Abstract
A series of tertiary amine analogues derived from lead azaaromatic quaternary ammonium salts has been designed and synthesized. The preliminary structure-activity relationships of these new analogues suggest that such tertiary amine analogues, which potently inhibit nicotine-evoked dopamine release from rat striatum, represent drug-like inhibitors of α6-containing nicotinic acetylcholine receptors. The bis-tertiary amine analog 7 exhibited an IC50 of 0.95 nM, while the tris-tertiary amine analog 19 had an IC50 of 0.35 nM at nAChRs mediating nicotine-evoked dopamine release.
Keywords: nAChR antagonists, smoking cessation, tobacco dependency
Nicotine, the principal alkaloidal component in tobacco, is believed to be primarily responsible for producing the rewarding effects of smoking. Nicotine produces this effect via its interaction with neuronal nicotinic acetylcholine receptors (nAChRs) in the brain to stimulate dopamine (DA) release from nucleus accumbens and striatum.1–2 Stimulation of the brain DA pathways is believed also to be involved in the mechanism of action of many other drugs of abuse.3 Thus, nAChR antagonists that inhibit nicotine-evoked DA release have been suggested to have potential as efficacious therapies for the treatment of tobacco dependence.4–5
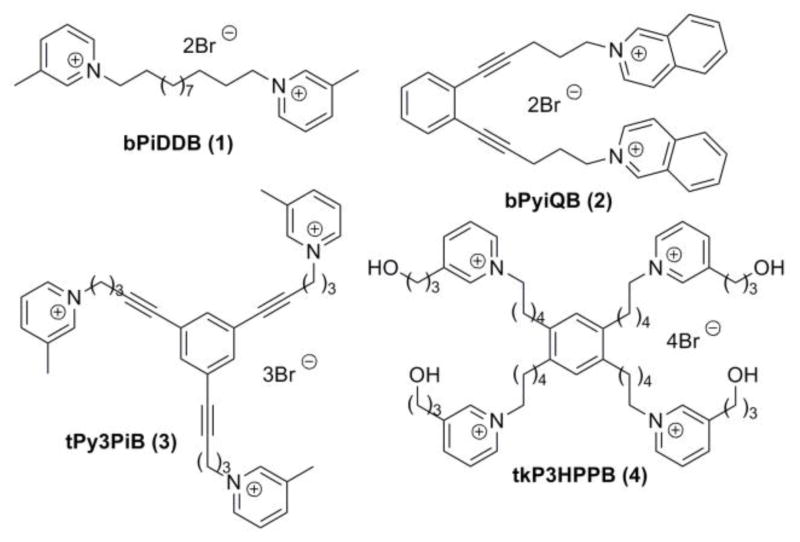
As part of a continuing drug discovery effort in our laboratories to identify novel nAChR antagonists that may have potential as smoking cessation agents,6–14 we found that N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB; 1; Fig. 1), potently inhibited (α6-containing nAChR subtype(s) mediating nicotine-evoked [3H]DA release from superfused rat striatal slices (IC50 = 2 nM).15 In vivo microdialysis studies showed that bPiDDB dose-dependently reduced the increase in extracellular DA in rat nucleus accumbens following acute or repeated nicotine administration.16 Also, behavioral studies in rats showed that bPiDDB dose-dependently decreased nicotine self-administration, but not sucrose-maintained responding, suggesting a specific inhibition of nicotine reward.17 The ability of bPiDDB to inhibit the effect of nicotine in nucleus accumbens and decrease nicotine self-administration in rats provides support for considering bPiDDB as a potential lead compound in the development of novel pharmacotherapies for nicotine dependence.
Figure 1.
Structures of quaternary ammonium leading compounds.
