Abstract
The production of antimicrobial peptides is essential for protection against a wide variety of microbial pathogens and plays an important role in the pathogenesis of several diseases. The mechanisms responsible for expression of antimicrobial peptides are incompletely understood, but a role for vitamin D as a transcriptional inducer of the antimicrobial peptide cathelicidin has been proposed. We show that 1,25-dihydroxyvitamin D3 (1,25-D3) acts together with parathyroid hormone (PTH), or the shared amino-terminal domain of PTH-related peptide (PTHrP), to synergistically increase cathelicidin and immune defense. Administration of PTH to mouse skin decreased susceptibility to skin infection by group A Streptococcus. Mice on dietary vitamin D3 restriction that responded with an elevation in PTH have an increased risk of infection if they lack 1,25-D3. These results identify PTH/PTHrP as a variable that serves to compensate for inadequate vitamin D during activation of antimicrobial peptide production.
INTRODUCTION
Antimicrobial peptides are a diverse group of molecules that prevent microbial invasion and infection in several tissues (1). In particular, cathelicidin and β-defensins are antimicrobial peptides that kill a broad spectrum of bacteria, fungi, and viruses (2). Antimicrobial peptides are produced by keratinocytes in the epidermis and can be further induced at sites of microbial entry to act as an impediment to infection. In mice, cathelicidin expression is essential for defense against invasive skin infection by group A Streptococcus (3), as well as protection against a wide variety of other bacterial, viral, and fungal pathogens (1). In humans, the importance of antimicrobial peptides to resist infection is seen in atopic dermatitis, where the expression of cathelicidin and β-defensins is diminished in skin lesions compared to skin lesions from other inflammatory disorders (4). This defect in antimicrobial peptide production is associated with increased susceptibility to skin infections due to group A Streptococcus, Staphylococcus aureus, and some viruses. Other diseases, such as psoriasis and rosacea, have been associated with abnormally high expression of antimicrobial peptides (1). Thus, understanding mechanisms that control antimicrobial peptide expression will be useful for several human diseases.
The observation that vitamin D can directly induce the production of cathelicidin in monocytes and epithelial cells (5, 6) provided an explanation for the association between increased rates of tuberculosis in patient populations with inadequate endogenous vitamin D synthesis (7). Supporting this finding, laboratory studies have shown potential biological mechanisms for a role of vitamin D in the immune response (8). These observations include findings that the vitamin D receptor exists in high concentrations on immune cells (9, 10) and that 25-hydroxyvitamin D3 (25-D3) 1α-hydroxylase (CYP27B1) is also located in these cells (7, 11). This enzyme represents a critical step in regulation of vitamin D function as it converts 25-D3 to 1,25-D3 (1,25-dihydroxyvitamin D3), the most potent form that activates the vitamin D receptor and can influence both adaptive and innate immune functions in vitro (12–14). Further support for an immunological role of vitamin D has been suggested by animal models of autoimmunity and inflammatory bowel disease (15, 16), and by observations in humans that polymorphisms in the vitamin D receptor are associated with increased susceptibility to infection with Mycobacterium tuberculosis (17).
Despite supportive laboratory evidence, only a minority of human clinical trials have shown that vitamin D supplementation provides protection against bacterial infection (18). Noncalcemic actions for vitamin D such as immune regulation have not yet been consistently proven in observations comparing only vitamin D intake or serum levels of 25-D3 (19). This inconsistency between in vitro model systems and human clinical studies suggests that there is an incomplete understanding of the variables that affect interactions between vitamin D and the immune system. Additional model systems are needed to understand these complex interactions.
Parathyroid hormone (PTH) is an important compensatory response to vitamin D deficiency (20, 21). The PTH-related peptide (PTHrP) is expressed in skin and other tissues, and its N-terminal peptide can bind similar receptors to PTH. Here, we investigated how PTH/PTHrP may act with vitamin D to influence immune defense against skin infection. Results uncover an unexpected role for PTH/PTHrP for enhancing antimicrobial peptide expression and for helping to protect against infection.
RESULTS
PTHrP is induced by bacterial products
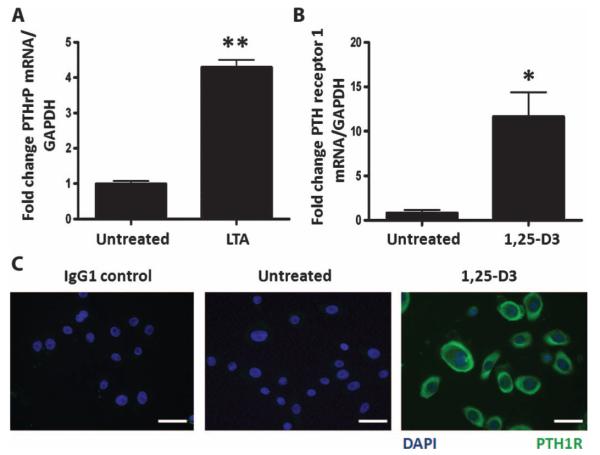
The expression of PTHrP was found to be inducible in keratinocytes by bacterial products. Stimulation of primary cultures of normal human keratinocytes with the Toll-like receptor 2 (TLR2) agonist lipoteichoic acid (LTA) enhanced PTHrP mRNA expression (Fig. 1A). PTH and PTHrP act through a common receptor [PTH receptor type 1 (PTH1R)], but previous work has reported inconsistent results regarding the expression of PTH1R in keratinocytes (22, 23). Treatment of normal human keratinocytes with 1,25-D3 (10−7 M) for 24 hours induced PTH1R mRNA (Fig. 1B) and abundant PTH1R protein expression (Fig. 1C).
Fig. 1.
