4 ± 3.6 SD) participated in the study. The Institutional Review Board at Massachusetts General Hospital approved the study and all subjects gave written informed consent.
Thermal pain stimulation
Thermal pain stimuli were delivered to the skin of the right volar forearm using a TSA-2001 Thermal Sensory Analyzer with a 3 cm × 3 cm probe (Medoc Advanced Medical Systems, RimatYishai, Israel). All stimuli were initiated from a baseline temperature of 32°C and increased to a target temperature. Each stimulus was presented for 12 seconds, including 2.5 seconds to ramp up to the target temperature and 2.5 seconds to ramp down to baseline. After each stimulus, subjects rated their pain according to the Gracely Sensory scale [20].
Experimental procedure
At the beginning of the study, subjects were told that the aim of the experiment was to investigate the brain’s response to different levels of thermal pain. Subjects were then familiarized with the visual presentation paradigm, including a pre-stimulus cue, a pain stimulus symbol, and a post-stimulus rating scale. In addition, subjects were told that the pre-stimulus cue (text saying either “HIGH” or “LOW”) would indicate the level of the subsequent pain stimulus.
All subjects who participated in the study also participated in an unrelated behavioral study to investigate the acute analgesic effect of different treatments (real and sham acupuncture treatment, and placebo pill). In that study, an ascending series of heat stimuli (starting at 38°C and increasing in increments of 1°C) was applied to both arms in the first session. The baseline temperature for the ascending series (32°C) was systemically increased to target temperatures in order to obtain subjective pain tolerance levels or to a maximum of 52°C. Temperatures that elicited subjective intensity ratings in the LOW pain range (~5; on the 0–20 Sensory Box Scale) and HIGH pain range (~15 on the 0–20 Sensory Box Scale) were selected for each subject and used in the treatment study as well as the present MRI study. The two studies were separated by at least two weeks. Thus, at the time of this MRI study on cue effects, subjects were familiar with the pain rating scales and heat pain administration. Immediately prior to the fMRI scan, a brief pain sensitivity test was performed to further confirm the subjective high and low temperatures applied in this study and adjustments were made where needed.
During fMRI scanning, after resting state fMRI data collection, three different series of pseudo-randomized pain sequences were applied on the right distal forearm. Subjects were instructed to focus on a small black fixation cross in the center of the screen in front of them. The first scan was a contextual learning scan where subjects were presented with a pre-stimulus cue, indicating (without deception) whether they would be administered a LOW or HIGH pain stimulus. The duration of the cue was 2 seconds and the time before onset of the pain stimulus varied between 4, 6, 8 and 10 seconds. The duration of the pain stimulus was always 12 seconds and the intensity of the stimulus for this first sequence always corresponded to the pre-stimulus cue. After a delay of 4, 6 or 8 seconds, the Sensory Box Scale was displayed on the screen for 8 seconds, and subjects rated the intensity of their subjective pain by moving a cursor along the scale. The interval between the end of the rating task and onset of the next stimulus cue ranged from 8 to 14 seconds, with an average of 12 seconds (see Figure 1a). In total, this learning sequence included 4 LOW and 4 HIGH pain stimuli.
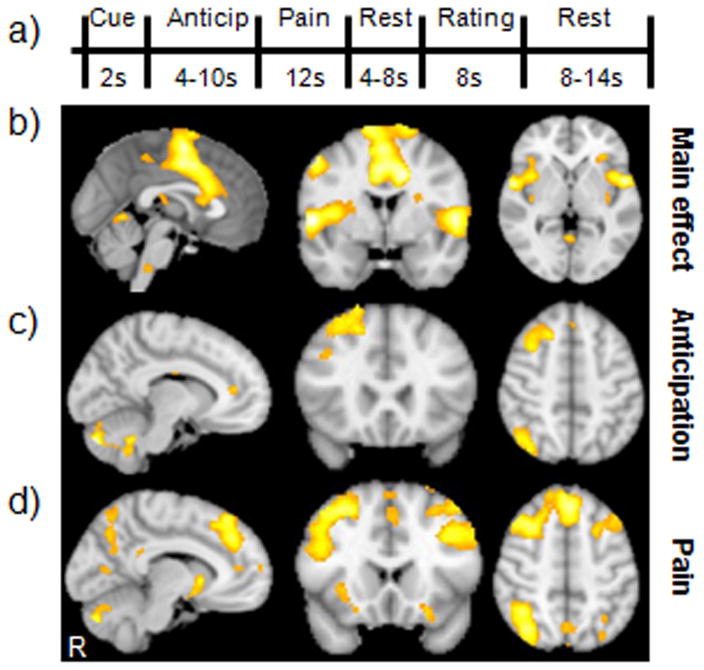
Figure 1. Experimental paradigm and corresponding BOLD responses.
(A) Each experimental heat pain trial included a cue (indicating either High or Low pain), an anticipation period (black crosshair), a painful stimulus (red crosshair), a resting period (black crosshair), a pain rating scale (0–20), and an inter-stimulus interval (black crosshair). (B) The brain networks associated with experience of pain (contrast: high pain (HP) > low pain (LP)) across three functional runs; (C) Brain regions involved in the effects of predictive cue modulation during anticipation of pain (low-cue high pain (LC) >high-cue high pain(HC)); (D) Brain regions involved in cue modulation effects during pain administration (LC > HC).
