Summary
Background
Huntington’s disease (HD) is an autosomal dominant, fully penetrant, neurodegenerative disease that most commonly affects adults in mid-life. Our aim was to identify sensitive and reliable biomarkers in premanifest carriers of mutated HTT and in individuals with early HD that could provide essential methodology for the assessment of therapeutic interventions.
Methods
This multicentre study uses an extensive battery of novel assessments, including multi-site 3T MRI, clinical, cognitive, quantitative motor, oculomotor, and neuropsychiatric measures. Blinded analyses were done on the baseline cross-sectional data from 366 individuals: 123 controls, 120 premanifest (pre-HD) individuals, and 123 patients with early HD.
Findings
The first participant was enrolled in January, 2008, and all assessments were completed by August, 2008. Cross-sectional analyses identified significant changes in whole-brain volume, regional grey and white matter differences, impairment in a range of voluntary neurophysiological motor, and oculomotor tasks, and cognitive and neuropsychiatric dysfunction in premanifest HD gene carriers with normal motor scores through to early clinical stage 2 disease.
Interpretation
We show the feasibility of rapid data acquisition and the use of multi-site 3T MRI and neurophysiological motor measures in a large multicentre study. Our results provide evidence for quantifiable biological and clinical alterations in HTT expansion carriers compared with age-matched controls. Many parameters differ from age-matched controls in a graded fashion and show changes of increasing magnitude across our cohort, who range from about 16 years from predicted disease diagnosis to early HD. These findings might help to define novel quantifiable endpoints and methods for rapid and reliable data acquisition, which could aid the design of therapeutic trials.
Funding
CHDI/High Q Foundation.
Introduction
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder with complete penetrance. HD is caused by a CAG repeat expansion in HTT, the gene that encodes huntingtin, which is on chromosome 4.1 Individuals who have inherited the expanded CAG trinucleotide repeat can be identified before symptom onset by predictive genetic testing. The prevalence of HD is about 4–10 per 100 000 in the general population of the western hemisphere, but many more are at risk of developing the disease. HD is characterised by a triad of signs: progressive motor dysfunction, cognitive decline, and psychiatric disturbance. The formal diagnosis of HD is made on the basis of motor signs that are regarded as diagnostic for HD in a person with a positive family history, confirmed by gene testing. The concept of “motor onset” or “phenoconversion” is defined as the unambiguous presence of an otherwise unexplained movement disorder;2 however, this does not account for the many individuals who show cognitive or behavioural disturbances several years before the onset of motor symptoms.3
The mean age at formal diagnosis is in the mid-40s, and most people with the CAG repeat expansion seem healthy until adulthood. The length of the CAG repeat accounts for 50 –70% of the variability in age at clinical onset, whereby individuals with longer repeat lengths commonly have an earlier onset than those with shorter repeat lengths.4 However, subclinical changes precede the onset of overt clinical manifestations; cross-sectional evidence shows that individuals with clinically premanifest HD perform significantly worse on a variety of cognitive measures5 and motor and oculomotor assessments,6,7 and have increased psychopathology.8 Structural neuropathology during the decade before the estimated age of symptom onset, including volume reduction in the striatum,9 loss of grey and white matter,10,11 and cortical thinning,12 have been reported as well as reduced neural activation13 and a reduction in raclopride (D2 dopamine receptor) binding.14
Much remains unknown about the mechanisms that underlie the considerable variation in age of onset, rate of progression, and clinical phenotypical characteristics. Extrapolation from existing studies is problematic because many did not exclude individuals with premanifest HD who might have shown some soft motor signs; rather, these studies relied solely on the unified HD rating scale (UHDRS) diagnostic confidence score to measure a patient’s prediagnosis status.2 This stratification score has high inter-rater variability and might also include individuals who do not have true pre-motor symptoms.
The optimum point at which to introduce neuroprotective drugs to delay symptom onset or slow the rate of disease progression is likely the premanifest stage, before the onset of rapid neuronal degeneration and the emergence of clinical symptoms. Data from a conditional HD mouse model suggest the potential reversibility of this neuronal dysfunction, leading to a full recovery when expression of the mutant gene is halted.15 To test potential treatments, sensitive and stable markers of change in individuals with premanifest and early HD must be identified. However, despite some encouraging results from in vitro studies and animal trials, disease-modifying therapeutic trials in HD are limited by a paucity of robust endpoints by which disease progression can be tracked. Current clinical rating scales lack sensitivity, have floor or ceiling effects, particularly in premanifest individuals, and require long observation periods to unequivocally show a change. Improvements in the efficiency and precision of objective measurements of disease progression in individuals in the premanifest and early stages of HD could lead to biomarkers that are better able to assess disease progression and measure the effects of therapeutic interventions.
The full penetrance of the HD mutation in people with an HD CAG expansion of more than 40 provides a unique opportunity to examine the pattern of signs, symptoms, and neurobiological changes as they emerge. In TRACK-HD we aim to exploit the certainty of disease manifestation to ascertain the biomarkers and endpoints required to test therapeutic interventions early in the disease.
TRACK-HD is a multinational longitudinal observational study designed with similar principles to a clinical trial, with rapid study preparation and data acquisition, rigorous quality assurance and quality control, and blinded data analysis. We report the baseline data from the TRACK-HD cohort with the aim of identifying the changes that occur from health to early stage 2 disease by assessing a wide range and novel combination of measurements (ie, genetic, clinical, cognitive, quantitative motor, oculomotor, neuropsychiatric, and 3T MRI). The cohort is evenly divided among HTT expansion carriers with no or minimum motor signs (pre-motor) who are up to about 16 years away from a predicted disease diagnosis,16 early manifest HD individuals, and age-matched and sex-matched controls.
Methods
Participants
We report the baseline cross-sectional data from a longitudinal study with three annual timepoints. Full enrolment and testing was completed over a period of 8 months. The first participant was enrolled in January, 2008, and all assessments were completed by August, 2008. Individuals with expansion of the HD gene were recruited from the National Hospital for Neurology and Neurosurgery, London, UK, the Department of Medical Genetics at the University of British Columbia, Vancouver, Canada, the Department of Genetics and Cytogenetics at the Hôpital de la Salpêtrière-Université Pierre and Marie Curie, Paris, France, and the Department of Neurology at Leiden University Medical Centre, Leiden, Netherlands (table 1). Recruitment targets were 90 individuals per centre, comprising 30 controls, 30 individuals with premanifest HD, and 30 individuals with early HD. Premanifest gene carriers required a burden of pathology score greater than 250, on the basis of their medical records at the time of recruitment, and a total motor score of 5 or less in the motor assessment of the United Huntington’s Disease Rating Scale (UHDRS), indicating no substantial motor signs.
Table 1.
Demographics of the TRACK-HD participants at baseline
| Controls (n=123) | PreHD | HD | |||||
|---|---|---|---|---|---|---|---|
| PreHD-A (n=62) | PreHD-B (n=58) | Combined (n=120) | HD1 (n=77) | HD2 (n=46) | Combined (n=123) | ||
| Age (years) | 46·1 (10·2, 23·0–65·7) | 41·1 (8·6, 18·6–59·4) | 40·6 (9·2, 22·3–64·1) | 40·8 (8·9, 18·6–64·1) | 47·2 (10·3, 22·8–64·1) | 51·4 (8·6, 33·3–63·3) | 48·8 (9·9, 22·8–64·1) |
| Women | 68 (55%) | 33 (53%) | 33 (57%) | 66 (55%) | 46 (60%) | 21 (46%) | 67 (55%) |
| Education (years) | 4·0 (1·3) | 4·1 (1·1) | 3·8 (1·3) | 3·9 (1·2) | 3·8 (1·3) | 3·2 (1·4) | 3·6 (1·3) |
| Disease-burden score* | ·· | 259·1 (30·1) | 333·1 (30·0) | 294·8 (47·7) | 364·1 (74·3) | 397·6 (67·5) | 376·6 (73·3) |
| Centres | |||||||
| Leiden | 30 | 16 | 14 | 30 | 16 | 14 | 30 |
| London | 30 | 14 | 16 | 30 | 19 | 11 | 30 |
| Paris | 30 | 14 | 16 | 30 | 26 | 4 | 30 |
| Vancouver | 33 | 18 | 12 | 30 | 16 | 17 | 33 |
Data are mean (SD, range) or number (%).
Disease-burden score=age×(CAG length−35·5).