LTA induces PTHrP and 1,25-D3 induces its receptor, PTH1R, in keratinocytes. (A) Expression of PTHrP mRNA in normal human epidermal keratinocytes 24 hours after treatment with the TLR2 agonist LTA (10 μg/ml). (B) Expression of PTH1R mRNA in normal human epidermal keratinocytes treated with 1,25-D3 (10−7 M) for 24 hours. (C) Immunofluorescence staining of keratinocytes using IgG1 control antibody (left panel) or anti-PTH1R antibody (green) on untreated (middle panel) or 1,25-D3–treated cells (right panel). Blue, staining of nuclei with 4′,6-diamidino-2-phenylindole (DAPI). Scale bars, 20 μm. Data are means ± SEM of triplicate independent cultures and are representative of two independent experiments. *P < 0.05, **P < 0.01 by unpaired t test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
PTHrP and PTH cooperate with vitamin D3 to induce cathelicidin expression
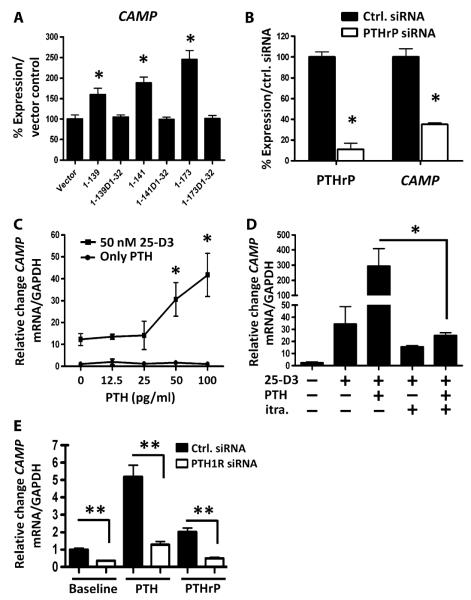
Given that keratinocytes were observed to produce PTHrP and its receptor under the influence of LTA and vitamin D, and that cathelicidin has previously been shown to also respond to these stimuli, we sought to determine whether the endogenous production of PTHrP could induce the cathelicidin gene (CAMP). PTHrP exists as three isoforms: PTHrP(1–139), PTHrP(1–141), and PTHrP(1–173) (24). Transfection of the human keratinocyte line HaCaT with constructs of each isoform increased CAMP expression, but transfection with vector alone, or constructs of each isoform that lack the N-terminal PTHrP 1 to 32 amino acids, did not (Fig. 2A). Transfection of HaCaT with PTHrP small interfering RNA (siRNA) was successful in suppressing endogenous PTHrP by 89% and simultaneously decreased the expression of CAMP mRNA (Fig. 2B).
Fig. 2.
PTHrP and PTH cooperate with vitamin D3 to induce cathelicidin antimicrobial peptide expression. (A) Cathelicidin (CAMP) mRNA expression in HaCaT keratinocytes transfected for 48 hours with different PTHrP expression plasmids. N-terminal amino acids encoded by each construct are designated. Constructs labeled D1-32 lack amino acids 1 to 32 of PTHrP and were inactive. Data are means ± SEM of triplicate independent cultures and are representative of two independent experiments. *P < 0.05 by unpaired t test. (B) HaCaT keratinocytes transfected with control siRNA (black bars) or PTHrP siRNA duplexes (white bars) and grown for 48 hours. PTHrP was measured in conditioned media by immunoassay, and cathelicidin (CAMP) mRNA was measured in cell extracts by qPCR. Data are means ± SEM of triplicate independent cultures and are representative of two independent experiments. *P < 0.05 versus vector control (unpaired t test). (C) CAMP mRNA expression in normal human epidermal keratinocytes after treatment with PTH (0 to 100 pg/ml) alone or cotreated with 25-D3 (50 nM). *P < 0.05 by Kruskal-Wallis with Dunn’s post hoc nonparametric test. Data are means ± SEM of triplicate independent cultures and are representative of three independent experiments. (D) Normal human epidermal keratinocyte CAMP mRNA expression after pretreatment with the CYP27B1 inhibitor itraconazole (itra., 10−7 M) for 1 hour before pretreatment with 25-D3 (50 nM) for 24 hours and PTH (10−11 M) for another 24 hours. *P < 0.05 by unpaired t test. Data are means ± SEM of triplicate independent cultures and are representative of three independent experiments. (E) Normal human epidermal keratinocyte CAMP mRNA expression after knockdown using PTH1R siRNA (open bars) or control siRNA (Ctrl. siRNA, solid bars) at baseline (left) or after pretreatment for 24 hours with PTH (middle, 10−11 M) or PTHrP (right, 10−11 M). Data are means ± SEM of triplicate independent cultures and are representative of two independent experiments. **P < 0.01 by Mann-Whitney U test.
To investigate whether exogenous PTH could also influence cathelicidin expression, we directly added PTH to the culture medium. This experiment was conducted with and without simultaneous addition of 25-D3 to induce PTH1R. Pilot experiments with normal human epidermal keratinocytes were performed in medium containing several Ca2+ concentrations to induce differentiation. CAMP was significantly induced at all Ca2+ concentrations in the presence of both low-dose 25-D3 and PTH (fig. S1A). Normal human epidermal keratinocytes under basal conditions were chosen for further experiments. At a 25-D3 concentration of 50 nM, costimulation with physiological doses of PTH (50 to 100 pg) for 24 hours resulted in a dose-dependent increase in CAMP (Fig. 2C). The action of PTH to induce CAMP was dependent on conversion of 25-D3 to 1,25-D3, because inhibition of keratinocyte endogenous hydroxylase activity with itraconazole blocked the response after addition of 25-D3 (Fig. 2D). The response to PTH and PTHrP by keratinocytes was dependent on the expression of PTH1R, because addition of PTH1R siRNA suppressed the cathelicidin response (Fig. 2E). PTH1R knockdown efficiency was 92%. In addition to CAMP, CD14 also responded to PTH and vitamin D (fig. S1, B and C). The murine cathelicidin gene Camp was also induced by PTH, but the addition of 1,25-D3 had no additional effect (fig. S1D).
