Abstract
Improved blood banking practices and the development and implementation of increasingly sensitive serological and nucleic acid amplification technology (NAT) assays for screening donors for HCV over the past few decades have helped minimize the residual risk from transfusion-transmitted HCV in the developed world. Furthermore, studies of transfusion-transmitted infections and of donors identified as infected by routine screening have provided significant insights into HCV transmission, epidemiology and pathogenesis. However, transfusion-transmission of HCV is still a significant route of infection in the developing world. Key preventive mechanisms to ensure safe blood include elimination of paid donors and development of national donor pools comprised of volunteer repeat blood donors, combined with implementation of standardized and maximally sensitive screening assays for HCV. There is also a need to develop up-to-date data on HCV disease burden on a global scale, in part derived from systematic screening of donors for HCV. We suggest the creation of blood donor databases and specimen repositories, both at national and international levels, to facilitate epidemiological surveillance and pathogenesis and treatment studies in the future.
Declining risk of transfusion-transmitted HCV in developed countries
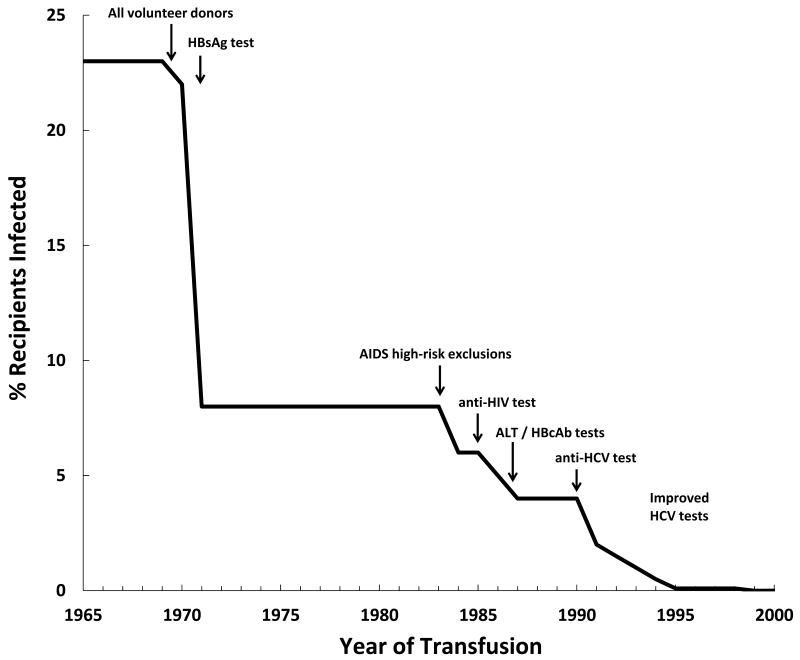
The discovery of HCV in 1989 and the implementation of HCV antibody (Ab) screening in 1990 allowed for a major breakthrough in the prevention of transfusion-transmitted (TT) HCV [1]. Retrospective studies of blood donor and recipient repositories from the mid 1960’s and early 1970’s demonstrated that almost 25% of recipients in the US were infected with HCV [2, 3] (Figure 1). Several key events in the 70’s, such as moving to a 100% volunteer blood donation as opposed to paid donors and implementation of screening and exclusion of Hepatitis B surface antigen (HBsAg) positive blood, allowed for a reduction in TT HCV to about 7% of recipients [4-6]. In addition, in the early 1980’s the recognition of TT HIV led to more stringent exclusion criteria for blood donations and a reduction in TT HCV. However, it was the discovery of HCV as the causative agent of non-A/non-B (NANB) hepatitis that facilitated the development of specific enzyme-immunoassays (EIA) for blood screening [7]. Throughout the 1990’s, the development of EIAs with improved performance along with supplemental recombinant immunoblot assays (RIBA) allowed for the rapid reduction in TT HCV to 0.01% (per unit transfused) in the latter part of the decade [8].