Further structural elaboration of the bPiDDB molecule led to the discovery of 1,2-bis-(5-isoquinolinium-pent-1-ynyl)benzene dibromide (bPyiQB; 2; Fig. 1), a conformationally constrained bis-quaternary ammonium salt;12 1,3,5-tri-{5-[1-(3-picolinium)]-pent-1-ynyl}benzene tribromide (tPy3PiB; 3, Fig. 1), a tris-quaternary ammonium compound;13 and 1,2,4,5-tetrakis –{5-[(3-(3-hydroxypropyl)-pyridinium)]-pentanyl}-benzene tetrabromide (tkP3HPPB; 4; Fig. 1), a tetrakis-quaternary ammonium compound;14 all of these compounds exhibited high potency and selectivity for nAChR subtype(s) mediating nicotine-evoked [3H]DA release with IC50 values ranging from 0.2–3.0 nM.12,13,14
The blood-brain barrier (BBB) is comprised of juxtaposed brain capillary endothelial cells producing tight cell membrane junctions which are essentially impermeable to hydrophilic compounds.18 Because of the cationic nature, high polarity and water-solubility of the bPiDDB molecule, access to brain by passive transport is very limited. However, results from pharmacokinetic studies utilizing radiolabeled 14C-bPiDDB showed that bPiDDB was brain-bioavailable after subcutaneous delivery, due to its facilitated transport via the BBB choline transporter.19–20 Nevertheless, quaternary ammonium compounds are generally not suitable for oral delivery, and bPiDDB has been shown to have limited bioavailability when given by the oral route (Albayati et al., unpublished data). Since oral delivery is the preferred clinical route for development of pharmaceutical products, we sought to optimize our synthetic strategies to focus on the design of analogues with improved oral bioavailability while maintaining inhibitory potency at α6-containing nAChRs.
A quaternized pyridinium moiety is the common characteristic feature in bPiDDB, bPyiQB, tPy3PiB and tkP3HPPB molecules. Conceivably, ionic interactions of such cationic pyridinium moieties with the nAChR binding site(s) may be an important factor in understanding mechanism of inhibition. In this respect, the ionic interaction of a protonated tertiary amine with binding sites on nAChRs may involve similar binding characteristics as a quaternized pyridinium moiety when the protonated tertiary amine moieties are appended