Antimicrobial peptide expression is mediated by DNA methylation and protein kinase C
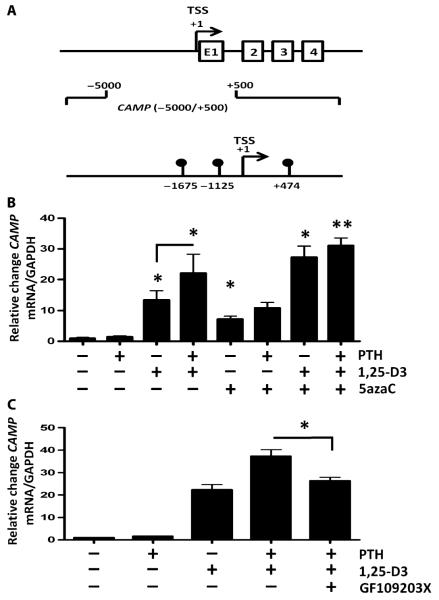
It is unclear how PTH/PTHrP enhances CAMP expression. Previously, PTH has been shown to control gene expression by demethylation (25), so we set out to determine whether methylation might also influence CAMP expression. Genome-wide methylation analysis of basal human keratinocytes using methylated DNA immunoprecipitation (MeDIP) revealed three methylated DNA sites in the vicinity of the CAMP transcription start site (TSS) (Fig. 3A). Treatment of keratinocytes with the methylation inhibitor 5-azacytidine (5azaC) enhanced 1,25-D3–induced CAMP expression to a degree similar to that of PTH-enhanced 1,25-D3. 5azaC did not further increase the combination of PTH and 1,25-D3 (Fig. 3B). In addition to methylation, PTH1R signaling involves protein kinase C (PKC) (25). Analysis of the effect of the PKC inhibitor GF109203X demonstrated that this abrogated the expression of CAMP induced by PTH (Fig. 3C).
Fig. 3.
CAMP expression by PTH/PTHrP involves DNA methylation. (A) Whole-genome MeDIP of primary human keratinocytes revealed three methylated DNA sites within −5000/+500 bp of the CAMP gene TSS at −1675, −1125, and +474 bp. Shown are exons 1 to 4 (E1, 2, 3, 4) of the cathelicidin gene. (B) CAMP mRNA expression in normal human epidermal keratinocytes after treatment with PTH (10−11 M), 1,25-D3 (10−7 M), and the DNA methyltransferase inhibitor 5azaC (10−6 M). (C) CAMP mRNA expression after treatment with PTH (10−11 M), 1,25-D3 (10−7 M), and the PKC inhibitor GF109203X (5 μM) for 24 hours. Results are means ± SEM of triplicate independent cultures and are representative of three independent experiments. *P < 0.05, **P < 0.01 by Mann-Whitney U test.
PTH and vitamin D3 protect against invasive bacterial skin infection in vivo
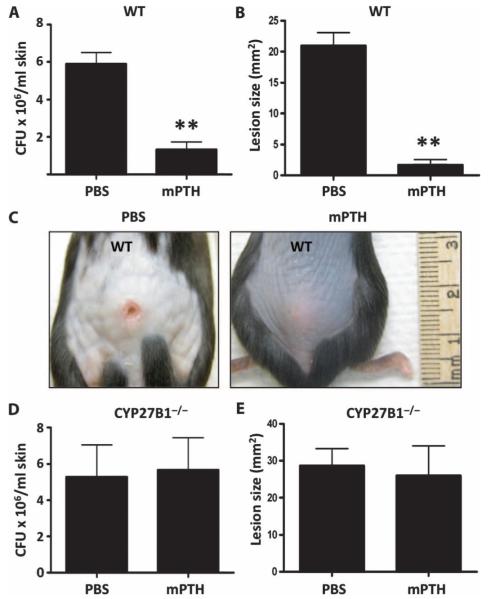
We next tested the functional relevance of PTH/PTHrP in enhancing protection against skin infection in vivo. Subcutaneous administration of mouse PTH (mPTH) 24 hours before challenge with group A Streptococcus protected mice against infection as observed by a modest reduction in group A Streptococcus recovered from the skin after 72 hours (Fig. 4A) and a significant reduction in the visible lesion size (Fig. 4, B and C). To test the co-dependence of mPTH and 1,25-D3 seen in Fig. 2, we also evaluated CYP27B1−/− mice lacking CYP27B1 and subsequently without 1,25-vitamin D3 after injection of mPTH. In contrast to wild-type mice, administration of mPTH before group A Streptococcus did not protect these mice against infection (Fig. 4, D and E).
Fig. 4.
PTH in the presence of vitamin D3 enhances protection against invasive bacterial skin infections in vivo. Wild-type (WT) mice and mice lacking the 1α-hydroxylase enzyme (CYP27B1−/−) were injected subcutaneously with mPTH(1–34) peptide (80 μg/kg) or PBS 24 hours before challenge with 107 CFU of group A Streptococcus NZ131. (A) Number of group A Streptococcus CFU retrieved from homogenized infected WT mouse skin after 72 hours. (B) Group A Streptococcus lesion size (mm2) in WT mice 72 hours after infection. **P < 0.01 by unpaired t test. (C) A representative photograph of group A Streptococcus lesion size in WT mice injected with mPTH or PBS at 72 hours. (D) Number of group A Streptococcus CFU retrieved from homogenized infected CYP27B1−/− mouse skin after 72 hours. (E) Group A Streptococcus lesion size (mm2) in CYP27B1−/− mice 72 hours after infection. All data are means ± SEM from six mice per group and are representative of three independent experiments.
