Introduction
The prevalence of allergy-related disorders, such as asthma, atopic dermatitis, and allergic rhinoconjunctivitis has increased dramatically in industrialized countries over the past several decades (1). Early identification of patients at high risk for allergic disorders could enhance the physician’s confidence in recommending early intervention and potential preventative measures. Cord blood IgE has been investigated as a possible early indicator of elevated risk for atopic disease, primarily during childhood with varying results (12,13).
The aim of this study was to determine whether elevated cord blood IgE is associated with subsequent elevated biomarkers of allergic disorders or of clinical allergic disease in young adult participants of an unselected birth cohort. We examined several outcomes at age 18 years including total IgE level, peripheral blood eosinophilia, pulmonary function indices, current or lifetime doctor-diagnosed asthma, atopic dermatitis, seroatopy (measurable allergen specific IgE to common aeroallergens) and self-reported allergic rhinitis.
Methods
The Childhood Allergy Study (CAS) is general risk, population-based birth cohort conducted among members of a health maintenance organization, the Health Alliance Plan (HAP) based in Detroit, Michigan. It is an ongoing study to evaluate environmental determinants of pediatric allergy and asthma. All pregnant HAP members that were at least 18 years of age, living in a predefined geographic area, had an estimated date of confinement between April 15, 1987 and August 31, 1989, who were seeing Henry Ford Health System (HFHS) providers at 7 different clinics and planned to deliver at area hospitals, were invited to participate in the study. Only full term infants (at least 36 weeks gestation) with valid cord blood IgE measurements were included. Mothers were interviewed prior to delivery to obtain information concerning maternal and paternal level of education, number of siblings, presence of allergies including history of hay fever and asthma, exposure to pets, and parental smoking habits.
Of the 1194 women who were eligible for CAS, 953 women consented to participate. Infants from 106 of these women were further excluded because their cord blood was not obtained. Of the remaining 847 infants, six had cord blood suspected to be contaminated by maternal blood and six more were determined to be ineligible at subsequent review of eligibility criteria. Mothers of the remaining 835 children were asked to complete interviews annually to discuss the health of the child for the previous year. Annual interviews were conducted through age 6–7 years at which time children were invited to complete a clinic visit.
Beginning in May 2005, the oldest children enrolled in CAS turned 18 years of age. All teens were contacted by telephone after their 18th birthday to obtain information on allergy, asthma, and other health and exposure histories including pets and tobacco smoke exposure from ages 6 years through 18 years. After the interview, teens were invited to attend a clinic visit for blood sample collection and spirometry measurements. All teens completed data collection between 18–21 years of age.
Interviews were completed on 670 young adult participants of the original birth cohort and 565 had serum samples collected between the ages of 18–21 years for measurement of total and specific IgE levels and peripheral blood eosinophil count. There were 430 participants who also completed pre- and post-bronchodilator spirometry. Written informed consent was obtained from each participant. This research was approved by the Henry Ford Health System Institutional Review Board.
Cord blood samples were collected in the delivery room. Serum was separated by centrifugation and frozen before analyses of total IgE. Venous blood was collected at age 18 for assessment of eosinophils and serum for total IgE levels and specific IgE levels was frozen and stored until assayed. Measurements for cord blood IgE, total IgE and allergen-specific IgE were performed following the standard manufacturer’s protocols using the Pharmacia UniCAP system (Phadia US, Portage, MI). Allergen-specific IgE was analyzed for dust mite (Dermatophagoides farinae - Der f), dog, cat, timothy grass (Phleum pretense), ragweed (Ambrosia elatior), Alternaria alternata, and peanut. One percent of all assays were repeated in a different assay run on a different day to provide estimates of interassay reliability. The geometric mean coefficient of interassay variation was 5.9% for all 7 allergens. Sensitization was defined as a positive allergen-specific IgE result of > 0.35 kU/L.
Lung function was recorded with a handheld SpiroPro spirometer (Jaeger, Hoechberg, Germany). Spirometry was performed in accordance with ATS standards and findings were considered to be acceptable if the subject made a good effort and if two forced exhalation maneuvers showed reproducibility (±5%) for FVC and FEV1.
