Abstract
Regiospecific and conformationally restrained analogs of melphalan and DL-2-NAM-7 have been synthesized and their affinities for the large neutral amino acid transporter (LAT1) of the blood–brain barrier have been determined to assess their potential for accessing the CNS via facilitated transport. Several analogs had Ki values in the range 2.1–8.5 μM with greater affinities than that of either L-phenylalanine (Ki = 11 μM) or melphalan (Ki = 55 μM), but lower than DL-2-NAM-7 (Ki = 0.08 μM). The results indicate that regiospecific positioning of the mustard moiety on the aromatic ring in these analogs is very important for optimal affinity for the large neutral amino acid transporter, and that conformational restriction of the DL-2-NAM-7 molecule in benzonorbornane and indane analogs leads to 25- to 60-fold loss, respectively, in affinity.
Keywords: Melphalan, Anticancer agents, Blood–brain barrier, Large neutral amino acid transporter
The treatment of malignant brain tumors represents a significant clinical challenge. Patients often present with disabling neurological syndromes, undergo rapid deterioration, and respond poorly to current therapies. Despite recent developments in treatment strategies, including combination therapy, tumor-targeted ligands, tissue-specific chemotherapy, and attempts at manipulating discrete molecular and cellular signaling pathways, therapeutic outcomes remain very poor.1–3 Although many effective anticancer drugs in numerous therapeutic classes are available for treating peripheral cancers, most anticancer drugs are ineffective for treating CNS tumors, in large part due to their inability to cross the blood–brain barrier (BBB) from the systemic circulation into the tumor and peritumoral areas in therapeutic concentrations.1,4
The permeability of the protective BBB to xenobiotics such as chemotherapeutic agents depends upon a number of factors, including molecular weight, lipid solubility, lipid and protein binding, metabolism, and efflux out of the CNS, as well as on prior treatments. 3,4 Attempts to compromise the integrity of the BBB in order to overcome its normal protective role and facilitate delivery of chemotherapy to the brain include MRI-guided ultrasound and transient hyper-osmotic disruption.4 Although transient disruption of the BBB shows promise in improving the CNS delivery of chemotherapeutic agents, there is some concern about potential tissue damage and neurological side effects. Another approach might be to take advantage of natural transporter proteins present at the intact BBB membrane in order to increase the CNS delivery of novel chemotherapeutic agents that are also designed as transporter substrates.
A number of amino acid transporters are expressed at the BBB in order to promote the uptake of amino acids from the systemic circulation into the brain. Such transporters are necessary for normal brain function. The large neutral amino acid transporter (System LAT1) facilitates the uptake of neutral amino acids, such as L-leucine and L-phenylalanine (1), in a saturable and stereospecific manner.5–7 The system LAT1 transporter is expressed on both the capillary luminal and abluminal membranes, has the greatest transport capacity of the BBB amino acid influx transporters, and mediates the uptake of the largest number of amino acids into brain.5–7 Thus, the system LAT1 transporter may be regarded as a versatile, high-capacity target for transporting appropriately designed therapeutic agents into the brain without disrupting the integrity of the BBB.