Dietary vitamin D3 restriction in CYP27B1−/− mice increases susceptibility to group A Streptococcus infection
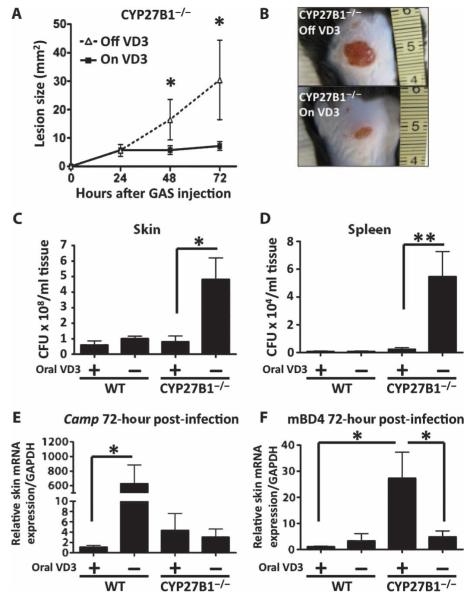
To understand the effect of vitamin D deficiency on defense against invasive bacterial infection of the skin, we maintained CYP27B1−/− or wild-type mice for 4 weeks on a diet not containing any source of vitamin D. These mice were then challenged by subcutaneous injection with group A Streptococcus. Dietary restriction of vitamin D3 in CYP27B1−/− mice increased susceptibility to invasive group A Streptococcus infection (Fig. 5, A to C). This group also had higher group A Streptococcus in skin and spleen (Fig. 5, D and E). However, no significant increase in susceptibility to infection was detectable in CYP27B1−/− mice maintained on a control diet or wild-type mice on a vitamin D–restricted diet.
Fig. 5.
Dietary vitamin D3 restriction in CYP27B1−/− mice increases susceptibility to group A Streptococcus infection. CYP27B1−/− mice were injected subcutaneously with 107 CFU of group A Streptococcus NZ131 after having been on a diet either containing vitamin D3 (2.2 IU/g) (“On VD3” or “+”) or not containing any vitamin D (“Off VD3” or “−”) for 4 weeks. (A) Lesion size of group A Streptococcus (GAS) skin infections (mm2) 24, 48, and 72 hours after injection. All data are means ± SEM from six mice per group and are representative of three independent experiments. *P < 0.05 by ANOVA with Bonferroni’s posttest. (B) A representative photograph of group A Streptococcus lesion size at 72 hours is shown. (C and D) Number of group A Streptococcus NZ131 CFU retrieved from homogenized skin (C) and spleen (D) 72 hours after infection. (E and F) Cathelicidin (Camp) (E) and mBD4 (F) mRNA expression in mouse skin 72 hours after group A Streptococcus injection. *P < 0.05, **P < 0.01 by Mann-Whitney U test.
Evaluation of serum PTH, calcium, 25-D3, and 1,25-D3 showed that dietary vitamin D3 restriction resulted in an expected compensatory increase in serum PTH and decrease in serum 25-D3. CYP27B1−/− mice lacked 1,25-D3 (fig. S2). As predicted by earlier in vitro analysis, skin Camp expression showed an increase that correlated with elevated PTH in wild-type mice if 1,25-D3 was present, but this response did not occur in CYP27B1−/− mice (Fig. 5E). Elevated mouse β-defensin 4 (mBD4) was found in CYP27B1−/− mice on the rescue diet (Fig. 5F). Increased group A Streptococcus infection was only observed in those mice that lacked an increase in Camp and mBD4.
DISCUSSION
The current study demonstrates that PTH/PTHrP and vitamin D both influence the expression of cathelicidin antimicrobial peptide and the capacity of mice to resist infection by group A Streptococcus. Previously, cell culture has suggested a link between infection and vitamin D (26, 27), but animal model data have been less compelling. A lack of a useful mouse model for dietary vitamin D deficiency has been explained by the difficulty in generating severe vitamin D deficiency in this species (15). Here, the action of PTH, which compensates for vitamin D deficiency by maintaining calcium homeostasis, was found to be a potent mechanism for inducing the expression of cathelicidin in both mice and human cells. These observations advance our understanding of how vitamin D3 influences immune defense by suggesting that the elevation of PTH in response to dietary vitamin D3 restriction may compensate for the loss of vitamin D and its role in immune functions.
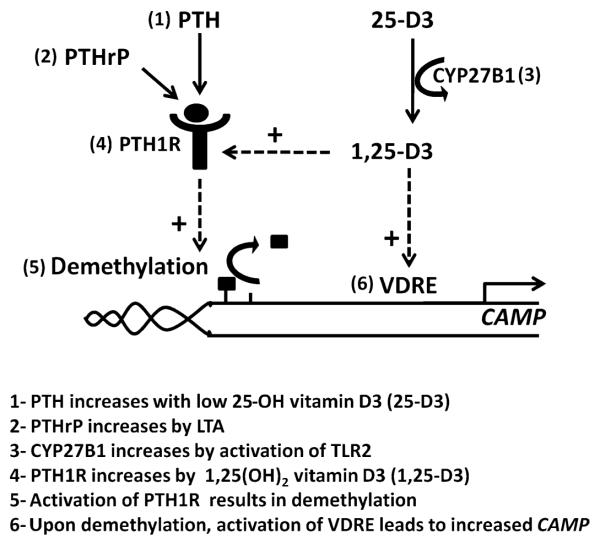
1,25-D3 and PTH/PTHrP appear to act together in human keratinocytes. Several interrelationships exist in the innate immune response between metabolic activation of vitamin D and regulation of PTH/PTHrP. An overview of the interactions between PTH/PTHrP, PTH1R, vitamin D, and cathelicidin expression is shown in Fig. 6. We found that 1,25-D3 induced PTH1R expression, thus enabling a response to PTH/PTHrP. 25-D3 enabled keratinocytes to respond to PTH/PTHrP because of the endogenous capacity of keratinocytes to hydroxylate 25-D3 to 1,25-D3. Inhibition of this activity by either addition of itraconazole in culture or targeted deletion of CYP27B1 in mice blocked the function of PTH/PTHrP. Furthermore, in the absence of 1,25-D3, the expression of PTH1R was low. Therefore, this result supported previous reports that 1,25-D3 acts as a stimulus of CAMP expression (5–7) and also suggests that it enables action of PTH/PTHrP. PTH can also increase 1,25-D3 levels in keratinocytes (28), suggesting a positive feedback system. We also observed that PTH/PTHrP and PTH1R also had an effect on keratinocyte CAMP expression in the absence of exogenous sources of vitamin D, suggesting that the PTH system has the capacity to act by itself or amplify other stimuli that regulate cathelicidin expression. Alternative pathways that induce cathelicidin expression exist in the mouse, where a vitamin D receptor element is absent from the Camp promoter. Therefore, the PTH/PTHrP enhancer system is active in the control of cathelicidin in mice even if the mouse promoter lacks a vitamin D receptor element.