Figure 1.
Events that led to the decline in transfusion-transmitted HCV in the US. Adapted from Tobler and Busch, 1997.
Although the serological tests were able to reduce the number of cases of TT HCV, the window period from viremia before seroconversion and detection of anti-HCV antibodies with third-geenration EIA remained around 7-8 weeks, but also may be delayed to 13 weeks (90 days). This long window period prior to detection of anti-HCV antibodies was an impetus to adopt nucleic acid amplification technology (NAT) by blood banks in the late 90’s [9]. The residual infectious window period for a 16-unit mini-pool NAT (MP-NAT) is 6 days and individual donation NAT (ID-NAT) is less than 3 days [10]. Routine MP-NAT screening (using Chiron/GenProbe’s TMA assay on 16-unit pools) was implemented in the US in 1999. A large number of additional countries adopted NAT testing over the subsequent decade [11]. As of 2010, 33 countries in the world reported that they had introduced NAT for HCV testing. The early implementation of NAT testing was based on in-house assays and semi-automated systems; however, currently most countries use the fully automated and commercially available NAT platforms either from Novartis/Gen-Probe or Roche.
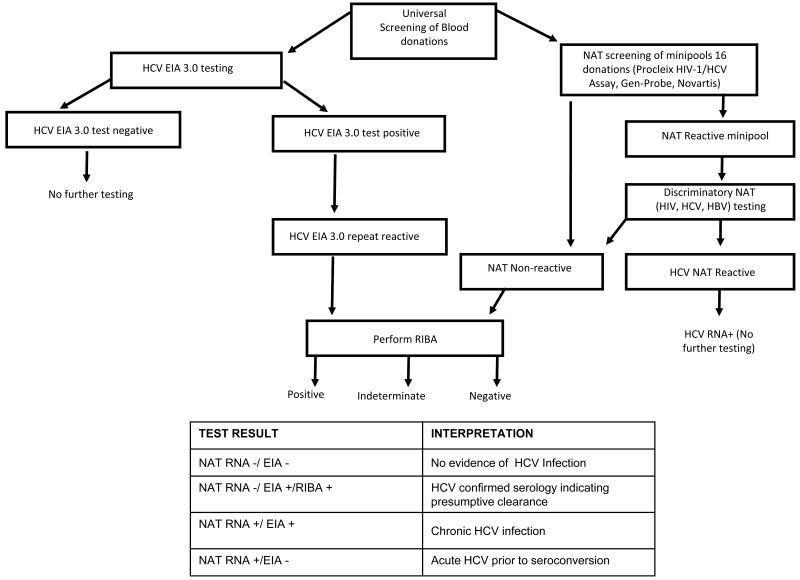
Over the years the data collected from blood donor screening has led to refined testing platforms including the development of multi-virus assays that simultaneously detect HIV and HBV in addition to HCV nucleic acids, and to improved screening and confirmatory algorithms. Figure 2 presents the algorithm that is currently used in US blood centers for serological and NAT screening and confirmatory testing for HCV [12]. The algorithms followed in different countries for screening blood donations vary from those followed in the US and are based on infectious disease prevalence and incidence rates in the respective countries. However, in almost all cases, serological assays are performed in parallel to NAT testing that allows discernment of the “stage” of HCV infected donors as acute (RNA-positive/Ab-negative), chronic (RNA-positive/Ab-positive) or presumptive resolved (RNA-negative/Ab-positive) infections.
Figure 2.
Flow chart summarizing routine screening and confirmatory testing for HCV at US blood centers, which enables classification of donors into disease outcome categories. NAT-Nucleic acid amplification testing; EIA-Enzyme immunoassay; RIBA-Recombinant immunoblot assay. Adapted from Selvarajah et al., 2010.