The initial contextual learning and conditioning scan was followed by two test scans in which the LOW cue was sometimes followed by the HIGH pain stimulus (HP) (the LC condition), representing a condition where subjects were expected to report less pain in response to a suggested low stimulus, and sometimes followed by the LOW pain stimulus. Both test scans included nine stimuli, where three of the stimuli were cued as HIGH pain and six were cued as LOW pain. Following all HIGH pain cues, a high pain stimulus was delivered (the HC condition). However, following three of the six LOW cues, a HIGH pain stimulus was delivered (the LC condition) instead of a LOW pain stimulus. The order of stimuli was jittered. All other timing aspects of the two test scans were identical to the first contextual learning/conditioning scan. The subjective pain ratings and fMRI signal changes evoked by the different cues (LC or HC with identical HIGH heat pain stimuli) in runs two and three were the primary outcomes of this study.
fMRI scanning
Measurements of brain activity were obtained using a 3 Tesla Siemens MRI System equipped for Echo Planar Imaging (EPI). Subjects were scanned with a high-resolution MPRAGE sequence and then a six-minute resting state fMRI. During the resting state fMRI scan, subjects were asked to look at a dark screen. The scan acquisition included 47 slices with slice thickness of 3 mm, TR 3000ms, TE 30ms, and a 3×3mm in-plane spatial resolution. The resting state scans were collected according to specific parameters that allowed us to contribute our resting state data to the “Superstructure project”, a project which aims to build a large resting state database of thousands of subjects.
Following this resting fMRI, three functional runs were collected during pain administration. For these runs, thirty axial interleaved slices (4mm thick with 1mm skip) parallel to the anterior and posterior commissure covering the whole brain were acquired with TR=2000ms, TE=40ms, flip angle= 90°, and a 3.13×3.13mm in-plane spatial resolution. The number of time points was 192 for the first functional run and 215 for both the second and third functional runs. In the scanner, cushions and earplugs were used to reduce head movement and dampen scanner noise. Visual presentation was performed using E-prime 2.0 software (Psychology Software Tools, Inc., USA) projected onto a screen in front of the subject.
Task related fMRI data analysis
Pre-processing and analyses of imaging data were performed using the Statistical Parametric Mapping 8 (SPM8) software (SPM8, Wellcome Trust Centre for Neuroimaging, London, UK) and Matlab 7.4 (Mathworks). All functional brain volumes were realigned, spatially normalized, and finally smoothed using an 8mm full-width at half-maximum (FWHM) isotropic Gaussian kernel. High-pass filtering of fMRI data (cutoff: 128s) and correction for temporal autocorrelations using AR(1) were also applied.
The univariate data analysis was performed using the general linear model (GLM). The individual design matrix for each subject (first-level matrix) included a total of twelve regressors. For the correctly cued stimuli, the following eight regressors were created: ‘low cue’, ‘low anticipation’, ‘low pain’, ‘low rating’ as well as ‘high cue’, ‘high anticipation’, ‘high pain’ and ‘high rating’. For the manipulated stimuli, where a low cue was followed by high pain, the following additional four regressors were created: ‘false low cue’, ‘false low anticipation’, ‘false low pain’ and ‘false low rating’. A file containing the movement parameters for each individual (three translation and three rotation axes) was obtained from the realignment step and saved for inclusion in the model. Regression coefficients for all twelve regressors were estimated using least squares within SPM8. Specific effects were tested by creating contrasts of the parameter estimates, resulting in a t-statistic for each voxel. After the individual first-level estimations, second-level analyses were performed using three separate paired t-tests between the low-cue high pain condition (LC) and high-cue high pain condition (HC) during three different temporal stages of the experiment: (1) measures of brain activity during anticipation of low pain (LC) versus high pain (HC), (2) measures of brain activity during the high pain stimulus application of HC versus LC, and (3) measures of brain activity during the post-stimulus rating of HC versus LC.
In addition, to further explore the relationship between cue-induced subjective pain rating changes and fMRI signal changes, we also performed a regression analysis between the pain rating difference evoked by different cues (HC and LC) and the corresponding fMRI signal changes during anticipation, pain administration, and pain rating.
In accordance with results from previous studies [1; 5; 16; 25; 30; 32; 43; 46; 55], brain regions believed to be involved in pain modulation, including the rostral anterior cingulate cortex (rACC), dorsal lateral prefrontal cortex (DLPFC), and insula were defined as ROIs. Similar to previous studies [16; 25; 55], for a priori ROIs, we used a threshold of voxel-wise p<0.005 uncorrected with 25 contiguous voxels, and p < 0.05 corrected on peak coordinate using 12 mm radius spheres for small volume correction (rACC (NMI coordinate x, y, z, 14 49 13) [60], DLPFC (−36 13 39) and insula (40 −6 −12) [55]). For non-ROI brain regions, we used a voxel-wise threshold p<0.005 uncorrected with 25 contiguous voxels and p<0.05 FEW corrected at cluster level.
fMRI resting state functional connectivity analysis
Pre-test resting state data were processed using FSL (FMRIB’s Software Library) [50] and AFNI [12] following the same processing steps (pipeline) as described previously [7]. Preprocessing of functional images included removal of non-brain structures, motion correction, temporal band-pass filtering at 0.01–0.1 Hz, spatial smoothing (6 mm FWHM Gaussian kernel), and nine parameters nuisance signal extraction. To co-register fMRI images to a standard space, functional images were first registered to each individual’s high-resolution T1 anatomical scan, and further registered to the MNI152 template using linear affine transformations with 12 degrees-of-freedom.