The burden of pathology score has been used in HD biomarker studies to assess the relation among variables of interest and the estimated burden of disease.17,18 Penney and co-workers19 showed that the degree of post-mortem striatal pathology was predicted by age at death and the length of the CAG repeat. On the basis of this observation, Sanchez-Pernaute and co-workers,20 who were studying striatal MRI abnormalities in vivo, found a similar relation between age and the length of the CAG repeat.
The burden of pathology score was proposed as an index of disease burden on the basis of these findings. The score is calculated from a formula (age×[CAG–35·5]),19 and functions are calculated as a simple a posteriori estimate of an individual’s lifetime exposure to mutant huntingtin, at any age, before and after motor onset. Other authors5,21 have reported that effect sizes could be estimated for different stages of preHD. We used disease-burden scores to optimise the recruitment of individuals with premanifest HD to compile a cohort with the best chance of showing detectible changes in one or more outcome measures over the course of the study. Because the score gives an estimate of pathological burden without making any prospective prediction about a patient’s future disease course, disease-burden score was the ethically preferred criterion for recruitment. By design, the cohort of individuals recruited according to this burden of pathology threshold were mostly closer to disease onset than would have been the case if we had recruited from the entire pool of pre-diagnosis individuals with gene expansion. A more broad-ranging preHD sample would result in smaller differences between the preHD groups and controls and would have required a larger sample size to detect group differences, a possibility that is prohibited by the limited time and funds available for the study.
Individuals in the premanifest group were divided at the group median for predicted years to diagnosis (10·8 years) into preHD-A (further from predicted diagnosis age) and preHD-B (nearer to predicted diagnosis age) on the basis of the survival analysis formula described by Langbehn and co-workers.16 Patients with early HD were divided into two subgroups—HD stage 1 (HD1) and HD stage 2 (HD2)—on the basis of their score on the total functional capacity scale.22 Controls were age-matched and gender-matched to individuals in the combined preHD and HD groups and were selected from the spouses or partners of individuals with premanifest or early HD or were gene-negative siblings, to ensure consistency of environments with carriers of the HTT gene expansion. The study was approved by the local ethics committees, and written informed consent was obtained from each participant. Additional details of the inclusion and exclusion criteria are provided in the webappendix.
Procedures
Participants were assessed with the UHDRS-99,23 which comprises medical and psychiatric history, current medications, HD history, clinical motor scores, cognitive impairment (eg, symbol digit modalities and Stroop word condition), and functional capacity.
CAG repeat size was measured at a central laboratory and was compared with the repeat size reported at recruitment. Details of the methods used for CAG repeat determination are described in the webappendix.
3T MRI data were acquired using T1 and T2 protocols, which were standardised for this study. Details of the preparatory-phase work for the multi-site 3T MRI acquisition are summarised in the webappendix. Rigorous quality control was done on all image datasets (IXICO, London, UK). Image data were archived at the Laboratory of Neuroimaging, University of California, Los Angeles (CA, USA). Four image analysis techniques were applied at three sites that specialise in image analysis: semiautomated measurements of intracranial volume and whole-brain volumes were calculated with MIDAS;24 fully automated segmentation of intracranial volume, whole brain, caudate, and putamen tissue volume were done on collected images with BRAINS (Brain Research: Analysis of Images, Networks, and Systems, Iowa City, Iowa, USA);25 cortical thickness analysis was done with the Freesurfer method of Fischl and colleagues;26 and voxel-based morphometry analysis was done with statistical parametric mapping (SPM) version 5.
We recorded horizontal eye position with the Saccadometer Advanced (Ober Consulting, Poznan, Poland) during a random, centrally cued mixed pro/anti saccade task.27 The isometric force during sustained tongue protrusion was recorded using a force transducer.28 The same force transducer system was used to record finger taps during a self-paced tapping task.28 Stride length variability was assessed during normal speed walking with a GAITrite system (CIR Systems, Havertown, Pennsylvania, USA).29
Tests for the TRACK-HD cognitive assessment were selected on the basis of the findings from the PREDICT-HD study5 and a meta-analysis of the results of studies in preHD. We report on three of the ten tests, including recognition of negative facial emotions, a visual working memory task (spot the change), and an abbreviated (20 item) version of the University of Pennsylvania Smell Identification Test (UPSIT; Sensonics, Haddon Heights, New Jersey, USA), which is a scratch and smell recognition test for common scents.
Neuropsychiatric assessment was a brief, structured interview that was a shortened form of the problem behaviour assessment (PBA-s)30 administered by raters who were trained to meet reliability thresholds. Functional and quality-of-life assessments were used to assess functional capacity with both clinician-based assessments (the UHDRS TFC scale) and participant self-reported measures (short-form 36 [SF36] and the quality of life index [QoLI]).
In about 90% of individuals, data were collected at one visit. Detailed methodological information is included in the webappendix with full information on quality control procedures and handling of missing data.
Statistical analysis
TRACK-HD was designed to investigate the feasibility of measuring potential biomarkers across a wide range of tasks and domains in HD. Before the data were analysed, key primary outcome variables for analysis were specified and these are listed in the statistical methods in the webappendix. The results presented here are a subset of these primary variables selected to show the most substantial findings in each assessment group. The five HD subgroups (control, preHD-A, preHD-B, HD1, and HD2) were the a priori predictor variables in our primary analysis. These five groups form a natural ordinal sequence and are treated as such when appropriate. Potential confounders, such as age, sex, study site, and education level (as a proxy for premorbid intelligence), were controlled for in all analyses, with the exception of whole-brain volume measures, for which age, sex, study site, and intracranial volume were controlled for.
We estimated adjusted differences among HD subgroups and controls with linear models. Where residual variance differed markedly between HD subgroups, iteratively reweighted least squares, rather than ordinary least squares, analysis was used. Bias-corrected and accelerated (2000 replications) bootstrap 95% CIs are reported where normality assumptions were materially violated. All outcome measures were continuous or suitably quasi-continuous for this approach. Inclusion in the HD subgroup, study site, and sex were treated as categorical variables. Primary comparisons of interest were formed by linear contrasts of subgroup membership. Age and education level were modelled as linear effects. Any potential interactions between HD subgroup and age were checked. There were no missing data for any of the predictor variables, and participants with missing outcome variables were excluded (webappendix).
Voxel-based morphometry was used to detect differences in grey and white matter among groups. Age, sex, intracranial volume, and study site were included as covariates. For cortical thickness, a vertex-by-vertex analysis was done with a multivariate general linear model with adjustments for age and sex.
TRACK-HD was designed to quantify clinically meaningful longitudinal changes over a 2-year period. Sample size was calculated on the basis of projected uncertainty in the resulting sample size recommendations for future clinical trials. The sample size provides ample power to detect cross-sectional differences among the HD subgroups.
Role of the funding source
The sponsor of the study contributed to the conception of the study and provided scientific advice and guidance on study design and data interpretation. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Results
385 potential participants were screened and 381 met the inclusion criteria and were enrolled. 14 of these were subsequently excluded: one owing to stage 3 disease and the rest because they were unable to undergo MRI; one further participant withdrew their consent. Owing to difficulties in recruiting the required number of individuals with premanifest HD who had total motor scores of 5 or less and a burden of pathology score of more than 250, waivers were granted for the inclusion for nine individuals (Leiden=2, London=2, Vancouver=5) who had burden of pathology scores of less than 250. Thus, the final sample comprised 366 participants, of whom 123 were controls, 123 had HD, and 120 had preHD. Table 1 shows their demographic data and pathological disease-burden scores.19 As expected, owing to the progressive nature of HD, the preHD group was younger than the HD group. Groups were sex-matched by design, and controls were age-matched to the combined preHD and HD distribution. Although the HD2 group had lower education levels, there were no other significant differences across groups.
Measurement of repeat size at recruitment relied on historical diagnostic genotyping with various techniques and size markers, and depended on the standard operating procedures at each local diagnostic laboratory. 44 (37%) participants with premanifest HD had CAG measurements that were 1 or 2 repeats shorter than were previously determined. As a result of centralised resizing of CAG repeats, 13% of the preHD group did not reach the severity threshold for disease burden (>250).
The number of participants on pharmacotherapy was similar among all groups—controls, premanifest, and early HD—and across centres. The use of neuroleptic medication was more common in participants with manifest HD (HD1 and HD2), and anti-depressant use was common in all groups, including the controls. Medication use is summarised in the webappendix.
Table 2 shows adjusted between-group and within-group differences. The unadjusted means and standard deviations are shown in the webappendix. Unadjusted and adjusted means were similar for all assessments, indicating no major confounding factors in our analyses.
Table 2.