Fig. 6.
Schematic showing how cathelicidin expression, PTH/PTHrP, and vitamin D may be connected. The human cathelicidin gene CAMP is induced by vitamin D, and its expression is further enhanced by the action of PTH/PTHrP. (1) PTH levels are elevated in response to low 25-D3 such as occurs with limited vitamin D intake. (2) PTHrP is increased locally in keratinocytes by the TLR2 agonist LTA. (3) The most active metabolite of vitamin D, 1,25-D3, is increased when TLR2 activation induces CYP27B1 to enhance conversion of 25-D3 to 1,25-D3. (4) 1,25-D3 enhances expression of PTH1R. (5) Activation of PTH1R results in demethylation of the CAMP promoter, thus enhancing expression of cathelicidin. (6) CAMP contains a functional vitamin D response element that induces its expression. These interactions define a potential mechanism to compensate for low serum 25-D3 by increasing the amount of PTH available for activation of CAMP, and also show how recognition of bacterial products can amplify cathelicidin expression by increasing both local 1,25-D3 and PTH1R.
Our current findings provide evidence that DNA methylation is involved in the control of cathelicidin. Previous studies have shown that transcriptional suppression through DNA methylation is derepressed upon PTH1R activation (25). We find that the cathelicidin promoter in human keratinocytes is methylated and that chemical inhibition of methylation can increase CAMP expression to a degree similar to that induced by PTH/PTHrP. These associations, as well as the role of PKC, support the notion that activation of PTH1R can induce CAMP. Future work is needed to investigate this system and expand on the hypothesis that PTH1R acts on CAMP through epigenetic mechanisms involving DNA methylation.
The exact functions of PTH/PTHrP in skin are poorly understood. PTHrP may have a role in the hair growth cycle (29), angiogenesis (30), epidermal cell proliferation, and differentiation (31). PTHrP modulates intracellular calcium in a number of cell types, and calcium has been implicated as an early signal in the differentiation of keratinocytes (32). Given that keratinocytes under high-calcium conditions show enhanced expression of skin antimicrobial peptides and their processing enzymes (33, 34), this may be an alternate mechanism of action. Furthermore, a recent study has shown that expression of CAMP can be induced by CREB (cyclic adenosine monophosphate response element–binding protein) (35), a downstream transcriptional regulator activated by engagement of the PTH1R (36). Thus, several cellular signaling mechanisms may enable PTH/PTHrP to influence innate immunity.
The relevance of PTH/PTHrP in vivo was demonstrated by observations that mice injected with PTH increase their resistance to invasive group A Streptococcus infection. Although the decrease induced by PTH in the absolute number of group A Streptococcus colony-forming units (CFU) measured in the skin was relatively modest, a large decrease in absolute lesion size was observed, therefore suggesting that a local increase in PTH is important to enhance resistance. Furthermore, the potential consequences of diet were illustrated in mice on dietary vitamin D restriction. Mice with intact 1,25-D3 levels but restricted dietary vitamin D had elevated PTH and Camp, and maintained normal protection against infection. On the basis of our results in culture, it is tempting to speculate that the increase in Camp was a result of the increase in PTH. Supporting this were additional observations that mice lacking CYP27B1 expression did not increase Camp despite elevated PTH. Only this group showed increased susceptibility to infection by group A Streptococcus. This is consistent with our findings in culture that response to PTH is optimal in the presence of 1,25-D3 and that mice injected with mPTH do not increase resistance to infection if they lack CYP27B1.
The antimicrobial responses of mice on vitamin D restriction support the notion of a role for PTH in innate immunity. Wild-type mice on dietary vitamin D3 restriction had a 600-fold increase in cathelicidin that accompanied the rise in PTH. We believe that this increase may have been induced by the elevated PTH and protected these mice against increased infection. The unexpected response in this system was that CYP27B1−/− mice on a normal diet showed a large increase in mBD4 and also did not have enhanced skin infection. This finding suggests that 25-D3, in the absence of 1,25-D3, has other influences on innate immunity beyond cathelicidin. Given that vitamin D3 and PTH induce CD14, one may speculate that enhanced pattern recognition through CD14 may result in enhanced mBD4 and thus offer protection in the absence of cathelicidin. Future work is required to better understand this potential alternate system.
We have shown that either PTH or PTHrP can induce an antimicrobial peptide response and enhance immune defense. The current study also shows a role of dietary vitamin D3 in the prevention of group A Streptococcus skin infection. We find that multiple factors can cooperate with dietary vitamin D3 to influence innate immune responses in the skin: vitamin D3 metabolism, PTH1R expression, and pattern receptor activation. These observations provide a new understanding of the relationship between vitamin D3 status and infection.