Clinical outcomes evaluated by interview included asthma, atopic dermatitis and allergic rhinitis. Asthma diagnoses were defined in two ways; ever and current asthma. “Ever” asthma was defined as a patient report of ever having received a doctor diagnosis of asthma. Current asthma, a subset of “ever asthma”, was defined as a participant report of a previous doctor diagnosis of asthma, or a positive response to the question, “Have you had any asthma symptoms in the past 4 weeks?,” along with report of an asthma attack in the last 12 months or having taken medicine for asthma in the last 12 months.
Atopic dermatitis (AD) diagnoses were classified three ways based on participant report; self report of ever having AD, doctor-diagnosis of ever having AD and current AD. Self-report or ever having had AD was defined by a positive response to the question, “Have you ever had eczema or atopic dermatitis?” Doctor-diagnosed AD was defined by a positive response to the question, “Did a doctor ever diagnose you with eczema or atopic dermatitis?” Current AD was defined by a positive response to the question, “Do you still have eczema or atopic dermatitis?”
The outcome of allergic rhinitis was divided into self-reported symptoms and doctor-diagnosed nose or eye allergies as indicated by interview responses. Self-reported allergic rhinitis was defined by a positive response to the question, “Do you have nasal allergies, including hay fever, or allergic rhinitis?” Doctor-diagnosed allergic rhinitis was defined by a positive response to the question, “Did a doctor ever tell you that you had nose or eye allergies?”
Possible effect modifiers and confounders evaluated were gender, birth order, maternal age at delivery, birth weight, season of birth, season of blood draw at age 18, maternal or parental history of asthma or allergy (based on maternal report at baseline), pet exposure (dog/cat), household smoke exposure, oral antibiotic use in the first six months of life, fever defined as a rectal temperature of 38.3 °C (101°F) or greater or its equivalent measured at another site in the first year of life, ever breastfed, and childcare attendance in the first year of life.
Lifetime pet and smoke exposure were classified several different ways including exposure in the first year of life, exposure in early life (between age 1 and 5), exposure in midlife (between age 6 and 12), exposure in teen years (between age 13 and 18), exposure ever, and number of total years exposed. Furthermore, smoke exposure inside the home, maternal, paternal and household exposures were identified as different variables for each category. Pet exposure was defined as ever living with a dog or cat.
Statistical Analysis
The cord blood IgE data was skewed and non-normally distributed. Due to the nature of the data, non-parametric statistical methods or log transformations were necessary. Spearman’s correlations were used to describe associations between cord IgE and other continuous variables. Wilcoxon rank sum tests were used to compare cord IgE levels within binary variables such as ever diagnosed with asthma (yes, no). Geometric means and their accompanying 95% confidence intervals (95% CI) are presented to describe the data.
Cord blood IgE was log transformed prior to inclusion in any regression (linear or logistic) model. Linear regression was used to test for interactions between cord blood IgE and potential effect modifiers. Logistic and linear regression models were used for multivariable analyses.
Previous publications have used somewhat arbitrary cutpoints in analyses of cord blood IgE and outcomes later in life (4,13,18). For this study, receiver operating characteristic (ROC) curves were constructed relating the level of cord IgE to the outcomes of seroatopy at age 18 years and current asthma at age 18 years. This allowed the selection of a cutpoint based on maximum sensitivity and specificity with sensitivity as the probability that the cord IgE is greater than or equal to a cutpoint for the presence of the outcome and specificity as the probability that the cord IgE is less than a cutpoint for absence of the outcome (20). The area under the curve (AUC) is a summary measure reflected in an ROC curve. If the AUC=0.5, for example, the predictor of interest (cord blood IgE in our case) is no better than chance at predicting the outcome and with an AUC=1.0 reflecting a “perfect” association. SAS version 9.2 was used for all analyses.
RESULTS
Subject Characteristics
There were 565 teens that had complete interviews and blood specimen results. Table 1 lists the major demographic and clinical characteristics of the study population.
Table 1.