The current risk of TT HCV in the US is 1 in a million (0.0001%) per unit transfused, and the risk is similarly low in most regions of the developed world. The residual infectious window period prior to HCV RNA detection by current donor NAT screening assays appears to be very brief (3-5 days). Of relevance to transfusion safety, it appears that blood transfusions from donors in the early stage of acute HCV infection can be infectious prior to the time of RNA detection by routinely performed NAT screening, including the most sensitive ID-NAT test currently available, which has a 50% limit of detection of 12.1 copies/mL (95% CI 11.1-13.2). For example, data from our study which transfused 50 mL of plasma from donors in the immediate pre-ID-NAT positive window period into chimpanzees, and from other chimpanzee experimental studies using serially diluted ramp-up phase plasma infusions, as well as rare clinical case reports of “breakthrough” HCV transmission by NAT screened transfusions, demonstrate that ID-NAT cannot completely eliminate the risk of TT of HCV due to the high level infectivity of early ramp-up phase virus [13-16]. Hence, improving the sensitivity of the currently employed MP-NAT or even transitioning to ID-NAT screening may not close the infectious window period completely and may not be warranted given very low incremental yield and poor cost-effectiveness. Continuing to screen blood donations using the current serological assays and NAT platforms is considered to be sufficiently powerful to minimize residual risk of TT HCV.
HCV prevalence, demographics and genotypes in infected blood donors in the US
The American Red Cross (ARC) analyzed HCV prevalence and NAT yield rates from 66 million donations in the US tested during the period of 1999 to 2008 in order to understand the benefits of adopting NAT testing in the US in the last decade. While over 30,000 HCV antibody positive donations were detected and interdicted prior to transfusion, only 244 (1 per 270,000) donations were “NAT yield” units (HCV RNA detection prior to seroconversion) over the ten-year study period [17, 18]. The NAT yield rate among first time donors was 0.872 (1 per 115,000) and for repeat donors was 0.038 (1 per 2,660,000). Males and females were equally represented among the HCV NAT yield donors, and the highest yield occurred in donors between the ages of 16-29 years and those who lived in the Southern portion of the US. Analyses such as the one performed by ARC on HCV positive blood donor datasets are essential to help understand the residual risk of TT HCV as well as the demographics of HCV in the screened populations.
Our research group has performed several epidemiologic investigations of HCV among blood donors. We used the Retrovirus Epidemiology Donor Study (REDS) database of blood donor demographic and virologic test data to report on the demographic correlates of HCV infection at blood banks in five large U.S. cities as well as the relationship of gender and race/ethnicity on spontaneous viral clearance [19, 20]. Recently, as part of REDS II, we characterized rates of HCV among 34 million allogeneic donations collected in the US between 2006 and 2009 to understand HCV infection rates relative to donor status (first-time or repeat donors) and donor demographic characteristics such as gender, race/ethnicity, geographic region and age (Table 1 [21]). In addition, by sequencing 196 prevalent (number of positive donations over the total number of donations tested) and 124 incident (number of observed new infections over the total number of persons-years observed) HCV viremic donations, we found eight subtypes of HCV, with 1a the predominant subtype in both incident and prevalent cases. The subtype distribution was 55% 1a, 15% 1b, 13% 3a, 11% 2b, 3% 2a, 2% 4a, and less than 1% 6d or 6e. Incident donors had significantly more subtype 3a strains (21% versus 9%) but significantly fewer subtype 1b strains (8% versus 18%) as compared to prevalent donors (p=0.04). The HCV genotype distribution among blood donors in the US is similar to what has been previously reported for intravenous drug users (IDU), even though the HCV prevalence and incidence rates vary between these two groups [22], indicating the relevance of infectious disease molecular surveillance data derived from donors to broader public health surveillance efforts [23].
Table 1.