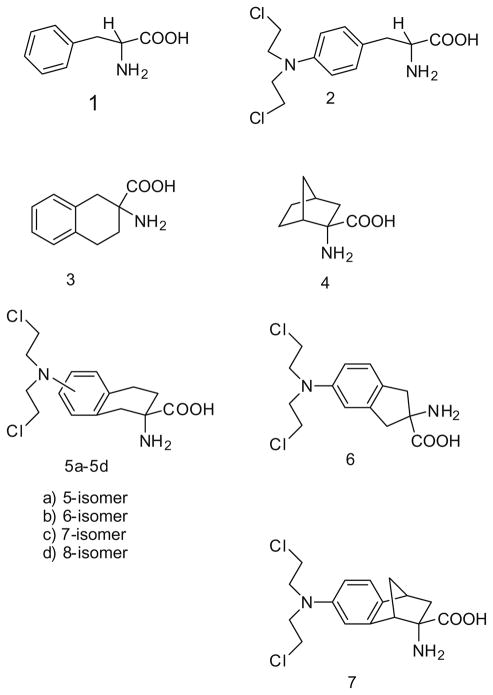
Melphalan (2) is an established anticancer agent and nitrogen mustard derivative of the system LAT1 substrate L-phenylalanine (Fig. 1). However, as a moderately low-affinity substrate for system LAT1, with an estimated Km of ~90–150 μM,8,9 it shows poor brain penetration and is an inferior candidate for the treatment of brain tumors. Conformational restriction of the phenylalanine molecule to afford DL-2-amino-1,2,3,4,-tetrahydronaphthoic acid (3) improved affinity for the system LAT1 transporter (Km = 7.1 μM).10 The rigid amino acid, BCH (4), also has good affinity for the system LAT1 transporter.10,11 Subsequent studies have suggested that other mustard analogs may be designed with improved affinity for system LAT1 compared to melphalan.10–12 Thus, it may be possible to improve CNS delivery through design and development of novel chemotherapeutic agents structurally related to melphalan that incorporate a more conformationally restrained amino acid scaffold, such as 3, thereby improving system LAT1 transporter affinity.12 The melphalan analogs 5a and 5c have each been reported to have higher affinity for the system L transporter (Ki = ~25 and ~0.2 μM, respectively) than melphalan.10 Also, 5c (DL-2-NAM-7) possesses enhanced in vitro antitumor activity and reduced myelosuppressive activity when compared to melphalan. 11 Furthermore, studies have shown that 5c is rapidly taken up into brain by the blood–brain barrier system LAT1 transporter (Vmax = 0.26 nmol/min/g; Km = 0.19 μM).10
Figure 1.
Structures of phenylalanine (1), melphalin (2), DL-2-NAM-7 (5c) and structurally related compounds.
Placing the mustard moiety at the C-7 position in 5 affords an isomer (5c) that has significantly higher affinity for system LAT1 than the C-5 compound 5a. However, it is not known whether the optimal position for the mustard moiety is at C-7, since the affinity of the C-6 and C-8 isomers 5b and 5d, respectively, have not been reported.
The goals of the current study were to prepare all the isomeric forms of DL-2-NAM-7 (5c) in order to determine the aromatic substitution position of the nitrogen mustard moiety that affords optimal affinity for the system LAT1 transporter, and to also prepare a number of more conformationally defined analogs of DL-2-NAM, in order to assess the effect of further conformational restriction on system LAT1 transporter affinity. In this respect the more conformationally restrained indane analog of DL-2-NAM-7, compound 6, was considered worthy of evaluation as a system LAT1 transporter ligand. Also, compounds incorporating the extremely rigid amino acid 4 into their structure were believed to be of potential interest due the high affinity of this amino acid for the system LAT1 transporter.13,14
In this study, all four isomers (5a–5d) of DL-2-NAM were prepared, as well as the structurally related analogs 6 and 7, and their affinities for the system LAT1 transporter were assessed by competitive L-[14C]-leucine uptake inhibition utilizing a modification15 of the in situ rat brain perfusion method of Takasato et al.16
Mustard analogs of the various amino acid targets were synthesized from precursor hydantoins, which were nitrated to afford a mixture of isomeric products. These isomeric mixtures were not separated, but catalytically reduced to the corresponding isomeric aromatic amines. These mixtures were then reacted with ethylene oxide to afford a mixture of the N-[bis-(ethylhydroxy)-amino isomers.