Adjusted between-group and within-group differences for measurements
| PreHD vs controls | HD vs controls | PreHD vs HD | PreHD-A vs controls |
PreHD-B vs preHD-A |
HD1 vs PreHD-B | HD2 vs HD1 | |
|---|---|---|---|---|---|---|---|
| Neuroimaging measures | |||||||
| Whole brain volume | −1·84% | −5·94% | −4·09% | −0·68% | −2·41% | −2·23% | −1·61% |
| (−2·70 to −0·98) | (−6·79 to −5·09) | (−4·99 to −3·20) | (−1·71 to 0·35) | (−3·6 to −1·21) | (−3·40 to −1·06) | (−2·87 to −0·34) | |
| p<0·0001 | p<0·0001 | p<0·0001 | p=0·20 | p<0·0001 | p=0·0002 | p=0·01 | |
| Quantitative motor and oculomotor measures | |||||||
| Antisaccade error rate (%) | 3·45 | 26·78 | 23·34 | −0·50 | 8·17 | 14·16 | 13·64 |
| (−1·97 to 8·87) | (21·44 to 32·13) | (17·72 to 28·96) | (−7·02 to 6·02) | (0·62 to 15·71) | (6·84 to 21·48) | (5·61 to 21·67) | |
| p=0·21 | p<0·0001 | p<0·0001 | p=0·88 | p=0·03 | p=0·0002 | p=0·0009 | |
| Tongue force heavy (log coefficient | 0·30 | 1·10 | 0·80 | 0·19 | 0·23 | 0·64 | 0·13 |
| of variance) | (0·17 to 0·42) | (0·97 to 1·22) | (0·67 to 0·93) | (0·03 to 0·34) | (0·05 to 0·40) | (0·46 to 0·81) | (−0·06 to 0·32) |
| p<0·0001 | p<0·0001 | p<0·0001 | p=0·02 | p=0·01 | p<0·0001 | p=0·18 | |
| Self-paced tapping precision | −9·84 | −27·53 | −17·69 | −5·64 | −8·54 | −11·10 | −6·04 |
| non-dominant hand* (1/SD) | (−12·92 to −6·77) | (−30·43 to −24·64) | (−20·43 to −14·95) | (−9·34 to −1·94) | (−12·43 to −4·65) | (−14·66 to −7·55) | (−9·57 to −2·51) |
| p<0·0001 | p<0·0001 | p<0·0001 | p=0·003 | p<0·0001 | p<0·0001 | p=0·0009 | |
| Gaitrite stride length normal speed | 0·12 | 0·36 | 0·24 | 0·08 | 0·09 | 0·11 | 0·22 |
| (log mean coefficient of variance) | (0·04 to 0·21) | (0·28 to 0·44) | (0·15 to 0·32) | (−0·02 to 0·18) | (−0·03 to 0·21) | (−0·003 to 0·22) | (0·1 to 0·34) |
| p=0·004 | p<0·0001 | p<0·0001 | p=0·12 | p=0·13 | p=0·06 | p=0·0004 | |
| Cognitive measures | |||||||
| Negative emotion recognition | −2·76 | −9·08 | −6·31 | −2·36 | −0·87 | −5·44 | −1·13 |
| (number correct) | (−4·21 to −1·32) | (−10·52 to −7·64) | (−7·82 to −4·81) | (−4·08 to −0·64) | (−2·88 to 1·15) | (−7·42 to −3·47) | (−3·29 to 1·03) |
| p=0·0002 | p<0·0001 | p<0·0001 | p=0·007 | p=0·40 | p<0·0001 | p=0·30 | |
| Spot the change 5 sec (“K” number | −0·47 | −1·39 | −0·93 | −0·25 | −0·44 | −0·66 | −0·10 |
| correct corrected for guessing) | (−0·76 to −0·17) | (−1·69 to −1·10) | (−1·24 to −0·62) | (−0·61 to 0·10) | (−0·85 to −0·03) | (−1·07 to −0·26) | (−0·54 to 0·35) |
| p=0·002 | p<0·0001 | p<0·0001 | p=0·16 | p=0·03 | p=0·001 | p=0·67 | |
| UPSIT smell identification* | −0·88 | −3·36 | −2·48 | −0·52 | −0·75 | −2·05 | −0·11 |
| (number correct) | (−1·42 to −0·35) | (−4·00 to −2·73) | (−3·18 to −1·78) | (−1·12 to 0·08) | (−1·58 to 0·07) | (−2·97 to −1·13) | (−1·30 to 1·07) |
| p=0·001 | p<0·0001 | p<0·0001 | p=0·09 | p=0·08 | p<0·0001 | p=0·85 | |
| Neuropsychiatric measures | |||||||
| PBA apathy† (points on PBA scale) | 0·72 | 2·38 | 1·65 | 0·47 | 0·54 | 0·68 | 1·79 |
| (0·18 to 1·27) | (1·77 to 3·03) | (0·89 to 2·39) | (−0·06 to 1·29) | (−0·34 to 1·61) | (−0·52 to 1·66) | (0·49 to 3·21) | |
| p<0·01 | p<0·001 | p<0·001 | p>0·05 | p>0·05 | p>0·05 | p=0·01 | |
| PBA irritability†(points on PBA | 1·50 | 3·89 | 2·38 | 1·56 | −0·12 | 1·50 | 2·52 |
| scale) | (0·57 to 2·63) | (2·82 to 5·09) | (0·96 to 3·76) | (0·51 to 3·20) | (−2·09 to 1·66) | (−0·41 to 3·34) | (0·50 to 4·59) |
| p<0·01 | p<0·001 | p<0·001 | P<0·01 | p>0·05 | p>0·05 | p<0·01 | |
| PBA affect† | 1·23 | 1·92 | 0·68 | 1·53 | −0·62 | 0·38 | 1·65 |
| (points on PBA scale) | (−0·15 to 2·58) | (0·49 to 3·31) | (−0·92 to 2·18) | (−0·16 to 3·68) | (−3·01 to 1·24) | (−1·47 to 2·25) | (−0·48 to 4·08) |
| p=0·08 | p<0·01 | p>0·10 | p=0·09 | p>0·10 | p>0·10 | p>0·10 | |
| Functional measures | |||||||
| Quality of life (total score) | −0·32 | −0·79 | −0·47 | −0·30 | −0·04 | 0·10 | −1·53 |
| (−1·35 to 0·72) | (−1·80 to 0·23) | (−1·54 to 0·60) | (−1·54 to 0·95) | (−1·47 to 1·40) | (−1·30 to 1·50) | (−3·08 to 0·02) | |
| p=0·55 | p=0·13 | p=0·39 | p=0·64 | p=0·96 | p=0·89 | p=0·05 | |
| SF-36 (total score) | −2·75 | −10·92 | −8·17 | −2·32 | −0·89 | −0·64 | −19·82 |
| (−7·06 to 1·56) | (−15·18 to −6·65) | (−12·64 to −3·69) | (−7·49 to 2·85) | (−6·89 to 5·10) | (−6·49 to 5·21) | (−26·34 to −13·29) | |
| p=0·21 | p<0·0001 | p=0·0004 | p=0·38 | p=0·77 | p=0·83 | p<0·0001 | |
Data are mean estimates (95% CI). All scores are adjusted for age, gender, study site, and education level, with the exception of whole-brain volume, which is adjusted for age, gender, study site, and intracranial volume. Whole-brain volume=% of total intracranial volume. All analyses done by ordinary least squares unless otherwise noted. PBA=problems behaviours assessment. SF36=short-form questionnaire 36.
Weighted least squares analysis.
Highly skewed variable; confidence intervals and p values estimated by bias-corrected and accelerated bootstrap of weighted least squares regression.