Numbers and rates of total allogeneic donations from HCV confirmed-positive donors by donor status (first-time, repeat) and donor demographic characteristics (gender, race-ethnicity, geographic region, and age) collected from January 2, 2006 through December 31, 2009. [21]
| Total Allogeneic Donations |
All HCV Positive |
Rate per 100,000 Donations |
||
|---|---|---|---|---|
| Total | Total | 33947146 | 8015 | 23.6 |
| Donor Status 1 |
First time
donors |
5968986 | 6741 | 112.9 |
|
Repeat
donors |
27950520 | 1274 | 4.6 | |
| Gender 1 | Female | 15850421 | 3047 | 19.2 |
| Male | 18054540 | 4968 | 27.5 | |
| Race/Ethnicity 1 | White | 27112643 | 4399 | 16.2 |
| Asian | 510633 | 81 | 15.9 | |
| Black | 1242416 | 916 | 73.7 | |
| Hispanic | 1354391 | 668 | 49.3 | |
| Other | 628302 | 211 | 33.6 | |
|
Not
Available |
3071121 | 1740 | 56.7 | |
| CDC Regions 1 | Midwest | 10523749 | 900 | 8.6 |
| Northeast | 8226559 | 1957 | 23.8 | |
| South | 8911822 | 3327 | 37.3 | |
| West | 5901802 | 1760 | 29.8 | |
| Other | 353635 | 66 | 18.7 | |
|
Age
Categories 1 |
<20 | 4782307 | 422 | 8.8 |
| 20-29 | 4547134 | 779 | 17.1 | |
| 30-39 | 4367417 | 1023 | 23.4 | |
| 40-49 | 7080519 | 2486 | 35.1 | |
| 50-59 | 7730474 | 2696 | 34.9 | |
| 60-69 | 3927177 | 478 | 12.2 | |
| 70+ | 1482979 | 131 | 8.8 | |
Donations that did not have information for donor status, gender, race/ethnicity, CDC region, or age were excluded.
Need to prevent HCV risk by transfusion-transmission in the developing world
HCV has an estimated prevalence of 2.5% globally, with ~170 million people infected [24, 25]. However, epidemiological studies pertaining to HCV prevalence and risk factors in countries around the world is patchy. A similar conclusion was derived through systematic analyses of the literature on the epidemiology of global HCV infections as reported in a recent supplement of Liver International [26-29]. There is an urgent need to better understand HCV prevalence and incidence rates in different countries around the world to help curb transmission from preventable causes, and especially through blood transfusion. Blood transfusion and other parenteral exposures in health care settings are still major routes of HCV transmission among individuals in developing countries compared to the rest of the world where the major route of transmission is currently IDU. As discussed above safe donor selection and screening can almost completely prevent TT HCV. However, basic methods used for ensuring safe blood are not up to par in some parts of the world.
There are several reasons for the high residual risk of TT HCV in parts of the developing world, such as Indian-subcontinent, China, South-East Asia and Africa. One reason is that policies and systems that encourage repeat blood donation by volunteers have not been successfully implemented in many developing countries, where the majority of blood donors are first time donors, often including replacement donors, either family or paid. Second, although based on WHO studies the EIA kits that are available at lower cost in less affluent countries are able to perform with similar sensitivity to assays used in developed countries, blood banks in developing countries are poorly regulated and lack systems to assure that standardized testing is performed [30]. Third, the long window period before EIA reactivity for HCV would be reduced with the addition of NAT testing as discussed above, but NAT testing is less amenable than EIA screening in countries with reduced resources to support technical training and reagent costs. Fully automated and commercialized NAT platforms requiring minimal technical expertise is a possibility but that too is limited due to increased equipment and reagent costs and infrastructure issues.
The events that led to the reduction of TT HCV in the developed world are good benchmarks and warrant consideration by governments and public health and blood banking officials in developing countries. Moving towards an all-volunteer and repeat blood donor pool can have a major impact, as well as stringent assessment, adoption and performance of anti-HCV EIA screening assays. In addition, improved basic blood banking practices, such as accurate record keeping, are integral to maintaining blood safety, preventing release of contaminated blood components and allowing for effective deferral of infected donors. At the time of identification and deferral, blood donors may be notified of their HCV status, including RNA and antibody confirmatory test results that may help HCV infected individuals to receive timely therapy to deter disease progression. In the era of new and potent antiviral agents against HCV, timely intervention can help prevent future public health burden associated with HCV-related liver diseases.