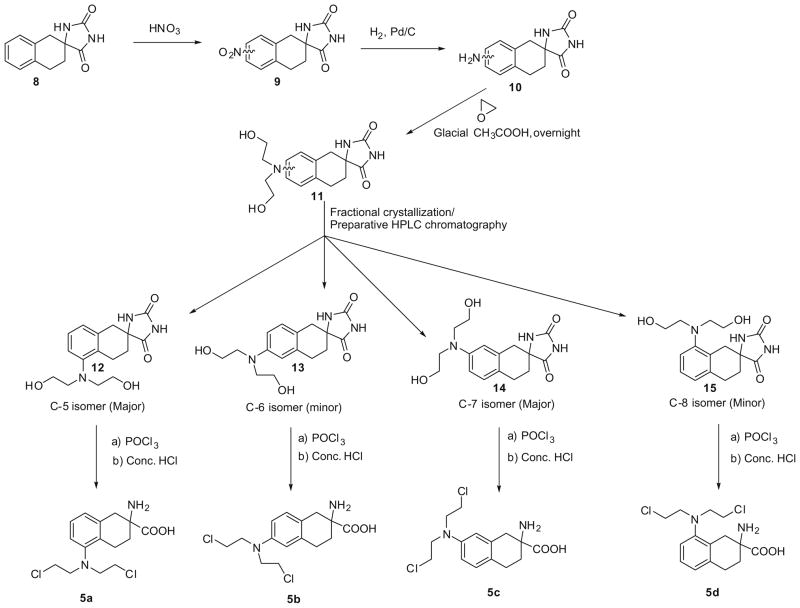
In the synthesis of compounds 5a–5d (Scheme 1), initial nitration of hydantoin 817 afforded a mixture of all 4-nitro isomers, which were reduced to their amino derivatives (10) followed by conversion to a mixture of the bis-(2-hydroxyethyl)-amino analogs 11 with ethylene oxide. Isomer 14 could be obtained in a pure form by fractional crystallization, and the resulting mother liquors could be fractionated by preparative HPLC chromatography to afford isomers 12, 13 and 15. Each bis-(2-hydroxyethyl)-amino isomer was then converted to the corresponding amino acid mustard by reaction with phosphoryl chloride followed by acid hydrolysis. The final mustard products, 5a–5d, were further purified by preparative HPLC prior to biological evaluation.18
Scheme 1.
Synthesis of the 5-, 6-, 7- and 8-[bis(2-chloroethyl)amino]-isomers of (±)-2-amino-1,2,3,4-tetrahydro-2-naphthoic acid.
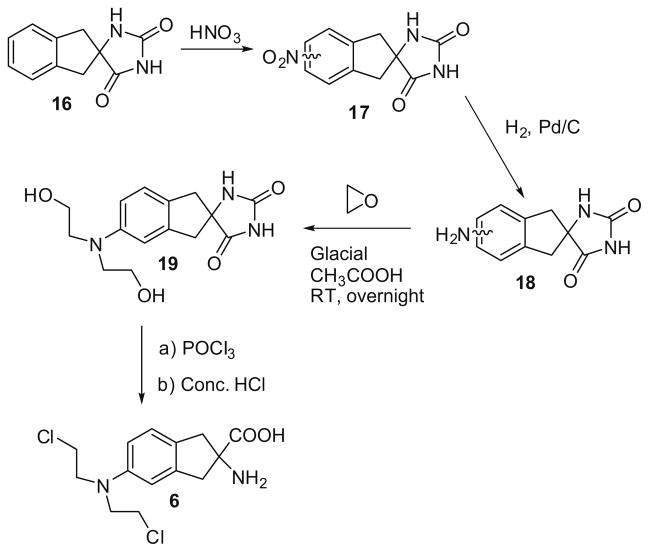
In the synthesis of the indane mustard 6 (Scheme 2), initial nitration of hydantion 1617 afforded a mixture of the 4-nitro (minor) and 5-nitro (major) analogs (17), which were not separated, but converted to their corresponding amino analogs (18) utilizing H2/Pd–C 10%/DMP, and then to their bis(2-hydroxyethyl)-amino analogs. Isomer 19 was obtained pure by silica gel chromatography. However, an isomerically pure sample of the corresponding 4-isomer could not be obtained. Mustard 6 was obtained from 19 as described above for products 5a–5d, and was purified by preparative HPLC prior to biological evaluation.18
Scheme 2.
Synthesis of 2-amino-(bis-2-chloroethyl)-5-aminoindane-2-carboxylic acid (6).