to a common structural scaffold. Based on this premise, we hypothesized that analogues derived from the above quaternized ammonium lead compounds, in which the quaternary pyridinium moieties had been replaced with tertiary amine moieties (capable of being protonated at physiological pH) may retain their inhibitory interactions with nAChRs mediating nicotine-evoked DA release from striatum.
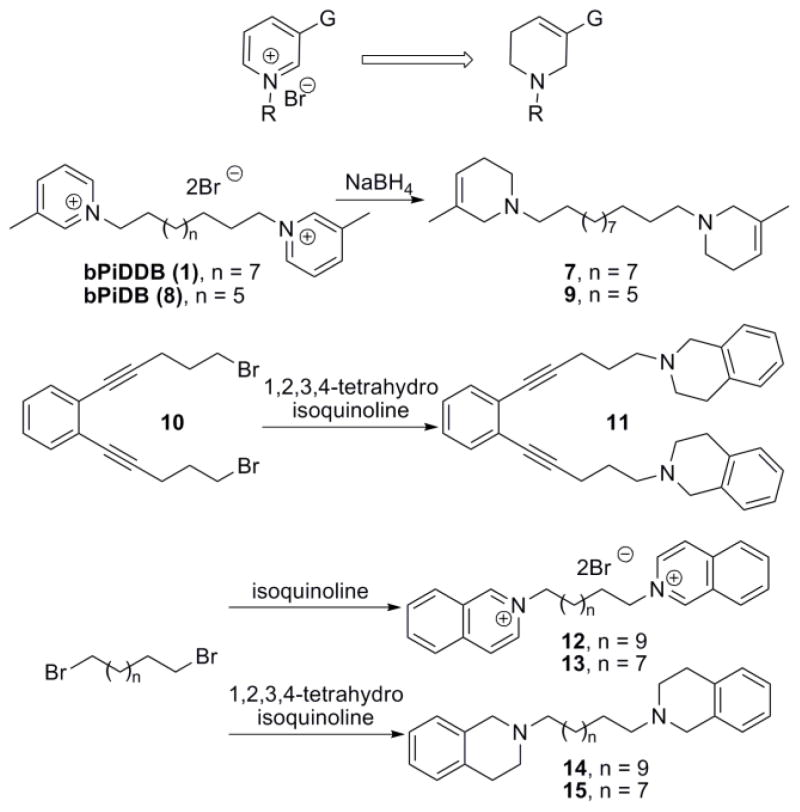
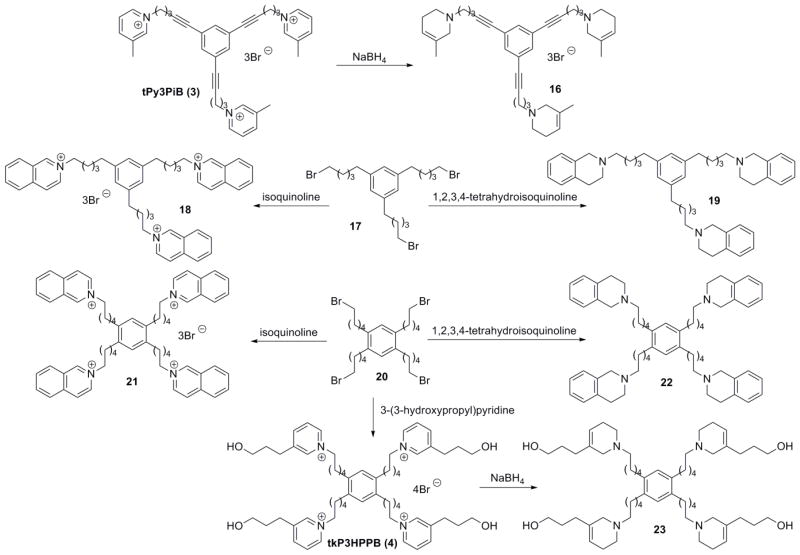
In our previous report,21 we have shown that replacing the quaternary ammonium head groups in compound 1 and 3 with classical nAChR antagonists, mecamylamine or TMP (e.g. compounds 5 and 6, respectively; Fig. 2) resulted in a retention of inhibitory potency. Since bPiDDB, bPyiQB, tPy3PiB, and tkP3HPPB were identified as the most important leads in the search for inhibitors of nicotine-evoked DA release, we designed tertiary amino analogues of these closely related compounds, viz: 7 (Scheme 1), 11 (Scheme 1), 16 (Scheme 2), and 23 (Scheme 2), in which the 3-picolinium, isoquinolinium, or 3-(3-hydroxypropyl)-pyridinium headgroups in these lead compounds have been reductively transformed into their corresponding tertiary amine headgroups: 3-methyl-1,2,5,6-tetrahydropyridine, 1,2,3,4-tetrahydro-isoquinoline, and 3-(3-hydroxypropyl)-1,2,5,6-tetrahydropyridine, respectively. In these structural modifications, the central structural scaffold is retained, while the head groups are de-aromatized. Initial designs in these tertiary amino analogues included retention of one double bound in the resulting piperidine ring, in order to eliminate the introduction of a chiral center into the azaheterocyclic ring, which would have led to multiple enantiomeric and diastereomeric products. The design also maintains to some degree the planar characteristics of the pyridinium moiety in the lead molecules. Additionally, compounds 9, 14, 15, 19, and 22 were synthesized; these compounds were generated from reduction of the 3-picolinium and isoquinolinium head groups in compounds 8, 12, 13, 18, and 21, affording the corresponding analogues containing 3-methyl-1,2,5,6-tetrahydropyridine and/or 1,2,3,4-tetra-hydroisoquinoline head groups (Schemes 1 and 2).
Figure 2.