Probabilistic independent component analysis (PICA)at low dimensionality (20 components) was performed (MELODIC, FSL [3]) to derive the group’s (n=46) resting-state networks. The network identification was based on their spatial similarity to functional networks described in earlier studies [7; 15; 49]. Spatial correlations between our group-level networks and the template networks derived from 1414 healthy subjects [7] were calculated. The group-derived networks that showed the highest spatial overlap with the template network were assigned to that particular functional network.
In this study, the sensory-motor network, executive-control network and the right frontoparietal network were identified a priori networks for further analysis. We chose these three networks because they are known to be associated with pain sensation and modulation [49].
In order to perform group-level analyses of the association between individual responses to cue modulation and resting-state networks derived from ICA, we used a dual regression technique used in previous studies [7; 18]. In brief, the three a priori defined networks as mentioned above were first used as spatial regressors in a general linear model (GLM) to extract temporal dynamics associated with each spatial map from each subject. The resulting time-courses were then implemented as temporal regressors in a GLM to generate subject-specific maps of whole brain for each subject. Finally, a regression analysis was performed between the whole brain subject-specific network maps from the second GLM (representing the strength of functional connectivity of each voxel with sensory-motor network, executive-control network and the right frontoparietal network) and the subject’s pain rating change (HC – LC) separately; cluster correction threshold at Z>2.3, p<0.05 was applied.
Results
Of the forty-eight subjects in total who enrolled in the study, two subjects were dropped due to abnormal brain structures observed during MRI scanning. Behavioral data of the remaining forty-six subjects showed that the subjective pain ratings for the low-cue low pain condition averaged 5±2.3 (mean± SD), the low-cue high pain condition (LC) averaged 11±2.9, and the high-cue high pain condition (HC) averaged 14±2.0during the test sequences (sequences 2 and 3). A one-way mixed model was applied with mean pain ratings as the response, subject as a random effect, and conditions (low-cue low pain, low-cue high pain and high-cue high pain) as a fixed effect for the two test sequences. The result of the mixed model showed that the contrasts low-cue low pain versus high-cue high pain, and low-cue high pain versus high-cue high pain were both highly significant (P < 0.0001). Our results indicate that when identical high pain stimuli were applied, the subjective pain ratings were significantly different depending on the preceding cues. The differences between the low-cue high pain condition and high-cue high pain condition across different individuals ranged from −0.5 to 7.6 (median = 3.0), indicating significant individual variability.
Task Related fMRI (Cue Effects) Results
In order to elucidate the brain regions associated with different pain intensities during task related fMRI analyses, we compared the high and low pain conditions with correctly matched cues (low-cue with low pain and high-cue with high pain) across the three functional runs. Consistent with previous studies [24–26; 28; 55], results showed significant brain activations (high pain > low pain) in the entire network of pain intensity-associated brain regions, including the bilateral insular/opercular cortices, secondary somatosensory cortex, dorsal anterior cingulate cortex (dACC)/medial frontal gyrus, dorsal lateral prefrontal cortex, thalamus, caudate, cerebellum, brain stem, left primary somatosensory cortex/primary motor cortex (Figure 1b). Corresponding subjective pain ratings were 5 ± 2.3 and 14 ± 1.8for LOW and HIGH intensity pain, respectively.
Next, we compared the high-cue high pain (HC) and low-cue high pain (LC) conditions, in which subjects received identical levels of high pain stimuli, for each of the three pain processing phases (anticipation, pain administration, and pain rating). During the anticipation stage, the LC condition (LC anticipation > HC anticipation) evoked significantly more fMRI signal increases than the HC condition in the left rACC and cerebellum, right middle frontal gyrus, inferior frontal gyrus, and inferior parietal lobule (see Table 1 and Figure 1c). Notably, no brain regions showed significant fMRI signal changes in the opposite comparison (HC anticipation > LC anticipation).
Table 1.