Creation of blood donor specimen and data repositories to help understand HCV epidemiology globally
Much of what is known about very early events in HCV infection was learned from studies of archived samples from recipients who acquired transfusion-associated infections prior to the discovery of HCV, and from experimentally infected chimpanzees [31-34]. For example, the Transfusion-Transmitted Viruses Study (TTVS) was designed in the 1970’s to prospectively identify cases of NANB hepatitis in a cohort of transfused patients and to create a repository of linked donor and recipient samples which was later tested to establish the rate and correlates of virus transmission through blood transfusion as well as the course and outcomes of infections in recipients [33-37]. The TTVS repository is an outstanding example of how NHLBI Biologic Specimen Repositories have played a significant role over the years in improving our understanding of infectious risks of transfusions as well as NANB/Hepatitis C virus biology [38]. Similarly, the, ARC, REDS and International Society for Blood Transfusion (ISBT) databases have been used successfully to demonstrate rates and correlates of HCV infections in donors and residual risk and consequences of TT HCV.
There is potential for public and private sector funding to help create databases with blood donor screening results and repositories of samples from HCV infected donors, and particularly NAT yield donations, on a global scale. Data from such databases and repositories can help understand the global burden of the disease and possible methods of intervention. In addition, individuals identified by donor screening as having persistent or resolved HCV infections represent an immense subject pool from which to obtain the sample sizes necessary to identify and subsequently confirm immune and genetic correlates of spontaneous clearance and disease progression, while minimizing selection bias [12]. Blood and plasma centers in the US collect approximately 2.5 million units of whole blood and apheresis components every year. Given the current seroprevalence of HCV among donors, there are approximately 5,000 newly diagnosed HCV infections detected in the US blood donor setting annually, and over 50,000 globally. The blood donation system therefore represents the largest systematic screening program for TT infectious diseases. The blood donor specimen and data repositories within a region or country could thus be an important source for studying the evolving epidemiology of HCV.
Conclusion
Stringent blood bank practices as well as serological assays and NAT in the developed world have successfully minimized the residual risk from TT HCV. Continuing to screen blood for HCV and other TT infectious diseases is a priority and safe blood is considered an individual right. However, a similar situation in the developing world will require a concerted effort both at national and international levels with support from public and private funds.
Footnotes
Disclosure statement
The authors declare no competing interests.
References
- 1.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 2.Grady GF, Chalmers TC. Risk of Post-Transfusion Viral Hepatitis. N Engl J Med. 1964;271:337–342. doi: 10.1056/NEJM196408132710702. [DOI] [PubMed] [Google Scholar]
- 3.Tobler LH, Busch MP. History of posttransfusion hepatitis. Clin Chem. 1997;43:1487–1493. [PubMed] [Google Scholar]