MATERIALS AND METHODS
Mice
Mice heterozygous for the CYP27B1 null mutation, CYP27B1−/+, outbred to C57BL/6 as previously described (37), were bred to provide wild-type (CYP27B1+/+) and homozygous mutant CYP27B1−/− mice. All animal experiments were approved by the University of California, San Diego, Institutional Animal Care and Use Committee. CYP27B1−/− mice and their wild-type littermates were used at the age of 12 weeks and were raised on a “rescue diet” (TD.96348, Teklad) containing 2.2 IU of vitamin D3 per gram of diet, 2% calcium, 1.25% phosphorus, and 20% lactose (38). The rescue diet was the control diet in all experiments. For dietary vitamin D restriction, mice were switched to a vitamin D–deficient diet (TD.87095, Teklad) 4 weeks before starting the experiments. The vitamin D–deficient diet differs from the rescue diet only in that it does not contain any source of vitamin D.
Bacterial skin infection
Skin infection experiments with group A Streptococcus NZ131 were done as described before (26). In brief, group A Streptococcus was grown to mid-log phase in Todd Hewitt broth (Sigma). Group A Streptococcus (107 CFU) in 100 μl of phosphate-buffered saline (PBS) was injected subcutaneously into mouse back skin. Lesion size was assessed after 24, 48, and 72 hours. After 72 hours, a 5-mm skin punch biopsy comprising the center of infection was harvested, homogenized with 2-mm zirconia beads in a Mini-Beadbeater (BioSpec Products Inc.), serially diluted, plated onto Todd Hewitt agar, and enumerated after 18 hours to quantify the CFU per milliliter.
Cell culture
Normal human keratinocytes (Life Technologies Inc.) were cultured in EpiLife medium (Cascade Biologics) supplemented with 0.06 mM calcium, EpiLife Defined Growth Supplement (EDGS) (Cascade Biologics), penicillin (50 U/ml), and streptomycin (50 mg/ml) (Cellgro Mediatech). HaCaT keratinocytes were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich). Cells at 70 to 80% confluence were stimulated with 25-D3, 1,25-D3 (10−10 to 10−7 M; Sigma), or control [0.1% ethanol, 15% (v/v) trypticase soy broth], human PTH [hPTH(1–34), 12.5 to 100 pg/ml or 10−11 M; Sigma], mPTH(1–34) (10−11 M; Sigma), or LTA from S. aureus (10 μg/ml; Sigma) for 24 hours. In addition, normal human epidermal keratinocytes were pretreated with the CYP27B1 enzyme inhibitor itraconazole (10−7 M) (5), the vitamin D receptor antagonist VAZ (ZK159222; provided by U. Zügel from Schering AG; 10−7 M), DNA methyltransferase inhibitor 5azaC (10−6 M; Sigma), or PKC inhibitor GF109203X (5 μM; Sigma) 1 hour before stimulation with vitamin D3. Furthermore, normal human epidermal keratinocytes were differentiated in high calcium (0.5 to 2.0 mM) for 48 hours before stimulation with PTH and 25-D3.
Quantitative real-time polymerase chain reaction
Total RNA was isolated with TRIzol reagent (Invitrogen), and 1 μg of RNA was reverse-transcribed with iScript (Bio-Rad). Expression of the cathelicidin gene in human (CAMP) and murine (Camp) cells was evaluated with the FAM-CAGAGGATTGTGACTTCA-MGB probe with primers 5′-CTTCACCAGCCCGTCCTTC-3′ and 5′-CCAGGACGACACAGCAGTCA-3′. TaqMan gene expression assays detecting human CD14 (assay ID: Hs00169122_g1), PTH1R (Hs00174895_m1), PTHrP (Hs00174969_m1), and mBD4 (Mm00731768_m1) were purchased from Applied Biosystems. Gene expression was normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH). For GAPDH expression, a VIC-CATCCATGACCACCCCTGGCCAAGMGB probe with primers 5′-CTTAGCACCCCTGGCCAAG-3′ and 5′-TGGTCATGAGTCCTTCCACG-3′ was used. The quantitative real-time polymerase chain reaction (qPCR) assays were performed in triplicate, and assays with mouse skin RNA were used at least five mice per group and were repeated twice.
MeDIP and DNA methylation profiling
MeDIP was performed as published before (39). In brief, genomic DNA was isolated from normal human epidermal keratinocytes with the DNeasy kit (Qiagen), sonicated to produce fragments from 300 to 1000 base pairs (bp), and subjected to immunoprecipitation with a monoclonal antibody against 5-methylcytosine (Eurogentec). DNA enriched for 5-methylcytosine was subjected to amplification with the Whole Genome Amplification kit (Sigma), labeled, and hybridized to Nimblegen’s HG18 tiled promoter array (Roche). The CAMP gene promoter was tiled 5000 bp upstream and 500 bp downstream. The one-sided Kolmogorov-Smirnov test was applied to determine significant intensity ratios.
Knockdown of PTH1R by siRNA in normal human epidermal keratinocytes
Normal human epidermal keratinocytes (5 × 106) were trypsinized and nucleofected with 1 nmol of ON-TARGETplus SMARTpool siRNA (Dharmacon, Thermo Scientific) for PTH1R or control siRNA with the Amaxa Human Keratinocyte Nucleofector Kit and an Amaxa Nucleofector II electroporator (Lonza) according to the manufacturers’ protocols. Assessment of knockdown efficiency and subsequent experiments were performed 72 hours after nucleofection.
Immunostaining
For immunocytofluorescence staining, keratinocytes were grown on chamber slides, fixed in 4% paraformaldehyde, blocked with 3% bovine serum albumin (BSA) in PBS, and incubated with mouse monoclonal anti-PTH1R antibody (3D1.1) or normal mouse immunoglobulin G (IgG) control (Santa Cruz Biotechnology) at 1:200 dilution at 4°C overnight. Fluorescein isothiocyanate (FITC)–conjugated goat anti-mouse IgG antibody (Sigma) was used as secondary antibody. Sections were mounted in ProLong Gold Antifade reagent with 4′-6-diamidino-2-phenylindole (Molecular Probes/Invitrogen). Images were obtained with an Olympus BX41 fluorescence microscope (Scientific Instrument Company).