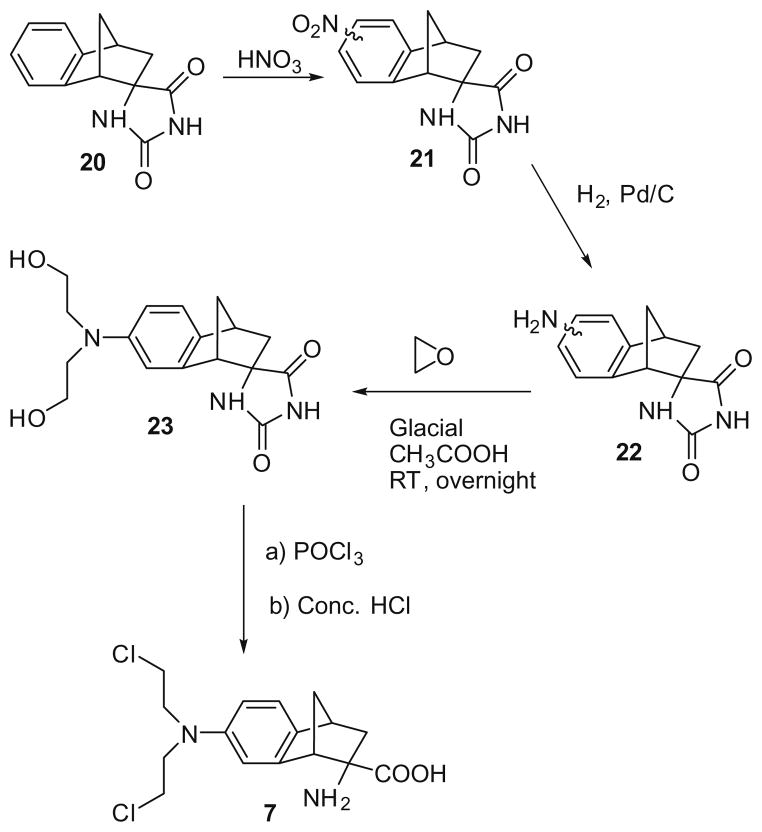
The synthesis of mustard 7 from hydantoin 2019 utilized a similar procedure to that described for the indane mustard 6 (Scheme 3), affording a mixture of the 5-(minor) and 7-(major)-nitro isomers (21). Isomer 23 could be obtained by fractional crystallization of the isomeric mixture obtained from the reaction of ethylene oxide with 22 and was then converted to mustard 7 as previously described. An isomerically pure sample of the 5-isomer of 22 could not be obtained.
Scheme 3.
The synthesis of (±)-2-endo-amino-bis(2-chloroethyl)-7′-aminobenzobicyclo[ 2.2.1]heptane-2-exo-carboxylic acid (7).
The structures of all the amino acid mustards were confirmed by 1H NMR, and HRMS.20
System LAT1 transporter affinities of the tested compounds were determined using the in situ rat brain perfusion technique12, which measured the concentration-dependent inhibition of L-[14C]-leucine uptake into rat brain by the amino acid analogues. The relative capacity of the tested compounds to inhibit L-[14C]-leucine uptake across the blood–brain barrier was determined as Ki (IC50) values, and the data were summarized in Table 1.
Table 1.
Relative affinities of amino acid and melphalan analogs for the system LAT1 transporter, expressed as the Ki for inhibition of the uptake of L-[14C]-leucine into rat brain via the blood–brain barrier.
| Amino acid | Nitrogen mustard | Kia (μM ± S.E.M) |
|---|---|---|
| L-Phenylalanine | 2 | 55 ± 4 |
| (±)-2-Amino-1,2,3,4-tetrahydro-2-naphthoic acid | 7.7 ± 0.8 | |
| 5a | 8.5 ± 0.6 | |
| 5b | 68 ± 9 | |
| 5c | 0.079 ± 0.006 | |
| 5d | 252 ± 44 | |
| (±)-2-Aminoindane-2 carboxylic acid | 12.5 ± 1.1 | |
| 6 | 5.0 ± 0.6 | |
| (±)-2-Aminobenzo-bicyclo-[2.2.1]heptane-2′-exo-carboxylic acid | 26 ± 1 | |
| 7 | 2.1 ± 0.2 |
n = 9–12.