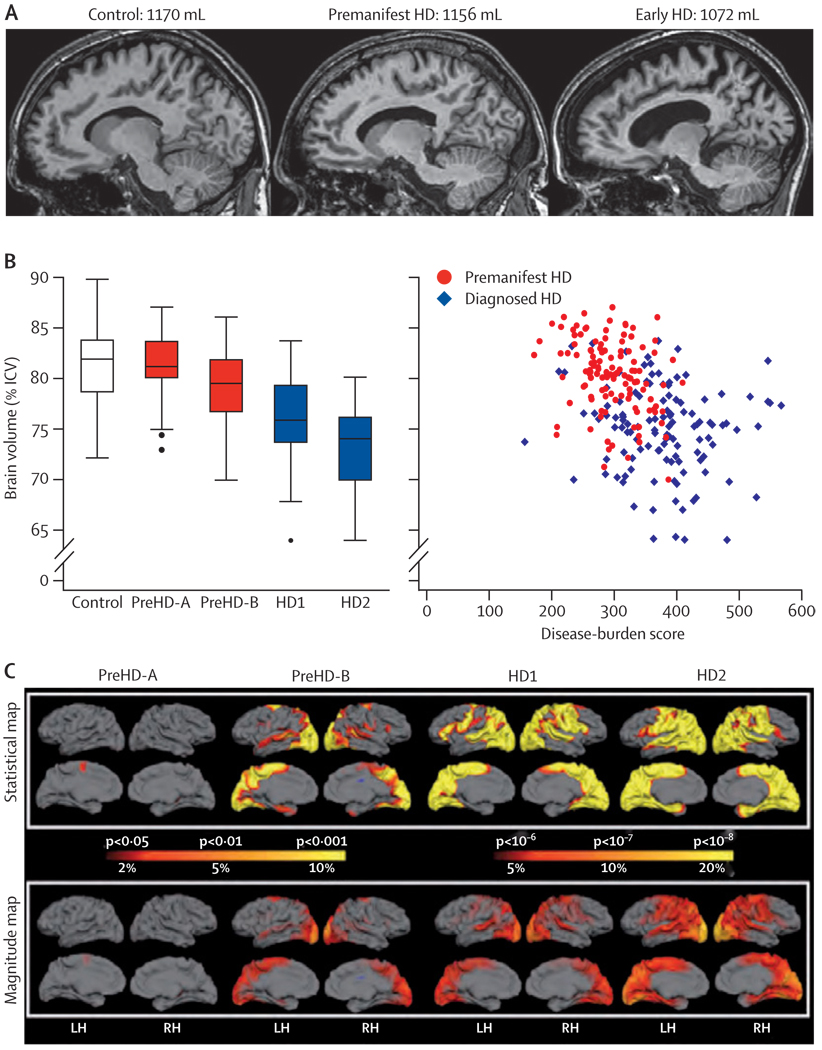
Simple visual inspection of representative MRIs showed shrinkage of the caudate nuclei and expansion of the CSF spaces in participants with preHD or early HD compared with controls (figure 1). Semi-automated volumetric analysis showed that intracranial volume did not differ significantly between the groups. Automated volumes of the striatum (total), caudate nuclei, and putamen (as percentages of intracranial volume) were significantly reduced compared with controls, even at the preHD-A stage, which was the earliest stage we studied (webappendix). The volume reduced across individuals with preHD-B, HD1, and HD2, respectively, although striatal volumes were not significantly different between HD1 and HD2 (webappendix). Semi-automated whole-brain measures (as a percentage of intracranial volume) showed a stepwise decline across the groups, with significantly reduced volumes in participants in the preHD-B and HD groups compared with the controls (figure 1). The size of these volume reductions compared with controls were 0·8%, 3·8%, 6·5%, and 8·5% in the preHD-A, preHD-B, HD1, and HD2 groups, respectively. Figure 1 shows the association between disease burden and whole-brain volume.
Figure 1. Whole brain and regional atrophy in the controls, premanifest, and early Huntington’s disease groups.
(A) 3T volumetric MRI scan in a 50-year-old control, a 55-year-old individual with preHD, and a 49-year-old with early Huntington’s disease. Brain volumes are corrected for intracranial volume. (B) Brain volume as a percentage of intracranial volume across all groups (horizontal lines are median; boxes are upper and lower quartiles; bars are range; dots are outliers) and scatter plot of brain volume as a percentage of intracranial volume against disease burden. (C) Cortical thinning in the Huntington’s disease groups compared with controls. The top panel shows statistical maps corrected with the false discovery rate; magnitude maps are shown below. All results are adjusted for age and sex. ICV=intracranial volume. LH=left hemisphere. RH=right hemisphere.
Cortical thinning increased in magnitude from the participants with premanifest HD to those with HD (figure 1). Individuals with preHD-A had localised thinning in the posterior frontal region, whereas there was involvement of the occipital, parietal, superior temporal, and superior frontal lobes in the participants with preHD-B. Participants with HD1 and HD2 showed similar patterns, with extensive thinning throughout the cortex and relative sparing of the anterior frontal and lateral temporal regions.
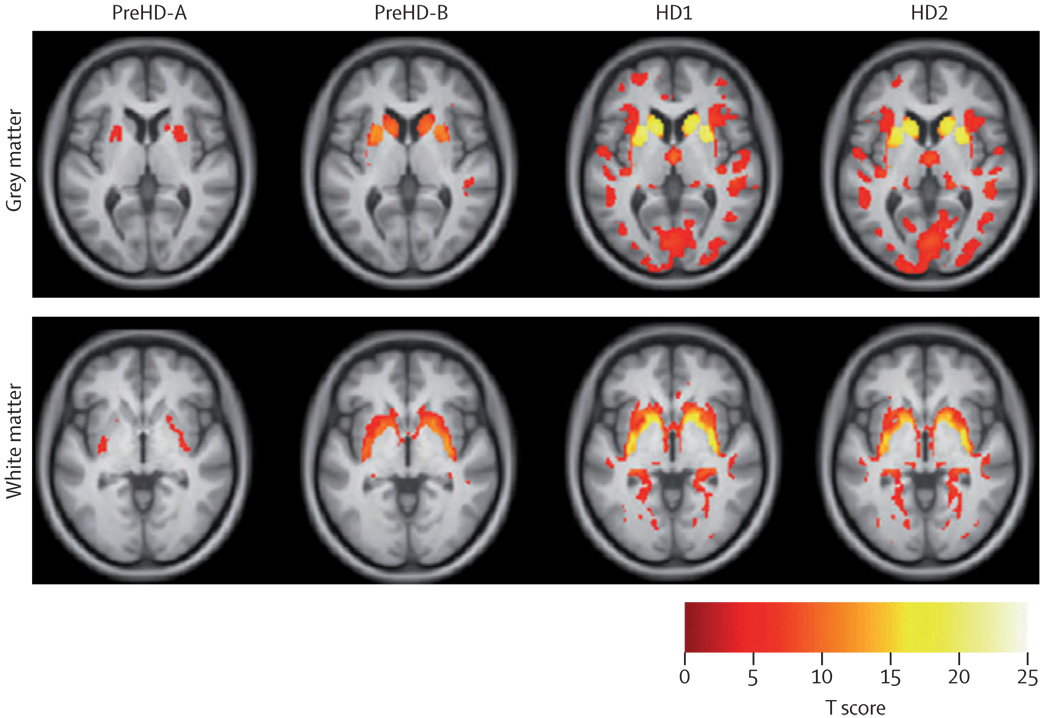
Voxel-based morphometry comparisons among the HD subgroups and the controls implied there were progressive abnormalities in both the grey and white matter in all groups (figure 2; webappendix). In the preHD-A group, grey matter loss was localised to the putamen, but was also beginning in the caudate. This pattern became more apparent in the individuals with preHD-B, and there was increasingly widespread grey matter loss in HD1 and HD2 that extended well beyond the neostriatum into the cingulate, pre-central and pre-frontal cortices and into the occipital, parietal, and temporal cortices. White matter loss in the posterior-frontal regions was also apparent early in the disease process; participants at the preHD-B stage showed more widespread involvement than those at the preHD-A stage and there was more white matter loss and similar involvement still in those with HD1 and HD2.
Figure 2. Voxel-based morphometry.
Statistical parametric maps of grey and white matter differences among groups compared with controls. Data adjusted for age, sex, study site, and intracranial volume. Results are corrected for multiple comparisons using familywise error at the p<0·05 level.
Overall, the findings across these four independent quantitative neuroimaging techniques are noteworthy for the consistency of the evidence they provide. Brain MRIs indicate that abnormalities occur before diagnosis, in the absence of overt motor signs in the grey and white matter, and involve both cortical and subcortical regions.