Transfections
HaCaT cells were transfected with 1 μg of plasmid per well with Lipofectamine 2000 (Life Technologies Corp.). Human prepro PTHrP(1–139), PTHrP(1–141), and PTHrP(1–173) and the respective 1 to 32 deletions were directionally subcloned into the pCi-neo mammalian expression vector (Promega Corp.) as previously described (40). Individual G418-resistant colonies were isolated and selected for expansion on the basis of detection of elevated immunoassay levels of PTHrP in conditioned media. HaCaT cells were then transfected with 30 pmol of siRNA duplexes per well (Qiagen) targeted to PTHrP(16–22) or nonsilencing control siRNA duplexes targeted to a nonmammalian protein from Thermotoga maritimia with Lipofectamine RNAiMAX (Life Technologies), according to the manufacturer’s recommendations.
PTHrP immunoassays
To evaluate PTHrP levels, we used one- and two-site immunoassays and assayed the samples in multiple dilutions that paralleled the corresponding PTHrP standard curves (41). Both immunoassays measured all three forms of PTHrP(1–139), PTHrP(1–141), and PTHrP(1–173), but only the one-site immunoassay was able to measure also the respective PTHrP forms with deletions 1 to 32.
Statistical analysis
Statistical analysis was performed with GraphPad Prism software v5.0 (GraphPad Software Inc.). The means and SEM were calculated for each data set. Data were analyzed by Mann-Whitney U test, an unpaired t test, or analysis of variance (ANOVA) with posttests when appropriate. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank R. Horst for measurements of serum vitamin D3, D. Ditto for measurement of serum calcium, K. Radek for help in designing the experiments, T. Jaleel and C. Widjaja for assistance with the experiments, and J. Fierer and L. E. French for scientific advice. Funding: Supported by the Atopic Dermatitis Research Network (HHSN272201000020C) and NIH grants R01 AR052728, R01 AI052453, and R01 AI0833358 to R.L.G., the Veterans Administration Merit Award (ID:1145995) and State of California Tobacco-Related Disease Research Program (#18XT-0182) to L.J.D., and the Swiss National Science Foundation (PBZHP3-125571 and PASMP3_140073) to B.M.
Footnotes
SUPPLEMENTARY MATERIALS www.sciencetranslationalmedicine.org/cgi/content/full/4/135/135ra66/DC1
Materials and Methods
Fig. S1. PTH induces cathelicidin in Ca2+-differentiated human keratinocytes and basal mouse keratinocytes but requires the presence of vitamin D3 in basal human keratinocytes.
Fig. S2. Dietary vitamin D3 restriction increases serum PTH and decreases 25-D3.
Reference
Author contributions: B.M., D.D.B., C.A., G.L.S., D.W.B., L.J.D., and R.L.G. designed the experiments; B.M. performed the mouse experiments and the experiments with cultured cells; C.A. performed the mouse experiments; G.L.S. performed the DNA methylation experiments; D.W.B. performed the transfection experiments with PTHrP; B.M., D.D.B., D.W.B., L.J.D., G.L.S., and R.L.G. analyzed the data; and B.M. and R.L.G. wrote the paper.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Lai Y, Gallo RL. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braff MH, Bardan A, Nizet V, Gallo RL. Cutaneous defense mechanisms by antimicrobial peptides. J. Invest. Dermatol. 2005;125:9–13. doi: 10.1111/j.0022-202X.2004.23587.x. [DOI] [PubMed] [Google Scholar]
- 3.Lai Y, Cogen AL, Radek KA, Park HJ, Macleod DT, Leichtle A, Ryan AF, Di Nardo A, Gallo RL. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J. Invest. Dermatol. 2010;130:2211–2221. doi: 10.1038/jid.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 5.Schauber J, Dorschner RA, Coda AB, Büchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zügel U, Bikle DD, Modlin RL, Gallo RL. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D–dependent mechanism. J. Clin. Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-Dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 7.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 8.Lagishetty V, Liu NQ, Hewison M. Vitamin D metabolism and innate immunity. Mol. Cell. Endocrinol. 2011;347:97–105. doi: 10.1016/j.mce.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kream BE, Reynolds RD, Knutson JC, Eisman JA, DeLuca HF. Intestinal cytosol binders of 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D. Arch. Biochem. Biophys. 1976;176:779–787. doi: 10.1016/0003-9861(76)90222-8. [DOI] [PubMed] [Google Scholar]
- 10.Narbaitz R, Sar M, Stumpf WE, Huang S, DeLuca HF. 1,25-Dihydroxyvitamin D3 target cells in rat mammary gland. Horm. Res. 1981;15:263–269. doi: 10.1159/000179465. [DOI] [PubMed] [Google Scholar]
- 11.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin D3-1α-hydroxylase. J. Clin. Endocrinol. Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 12.Peric M, Koglin S, Dombrowski Y, Gross K, Bradac E, Ruzicka T, Schauber J. VDR and MEK-ERK dependent induction of the antimicrobial peptide cathelicidin in keratinocytes by lithocholic acid. Mol. Immunol. 2009;46:3183–3187. doi: 10.1016/j.molimm.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka H, Abe E, Miyaura C, Kuribayashi T, Konno K, Nishii Y, Suda T. 1α,25-Dihydroxycholecalciferol and a human myeloid leukaemia cell line (HL-60) Biochem. J. 1982;204:713–719. doi: 10.1042/bj2040713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu S, Liao AP, Xia Y, Li YC, Li JD, Sartor RB, Sun J. Vitamin D receptor negatively regulates bacterial-stimulated NF-κB activity in intestine. Am. J. Pathol. 2010;177:686–697. doi: 10.2353/ajpath.2010.090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deluca HF, Cantorna MT. Vitamin D: Its role and uses in immunology. FASEB J. 2001;15:2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 16.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol. Endocrinol. 2003;17:2386–2392. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: A case-control study. Lancet. 2000;355:618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 18.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med. Indones. 2006;38:3–5. [PubMed] [Google Scholar]