Characteristics of study subjects and their respective interactions on the correlation of cord blood IgE and total IgE at age 18–21.
| Strata | n | Correlation of Cord IgE to Total IgE at age 18–21 | P-value for interaction |
|---|---|---|---|
| Gender | |||
| - Female | 296 (52%) | 0.19 | 0.82 |
| - Male | 269 (48%) | 0.17 | |
| Firstborn | |||
| - Yes | 256 (45%) | 0.18 | 0.32 |
| - No | 309 (55%) | 0.16 | |
| Mother’s age | |||
| - ≥ 30 yrs | 248 (44%) | 0.16 | 0.27* |
| - < 30 yrs | 317 (56%) | 0.19 | |
| Birth weight | |||
| - ≥ 7.4 lbs | 293 (53%) | 0.19 | 0.55* |
| - < 7.4 lbs | 259 (47%) | 0.18 | |
| Season of birth (cord blood draw) | |||
| - Winter | 101 (18%) | 0.12 | 0.74 |
| - Spring | 187 (33%) | 0.20 | |
| - Summer | 171 (30%) | 0.16 | |
| - Fall | 106 (19%) | 0.16 | |
| Season of blood draw at 18 | |||
| - Winter | 144 (25%) | 0.07 | |
| - Spring | 151 (27%) | 0.28 | 0.96 |
| - Summer | 178 (32%) | 0.14 | |
| - Fall | 92 (16%) | 0.18 | |
| Maternal history of allergy and/or asthma? | |||
| - Yes | 186 (33%) | 0.18 | 0.97 |
| - No | 379 (67%) | 0.16 | |
| Paternal history of allergy and/or asthma? | |||
| - Yes | 178 (33%) | 0.26 | 0.29 |
| - No | 364 (67%) | 0.12 | |
| Pet first year of life | |||
| - Yes | 251 (44%) | 0.11 | 0.19 |
| - No | 314 (56%) | 0.23 | |
| Household smoke exposure 1st year of life | |||
| - Yes | 183 (32%) | 0.25 | 0.20 |
| - No | 382 (68%) | 0.14 | |
| Antibiotic use 1st 6 months of life | |||
| - Yes | 268 (51%) | 0.08 | 0.014 |
| - No | 259 (49%) | 0.29 | |
| Fever 1st year of life | |||
| - Yes | 236 (44%) | 0.09 | 0.11 |
| - No | 297 (56%) | 0.23 | |
| Breastfed | |||
| - Yes | 336 (67%) | 0.18 | 0.84 |
| - No | 166 (33%) | 0.13 | |
| Daycare | |||
| - Yes | 215 (45%) | 0.11 | 0.16 |
| - No | 264 (55%) | 0.24 |
Interaction term was tested using mother’s age and birth weight as continuous variables.
These indices are dichotomized in this table for descriptive purposes.
Relationship of cord blood IgE to allergic biomarkers
Table 2 lists correlations of cord blood IgE level to selected allergic biomarkers and pulmonary function indices in the participants. There was a statistically significant but weak correlation between cord blood IgE level and total IgE level (r=0.18, p<0.001). The correlation between cord blood IgE and total IgE at age 18 years was determined for subgroups of participants. The impact of participant characteristics on this correlation is listed in the third and fourth columns of Table 1. Interestingly, antibiotic usage in the first six months of life appeared to be an important effect modifier of the association of cord IgE with total IgE in young adulthood, with a correlation primarily seen among those who had not had early antibiotics (r=0.29, p<0.001) rather than those who had (r=0.08, p=0.22). No other participant characteristics significantly modified this correlation.
Table 2.
Correlation of cord blood IgE levels to the outcomes of allergy biomarkers and lung function at age 18 years
| Outcomes | Correlation r | P-value |
|---|---|---|
| Total IgE (kU/L) | 0.18 | < 0.001 |
| Number of positive allergen specific IgE tests (panel of 7) (kU/L) | 0.10 | 0.016 |
| Eosinophil count | 0.05 | 0.26 |
| FEV1 | 0.03 | 0.56 |
| % predicted FEV1 | 0.02 | 0.62 |
| % predicted FVC | 0.02 | 0.72 |
| FEV1/FVC | 0.02 | 0.73 |
| % predicted FEF 25–75% | 0.02 | 0.61 |
Table 2 shows a statistically significant but weak correlation between cord blood IgE and number of positive allergen-specific tests out of the panel of seven allergens (r=0.10; p=0.016). There was no association between cord blood IgE and peripheral blood eosinophil counts in young adulthood (r=0.05, p=0.26). However, gender appeared to modify this relationship and in females a weak positive correlation of cord IgE with eosinophil counts (r=0.16, p=0.005) was noted; an association that was not present in males (r= −0.08, p=0.21).