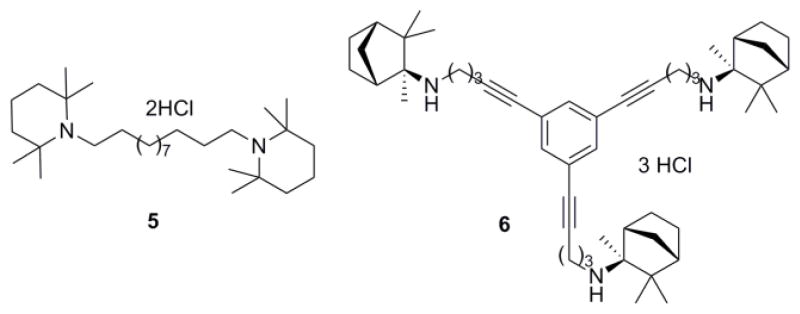
Structures of the TMP and mecamylamine containing compounds.
Scheme 1.
Synthesis of compounds 7, 9, 11, 14 and 15.
Scheme 2.
Synthesis of compounds 16, 18, 19, and 21-23.
The synthesis of the non-quaternary analogue 7 was achieved through NaBH4 reduction of bPiDDB (Scheme 1). A similar reductive procedure was used to synthesize analogues 9, 16, and 23 from the corresponding quaternary ammonium analogues, bPiDB (8), tPy3PiB (3) and tkP3HPPB (4) (Scheme 1 and 2, Table 1). The corresponding tertiary amine analogues of bPyiQB (2), i.e. compound 11, was prepared from dibromide 10 through direct substitution with 1,2,3,4-tetrahydroisoquinoline (Scheme 1). A similar method to that utilized in the synthesis of compound 11 was applied to the synthesis of analogues 14, 15, 19, and 22 (Scheme 1 and 2, Table 1). The bromide precursors 10, 17, and 20, were prepared according to previously reported procedures.12–14
Table 1.
Inhibition of nicotine-evoked [3H]DA release from superfused rat striatal slices.
| Compound | DA Release | |||
|---|---|---|---|---|
| Head group | Linker | Inhibition (100 nM)a | IC50 (nM) and Imaxbb | |
| bPiDDB 1 |
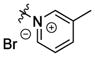
|
bis-1,12-dodecane | NDc | 2.0±1.0d 63% |
| bPiDB 8 | bis-1,10-decane | ND | 180±110 63% |
|
| tPy3PiB 3 | tris-linker (unsaturated) | 40±12% | 0.2±0.07e 67% |
|
| 7 |
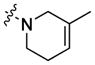
|
bis-1,12-dodecane | 50±21% | 0.95±0.30 60% |
| 9 | bis-1,10-decane | 36±12% | 37.4±18.7 65% |
|
| 16 | tris-linker (unsaturated) | 83±2% | 3.22±1.36 67% |
|
| bPyiQB 2 |
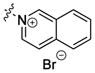
|
rigid bis-linker | ND | 63±39f 59% |
| 12 | bis-1,12-dodecane | ND | 40±30 53% |
|
| 13 | bis-1,10-decane | ND | 70±50 95% |
|
| 18 | tris-linker (saturated) | 28±11% | ND | |
| 21 | tetrakis-linker | ND | 56±45 52% |
|
| 11 |
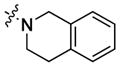
|
rigid bis-linker | 18±18% | ND |
| 14 | bis-1,12-dodecane | ND | 8.59±3.27 76% |
|
| 15 | bis-1,10-decane | ND | 9.91±9.23 74% |
|
| 19 | tris-linker (saturated) | 58±9% | 0.35±0.09 58% |
|
| 22 | tetrakis-linker | 84±4% | 205±132 64% |
|
| tkP3HPPB 4 |
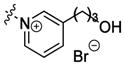
|
tetrakis-linker | 41±15% | 3.0±3.0g 63% |
| 23 |
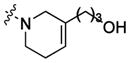
|
tetrakis-linker | 40±23% | 30±16 64% |
Percentage inhibition at 100 nM is presented unless otherwise specified. Each value represents data from at least 3 independent experiments, each performed in duplicate.