The data in Table 1 clearly indicate that in the 2-NAM series of compounds, the optimal position for the mustard moiety is at C-7 of the 1,2,3,4-tetrahydronaphthalene ring. The previously unreported isomers 5b and 5d had >1000 times lower affinity for the system LAT1 transporter than DL-2-NAM-7 (5c) and isomer 5a had 100 times less affinity for the transporter than 5c. Further restriction of the conformational flexibility of the 2-NAM scaffold appeared to be detrimental to system LAT1 binding, since the indane analog 6 and the rigid analog 7 had 60 and 25 times less affinity, respectively, for the transporter than 5c, but approximately matched affinity for 5a. It is important to note that both 6 and 7 were superior ligands than melphalan (2) at the system LAT1 transporter and may show improved delivery to brain and activity against brain tumors.
Acknowledgments
This work was supported by grants from the Department of Defense Breast Cancer Program (W81XWH-062-0033) and NIH/NINDS (R01 NS052484).
References and notes
- 1.Argyriou AA, Antonacopoulou A, Iconomou G, Kalofonos HP. Crit Rev Hematol Oncol. 2008 Jul 3; doi: 10.1016/j.critrevonc.2008.05.005. Epub. [DOI] [PubMed] [Google Scholar]
- 2.Feun L, Savaraj N. Expert Rev Anticancer Ther. 2008;8:707. doi: 10.1586/14737140.8.5.707. [DOI] [PubMed] [Google Scholar]
- 3.Gerstner ER, Fine RL. J Clin Oncol. 2007;25:2306. doi: 10.1200/JCO.2006.10.0677. [DOI] [PubMed] [Google Scholar]
- 4.Muldoon LL, Soussain C, Jahnke K, Johanson C, Siegal T, Smith QR, Hall WA, Hynynen K, Senter PD, Peereboom DM, Neuwelt EA. J Clin Oncol. 2007;25:2295. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 5.Oldendorf WH. Am J Physiol. 1971;221:1629. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- 6.Smith QR, Momma ASM, Rapoport SI. J Neurochem. 1987;49:165. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 7.Smith QR. J Nutr. 2000;130:1016S. doi: 10.1093/jn/130.4.1016S. [DOI] [PubMed] [Google Scholar]
- 8.Greig NH, Momma S, Sweeney DJ, Smith QR, Rapoport SI. Cancer Res. 1987;47:1571. [PubMed] [Google Scholar]
- 9.Takada Y, Greig NH, Vistica DT, Rapoport SI, Smith QR. Cancer Chemother Pharmacol. 1991;29:89. doi: 10.1007/BF00687316. [DOI] [PubMed] [Google Scholar]
- 10.Takada Y, Vistica DT, Grieg NH, Purdon D, Rapoport SI, Smith QR. Cancer Res. 1992;52:2191. [PubMed] [Google Scholar]
- 11.Haines D, Fuller RW, Ahmad S, Vistica DT, Marquez VE. J Med Chem. 1987;30:542. doi: 10.1021/jm00386a017. [DOI] [PubMed] [Google Scholar]
- 12.Smith QR. Int Congress Series. 2005;1277:63. [Google Scholar]
- 13.Lewensohn R, Hansson J, Ringborn U, Ehrsson H. Eur J Cancer Clin Oncol. 1987;23:783. doi: 10.1016/0277-5379(87)90279-3. [DOI] [PubMed] [Google Scholar]
- 14.del Amo EM, Urtti A, Yliperttula M. Eur J Pharm Sci. 2008;35:161. doi: 10.1016/j.ejps.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Brain perfusion and system L transporter affinity studies: Transport affinity was evaluated from the concentration-dependent inhibition of L-[14C]leucine uptake into rat brain during perfusion at tracer leucine concentration (<0.3 μM) and in the absence of competing amino acids. This perfusion technique enabled the determination of inhibitory transport activity of the compounds against the in vivo blood–brain barrier system LAT1 transporter in the absence of endogenous competitors and normal plasma proteins. The circulation to the brain is taken over for a brief period of time (<30 s) by infusing physiologic saline into the carotid artery at a physiological rate. The