There was also no significant correlation between cord IgE levels and lung function parameters (FEV1/FVC, % predicted FEV1, % predicted FVC or % predicted FEF25-75), (Table 2).
Relationship of cord blood IgE to clinical allergy
Table 3 includes results demonstrating that the distribution of cord blood IgE levels were not significantly different among those who had developed clinical allergic disorders as compared to those who had not. There was no overall difference in cord blood IgE levels between those with and without current asthma. However, this observation varied by first-born status. Among the subgroups of participants that had older siblings, those with current asthma tended to have higher cord blood IgE compared with those who did not have current asthma, (0.35 kU/L, 95% CI, 0.22–0.54 versus 0.20 kU/L, 95% CI, 0.18–0.23; p=0.039). This was not duplicated among those who were first-born.
Table 3.
Relationship of cord blood IgE levels to clinical outcomes at age 18–21
| Clinical outcomes | n | Cord blood IgE (kU/L) Geometric Mean (95% CI) | Wilcoxon rank sum p-value | |
|---|---|---|---|---|
| Current Asthma | - Yes | 54 | 0.27 (0.19, 0.38) | 0.17 |
| - No | 610 | 0.21 (0.19, 0.23) | ||
|
| ||||
| Ever diagnosed with asthma by age 18 | - Yes | 145 | 0.22 (0.18, 0.27) | 0.49 |
| - No | 519 | 0.21 (0.19, 0.23) | ||
|
| ||||
| Self report of ever having eczema/atopic dermatitis | - Yes | 73 | 0.24 (0.18, 0.31) | 0.43 |
| - No | 586 | 0.21 (0.19, 0.23) | ||
|
| ||||
| Ever diagnosed with eczema or atopic dermatitis | - Yes | 57 | 0.25 (0.19, 0.34) | 0.29 |
| - No | 599 | 0.21 (0.19, 0.23) | ||
|
| ||||
| Current eczema or atopic dermatitis (self- report or doctor diagnosis) | - Yes | 34 | 0.29 (0.19, 0.44) | 0.20 |
| - No | 621 | 0.21 (0.19, 0.23) | ||
|
| ||||
| Self report of allergic rhinitis | - Yes | 70 | 0.24 (0.19, 0.31) | 0.14 |
| - No | 579 | 0.21 (0.19, 0.23) | ||
|
| ||||
| Doctor diagnosis of allergic rhinitis | - Yes | 34 | 0.22 (0.15, 0.32) | 0.82 |
| - No | 609 | 0.21 (0.15, 0.32) | ||
Cord blood IgE was not associated with ever being diagnosed with asthma, atopic dermatitis (ever, doctor diagnosis, or current), or with allergic rhinitis (self-report or doctor diagnosis), even after considering the potential effect modifiers and confounders (Table 3).
Table 4 shows a small association between cord blood IgE and allergic sensitization as defined by at least one positive allergen specific IgE test to the seven allergens tested (OR=1.18, 95% CI, 1.02 – 1.37; p=0.031). Analysis of this data indicates that there is a 15.7% increase in the likelihood of sensitization for every 1.5 fold (50%) increase in cord blood IgE. Interestingly, this relationship was stronger within the subgroup with no pet exposure in the first year of life (OR=1.43, 95% CI, 1.16 – 1.77; p=0.001) as compared to no significant association within the subgroup with pet exposure (OR=0.94, 95% CI, 0.76 – 1.18; p=0.61).
Table 4.