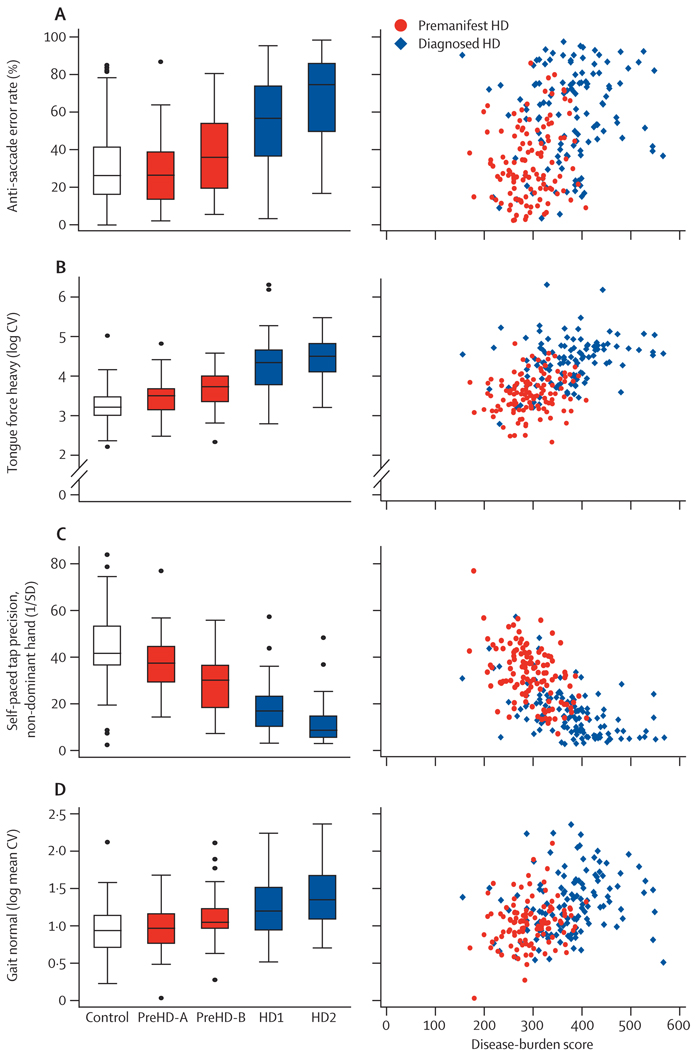
Antisaccade error rates showed stepwise increases across groups, with more errors made in preHD-B than in preHD-A, in HD1 than in preHD-B, and in HD2 compared with HD1 (figure 3). Antisaccade error rates in the participants with preHD-A did not differ from those in the controls. High error rates were associated with high disease-burden scores (figure 3).
Figure 3. Quantitative and oculomotor measures.
(A) Antisaccade error rate. (B) Static tongue force variability. (C) Self-paced tapping. (D) Gait as normal speed stance. Box plots: horizontal lines are median; boxes are upper and lower quartiles; bars are range; and dots are outliers. CV=coefficient of variance.
Deficits in tongue protrusion force coordination, expressed as tongue force variability, also showed stepwise increases across groups (figure 3). Variability (logarithmic) was greater in participants with preHD-A than in controls, in preHD-B compared with preHD-A, and in HD1 compared with preHD-B. Variability did not differ between HD2 and HD1. These results suggest that variability in tongue protrusion force is sensitive enough to detect a motor phenotype early in preHD, although tongue force variability might be too severely impaired to distinguish between stages of early symptomatic disease. Tongue force variability was also clearly associated with disease-burden scores (figure 3).
The precision of self-paced tapping (defined as 1/SD of the deviation of taps from the training tap rate) was a sensitive measure that showed differences between all adjacent group pairs (figure 3). Specifically, precision was lower in preHD-A than it was in controls, lower in preHD-B than in preHD-A, lower in HD1 than in preHD-B, and lower in HD2 than in HD1. Self-paced tapping was also clearly associated with disease burden (figure 3), and was sensitive from the earliest timepoints we assessed.
Gait, measured as the coefficient of variation for stride length at normal walking speed, was less sensitive than the other quantitative motor measures across all groups, as shown by small effect sizes (figure 3). Only the HD2 group differed significantly from controls, although when composite groups were compared (HD to controls, preHD to controls, and HD to preHD), these comparisons were significant (table 2).
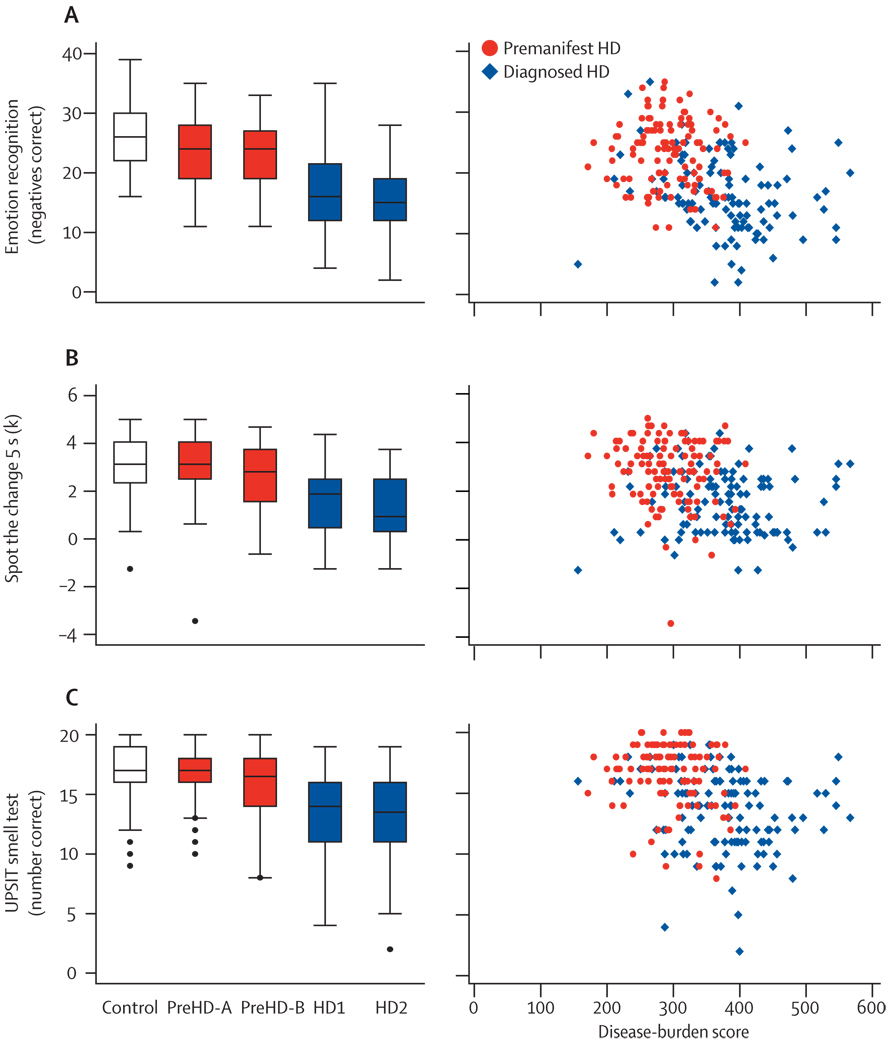
To distinguish cognitive measurements clearly from the quantitative motor assessment, we selected three tasks from a larger set of cognitive measures, on the basis of their motor demands being minimal. These tasks included recognition of facial expressions of emotion, visual working memory, and smell identification. All three of these tests showed sensitivity compared with the controls, before and after diagnosis (table 2). However, findings in pairwise group comparisons were inconsistent. For recognition of facial expressions of emotions (all negative emotions), participants with preHD-A did worse than controls, and those with HD1 did worse than those with preHD-B (figure 4). For visual working memory, the participants with preHD-B differed from those with preHD-A or HD1 (figure 4). For smell identification, we only detected significant pairwise differences between the participants with HD1 and those with preHD-B (figure 4). Remaining adjacent group comparisons did not differ. All three cognitive measures showed convincing associations between disease-burden scores and performance (figure 4).
Figure 4. Cognitive measures.
(A) Recognition of facial expressions of negative emotions, scored as number correct out of 50. (B) Spot the change visual working memory Set Size 5 condition; “K” is the number correct, corrected for guessing (calculated as 5 [for the set size]×[the number of hits plus the number of correct rejections –1]. (C) University of Pennsylvania Smell Identification Test (number correct out of 20). Box plots: horizontal lines are median; boxes are upper and lower quartiles; bars are range; and dots are outliers.