IC50 and Imax from full concentration response assays; data from 4–6 independent experiments.
Not determined;
taken from results published in ref 15;
taken from results published in ref 13;
taken from results published in ref 12;
taken from results published in ref 14.
The resulting analogues22 were initially evaluated for inhibition of nAChRs mediating nicotine-evoked [3H]DA release from superfused rat striatal slices using a probe concentration of 100 nM. [3H]DA release assays were performed according to a previously published method.7, Analogue-induced inhibition of nicotine-evoked [3H]DA release was determined using 10 μM nicotine and 100 nM analogue concentrations. The amount of inhibition is presented as a percentage of the response to nicotine under control conditions (i.e. in the absence of analogue) and the values are provided in Table 1. The most active compounds (>40% inhibition) were then evaluated across a full concentration range, to determine IC50 and Imax values for inhibition of nicotine-evoked [3H]DA release (Table 1).
With the exception of compound 11, all of the tertiary amine analogues that were directly derived from the corresponding quaternary ammonium lead compounds demonstrated high potency in inhibiting nicotine-evoked DA release from rat striatal slices. These results support the validity of the hypothesis that both the quaternary ammonium lead compounds and their reduced tertiary amine analogues possibly interact at a common site on α6-containing nAChRs, and that the tertiary amine analogues likely interact at these sites in their protonated forms via ionic interactions. It is important to note that the IC50 values of these tertiary amine derivatives are generally within an order of magnitude of the IC50 values of their corresponding parent quaternary ammonium molecules. The bis-analog 7 and the tris-analog 19 were the most potent inhibitors in the tertiary amine series of compounds, with IC50 values of 0.91 nM and 0.35 nM, respectively.
All of the tertiary amino compounds evaluated exhibited incomplete inhibition of nicotine-evoked DA release, as indicated by their Imax values, which ranged from 58 to 76%. These results are consistent with previous literature, which indicates that multiple nAChRs mediate nicotine-evoked DA release. Thus, these analogues are likely acting as antagonists at only a subset of nAChR subtypes mediating nicotine-evoked DA release, and may have a unique selectivity for specific nAChR subtypes in brain.23,24
Due to high polarity, the quaternary ammonium lead compounds are not able to pass through the BBB through passive diffusion. However, bPiDDB has been demonstrated to be transported from the periphery into the brain by facilitated transport via the BBB choline transporter.19,25 On the other hand, the tertiary amine analogues are expected to readily pass the BBB via passive diffusion due to improved drug-like properties such as enhanced membrane permeation properties and improved LogP values, resulting in better brain distribution, as well improved oral bioavailability. Thus, the results from this study reveal a new direction for the discovery of more drug-like derivatives of bPiDDB and its analogues in the search for potent agents that inhibit nicotine-evoked DA release.
In conclusion, in the search for potent agents that inhibit nicotine-evoked DA release from striatum, a series of tertiary amine analogues derived from lead azaaromatic quaternary ammonium salts has been identified as having potential as tobacco cessation agents. The preliminary results suggest that these novel tertiary amine analogues, which are protonated at physiological pH, may interact with the same target nAChRs as their parent quaternary ammonium compounds. However, in contrast to their parent compounds, the tertiary amine analogues are expected to pass the BBB easily through passive diffusion, and may exhibit improved plasma and brain bioavailability via the oral route of administration.