Relationship of cord blood IgE levels to allergen sensitization at age 18–21
| Strata | Sensitization (≥ 1 positive allergen-specific IgE test) | n | Cord blood IgE (kU/L) Geometric Mean (95% CI) | OR* (95% CI) p-value |
|---|---|---|---|---|
| All | - Yes | 312 | 0.24 (0.21, 0.27) | 1.18 (1.02, 1.37) |
| - No | 253 | 0.19 (0.17, 0.22) | p=0.031 | |
|
| ||||
| Pet exposure during first year of life | - Yes | 136 | 0.21 (0.18, 0.26) | 0.94 (0.76, 1.18) |
| - No | 115 | 0.23 (0.18, 0.28) | p=0.61 | |
|
| ||||
| No pet exposure during first year of life | - Yes | 176 | 0.26 (0.22, 0.31) | 1.43 (1.16, 1.77) |
| - No | 138 | 0.17 (0.14, 0.20) | p=0.001 | |
OR = Odds ratio
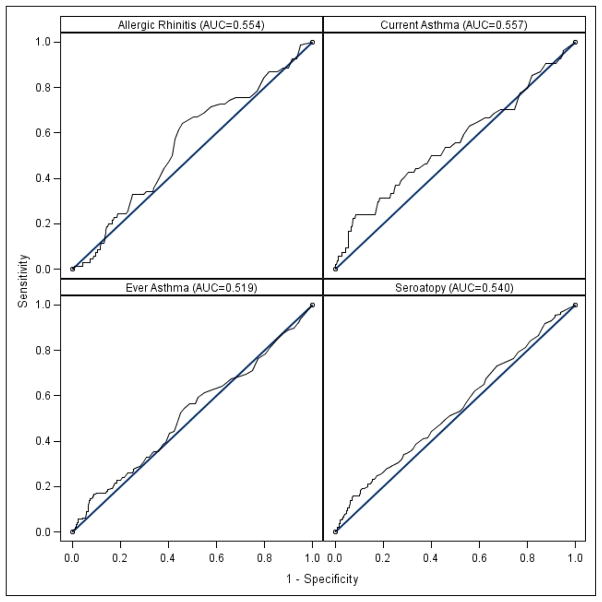
Since previous publications have suggested that there may be a particular threshold level of cord blood IgE that is useful in prediction of allergic outcomes, ROC curves were generated for the dichotomous outcomes of absence or presence of seroatopy, self-report of allergic rhinitis, current asthma and ever asthma (Figure 1). These ROC curves demonstrate that cord blood IgE remains a poor predictor of these allergic outcomes across a spectrum of cutoff levels. The AUC’s were 0.54, 0.55, 0.56 and 0.52, respectively.
Figure 1.
ROCs for cord blood IgE and presence of (a) self-reported allergic rhinitis, (b) current asthma, (c) ever asthma and (d) allergen sensitization (seroatopy).
Discussion
Cord blood IgE has been discussed as a useful marker in identifying children at high risk of developing allergic outcomes such as atopic dermatitis, asthma and allergic sensitization during their childhood years (2–5, 8, 10, 14, 18–19). Our data suggests that although cord blood IgE is modestly associated with total IgE levels at age 18–21 years, there is no consistent association of cord blood IgE and clinical outcomes.
In addition, we found a weak association between higher cord blood IgE levels and the likelihood of allergen sensitization in young adulthood although this association was driven by the subset of subjects that lived in pet-free homes during infancy. Although several studies over the past three decades have suggested that cord blood IgE is predictive of atopic disease, these analyses were limited to early childhood with manifestations up to age 10 years in one study (2, 3, 16). In addition, cord blood IgE was analyzed using predetermined cut-off values. Similar to total serum IgE, at other time points, cut-off values of IgE in cord blood associated with allergic disease risk, if they exist, have not been clearly defined (17).
In contrast to our findings, Pesonen et al. recently reported in a Finnish population that cord blood IgE was associated with a higher rate of allergic rhinoconjunctivitis (p=0.04) among 164 young adults at age 20 (4). This discrepancy with our results is likely due to the author’s use of a specific value for cord blood IgE level designated as elevated (>0.5). We did not find that using such a level was discriminatory for either self-reported or doctor-diagnosed allergic rhinitis (Figure 1). Similar to our study, they did find a correlation between cord blood IgE and total IgE levels in adulthood.
Our results in young adults are consistent with several studies that suggest that cord blood IgE is a poor clinical predictor of atopic disease in childhood (6–9, 12, 13). Although Tariq et al suggested that cord blood IgE >0.5 kU/L was related to sensitization to aeroallergens at age 4, they did not find a correlation with clinical outcomes (19). Interestingly, in our study, cord blood IgE was modestly associated with the presence of allergen sensitization. Yet, we could not identify a specific cut-off value for cord blood IgE that had sufficient sensitivity or specificity to be considered as a clinical predictor. This situation is analogous to the recognized lack of a specific total IgE level as a useful screening test for asthma in spite of a clear association of higher IgE levels with asthma. Similarly cord blood IgE may be associated with atopy but is not necessarily useful as an early predictor.