- 4.Walsh JH, Purcell RH, Morrow AG, Chanock RM, Schmidt PJ. Posttransfusion hepatitis after open-heart operations. Incidence after the administration of blood from commercial and volunteer donor populations. JAMA. 1970;211:261–265. doi: 10.1001/jama.211.2.261. [DOI] [PubMed] [Google Scholar]
- 5.Gocke DJ, Greenberg HB, Kavey NB. Hepatitis antigen. Detection of infectious blood donors. Lancet. 1969;2:248–249. doi: 10.1016/s0140-6736(69)90011-7. [DOI] [PubMed] [Google Scholar]
- 6.Alter HJ, Holland PV, Purcell RH, Lander JJ, Feinstone SM, Morrow AG, Schmidt PJ. Posttransfusion hepatitis after exclusion of commercial and hepatitis-B antigen-positive donors. Ann Intern Med. 1972;77:691–699. doi: 10.7326/0003-4819-77-5-691. [DOI] [PubMed] [Google Scholar]
- 7.Alter HJ, Purcell RH, Shih JW, Melpolder JC, Houghton M, Choo QL, Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989;321:1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- 8.Courouce AM, Le Marrec N, Girault A, Ducamp S, Simon N. Anti-hepatitis C virus (anti-HCV) seroconversion in patients undergoing hemodialysis: comparison of second- and third-generation anti-HCV assays. Transfusion. 1994;34:790–795. doi: 10.1046/j.1537-2995.1994.34994378281.x. [DOI] [PubMed] [Google Scholar]
- 9.Stramer SL, Glynn SA, Kleinman SH, Strong DM, Caglioti S, Wright DJ, Dodd RY, Busch MP. Detection of HIV-1 and HCV infections among antibody-negative blood donors by nucleic acid-amplification testing. N Engl J Med. 2004;351:760–768. doi: 10.1056/NEJMoa040085. [DOI] [PubMed] [Google Scholar]
- 10.Glynn SA, Wright DJ, Kleinman SH, Hirschkorn D, Tu Y, Heldebrant C, Smith R, Giachetti C, Gallarda J, Busch MP. Dynamics of viremia in early hepatitis C virus infection. Transfusion. 2005;45:994–1002. doi: 10.1111/j.1537-2995.2005.04390.x. [DOI] [PubMed] [Google Scholar]
- 11.Roth WK, Busch MP, Schuller A, Ismay S, Cheng A, Seed CR, Jungbauer C, Minsk PM, Sondag-Thull D, Wendel S, Levi JE, Fearon M, Delage G, Xie Y, Jukic I, Turek P, Ullum H, Tefanova V, Tilk M, Reimal R, Castren J, Naukkarinen M, Assal A, Jork C, Hourfar MK, Michel P, Offergeld R, Pichl L, Schmidt M, Schottstedt V, Seifried E, Wagner F, Weber-Schehl M, Politis C, Lin CK, Tsoi WC, O’Riordan J, Gottreich A, Shinar E, Yahalom V, Velati C, Satake M, Sanad N, Sisene I, Bon AH, Koppelmann M, Flanagan P, Flesland O, Brojer E, Letowska M, Nascimento F, Zhiburt E, Chua SS, Teo D, Stezinar SL, Vermeulen M, Reddy R, Park Q, Castro E, Eiras A, Gonzales Fraile I, Torres P, Ekermo B, Niederhauser C, Chen H, Oota S, Brant LJ, Eglin R, Jarvis L, Mohabir L, Brodsky J, Foster G, Jennings C, Notari E, Stramer S, Kessler D, Hillyer C, Kamel H, Katz L, Taylor C, Panzer S, Reesink HW. International survey on NAT testing of blood donations: expanding implementation and yield from 1999 to 2009. Vox Sang. 2012;102:82–90. doi: 10.1111/j.1423-0410.2011.01506.x. [DOI] [PubMed] [Google Scholar]
- 12.Selvarajah S, Tobler LH, Simmons G, Busch MP. Host genetic basis for hepatitis C virus clearance: a role for blood collection centers. Curr Opin Hematol. 2010;17:550–557. doi: 10.1097/MOH.0b013e32833e7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shata MT, Tricoche N, Perkus M, Tom D, Brotman B, McCormack P, Pfahler W, Lee DH, Tobler LH, Busch M, Prince AM. Exposure to low infective doses of HCV induces cellular immune responses without consistently detectable viremia or seroconversion in chimpanzees. Virology. 2003;314:601–616. doi: 10.1016/s0042-6822(03)00461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katayama K, Kumagai J, Komiya Y, Mizui M, Yugi H, Kishimoto S, Yamanaka R, Tamatsukuri S, Tomoguri T, Miyakawa Y, Tanaka J, Yoshizawa H. Titration of hepatitis C virus in chimpanzees for determining the copy number required for transmission. Intervirology. 2004;47:57–64. doi: 10.1159/000076643. [DOI] [PubMed] [Google Scholar]