infusion rate is adjusted so that perfusion pressure matches normal systemic blood pressure (80–110 mm Hg) and allows the delivery of a constant, defined concentration of the test compound in the perfusion fluid to the brain in the absence of plasma proteins, ions and other substrates that may compete for transport. Furthermore, delivery of the test compounds to the brain occurs without prior exposure to the peripheral organs and metabolic enzymes, minimizing the confounding effects of radioactive metabolites. Although metabolism may occur in the brain, due to the limited time exposure of the perfusion, there is minimal loss of tracer products, such as 14C–carbon dioxide. Adult male Sprague–Dawley rats weighing between 240 and 450 g were used for the experiments. The rats were anesthetized with sodium pentobarbital (50 mg/kg ip). The occipital and superior thyroid arteries were cauterized and cut. A catheter (PE-60) was placed in the common carotid artery and the perfusate (bicarbonate-buffered physiologic saline, pH 7.4) was infused into the left common carotid artery at the rate of 20 ml/min using a pump. Prior to the perfusion, the external carotid artery was ligated just proximal to the bifurcation of the common carotid artery. The heart was stopped prior to perfusion (2–3 s) by severing the left ventricle so as to eliminate potential flow contributions from the systemic circulation. After the catheter is placed in the common carotid artery, blood flow to the ipsilateral cerebral hemisphere is maintained by crossover at the circle of Willis from the contralateral circulation. The pterygopalatine artery is kept open. The cannulation procedure of the common carotid artery is simple to perform and generally takes <15 min. In this technique, the thoracic cavity is opened at the beginning of the perfusion, and the cardiac ventricles are severed to stop blood flow from the systemic circulation. The infusion rate of the perfusion into the common carotid artery is usually sufficient to perfuse both cerebral hemispheres as well as the brain stem. The composition of the perfusate was 128 mM sodium chloride, 24 mM sodium bicarbonate, 4.2 mM potassium chloride, 1.5 mM calcium chloride, 0.9 mM magnesium chloride, 2.4 mM sodium phosphate and 9mM D-glucose in order to supply sufficient nutrients and salts to maintain the metabolic and structural integrity of the brain. To this was added ~0.1 μCi L-[14C]leucine/mL, ~0.1 μCi [3H]-diazepam/mL (to measure cerebral perfusion fluid flow), 0–1000 μM unlabelled amino acid analog (for the inhibition studies) and/or [3H]-sucrose (for vascular volume measurements). All solutions were filtered, warmed to 37 °C, and saturated with 95% air and 5% carbon dioxide prior to their use. The body temperature of the rats was maintained at 36–37 °C throughout the experiments with a heating pad. Perfusions were stopped after 15–20 s. The brain was removed from the skull and dissected on ice. Tissue samples were then removed from the ipsilateral cerebral hemisphere after the removal of the meninges and surface blood vessels. Two 20–30 μL aliquots of perfusion fluid were also collected for determination of perfusate tracer concentrations. The tissue samples were weighed, digested overnight in 1 M piperidine (1 mL/sample) and then dissolved in scintillation cocktail (10 mL/sample: Ready Solv-MP Beckman, Fullerton, California). 3H- and 14C dpm values were determined after correction for background, quench, and efficiency using dual-label liquid scintillation counting.