Acknowledgments
This work was supported by NIH. (Grant U19DA017548)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Dani JA, De Biasi M. Pharmacol Biochem Behav. 2001;70:439. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- 2.Benowitz NL. Am J Med. 2008;121:S3. doi: 10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Pierce RC, Kumaresan V. Neurosci Biobehav Rev. 2006;30:215. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Dwoskin LP, Crooks PA. J Pharmacol Exp Ther. 2001;298:395. [PubMed] [Google Scholar]
- 5.Dwoskin LP, Sumithran SP, Zhu J, Deaciuc AG, Ayers JT, Crooks PA. Bioorg Med Chem Lett. 2004;14:1863. doi: 10.1016/j.bmcl.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 6.Dwoskin LP, Wilkins LH, Pauly JR, Crooks PA. Ann N Y Acad Sci. 1999;868:617. doi: 10.1111/j.1749-6632.1999.tb11334.x. [DOI] [PubMed] [Google Scholar]
- 7.Wilkins LH, Jr, Haubner A, Ayers JT, Crooks PA, Dwoskin LP. J Pharmacol Exp Ther. 2002;301:1088. doi: 10.1124/jpet.301.3.1088. [DOI] [PubMed] [Google Scholar]
- 8.Ayers JT, Dwoskin LP, Deaciuc AG, Grinevich VP, Zhu J, Crooks PA. Bioorg Med Chem Lett. 2002;12:3067. doi: 10.1016/s0960-894x(02)00687-x. [DOI] [PubMed] [Google Scholar]
- 9.Wilkins LH, Jr, Grinevich VP, Ayers JT, Crooks PA, Dwoskin LP. J Pharmacol Exp Ther. 2003;304:400. doi: 10.1124/jpet.102.043349. [DOI] [PubMed] [Google Scholar]
- 10.Grinevich VP, Crooks PA, Sumithran SP, Haubner AJ, Ayers JT, Dwoskin LP. J Pharmacol Exp Ther. 2003;306:1011. doi: 10.1124/jpet.103.051789. [DOI] [PubMed] [Google Scholar]
- 11.Sumithran SP, Crooks PA, Xu R, Zhu J, Deaciuc AG, Wilkins LH, Dwoskin LP. Aaps J. 2005;7:E201. doi: 10.1208/aapsj070119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng GR, Zhang ZF, Pivavarchyk M, Deaciuc AG, Dwoskin LP, Crooks PA. Bioorg Med Chem Lett. 2007;17:6734. doi: 10.1016/j.bmcl.2007.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng GR, Sumithran SP, Deaciuc AG, Dwoskin LP, Crooks PA. Bioorg Med Chem Lett. 2007;17:6701. doi: 10.1016/j.bmcl.2007.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang ZF, Zheng GR, Pivavarchyk M, Deaciuc AG, Dwoskin LP, Crooks PA. Bioorg Med Chem Lett. 2008;18:5753. doi: 10.1016/j.bmcl.2008.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dwoskin LP, Wooters TE, Sumithran SP, Siripurapu KB, Joyce BM, Lockman PR, Manda VK, Ayers JT, Zhang Z, Deaciuc AG, McIntosh JM, Crooks PA, Bardo MT. J Pharmacol Exp Ther. 2008;326:563. doi: 10.1124/jpet.108.136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahman S, Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Neuropharmacology. 2007;52:755. doi: 10.1016/j.neuropharm.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Psychopharmacology (Berl) 2006;184:426. doi: 10.1007/s00213-005-0163-8. [DOI] [PubMed] [Google Scholar]