Brain regions involved in contextual conditioning of pain perception (LC: low-cue high pain condition; HC: High-cue high pain condition) using SPM8
| Stage | Contrast | Brain region | Z value | Cluster size | Peak coordinate |
|---|---|---|---|---|---|
| Anticipation | LC > HC | Left rostral ACC + | 3.36 | 42 | 12 40 10 |
| Right middle frontal gyrus | 3.60 | 742 | −20 24 50 | ||
| Right inferior frontal gyrus + | 3.43 | 395 | −24 64 18 | ||
| Right inferior parietal lobule | 4.26 | 984 | −48 −60 46 | ||
| Left cerebellum | 4.76 | 766 | 10 −82 −34 | ||
| HC >LC | No significant clusters | ||||
| Pain | LC > HC | Bilateral posterior medial prefrontal cortex | 4.48 | 5598 | −4 36 54 |
| Bilateral anterior medial prefrontal cortex | 3.03 | −2 58 20 | |||
| Right middle frontal gyrus | 4.25 | −20 60 8 | |||
| Right inferior frontal gyrus | 3.49 | −44 38 −10 | |||
| Right anterior insula | 3.61 | −34 22 −2 | |||
| Left anterior medial prefrontal gyrus | 3.27 | 589 | 14 64 10 | ||
| Left middle frontal gyrus | 4.31 | 26 60 6 | |||
| Left rostral ACC+* | 2.93 | 31 | 10 46 10 | ||
| Left middle frontal gyrus | 3.75 | 1425 | 38 16 34 | ||
| Right angular gyrus | 4.74 | 5236 | −38 −72 32 | ||
| Right inferior/superior parietal lobule | 4.08 | −36 −68 50 | |||
| Right middle temporal gyrus | 4.33 | −52 −66 30 | |||
| Left angular gyrus | 3.20 | 396 | 34 −68 30 | ||
| Left inferior/superior parietal lobule | 3.06 | 30 −64 56 | |||
| Bilateral cerebellum | 4.10 | 1211 | 8 −80 −34 | ||
| HC > LC | No significant clusters | ||||
| Rating | LC> HC | No significant clusters | |||
| HC > LC | No significant clusters |
p< 0.05 using small volume correction,
*P = 0.07 using small volume correction. All other brain regions showed significant difference after FWE correction at cluster level (p< 0.05).
During pain administration, the LC condition was associated with significant increases in fMRI signal (LC pain > HC pain) in brain regions including the bilateral pMPFC, anterior MPFC, middle prefrontal cortex, inferior & superior parietal lobule, angular gyrus, and cerebellum; left rACC, right inferior frontal gyrus, anterior insula, middle temporal gyrus, (Figure 1d and Table 1). No brain regions displayed significant fMRI signal increases in the opposite comparison (HC pain > LC pain).
Lastly, we compared the high-cue high pain (HC) and low-cue high pain (LC) conditions during the pain-rating task. The results of this comparison demonstrated that no brain regions displayed significant fMRI signal differences between the LC and HC conditions (Table 2).
Table 2.
Results from regression analyses between the subjective pain rating change (HC – LC) and the corresponding fMRI signal changes (LC: low cue, high pain condition, HC: High cue, high pain condition)
| Stage | Brain region | Z value | Cluster size | Peak coordinate |
|---|---|---|---|---|
| Anticipation | No significant clusters | |||
| Pain + | Left operculum/insula | 5.23 | 256 | 54 −6 12 |
| Left pre-central gyrus | 4.49 | 124 | 46 −10 34 | |
| Right middle frontal gyrus | 4.54 | 431 | −58 2 40 | |
| Right post-central gyrus | 4.13 | 131 | −26 −32 52 | |
| Left secondary somatosensory cortex | 4.41 | 114 | 50 −34 30 | |
| Right secondary somatosensory cortex | 4.61 | 299 | −52 −32 22 | |
| Right insula | 4.73 | 273 | −44 8 −2 | |
| Right inferior temporal gyrus | 5.0 | 113 | −48 −58 −4 | |
| Left superior temporal gyrus | 4.44 | 205 | 36 −28 −22 | |
| Right fusiform gyrus/cerebellum | 4.94 | 951 | −18 −54 −12 | |
| Left fusiform gyrus/cerebellum | 4.38 | 175 | 18 −52 −14 | |
| No significant clusters | ||||
| Rating +* | Left hippocampus/parahippocampus | 3.91 | 2110 | 28 −32 −10 |
| Left middle temporal gyrus | 3.91 | 52 −14 −24 | ||
| Bilateral brain stem | 4.45 | 0 −36 −36 | ||
| No significant clusters |
voxelwise p = 0.0001 was used to detangle large cluster,
*voxelwise p= 0.001 was used to detangle large cluster. All brain regions showed significant association after FWE correction at cluster level (p< 0.05).
A regression analysis between the pain rating difference evoked by different cues (HC and LC) and the corresponding fMRI signal changes showed significant positive associations in brain regions including bilateral insula/operculum, secondary somatosensory cortex, and fusiform gyrus/cerebellum, left precentral gyrus and superior temporal gyrus, right middle frontal gyrus, postcentral gyrus, and inferior temporal gyrus during pain administration. In addition, we also found significant associations in the left hippocampus/parahippocampus, middle temporal gyrus, and bilateral brain stem during pain rating stage.
To further validate the findings from studies using a similar cue model for pain modulation [1; 23; 30; 32] that have suggested fMRI signal decreases when comparing HC pain > LC pain, we performed an identical comparison of the high-cue high pain (HC) and low-cue high pain (LC) conditions in subgroups of our subjects: 16 “good responders” (top 1/3 of subjects based on the HC-LC pain rating ranking: mean± SD 4.8 ± 1.1) and 16 “poor responders” (bottom 1/3 of subjects based on the HC-LC ranking, 1.0 ± 1.0). The results of this comparison indicated that in good responders, LC was associated with significant fMRI signal decrease in left insula (p < 0.005, small volume correction using coordinate (40 −6 −12) [55] p = 0.022 peak) at 52 −4 10, with 178 voxels, compared to the HC condition. Poor responders, in contrast, showed no significant fMRI signal change when we compared HC and LC conditions.