- 15.Kleinman SH, Lelie N, Busch MP. Infectivity of human immunodeficiency virus-1, hepatitis C virus, and hepatitis B virus and risk of transmission by transfusion. Transfusion. 2009;49:2454–2489. doi: 10.1111/j.1537-2995.2009.02322.x. [DOI] [PubMed] [Google Scholar]
- 16.Busch MP, Murthy KK, Kleinman SH, et al. Infectivity in Chimpanzees (Pan troglodytes) of Plasma Collected before HCV RNA Detectability: Implications for Transfusion Safety and HCV Infection Outcomes. Blood. 2012;199:6326–6334. doi: 10.1182/blood-2011-12-393637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou S, Dorsey KA, Notari EP, Foster GA, Krysztof DE, Musavi F, Dodd RY, Stramer SL. Prevalence, incidence, and residual risk of human immunodeficiency virus and hepatitis C virus infections among United States blood donors since the introduction of nucleic acid testing. Transfusion. 2010 doi: 10.1111/j.1537-2995.2010.02622.x. [DOI] [PubMed] [Google Scholar]
- 18.Zou S, Stramer SL, Dodd RY. Donor Testing and Risk: Current Prevalence, Incidence, and Residual Risk of Transfusion-Transmissible Agents in US Allogeneic Donations. Transfus Med Rev. 2011 doi: 10.1016/j.tmrv.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Murphy EL, Bryzman SM, Glynn SA, Ameti DI, Thomson RA, Williams AE, Nass CC, Ownby HE, Schreiber GB, Kong F, Neal KR, Nemo GJ. Risk factors for hepatitis C virus infection in United States blood donors. NHLBI Retrovirus Epidemiology Donor Study (REDS) Hepatology. 2000;31:756–762. doi: 10.1002/hep.510310329. [DOI] [PubMed] [Google Scholar]
- 20.Busch MP, Glynn SA, Stramer SL, Orland J, Murphy EL, Wright DJ, Kleinman S. Correlates of hepatitis C virus (HCV) RNA negativity among HCV-seropositive blood donors. Transfusion. 2006;46:469–475. doi: 10.1111/j.1537-2995.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 21.Delwart E, Silkas E, Stramer SL, et al. Genetic diversity of recently acquired and prevalent HIV, hepatitis B virus, and hepatitis C virus infections in US blood donors. J Infect Dis. 2012;205:875–885. doi: 10.1093/infdis/jir862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, Tobler L, Andrews W, Avanesyan L, Cooper S, Busch MP. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis. 2009;200:1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz LM. How Can the Blood Transfusion Medicine Community Contribute to Public Health in Tough Economic Times? J Infect Dis. 2012 doi: 10.1093/infdis/jir866. [DOI] [PubMed] [Google Scholar]
- 24.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 25.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 26.Sievert W, Altraif I, Razavi HA, Abdo A, Ahmed EA, Alomair A, Amarapurkar D, Chen CH, Dou X, El Khayat H, Elshazly M, Esmat G, Guan R, Han KH, Koike K, Largen A, McCaughan G, Mogawer S, Monis A, Nawaz A, Piratvisuth T, Sanai FM, Sharara AI, Sibbel S, Sood A, Suh DJ, Wallace C, Young K, Negro F. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31(Suppl 2):61–80. doi: 10.1111/j.1478-3231.2011.02540.x. [DOI] [PubMed] [Google Scholar]