Hansen et al evaluated several cut-off values for cord blood IgE but did not find increased atopy in 18 month old children at any of the potential cutoff values analyzed (13). During a follow up study at the age of 5 years, the authors did find a statistically significant association between cord blood IgE levels and total IgE levels at age 5 (18). At this age, when using a cut off value of 0.3 kU/L, a statistically significant association was reported but the positive predictive value of this IgE level was only 26%, with a sensitivity of 33% (18).
Analysis of total IgE levels in children up to age 10 in the large German Multicenter Allergy Study, published in 2005, showed that total IgE levels vary over time even in the absence of atopy (17). In spite of this, our study confirms others that show that higher cord blood IgE levels modestly but persistently correlate with higher total IgE levels later in life. However, we could not confirm that cord blood IgE was consistently associated with the presence of allergic disorders. In our data, modest associations between cord blood IgE level and asthma outcomes within two “low risk” subgroups were seen. These subgroups were defined as children with older siblings and children not receiving antibiotics in the first six months of life.
This may reflect the known association of higher total IgE in adulthood and higher risk of asthma. However, further analysis using ROC curves shows that there is no particular cord blood IgE level that functions as a reasonable clinical predictor of subsequent asthma in the population overall
Compared to existing literature, our data reflect a larger sample size from a general risk birth cohort. To our knowledge, this is the only study evaluating cord blood and total serum IgE levels as continuous variables, analysis of specific threshold values as predictors of allergic risk and evaluation of both laboratory markers and clinical outcomes of atopic disease. A possible limitation of our study is the relatively low numbers of asthma diagnoses for outcomes. Furthermore, while our study did adjust for several possible confounding variables and effect modifiers, there may be other factors that influence cord blood IgE levels.
In addition, the CAS participants are a relatively homogeneous population of Caucasian, middle class, young adults. Compared to other reports, this may be advantageous by minimizing variation attributable to ethnicity or socioeconomic status, but may make the findings less applicable to more diverse populations.
In summary, cord blood IgE levels are associated with higher total IgE and the likelihood of allergen sensitization in young adults. However, a consistent association of cord blood IgE level to clinical outcomes in young adulthood was not apparent. Overall, our analyses suggest that cord blood IgE levels in an unselected population is a poor clinical predictor of subsequent allergy-related biomarkers or clinical outcomes when evaluated in young adults.
Acknowledgments
Sources of Funding: National Institute of Allergy and Infectious Disease Award Number: RO1AI51598; Fund for Henry Ford Hospital; Henry Ford Hospital Department of Medical Education Resident Research Award
Footnotes
Conflict of Interest:
- Purvee S. Shah – NONE
- Ganesa Wegienka – NONE
- Suzanne Havstad – NONE
- Christine Cole Johnson – NONE
- Dennis R. Ownby – NONE
- Edward M. Zoratti - NONE
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beasley R, Crane J, Lai CK, Pearce N. Prevalence and etiology of asthma. J Allergy Clin Immunol. 2000;105(suppl):S466–72. doi: 10.1016/s0091-6749(00)90044-7. [DOI] [PubMed] [Google Scholar]
- 2.Sadeghnejad A, Karmaus W, Davis S, Kurukulaaratchy RJ, Matthews S, Arshad SH. Raised cord serum immunoglobulin E increases the risk of allergic sensitization at ages 4 and 10 and asthma at age 10. Thorax. 2004;59:936–42. doi: 10.1136/thx.2004.024224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kjellman NI, Croner S. Cord blood IgE determination for allergy prediction – a folllow-up to seven years of age in 1651 children. Ann Allergy. 1984;53:167–71. [PubMed] [Google Scholar]