fMRI pre-test resting state ICA results
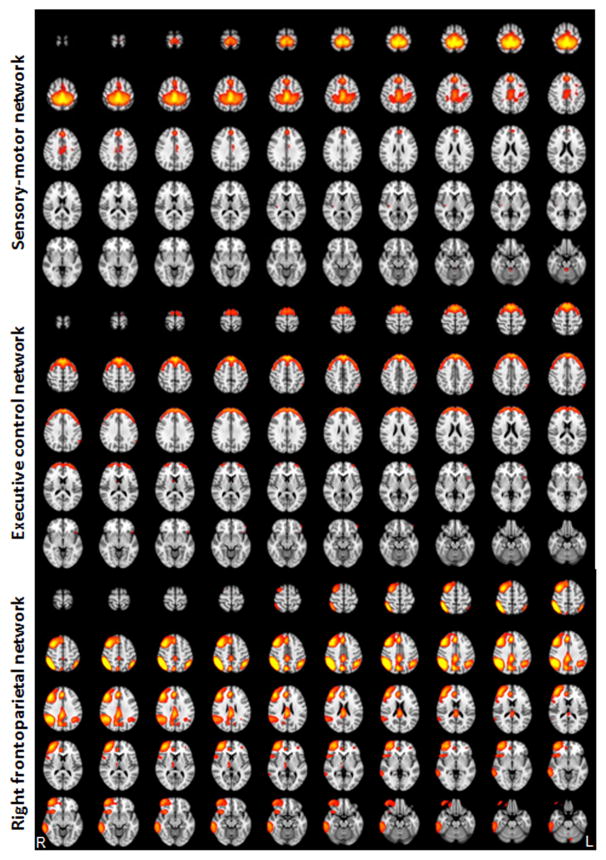
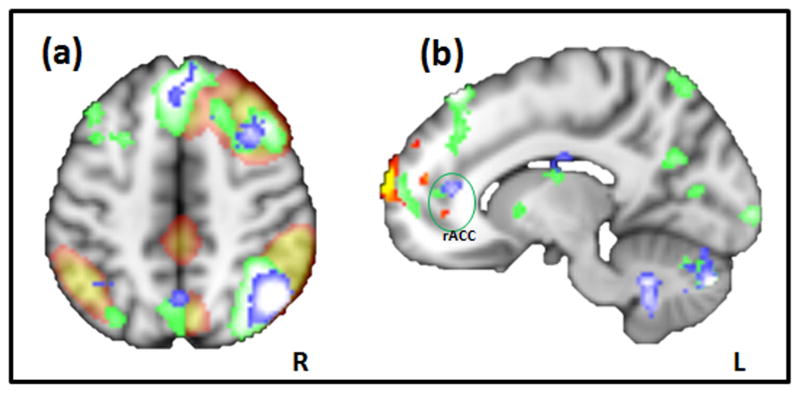
Based on all 46 subjects, twenty spatial and temporal components were obtained from the ICA analysis, similar to the results from previous ICA analyses [7; 18]. Three resting state networks, i.e., the sensory-motor network, executive-control network, and right frontoparietal network, were identified and validated based on previous studies that reliably observed these three networks [7; 18] (see Figure 2). Interestingly, the right frontoparietal network, which includes bilateral dorsal lateral prefrontal gyri, the inferior and superior parietal lobules, highly overlapped with the brain regions observed during the analysis of pain administration and anticipation for the contrast comparing LC and HC as mentioned above (Figure 3a).
Figure 2.
Three networks identified by ICA analysis (z = 4–10).
Figure 3.
(A) Spatial overlap of the frontoparietal networks (red) as observed by ICA analysis using fMRI resting state data, and LC > HC comparison during anticipation and pain administration (blue and green, respectively). (B) Sagittal view of the rACC locations from three different analyses. Red-yellow heat map represents results of the regression analysis between degree of functional connectivity with frontoparietal network and the cue effects, and the blue and green represent the LC > HC comparisons during anticipation and pain administration, respectively.
A regression analysis for each of the three subject-specific network maps derived from second GLM and the behavioral cue effects (HC – LC rating) was performed. We found a significant positive association between the second GLM map, which indicates the strength of functional connectivity with frontoparietal network, and behavioral cue effects in bilateral medial prefrontal cortex and left rACC (Table 3, Figure 4). Similarly, for the sensory-motor network, significant positive associations were observed in the cerebellum and brain stem (medulla) (Table 3). For the executive-control network, no significant association was observed.
Table 3.
Results from the regression analysis between the subjective pain rating change (HC – LC) and subject-specific spatial network maps from ICA using FSL
| Network | Brain region | Z value | Cluster size | Peak coordinate |
|---|---|---|---|---|
| Sensory-motor network | Left cerebellum | 4.40 | 1086 | −38 −46 50 |
| Right cerebellum | 3.52 | 12 −54 −48 | ||
| Right cerebellum/brain stem | 3.75 | 876 | 6 −46 −48 | |
| Executive control network | No significant clusters | |||
| Right frontoparietal network | Right MPFC | 4.08 | 2000 | 12 38 36 |
| Left MPFC | 3.67 | −12 70 −14 | ||
| Left rostral ACC | 3.5 | −16 52 14 |
Figure 4.