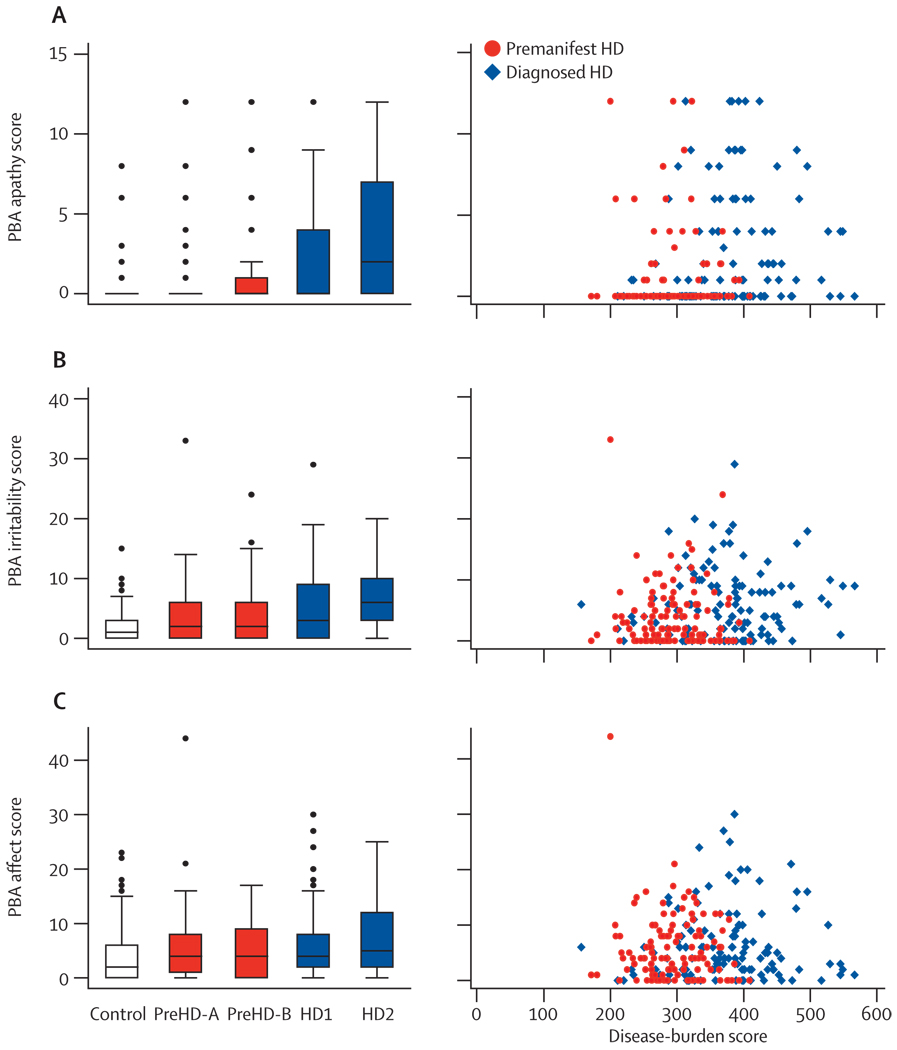
Findings from the PBA-s indicate that apathy and irritability are also sensitive markers before and after diagnosis, and show differences between preHD and controls and between HD and controls (table 2). PBA-s affect scores were significantly higher in individuals with HD, but not those with preHD, than the scores in controls. Apathy and irritability, but not affect, were also raised more in HD2 than in HD1, providing evidence that progression occurs across early diagnosed disease in two of these three symptoms (figure 5). The box plots show that many participants scored zero on these three measures and, compared with the quantitative motor and cognitive measures, correlations with disease burden were less apparent (figure 5).
Figure 5. Problem behaviour assessment.
(A) PBA apathy score (frequency×severity). (B) PBA irritability score (frequency×severity; sum of irritability, anger/aggression and perseverative thinking/behaviour items). (C) PBA affect score (frequency×severity; sum of depressed mood, suicidal ideation, and anxiety items). Box plots: horizontal lines are median; boxes are upper and lower quartiles; bars are range; dots are outliers. PBA=problem behaviour assessment.
Neither the quality of life index nor the short-form 36 questionnaire were sensitive to group effects in preHD (table 2). The quality of life index showed no effects, except a marginal difference between HD1 and HD2 (webappendix). The short-form 36 questionnaire responses were significantly different for HD compared with controls, HD compared with preHD, and between HD1 and HD2 (webappendix).
Discussion
In the TRACK-HD study, 243 HD expansion mutation carriers and 123 age-matched controls were studied over an 8-month recruitment and data collection period by the use of a collection of rigorous assessments that included novel multi-site applications of 3T MRI, quantitative motor, cognitive, and neuropsychiatric methods. Our findings indicate an increasing separation of the disease course of preclinical HD from healthy individuals at an early stage, in a cohort of premanifest HD gene carriers with no or minimum motor signs, and continuing into stage 2 disease. TRACK-HD builds on what has been learned from PREDICT-HD5,22 and other studies to show that preHD findings can be detected, even in the absence of early clinical motor signs, and that some measures are sensitive to disease effects across a broad range of stages, from health to different stages of clinical impairment. Our observations support the hypothesis that neuronal dysfunction occurs many years before the development of motor signs that are diagnostic of HD. The alterations we describe are likely to be secondary to progressive neuronal dysfunction or loss and will help to define quantifiable endpoints for future therapeutic interventions.
By use of advanced imaging techniques, we have shown structural changes in many brain regions. Even in the premanifest stages, atrophy is not confined to the striatum; we detected whole-brain volume loss in the preHD-B group, who are up to 10 years from predicted age of onset, as well as cortical thinning, particularly in the posterior regions of the brain. The 3·8% reduction in whole-brain volume in the preHD-B group is similar to the volume reduction reported in a different group of premanifest gene carriers.31 When Paulsen and co-authors divided this group into those “close to” and those “far from” the age of predicted onset, those that were far from onset had greater brain volumes than the controls. However, in TRACK-HD whole-brain volumes were reduced by 0·8%, even in those furthest from onset. Atrophy becomes increasingly widespread as the disease progresses through stages 1 and 2, which is in agreement with other reports.32,33 In accordance with previous studies,9,11,31 Voxel-based morphometry and volumetric measures showed volume reductions in the caudate and putamen in the preHD-A group, who are up to about 16 years from expected disease onset. We found no significant differences in the volume of the caudate or putamen between HD stage 1 and 2. The rate of volume seems to slow in the areas affected early in the disease. We also showed striking white matter loss that is evident before motor onset, which is consistent with other reports11 and suggests that a loss of connectivity might underlie many of the early clinical decrements. Our findings support the reduction in functional connectivity in premanifest individuals that has been reported previously.34 Overall, these findings suggest that focusing on the striatum in individuals with premanifest HD might miss other important structural brain changes, and that a focus on circuitry and systems might better describe the breadth and complexity of the alterations that occur in premanifest HD.
Our results show that quantitative neurophysiological techniques may objectively detect subtle deficits in motor coordination in individuals more than a decade before a diagnosis of HD. Variability in tongue force is a useful measure in the premanifest stage of HD, converting a clinical impression (unsteady tongue) into a quantitative measure. The variability in the timing of voluntary finger taps was significantly different between all sub-groups, showing that impairment of voluntary movements is a feature of the disease, even in premanifest HD gene carriers, up to 16 years before the predicted onset, as has been suggested previously.16 Gait analysis was a less sensitive measure for separating subgroups, although differences between controls, all premanifest, and symptomatic individuals were significant. Our results are in agreement with previous observations that variability in voluntary motor performance is the most sensitive measure of motor deficits,7 possibly as a consequence of impaired motor feedback.35 We show that individuals who are classified as “premanifest” on the basis of current definitions2 are not “pre-motor” in the sense that they have no reproducible, measurable motor abnormalities. The oculomotor antisaccade error rate also increased proportionally with disease progression. This is consistent with evidence that the frontal lobes and basal ganglia contribute more to the control of voluntary movements than to reflexive saccades.36 The premanifest individuals predicted to be furthest from clinical onset (preHD-A) performed at the same level as the controls. However, we noted a higher than expected error rate in the normal controls, which we are investigating further.
Additional evidence of the functional relevance of the brain alterations seen on MRI is shown in the cognitive findings, which point to damage to the frontostriatal pathways. Problems in the processing of negative emotional stimuli, implicating orbitofrontal and amygdala involvement, have been previously shown in individuals with premanifest HD.37,38 We have replicated these findings and also extended them into the post-diagnosis period. Visual working memory, another function that is clearly linked to the frontostriatal pathways, was also affected before and after diagnosis in the individuals in our sample. This finding is interesting in light of the common complaints of difficulties with multi-tasking, and also with TRACK-HD findings of occipital, frontal, and striatal changes in HD. Possibly, the use of a working memory task with a visual component added sufficient sensitivity, given the overall pattern of brain changes, which would include both areas involved in visual processing and in working memory. Findings of reduced odour recognition confirm those in several previous reports and are consistent with the possibility of changes in the olfactory cortex and the temporal lobe, which were seen in our voxel-based morphometry and cortical thinning imaging measures. Overall, these cognitive findings indicate that alterations can be detected across the spectrum, from preHD to stage 2 disease, even when tasks are chosen because they do not make substantial motor demands.