- 4.Pesonen M, Kallio M, Martti A, Elg P, Bjorksten F, Ranki A. Cord serum immunoglobulin E as a risk factor for allergic symptoms and sensitization in children and young adults. Pediatric Allergy and Immunology. 2009;20:12–18. doi: 10.1111/j.1399-3038.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 5.Perkin MR, Strachan DP. The predictive value of early life total immunoglobulin E measurement in identifying atopic children in a population-based birth cohort study. Pediatric Allergy and Immunology. 2006;17:118–124. doi: 10.1111/j.1399-3038.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- 6.Bonnelykke K, Pipper CB, Bisgaard H. Sensitization does not develop in utero. J Allergy Clin Immunol. 2008;121(3):646–651. doi: 10.1016/j.jaci.2007.12.1149. [DOI] [PubMed] [Google Scholar]
- 7.Eiriksson TH, Sigurgeirsson B, Ardal B, Sigfusson A, Valdimarsson H. Cord blood IgE levels are influenced by gestational age but do not predict allergic manifestations in infants. Pediatric Allergy Immunology. 1994;5:5–10. doi: 10.1111/j.1399-3038.1994.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 8.Bergmann RL, Edenharter G, Bergmann KE, et al. Predictability of early atopy by cord blood IgE and parental history. Clin Exp Allergy. 1997;27:752–760. [PubMed] [Google Scholar]
- 9.Lodrup Carlson KC, Carlsen KH, Nafstad P, Bakketeig L. Perinatal risk factors for recurrent wheeze in early life. Pediatric Allergy Immunology. 1999;10:89–95. doi: 10.1034/j.1399-3038.1999.00028.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaan A, Dimich-Ward H, Manfreda J, et al. Cord blood IgE: its determinants and prediction of development of asthma and other allergic disorders at 12 months. Annals of Allergy, Asthma and Immunology. 2000 Jan;84:37–42(6). doi: 10.1016/S1081-1206(10)62738-X. [DOI] [PubMed] [Google Scholar]
- 11.Businco L, Marchetti F, Pellegrini G, Perlini R. Predictive value of cord blood IgE levels in ‘at-risk’ newborn babies and influence of type of feeding. Clin Exp Allergy. 1990;13(6):503–508. doi: 10.1111/j.1365-2222.1983.tb02631.x. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz RG, Richards D, Kemeny DM, Price JF. Neonatal IgE: a poor screen for atopic disease. Clin Exp Allergy. 1991;21:467–72. doi: 10.1111/j.1365-2222.1991.tb01687.x. [DOI] [PubMed] [Google Scholar]
- 13.Hansen LG, Host A, Halken S, et al. Cord blood IgE II. Prediction of atopic disease. A follow-up at the age of 18 months. Allergy. 1992;47:397–403. doi: 10.1111/j.1398-9995.1992.tb02079.x. [DOI] [PubMed] [Google Scholar]
- 14.Allam JP, Zivanovic O, Berg C, Gembruch U, Bieber T, Novak N. In search for predictive factors for atopy in human cord blood. Allergy. 2005;60:743–750. doi: 10.1111/j.1398-9995.2005.00815.x. [DOI] [PubMed] [Google Scholar]
- 15.Scirica CV, Gold DR, Ryan L, et al. Predictors of cord blood IgE levels in children at risk for asthma and atopy. J Allergy Clin Immunol. 2007;119(1):81–88. doi: 10.1016/j.jaci.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Liu CA, Wang CL, Chuang H, Ou CY, Hsu TY, Yang KD. Prenatal prediction of infant atopy by maternal but not paternal total IgE levels. J Allergy Clin Immunol. 2003;112:899–904. doi: 10.1016/j.jaci.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 17.Nickel R, Illi S, Lau S, et al. Variability of total serum immunoglobulin E levels from birth to the age of 10 years. A prospective evaluation in a large birth cohort (German Multicenter Allergy Study) Clin Exp Allergy. 2005;35:619–623. doi: 10.1111/j.1365-2222.2005.02237.x. [DOI] [PubMed] [Google Scholar]
- 18.Hansen LG, Halken S, Host A, Moller K, Osterballe O. Prediction of allergy from family history and cord blood IgE levels. A follow-up at the age of 5 years. Pediatric Allergy and Immunology. 1993;4(1):34–40. doi: 10.1111/j.1399-3038.1993.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 19.Tariq SM, Arshad SH, Matthews SM, Hakin EA. Elevated cord serum IgE increases the risk of aeroallergen sensitization without increasing respiratory allergic symptoms in early childhood. Clin Exp Allergy. 1999;29:1042–1048. doi: 10.1046/j.1365-2222.1999.00594.x. [DOI] [PubMed] [Google Scholar]
- 20.Ware JH. The limitations of risk factors as prognostic tools. NEJM. 2006;355(25):2615–2617. doi: 10.1056/NEJMp068249. [DOI] [PubMed] [Google Scholar]