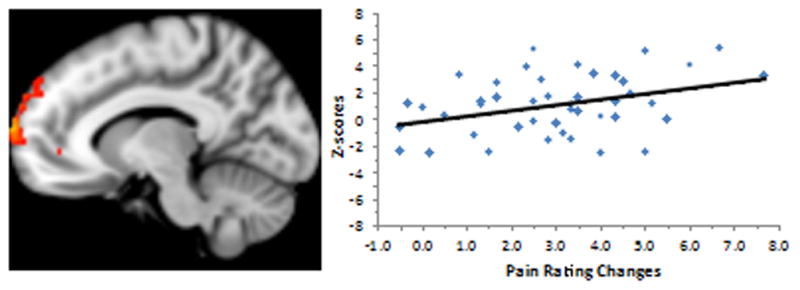
Resting state functional connectivity between the frontoparietal network and the rACC& MPFC is significantly associated with subjective pain rating changes evoked by visual cue. The x-axis of the bar graph (right) indicates the subjective pain rating changes (HC – LC); the y-axis of the bar indicates the z-score of functional connectivity between frontoparietal network and rACC at resting state.
To explore the relationship between the difference in high pain and low pain ratings in the first run (conditioning/learning run) and the cue effects as measured in run 2 and run 3, we performed a Pearson correlation analysis between the difference in high pain and low pain ratings in run 1 and the cue effects (HC-LC rating). Results showed that there is a moderate correlation between the two (r = 0.4, p = 0.002); however, when we performed an identical regression analysis between the difference in high pain and low pain ratings and functional connectivity maps of different networks, no brain regions showed significant differences, indicating that the pre-test resting state connectivity did not correlate with the response to the contextual learning scan.
Finally, we also investigated the association between three networks and the pain threshold and tolerance separately. The only significant association we observed was between the executive control network and pain threshold at bilateral middle cingulate cortex/MPFC. The network-threshold results showed no overlap with the regression analysis between the executive control network and cue effect.
Discussion
This study investigated the neural underpinnings of how predictive cues can modulate the subjective experience of pain, and the association between cue modulatory effects and pre-test resting state functional connectivity. We found that predictive cues could significantly modulate subjective pain ratings, reflected in brain regions such as the dorsal lateral prefrontal gyrus, and inferior parietal lobules and right rACC. Most importantly, we found a significant association between the individual ability to modulate pain and pre-test resting state functional coherence between the frontoparietal network and rACC and MPFC. Our results may deepen our understanding of the individual variability in pain modulatory responses, and ultimately contribute to the development of new treatment strategies using endogenous analgesia.
To the best of our knowledge, this is the first study to investigate the association between pre-test resting state functional connectivity and the cue modulation effect on pain across different individuals. Previous studies [8; 41] have suggested that pre-stimulus functional connectivity may predict subsequent somatosensory perception. These studies contributed to our understanding of how sensory processing could be modulated by brain activity seconds prior to stimulus application. However, these studies focused on trial-to-trial variability within individual subjects, not on the variability across different individuals, which is the focus of this study.
There is a large body of literature suggesting that the frontoparietal network, including the prefrontal, parietal cortex and MPFC/ACC, is involved in guiding decisions and performance adjustment [35; 52]. Studies suggest that the frontoparietal network may integrate information from the external environment with stored internal representations [34], control top–down attention during conflict processing of alternative responses [11], and control monitor conflict with subsequent adjustment in performance [10; 14; 33; 45].
In a recent study, investigators [37] manipulated the correspondence between anticipated and actual recognition evidence by presenting valid or invalid anticipatory cues before recognition judgments. They found a reliable correlation between participants’ response biases and activations in the frontoparietal network, including the pMPFC, suggesting that a mechanism of expectation and conflict regulation may be involved in cue modulatory effects on pain.
Previous studies also found that the frontoparietal network plays an important role in endogenous pain modulation. For example, Wager and colleagues [54] found that increased anticipatory activity in the frontoparietal control network may predict placebo responses. In the present study; however, we found significant fMRI signal increases in the frontoparietal network, including right frontal and parietal cortex during both anticipation and pain application, where the activation in the right frontal and parietal cortex was stronger than those on the left side. This activation pattern of the frontoparietal network, observed during pain anticipation and administration of cue modulation, highly overlapped with the frontoparietal network identified during pre-test resting state using ICA. Taken together, there are strong indications that the frontoparietal network plays an important role in expectancy-induced modulation of pain.
Interestingly, we found that the stronger the pre-test resting state intrinsic functional connectivity between the frontoparietal network and rACC and anterior MPFC, the more robust the cue effects of pain modulation. The rACC is frequently reported as an essential node within the descending pain modulatory system and it interacts closely with other brain regions such as the periaqueductal gray (PAG), amygdala, and nucleus accumbens [17]. A previous study from our group found that key regions of the pain descending control system, i.e., the rACC-PAG-rostral ventral medulla (RVM), are functionally connected during resting state [27]. Several studies have shown that placebo analgesia is associated with fMRI signal increases in the rACC, compared with a control condition [5; 16; 25; 39]. For instance, in a recent study [16], Eippert and colleagues found that the coupling between the rACC and PAG observed in placebo analgesia was modulated by naloxone, which supports the role of endogenous opioids and the rACC in top-down modulation of pain.