Neuropsychiatric burden is substantial in patients with HD, and the neuropsychiatric findings in the present study are consistent with previous reports that indicate that depression, irritable or aggressive behaviour, and apathy might precede the onset of motor abnormalities by many years.39 Scores for irritability and apathy were significantly higher in premanifest individuals than they were in spouse controls, despite the same home environments. We believe this implicates a neurobiological, rather than psychological or reactive, basis for these behavioural signs. Almost a half of our participants (155 of 366) were taking medication that had effects on the CNS that were likely to mask the prevalence of depression, and irritable or aggressive behaviour in particular. The use of these medications was particularly common in the individuals diagnosed with HD. In contrast to the clear abnormalities detected with many of our novel measures, two commonly used quality of life rating scales, which are not designed specifically for HD, did not show any impairment in our pre-HD population. Although this discrepancy might have been the result of the insensitivity of the measures used, this finding is consistent with several studies that have showed that HD gene carriers cope well after predictive genetic testing,40 and the common-sense expectation that instruments designed to measure illness-related effects on quality of life will not detect changes before the onset of clinically significant symptoms.
The strong association between pathological disease burden scores and most phenotypical features, and the biological (MRI) and clinical read-outs, suggest that many of our assessments show the underlying disease process and might therefore be suitable as biomarkers of disease progression. However, whether there might be a causal relation between particular parameters (eg, changes in performance in cognitive tests and regional brain atrophy) is beyond the scope of this publication.
Analysis of the quantitative motor and oculomotor data was done automated, blinded, and free from bias. The most sensitive outcome measures identified in the quantitative motor assessments can be tested within 5 min in each individual. All techniques are portable and can be used in an out-patient setting by trained technicians. Therefore, the use of these techniques is not restricted to specialised centres, but can easily be established in studies with many sites, with relatively low costs for equipment, maintenance, and assessment of blinded data. After longitudinal assessment during the next 2 years, TRACK-HD might identify whether quantitative motor assessments can supplement or replace less sensitive measures and those that are affected by the subjective performance of raters, such as the UHDRS motor score.
Overall, these data from the baseline TRACK-HD study confirm that many biological and clinical parameters in HD gene carriers differ markedly from controls, showing a clear and increasing separation from the controls in disease course across groups with increasing disease burden. Highly sensitive read-outs include 3T neuroimaging, quantitative motor, and cognitive assessments, and emphasise the multi-system nature of the abnormalities. Longitudinal follow-up data will enable us to ascertain the rate of change within specific groups and the variability in change among individuals in these groups. The variability in the rates of changes will ultimately define the usefulness of the parameters studied as markers of disease progression for enabling disease-modifying clinical trials.
The findings reported here have several major implications. First, they suggest that the current convention of defining the onset of HD as the onset of the movement disorder does not do justice to the full spectrum of presentations of this heterogeneous condition. Second, neuronal dysfunction while individuals are still functioning at a high level and in full employment suggests that disease-modifying interventions should be initiated during this period, while functional capacity is still within normal range and any deficits might be fully reversible. Last, the identification of a panel of markers to detect these early changes will enable testing of novel therapeutic drugs in this population.
The predictability of HD makes it perhaps the most governable of the neurodegenerative diseases from the standpoint of early intervention. The findings from TRACK-HD have key relevance for informing early intervention strategies that are under development for other, more prevalent, neurodegenerative disorders, such as sporadic Alzheimer’s disease or Parkinson’s disease, for which no highly predictive tests for the premanifest stage of the disease are available. We have identified early changes that occur before there is evidence of the clinically appreciable motor signs that are widely accepted as diagnostic for HD, in a similar way to how mild cognitive impairment can precede Alzheimer’s disease in a subset of patients. However, unlike in sporadic Alzheimer’s disease and other neurodegenerative diseases, a sensitive and specific gene test definitely predicts those who will develop HD. Together with this genetic test, the battery of assessments presented here moves us closer to being able to carry out clinical trials to evaluate treatments that can slow the neurodegeneration in this devastating group of diseases.
Acknowledgments
The authors wish to thank the TRACK-HD study participants, the CHDI/High Q Foundation, a not-for-profit organisation dedicated to finding treatments for HD, Sherry Lifer, Saiqah Munir, Azra Hassanali, Daniel van Kammen, Ethan Signer, Michael Hayden, Susan Creighton, 2mt Software GmbH, Anne Rosser, Andrea Nemeth, Emma Hobson, the Huntington’s disease clinic at Guy’s hospital, Centre d’Investigation Clinique Hospital de la Salpêtrière Paris, National Hospital for Neurology London, Leiden University Medical Centre, Katja Vitkin, Felix Mudoh Tita, Irina Vainer, Theresia Kelm, Biorep Technologies, Arthur Toga, Laboratory of Neuro Imaging UCLA (LONI) and IXICO for all their help in enabling all aspects of TRACK-HD to move forward, and also to Ray Young for graphics and the PREDICT-HD investigators for helpful advice. Some of this work was undertaken at University College London Hospital/University College London, which received funding from the Department of Health NIHR Biomedical Research Centres funding scheme.
Footnotes
Contributors
SJT is global principal investigator (PI) for TRACK HD and PI for the London site. She has responsibility for overseeing the entire study. She had a role in getting the funding, the study conception and design, writing the protocol, setting-up the study, data interpretation, and drafting the manuscript. DRL is a biostatistician and had a role in study design, protocol writing, statistical design, data analysis and interpretation, and writing the main draft of this paper. BRL is the site PI for Vancouver and was involved in study design, contributed to the interpretation and discussion of analysed data, and co-wrote the first draft of the manuscript. RACR is site PI for Leiden and contributed to study design, set-up, and data interpretation, and participated in writing the main article. AD is site PI for Paris and contributed to the interpretation and discussion of analysed data. DC is a neuropsychiatrist who was responsible for training the psychiatric raters and for quality control for the psychiatric data; he contributed to the study design, protocol writing, data analysis and interpretation, and drafting the manuscript. CK and SLH are the PI and co-investigator, respectively, for the Oculomotor Assessment Battery and had responsibility for the study design and conception, writing and editing of test protocols, analysis and interpretation of data in the context of the current literature, and drafting of sections of the manuscript. NCF contributed to the study concept and design, the MRI protocol, and analysis, design, and editing the manuscript. RIS was responsible for setup of the UCL image analysis team, contributed to the statistical design of the imaging analysis, was involved in data analysis and interpretation, and contributed to writing of the manuscript. BB is the sponsor’s science director and was involved in the study conception, design, set-up, and the interpretation of the data. AT had a role in the original conception and design of the study. HDR contributed to the development of the scanner sequences, directed the cortical thickness data analysis and quality control procedures, and interpreted the data. HJ is responsible for producing morphometric regional measures of the brain; he contributed to study setup and development of the scanner sequences, quality control procedures, and analysis protocols for medical imaging. RR is PI of the quantitative motor sub-study; he did the blinded quality control and analysis of quantitative motor data and participated in protocol writing, setting-up the study, data interpretation, and writing the paper. GBL contributed to the conception and design of the study, reviewed the protocol, had a role in adapting the electronic data capture system, oversaw the eCRF, database team, and the monitoring team, and contributed to data interpretation and drafting the manuscript. JCS had a role in obtaining funding, study design and conception, writing the protocol, setting-up the study, development and oversight of the cognitive component, data interpretation, and drafting the manuscript.
TRACK-HD study group
Canada—A Coleman, R Dar Santos, J Decolongon, A Sturrock (University of British Columbia, Vancouver). France—E Bardinet, C Jauffret, D Justo, S Lehericy, C Marelli, K Nigaud, R Valabrègue (APHP, Hôpital Salpêtriere, Paris). Germany—N Bechtel (University of Münster, Münster); A Hoffman, P Kraus (University of Bochum, Bochum). Netherlands—SJA van den Bogaard, E M Dumas, J van der Grond, EP t’Hart, C Jurgens, M-N Witjes-Ane (Leiden University Medical Centre, Leiden). UK—N Arran, J Callaghan (St Mary’s Hospital, Manchester); C Frost, R Jones (London School of Hygiene and Tropical Medicine, London); N Hobbs, N Lahiri, R Ordidge, G Owen, T Pepple, J Read, M Say, E Wild (University College London, London); S Keenan (Imperial College London, London); D M Cash (IXICO, London). USA—E Axelson, C Wang (University of Iowa, Iowa City, IA); S Lee, W Monaco (Massachusetts General Hospital, Harvard, MA); C Campbell, S Queller, K Whitlock (Indiana University, IN). Australia—C Campbell, M Campbell, E Frajman, C Milchman, A O’Regan (Monash University, Victoria).