- 19.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krall EA, Sahyoun N, Tannenbaum S, Dallal GE, Dawson-Hughes B. Effect of vitamin D intake on seasonal variations in parathyroid hormone secretion in postmenopausal women. N. Engl. J. Med. 1989;321:1777–1783. doi: 10.1056/NEJM198912283212602. [DOI] [PubMed] [Google Scholar]
- 21.McKenna MJ, Freaney R. Secondary hyperparathyroidism in the elderly: Means to defining hypovitaminosis D. Osteoporos. Int. 1998;8(Suppl. 2):S3–S6. doi: 10.1007/pl00022725. [DOI] [PubMed] [Google Scholar]
- 22.Errazahi A, Lieberherr M, Bouizar Z, Rizk-Rabin M. PTH-1R responses to PTHrP and regulation by vitamin D in keratinocytes and adjacent fibroblasts. J. Steroid Biochem. Mol. Biol. 2004;89–90:381–385. doi: 10.1016/j.jsbmb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Hanafin NM, Chen TC, Heinrich G, Segre GV, Holick MF. Cultured human fibroblasts and not cultured human keratinocytes express a PTH/PTHrP receptor mRNA. J. Invest. Dermatol. 1995;105:133–137. doi: 10.1111/1523-1747.ep12313466. [DOI] [PubMed] [Google Scholar]
- 24.Brandt DW, Wachsman W, Deftos LJ. Parathyroid hormone-like protein: Alternative messenger RNA splicing pathways in human cancer cell lines. Cancer Res. 1994;54:850–853. [PubMed] [Google Scholar]
- 25.Kim MS, Kondo T, Takada I, Youn MY, Yamamoto Y, Takahashi S, Matsumoto T, Fujiyama S, Shirode Y, Yamaoka I, Kitagawa H, Takeyama K, Shibuya H, Ohtake F, Kato S. DNA demethylation in hormone-induced transcriptional derepression. Nature. 2009;461:1007–1012. doi: 10.1038/nature08456. [DOI] [PubMed] [Google Scholar]
- 26.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 27.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–519. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bikle DD, Nemanic MK, Gee E, Elias P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J. Clin. Invest. 1986;78:557–566. doi: 10.1172/JCI112609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho YM, Woodard GL, Dunbar M, Gocken T, Jimènez JA, Foley J. Hair-cycle-dependent expression of parathyroid hormone-related protein and its type I receptor: Evidence for regulation at the anagen to catagen transition. J. Invest. Dermatol. 2003;120:715–727. doi: 10.1046/j.1523-1747.2003.12147.x. [DOI] [PubMed] [Google Scholar]
- 30.Bakre MM, Zhu Y, Yin H, Burton DW, Terkeltaub R, Deftos LJ, Varner JA. Parathyroid hormone–related peptide is a naturally occurring, protein kinase A–dependent angiogenesis inhibitor. Nat. Med. 2002;8:995–1003. doi: 10.1038/nm753. [DOI] [PubMed] [Google Scholar]
- 31.Holick MF, Ray S, Chen TC, Tian X, Persons KS. A parathyroid hormone antagonist stimulates epidermal proliferation and hair growth in mice. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8014–8016. doi: 10.1073/pnas.91.17.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abou-Samra AB, Jüppner H, Force T, Freeman MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV, Potts JT, Jr., Kronenberg HM, Segre GV. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormonerelated peptide from rat osteoblast-like cells: A single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc. Natl. Acad. Sci. U.S.A. 1992;89:2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu AY, Destoumieux D, Wong AV, Park CH, Valore EV, Liu L, Ganz T. Human β-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J. Invest. Dermatol. 2002;118:275–281. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- 34.Morizane S, Yamasaki K, Kabigting FD, Gallo RL. Kallikrein expression and cathelicidin processing are independently controlled in keratinocytes by calcium, vitamin D3, and retinoic acid. J. Invest. Dermatol. 2010;130:1297–1306. doi: 10.1038/jid.2009.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakraborty K, Maity PC, Sil AK, Takeda Y, Das S. cAMP stringently regulates human cathelicidin antimicrobial peptide expression in the mucosal epithelial cells by activating cAMP-response element-binding protein, AP-1, and inducible cAMP early repressor. J. Biol. Chem. 2009;284:21810–21827. doi: 10.1074/jbc.M109.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jüppner H, Abou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J, Kolakowski LF, Jr., Hock J, Potts JT, Jr., Kronenberg HM, Segre GV. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 37.Dardenne O, Prud’homme J, Arabian A, Glorieux FH, St-Arnaud R. Targeted inactivation of the 25-hydroxyvitamin D3-1α-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 2001;142:3135–3141. doi: 10.1210/endo.142.7.8281. [DOI] [PubMed] [Google Scholar]
- 38.Dardenne O, Prud’Homme J, Hacking SA, Glorieux FH, St-Arnaud R. Rescue of the pseudo–vitamin D deficiency rickets phenotype of CYP27B1-deficient mice by treatment with 1,25-dihydroxyvitamin D3: Biochemical, histomorphometric, and biomechanical analyses. J. Bone Miner. Res. 2003;18:637–643. doi: 10.1359/jbmr.2003.18.4.637. [DOI] [PubMed] [Google Scholar]
- 39.Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature. 2010;463:563–567. doi: 10.1038/nature08683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ditmer LS, Burton DW, Deftos LJ. Elimination of the carboxy-terminal sequences of parathyroid hormone-related protein 1-173 increases production and secretion of the truncated forms. Endocrinology. 1996;137:1608–1617. doi: 10.1210/endo.137.5.8612492. [DOI] [PubMed] [Google Scholar]
- 41.Deftos LJ. In: The Parathyroids: Basic and Clinical Concepts. Bilezikian JP, Marcus R, Levine MA, editors. Academic Press; San Diego, CA: 2001. pp. 143–166. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.