- 18.Pardridge WM. Nat Rev Drug Discov. 2002;1:131. doi: 10.1038/nrd725. [DOI] [PubMed] [Google Scholar]
- 19.Albayati ZAF, Dwoskin LP, Crooks PA. Drug Metab Dispos. 2008;36:2024. doi: 10.1124/dmd.108.020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockman PR, Manda VK, Geldenhuys WJ, Mittapalli RK, Thomas F, Albayati ZF, Crooks PA, Dwoskin LP, Allen DD. J Pharmacol Exp Ther. 2008;324:244. doi: 10.1124/jpet.107.130906. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Pivavarchyk M, Subramanian KL, Deaciuc AG, Dwoskin LP, Crooks PA. Bioorg Med Chem Lett. 2010 doi: 10.1016/j.bmcl.2009.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spectra data of selective compounds: 7, 1H NMR (300 MHz, CDCl3) δ 5.43 (ddd, J = 6.9, 3.3, 1.8 Hz, 2H), 2.82 (dd, J = 1.8, 0.9 Hz, 4H), 2.48 (t, J = 5.7 Hz, 4H), 2.38 (dd, J = 7.8, 4H), 2.14 (m, 4H), 1.64 (d, J = 1.5 Hz, 6H), 1.53 (m, 4H), 1.20–1.38 (m, 16H) ppm; 13C NMR (75 MHz, CDCl3) δ 132.29, 119.50, 58.95, 57.31, 50.14, 29.87, 27.96, 27.43, 26.32, 21.34 ppm; 16, 1H NMR (300 MHz, CDCl3) δ 7.23 (s, 3H), 5.42–5.44 (m, 3H), 2.85 (br, 3H), 2.49–2.57 (m, 12H), 2.43 (t, J = 7.2 Hz, 6H), 2.10–2.16 (m, 6H), 1.77–1.87 (m, 6H), 1.63 (b, 9H) ppm; 13C NMR (75 MHz, CDCl3) δ 133.71, 132.22, 124.39, 119.63, 90.90, 79.89, 57.67, 57.32, 50.17, 26.51, 26.28, 21.34, 17.85 ppm; 19, 1H NMR (300 MHz, CDCl3) δ 7.00–7.16 (m, 12 H), 6.84 (s, 3H), 3.64 (s, 6H), 2.86 (t, J = 6 Hz, 6H), 2.68 (t, J = 6.0 Hz, 6H), 2.62 (t, J = 6. 0 Hz, 6H), 2.48 t, J = 6.0 Hz, 6H), 1.62–1.68 (m, 12 H), 1.41–1.46 (m, 6H) ppm; 13C NMR (75 MHz, CDCl3) δ 142.75, 135.11, 134.56, 128.84, 126.82, 126.26, 126.12, 125.76, 58.87, 56.64, 51.40, 36.29, 31.96, 29.54, 27.83, 27.56 ppm; 22, 1H NMR (300 MHz, CDCl3) δ 7.08–17.10 (m, 16 H), 6.92 (s, 2H), 3.63 (s, 8H), 2.90 (t, J = 6 Hz, 8H), 2.73 (t, J = 6 Hz, 8H), 2.56 (t, J = 6 Hz, 8 H), 1.59–1.70 (m, 16 H), 1.41–1.49 (m, 8H) ppm; 13C NMR (75 MHz, CDCl3) δ 137.78, 135.03, 134.52, 130.05, 128.81, 126.77, 126.24, 125.72, 58.87, 56.59, 51.40, 32.72, 31.79, 29.48, 28.24, 27.57 ppm; 23, 1H NMR (300 MHz, CDCl3) δ 6.88 (s, 2H), 5.46 (br, 4H), 3.59 (t, J = 6 Hz, 8 Hz), 2.85 (s, 8H), 2.43–2.60 (m, 16H), 2.37–2.49 (m, 8H), 2.15 (m, 8H), 1.98–2.03 (m, 8H), 1.58–1.69 (m, 24H), 1.36–1.43 (m, 8H) ppm; 13C NMR (75 MHz, CDCl3) δ 137.69, 135.69, 130.03, 119.44, 62.59, 58.96, 56.02, 50.49, 32.61, 31.91, 31.67, 31.06, 28.19, 27.24, 26.17 ppm.
- 23.Graham JH, Papke RL, Buccafusco JJ. Curr Alzheimer Res. 2005;2:141. doi: 10.2174/1567205053585747. [DOI] [PubMed] [Google Scholar]
- 24.Papke RL, Craig AG, Heinemann SF. J Pharmacol Exp Ther. 1994;268:718. [PubMed] [Google Scholar]
- 25.Geldenhuys WJ, Lockman PR, Nguyen TH, Van der Schyf CJ, Crooks PA, Allen DD. Bioorg Med Chem. 2005;13:4253. doi: 10.1016/j.bmc.2005.04.020. [DOI] [PubMed] [Google Scholar]