In addition to its central role in the descending pain modulation system, the rACC is also involved in emotional modulation. The specific region of the rACC observed in our study, located in the affective subdivision of the ACC [9], has previously been linked to arousal during emotional/motivational processing [13]. Kalisch and colleagues [22] found that activation in the MPFC/rACC and lateral prefrontal cortex wa associated with modulation of anticipatory anxiety evoked by pain. Moreover, activity in the rACC has also been associated with positive affective states such as happiness [53]. Thus, in our study, the rACC may serve as a top-down modulator of negative emotional responses evoked by pain. Taken together, we believe that the frontoparietal network may influence the individual variability observed in the cue effect through its association with the rACC, which may activate the descending pain modulatory system and/or emotional modulation network. The elucidation of this mechanism may provide new clues for our understanding of individual variability of expectancy effects in response to pain.
One related question is whether the observed connectivity changes over time within the same subject, or whether the connectivity represents a trait or state neural factor? Previous studies have suggested that resting state functional connectivity measurements have moderate to high reliability [48; 62]; in parallel, studies also suggest that previous experience can significantly modify functional connectivity [31, Schultz, 2012 #2873; 58]. We speculate that the resting state functional connectivity measured immediately prior to the task may represent a combination of trait and state neural factors, however further study is needed at this point.
In addition to the frontoparietal network, we also found that resting state functional connectivity between the sensory-motor network and the brainstem (ventrolateral medulla)/cerebellum was significantly associated with cue-evoked changes in pain ratings. The brainstem/medulla [17; 21] is another important region in the descending pain modulatory network, which sends direct projections to dorsal horn laminae to modulate pain transmission. Although the role of the cerebellum in pain experience is still under debate, studies [36] have suggested that it can act as an integrator of multiple systems including affective processes, pain modulation, and sensorimotor processing. Thus, increased connectivity between the sensory-motor network and brainstem/cerebellum may imply enhanced capacity to modulate pain.
Although we did not find any fMRI signal decreases when we compared the HC and the LC conditions during pain administration across the whole cohort of subjects, regression analyses have showed significant positive associations between the cue effect and the corresponding fMRI signal changes in pain associated brain regions such as insula and S2. In addition, an identical analysis on subgroups of good and poor responders showed that the LC condition was associated with significant fMRI signal decrease as compared with HC condition, reflected in the left insula. This result indicates that the large individual variability in response to the present cue paradigm may explain the lack of significant fMRI signal decrease between the HC and LC comparison across the whole cohort.
In this study, we also found a positive correlation between the HC and LC pain rating difference and corresponding fMRI signal changes during pain rating in left hippocampus, parahippocampus, and middle temporal gyrus. It is well known that these brain regions are involved in semantic memory, which refers to knowledge about people, objects, actions, relations, self, and culture acquired through experience [4]. We speculate that this finding implies that recall of previous contextual conditioning experiences may subsequently influence the subjective pain intensity rating, a task that can be compared to a complicated cognitive decision making process [28].
One possible limitation of our experimental design is the omission of a high-cue low pain condition to balance the design. The reason we did not include this condition was to maintain a balance between sample size and total scan time (as each additional condition required more scan time). In this tradeoff, we chose a relatively large sample size in order to increase power to investigate individual variability; we were particularly interested in the association between resting state functional connectivity and the cue effects. The robust behavioral results (including the significant statistical difference between the LC and HC conditions), the task-related fMRI analysis as well as the resting state fMRI results validate that the sample size was sufficient for detecting cue-related effects and therefore appropriate for this design.
In summary, using a combined task-related and resting state experimental paradigm we found that specific brain regions, including frontal and parietal gyri and the rACC, are involved in cue modulation of pain during pain anticipation and pain application. Most importantly, we were able to demonstrate, for the first time, that pre-test intrinsic resting state connectivity between the frontoparietal network and the rACC/MPFC is associated with predictive cue modulation of pain. The elucidation of this mechanism may enhance our understanding of the individual variability of expectancy effects in response to pain.