- 27.Cornberg M, Razavi HA, Alberti A, Bernasconi E, Buti M, Cooper C, Dalgard O, Dillion JF, Flisiak R, Forns X, Frankova S, Goldis A, Goulis I, Halota W, Hunyady B, Lagging M, Largen A, Makara M, Manolakopoulos S, Marcellin P, Marinho RT, Pol S, Poynard T, Puoti M, Sagalova O, Sibbel S, Simon K, Wallace C, Young K, Yurdaydin C, Zuckerman E, Negro F, Zeuzem S. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 2011;31(Suppl 2):30–60. doi: 10.1111/j.1478-3231.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 28.Kershenobich D, Razavi HA, Sanchez-Avila JF, Bessone F, Coelho HS, Dagher L, Goncales FL, Quiroz JF, Rodriguez-Perez F, Rosado B, Wallace C, Negro F, Silva M. Trends and projections of hepatitis C virus epidemiology in Latin America. Liver Int. 2011;31(Suppl 2):18–29. doi: 10.1111/j.1478-3231.2011.02538.x. [DOI] [PubMed] [Google Scholar]
- 29.Negro F, Alberti A. The global health burden of hepatitis C virus infection. Liver Int. 2011;31(Suppl 2):1–3. doi: 10.1111/j.1478-3231.2011.02537.x. [DOI] [PubMed] [Google Scholar]
- 30.Scheiblauer H, El-Nageh M, Nick S, Fields H, Prince A, Diaz S. Evaluation of the performance of 44 assays used in countries with limited resources for the detection of antibodies to hepatitis C virus. Transfusion. 2006;46:708–718. doi: 10.1111/j.1537-2995.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- 31.Busch MP. Insights into the epidemiology, natural history and pathogenesis of hepatitis C virus infection from studies of infected donors and blood product recipients. Transfus Clin Biol. 2001;8:200–206. doi: 10.1016/s1246-7820(01)00125-2. [DOI] [PubMed] [Google Scholar]
- 32.Alter HJ, Conry-Cantilena C, Melpolder J, Tan D, Van Raden M, Herion D, Lau D, Hoofnagle JH. Hepatitis C in asymptomatic blood donors. Hepatology. 1997;26:29S–33S. doi: 10.1002/hep.510260705. [DOI] [PubMed] [Google Scholar]
- 33.Mosley JW, Operskalski EA, Tobler LH, Buskell ZJ, Andrews WW, Phelps B, Dockter J, Giachetti C, Seeff LB, Busch MP. The course of hepatitis C viraemia in transfusion recipients prior to availability of antiviral therapy. J Viral Hepat. 2008;15:120–128. doi: 10.1111/j.1365-2893.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu YK, Weiner AJ, Rosenblatt J, Wong DC, Shapiro M, Popkin T, Houghton M, Alter HJ, Purcell RH. Early events in hepatitis C virus infection of chimpanzees. Proc Natl Acad Sci U S A. 1990;87:6441–6444. doi: 10.1073/pnas.87.16.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Operskalski EA, Mosley JW, Tobler LH, Fiebig EW, Nowicki MJ, Mimms LT, Gallarda J, Phelps BH, Busch MP. HCV viral load in anti-HCV-reactive donors and infectivity for their recipients. Transfusion. 2003;43:1433–1441. doi: 10.1046/j.1537-2995.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- 36.Mosley JW, Operskalski EA, Tobler LH, Andrews WW, Phelps B, Dockter J, Giachetti C, Busch MP. Viral and host factors in early hepatitis C virus infection. Hepatology. 2005;42:86–92. doi: 10.1002/hep.20742. [DOI] [PubMed] [Google Scholar]
- 37.Selvarajah S, Keating S, Heitman J, et al. Detection of host immune responses in acute phase sera of spontaneous resolution versus persistent HCV infection. J Gen Virol. 2012;93:1673–1679. doi: 10.1099/vir.0.041277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Busch MP, Glynn SA. Use of blood-donor and transfusion-recipient biospecimen repositories to address emerging blood-safety concerns and advance infectious disease research: the National Heart, Lung, and Blood Institute Biologic Specimen Repository. J Infect Dis. 2009;199:1564–1566. doi: 10.1086/598860. [DOI] [PubMed] [Google Scholar]