- 16.Takasato Y, Rapoport SI, Smith QR. Am J Physiol. 1984;247:H484. doi: 10.1152/ajpheart.1984.247.3.H484. [DOI] [PubMed] [Google Scholar]
- 17.Deeks T, Crooks PA, Waigh RD. J Med Chem. 1983;26:762. doi: 10.1021/jm00359a024. [DOI] [PubMed] [Google Scholar]
- 18.Deeks T, Crooks PA, Waigh RD. J Pharm Sci. 1984;73:457. doi: 10.1002/jps.2600730408. [DOI] [PubMed] [Google Scholar]
- 19.High pressure liquid chromatography of the final mustard products was carried out on a system comprising a Labomatic® HD 200 high pressure metering pump, a Labomatic® Labocord-200 UV spectrophotometer and a Shimadzu-C-R4A™ (Columbia, MD) chromatopac integrator and printer. The column was a YMC Pack ODS-AM® column, 250 × 30 mm i.d., particle-S-5 μm; 120 A. The mobile phase was a 50:50 v/v water methanol mixture acidified to a pH of 2.75–2.80 with concentrated HCl. The flow rate was 15 mL/min. The fractions were collected manually, lyophilized to a dry powder and reinjected on a comparable analytical system to confirm isomeric purity.
- 20.Compounds 5a and 5c have been reported elsewhere.10,11 (±)-2-Amino-(bis-2-chloroethyl)-5-aminoindane-2-carboxylic acid (6): 1H NMR (D2O/DCl) 400 MHz δ 3.26–3.33 (2H, geminal coupling doublets, C1protons); 3.44–3.49 (4H, t,N–CH2 protons); 3.66–3.73 (2H, geminal coupling doublets, C3-protons); 3.91–3.96 (4H, t, –CH2–Cl); 7.34–7.46 (3H, m, aromatic protons), GC–MS, (BSTFA derivative) m/z 461 (M+), 425 (–Cl), 389 (–2Cl); HRMS: 316.0833, calcd for C14H18N202Cl2 316.0745. (±)-2-Amino-(bis-2-chloroethyl)-6-amino-1,2,3,4-tetrahydro-2-naphthoic acid dihydrochloride salt. 1H NMR (D2O/DCl) 400 MHz δ 1.90–2.0, 2.10–2.20 (2H, m, C3-protons); 2.64–2.74, 2.75–2.84 (2H, m, C4-protons); 2.86–2.92, 3.23–3.30 (2H, doublets, C1-protons); 3.28–3.34, 3.72–3.82 (8H, m, chloroethyl protons), 7.13–7.26 (3H, m, aromatic protons), GC–MS (BSTFA derivative) m/z 475 (M+), 440 (–Cl), 403 (–2Cl); tR HPLC19 27.91 min; HRMS: 330. 0893, calcd for C15H20N202Cl2 330.0901. (±)-2-amino-(bis-2-chloroethyl)-8-amino-1,2,3,4-tetrahydro-2-naphthoic acid dihydrochloride salt. 1H NMR (D2O/DCl) 400 MHz δ 1.80–1.90, 2.02–2.10 (2H, m, C2-protons); 2.55–2.64, 2.65–2.74 (2H, m, C4-protons); 2.76–2.84, 3.16–3.22 (2H, doublets, C1-protons); 3.20–3.26, 3.66–3.74 (8H, m, chloroethyl protons); 7.08–7.14 (3H, m, aromatic protons), GC–MS (BSTFA derivative) m/z 475 (M+), 440 (–Cl), 403 (– 2Cl); tR HPLC19 29.95 min; HRMS: 330.0951, calcd for C15H20N2O2Cl2 330.0901. (±)-2′-endo-Amino-bis-2-chloroethyl-7-aminobenzobicyclo-[2.2.1]heptane-2′-exo-carboxylic acid dihydrochloride. 1H NMR (D2O/DCl) 400 MHz δ 1.44–1.50 (1H, d of d, C3 axial proton); 1.80–1.88 (1H, d of d, C3 equatorial proton); 2.28– 2.34 (1H, m, C4-proton); 2.71–2.78 (1H, m, C1-proton); 3.48–3.56, 3.90–3.98 (8H, m, chloroethyl protons); 3.70–3.76 (2H, m, C9-protons); 7.34–7.38 (1H, d of d, C6 proton); 7.44–7.46 (1H, d, C8-proton): 7.50–7.52 (1H, d, C5-proton), GC–MS (BSTFA derivative), m/z 487 (M+), 451(–1 Cl), 417 (–2 Cl); HRMS: 342.0944, calcd for C16H20N202Cl2 342.0901.