Acknowledgments
This work was supported by KO1AT003883 (NCCAM), R21AT004497 (NCCAM), R03AT218317 (NIDA), R01AT006364 (NCCAM) to Jian Kong, R01AT005280 (NCCAM) to Randy Gollub, K24AT004095 (NCCAM) to Ted Kaptchuk, PO1-AT002048 (NCCAM) to Bruce Rosen, M01-RR-01066 and UL1 RR025758-01 for Clinical Research Center Biomedical Imaging Core from National Center for Research Resources (NCRR), and P41RR14075 for Center for Functional Neuroimaging Technologies from NCRR. Karin Jensen receives support from the Swedish Society for Medical Research (SSMF) and the Swedish Council for Working Life and Social Research (FAS). The authors also thank Dr. Tom Zeffiro for his valuable suggestions and consultation on data analysis methods and Mr. Magnus Gyllensward for his help in figure preparation
Footnotes
There is no conflict of interest to claim for all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30(39):12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker A, Sotje I, Paulmann C, Beckmann F, Donath T, Boese R, Prymak O, Tiemann H, Epple M. Calcium sulfate hemihydrate is the inorganic mineral in statoliths of Scyphozoan medusae (Cnidaria) Dalton Trans. 2005;(8):1545–1550. doi: 10.1039/b416246c. [DOI] [PubMed] [Google Scholar]
- 3.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 4.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120(1–2):8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 7.Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci U S A. 2007;104(29):12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 10.Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7(4):367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- 11.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 12.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 13.Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci U S A. 2004;101(17):6333–6334. doi: 10.1073/pnas.0401510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16(4):475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- 15.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63(4):533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5(7):565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 18.Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 20.Gracely RH, McGrath PA, Dubner R. Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: manipulation of affect by diazepam. Pain. 1978;5:19–29. doi: 10.1016/0304-3959(78)90021-0. [DOI] [PubMed] [Google Scholar]
- 21.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60(1):214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalisch R, Wiech K, Critchley HD, Seymour B, O’Doherty JP, Oakley DA, Allen P, Dolan RJ. Anxiety reduction through detachment: subjective, physiological, and neural effects. J Cogn Neurosci. 2005;17(6):874–883. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- 23.Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci. 2006;26(16):4437–4443. doi: 10.1523/JNEUROSCI.4463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong J, Gollub RL, Polich G, Kirsch I, Laviolette P, Vangel M, Rosen B, Kaptchuk TJ. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. J Neurosci. 2008;28(49):13354–13362. doi: 10.1523/JNEUROSCI.2944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26(2):381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong J, Loggia ML, Zyloney C, Tu P, Laviolette P, Gollub RL. Exploring the brain in pain: Activations, deactivations and their relation. Pain. 2010;148:257–267. doi: 10.1016/j.pain.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong J, Tu PC, Zyloney C, Su TP. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res. 2010;211(2):215–219. doi: 10.1016/j.bbr.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2006;27(8):715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyama MS, Di Martino A, Zuo XN, Kelly C, Mennes M, Jutagir DR, Castellanos FX, Milham MP. Resting-state functional connectivity indexes reading competence in children and adults. J Neurosci. 2011;31(23):8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A. 2005;102(36):12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A. 2009;106(41):17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lui F, Colloca L, Duzzi D, Anchisi D, Benedetti F, Porro CA. Neural bases of conditioned placebo analgesia. Pain. 2010;151(3):816–824. doi: 10.1016/j.pain.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto K, Tanaka K. Neuroscience. Conflict and cognitive control Science. 2004;303(5660):969–970. doi: 10.1126/science.1094733. [DOI] [PubMed] [Google Scholar]
- 34.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1(1):59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 35.Molinari E, Baraldi P, Campanella M, Duzzi D, Nocetti L, Pagnoni G, Porro CA. Human Parietofrontal Networks Related to Action Observation Detected at Rest. Cereb Cortex. 2012 doi: 10.1093/cercor/bhr393. [DOI] [PubMed] [Google Scholar]
- 36.Moulton EA, Schmahmann JD, Becerra L, Borsook D. The cerebellum and pain: Passive integrator or active participator? Brain Research Reviews. 2010 doi: 10.1016/j.brainresrev.2010.05.005. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Connor AR, Han S, Dobbins IG. The inferior parietal lobule and recognition memory: expectancy violation or successful retrieval? J Neurosci. 2010;30(8):2924–2934. doi: 10.1523/JNEUROSCI.4225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pessoa L. How do emotion and motivation direct executive control? Trends Cogn Sci. 2009;13(4):160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 40.Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21(24):9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci U S A. 2010;107(1):355–360. doi: 10.1073/pnas.0906186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, Nichelli P. Does anticipation of pain affect cortical nociceptive systems? J Neurosci. 2002;22(8):3206–3214. doi: 10.1523/JNEUROSCI.22-08-03206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127(1–2):63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 45.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 46.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55(2):325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 47.Seymour B, O’Doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K, Dolan R. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat Neurosci. 2005;8(9):1234–1240. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- 48.Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19(10):2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 (Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 51.Tian L, Ren J, Zang Y. Regional homogeneity of resting state fMRI signals predicts Stop signal task performance. Neuroimage. 2012;60(1):539–544. doi: 10.1016/j.neuroimage.2011.11.098. [DOI] [PubMed] [Google Scholar]
- 52.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6(7):533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci. 2011;31(2):439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Laviolette P, O’Keefe K, Putcha D, Bakkour A, Van Dijk KR, Pihlajamaki M, Dickerson BC, Sperling RA. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage. 2010;51(2):910–917. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L, Negreira A, LaViolette P, Bakkour A, Sperling RA, Dickerson BC. Intrinsic interhemispheric hippocampal functional connectivity predicts individual differences in memory performance ability. Hippocampus. 2010;20(3):345–351. doi: 10.1002/hipo.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Z, Liu J, Zhong N, Qin Y, Zhou H, Li K. Changes in the brain intrinsic organization in both on-task state and post-task resting state. Neuroimage. 2012;62(1):394–407. doi: 10.1016/j.neuroimage.2012.04.051. [DOI] [PubMed] [Google Scholar]
- 59.Zhu Q, Zhang J, Luo YL, Dilks DD, Liu J. Resting-State Neural Activity across Face-Selective Cortical Regions Is Behaviorally Relevant. J Neurosci. 2011;31(28):10323–10330. doi: 10.1523/JNEUROSCI.0873-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25(34):7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zubieta JK, Yau WY, Scott DJ, Stohler CS. Belief or Need? Accounting for individual variations in the neurochemistry of the placebo effect. Brain Behav Immun. 2006;20(1):15–26. doi: 10.1016/j.bbi.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49(3):2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]