Conflicts of interest
We have no conflicts of interest.
References
- 1.The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 2.Huntington Study Group. Unified Huntington’s disease rating scale: reliability and consistency. Mov Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 3.Marder K, Zhao H, Myers RH, et al. Rate of functional decline in Huntington’s disease. Neurology. 2000;54:452–458. doi: 10.1212/wnl.54.2.452. [DOI] [PubMed] [Google Scholar]
- 4.Duyao M, Ambrose C, Myers R, et al. Trinucleotide repeat length instability and age of onset in Huntington’s disease. Nat Genet. 1993;4:387–392. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- 5.Paulsen JS, Hayden M, Stout JC, et al. Preparing for preventive clinical trials: the Predict-HD study. Arch Neurol. 2006;63:883–890. doi: 10.1001/archneur.63.6.883. [DOI] [PubMed] [Google Scholar]
- 6.Kirkwood SC, Siemers E, Hodes ME, Conneally PM, Christian JC, Foroud T. Subtle changes among presymptomatic carriers of the Huntington‘s disease gene. J Neurol Neurosurg Psychiatry. 2000;69:773–779. doi: 10.1136/jnnp.69.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon AM, Quinn L, Reilmann R, Marder K. Coordination of prehensile forces during precision grip in Huntington‘s disease. Exp Neurol. 2000;163:136–148. doi: 10.1006/exnr.2000.7348. [DOI] [PubMed] [Google Scholar]
- 8.Berrios GE, Wagle AC, Markova IS, Wagle SA, Rosser A, Hodges JR. Psychiatric symptoms in neurologically asymptomatic Huntington‘s disease gene carriers: a comparison with gene negative at risk subjects. Acta Psychiatr Scand. 2002;105:224–230. doi: 10.1034/j.1600-0447.2002.0o456.x. [DOI] [PubMed] [Google Scholar]
- 9.Aylward EH, Sparks BF, Field KM, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63:66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- 10.Thieben MJ, Duggins AJ, Good CD, et al. The distribution of structural neuropathology in pre-clinical Huntington‘s disease. Brain. 2002;125:1815–1828. doi: 10.1093/brain/awf179. [DOI] [PubMed] [Google Scholar]
- 11.Ciarmiello A, Cannella M, Lastoria S, et al. Brain white–matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington‘s disease. J Nucl Med. 2006;47:215–222. [PubMed] [Google Scholar]
- 12.Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005;65:745–747. doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- 13.Zimbelman JL, Paulsen JS, Mikos A, Reynolds NC, Hoffmann RG, Rao SM. fMRI detection of early neural dysfunction in preclinical Huntington‘s disease. J Int Neuropsychol Soc. 2007;13:758–769. doi: 10.1017/S1355617707071214. [DOI] [PubMed] [Google Scholar]
- 14.Weeks RA, Piccini P, Harding AE, Brooks DJ. Striatal D1 and D2 dopamine receptor loss in asymptomatic mutation carriers of Huntington‘s disease. Ann Neurol. 1996;40:49–54. doi: 10.1002/ana.410400110. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington‘s disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- 16.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington‘s disease based on CAG length. Clinical Genetics. 2004;65:267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 17.Kloppel S, Draganski B, Golding CV, Chu C, Nagy Z, Cook PA, et al. White matter connections refl ect changes in voluntary-guided saccades in pre-symptomatic Huntington‘s disease. Brain. 2008;131:196–204. doi: 10.1093/brain/awm275. [DOI] [PubMed] [Google Scholar]
- 18.Golding CV, Danchaivijitr C, Hodgson TL, Tabrizi SJ, Kennard C. Identification of an oculomotor biomarker of preclinical Huntington disease. Neurology. 2006;67:485–487. doi: 10.1212/01.wnl.0000218215.43328.88. [DOI] [PubMed] [Google Scholar]
- 19.Penney JB, Jr, Vonsattel JP, MacDonald ME, Gusella JF, Myers RH. CAG repeat number governs the development rate of pathology in Huntington‘s disease. Ann Neurol. 1997;41:689–692. doi: 10.1002/ana.410410521. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Pernaute R, Garcia-Segura JM, Barrio Alba A, Viano J, de Yebenes JG. Clinical correlation of striatal 1H MRS changes in Huntington‘s disease. Neurology. 1999;53:806. doi: 10.1212/wnl.53.4.806. [DOI] [PubMed] [Google Scholar]
- 21.Paulsen JS, Langbehn DR, Stout JC, et al. Detection of Huntington‘s disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoulson I, Fahn S. Huntington disease: clinical care and evaluation. Neurology. 1979;29:1–3. doi: 10.1212/wnl.29.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Huntington Study Group. Unified Huntington‘s Disease Rating Scale-99. Huntington Study Group; 1999. [Google Scholar]
- 24.Freeborough PA, Fox NC, Kitney RI. Interactive algorithms for the segmentation and quantitation of 3-D MRI brain scans. Comput Methods Programs Biomed. 1997;53:15–25. doi: 10.1016/s0169-2607(97)01803-8. [DOI] [PubMed] [Google Scholar]
- 25.Magnotta VA, Harris G, Andreasen NC, O‘Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 26.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 27.Hicks SL, Robert MP, Golding CV, Tabrizi SJ, Kennard C. Oculomotor deficits indicate the progression of Huntington‘s disease. Prog Brain Res. 2008;171:555–558. doi: 10.1016/S0079-6123(08)00678-X. [DOI] [PubMed] [Google Scholar]
- 28.Reilmann R, Kirsten F, Bohlen S, Saemann P, Merl T, Auer D. Multimodal objective assessment of motor deficits in Huntington‘s disease using isometric force analysis. J Neurol Neurosurg Psychiatry. 2005;76:A33. [Google Scholar]
- 29.Rao AK, Muratori L, Louis ED, Moskowitz CB, Marder KS. Spectrum of gait impairments in presymptomatic and symptomatic Huntington‘s disease. Mov Disord. 2008;23:1100–1107. doi: 10.1002/mds.21987. [DOI] [PubMed] [Google Scholar]
- 30.Craufurd D, Thompson JC, Snowden JS. Behavioral changes in Huntington Disease. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:219–226. [PubMed] [Google Scholar]
- 31.Paulsen JS, Magnotta VA, Mikos AE, et al. Brain structure in preclinical Huntington‘s disease. Biol Psychiatry. 2006;59:57–63. doi: 10.1016/j.biopsych.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Henley SM, Wild EJ, Hobbs NZ, et al. Whole-brain atrophy as a measure of progression in premanifest and early Huntington‘s disease. Mov Disord. 2009;24:932–936. doi: 10.1002/mds.22485. [DOI] [PubMed] [Google Scholar]
- 33.Rosas HD, Liu AK, Hersch S, et al. Regional and progressive thinning of the cortical ribbon in Huntington‘s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- 34.Wolf RC, Vasic N, Schonfeldt-Lecuona C, Landwehrmeyer GB, Ecker D. Dorsolateral prefrontal cortex dysfunction in presymptomatic Huntington‘s disease: evidence from event-related fMRI. Brain. 2007;130:2845–2857. doi: 10.1093/brain/awm210. [DOI] [PubMed] [Google Scholar]
- 35.Smith MA, Brandt J, Shadmehr R. Motor disorder in Huntington‘s disease begins as a dysfunction in error feedback control. Nature. 2000;403:544–549. doi: 10.1038/35000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- 37.Henley SM, Wild EJ, Hobbs NZ, et al. Defective emotion recognition in early HD is neuropsychologically and anatomically generic. Neuropsychologia. 2008;46:2152–2160. doi: 10.1016/j.neuropsychologia.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 38.Johnson SA, Stout JC, Solomon AC, et al. Beyond disgust: impaired recognition of negative emotions prior to diagnosis in Huntington‘s disease. Brain. 2007;130:1732–1744. doi: 10.1093/brain/awm107. [DOI] [PubMed] [Google Scholar]
- 39.Julien CL, Thompson JC, Wild S, et al. Psychiatric disorders in preclinical Huntington‘s disease. J Neurol Neurosurg Psychiatry. 2007;78:939–943. doi: 10.1136/jnnp.2006.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tibben A. Genetic counselling and presymptomatic testing. In: Bates GP, Harper PS, Jones L, editors. Huntington‘s disease. Oxford: Oxford Medical Publishers; 2002. pp. 198–250. [Google Scholar]