Abstract
A series of novel 3-hydroxy-3-(2-imino-3-methyl-5-oxoimidazolidin-4-yl)indolin-2-one analogs (3) have been synthesized under microwave irradiation and conventional heating methods. These analogs were evaluated for in vitro cytotoxicity against a panel of 57 human tumor cell lines. Compound 3o had GI50 values of 190 nM and 750 nM against A549/ATTC non-small cell lung cancer and LOX IMVI melanoma cell lines, respectively, and both 3n and 3o exhibited GI50 values ranging from 2–5 μM against CCRF-CEM, HL-60(TB), K-562, MOLT-4, and RPMI-8226 leukemia cell lines. These results indicate that N-4-methoxybenzyl-3-hydroxy-(2-imino-3-methyl-5-oxo-4-yl)indolin-2-one analogs may be useful leads for anticancer drug development.
Keywords: N-alkyl isatins, creatinine, microwave irradiation, in vitro cytotoxicity
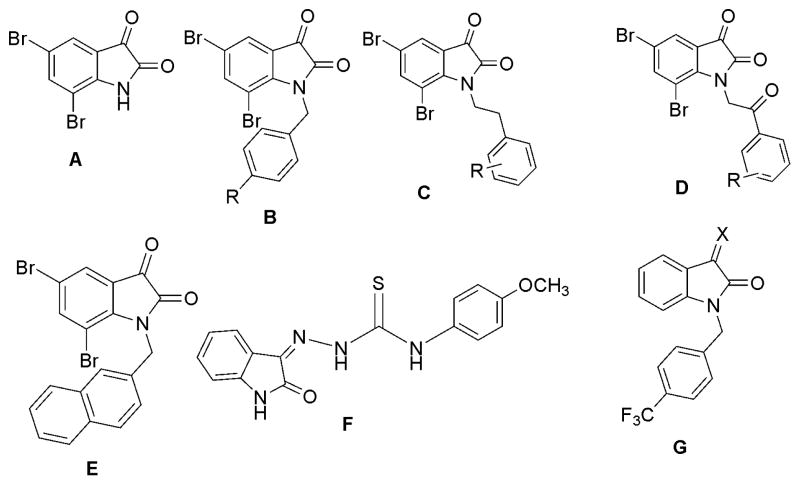
Many isatin analogs have been reported as potent and selective cytotoxic agents against cancer cells. Vine et al1 reported that the substitution of bromo groups at the C-5 and C-7 positions of isatin afforded an analog (Fig. 1. structure A) that exhibited potent cytotoxic activity. These investigators also reported that introduction of N-benzyl, N-phenethyl, N-phenethan-1-one, and N-2-naphthylmethyl moieties into the 5,7-dibromoisatin molecule (Fig. 1. structures B, C, D and E, respectively) significantly increased hydrophobicity and increased cytotoxicity toward lymphoma cells. These isatin analogs were also potent against a wide range of human cancer cell lines; including MDA-MB-231 metastatic breast adenocarcinoma cells and U937 human monocyte-like histiocytic lymphoma cells.1–3 Hall et al.4 studied the MDR1-selective mechanism of action of several isatin-β–thiosemicarbazones (Fig. 1. structure F) against a parental HeLa-derived cervical cancer cell line (KB-3-1), while Perrow et al.5 have described the cytotoxicity of a number of isatin derivatives conjugated to a cell targeting moiety via a spacer group (Fig. 1. structure G). In view of the general anticancer properties of these isatin analogs, and as part of an investigation devoted to the development of new anticancer agents derived from structural modification of indoles,6–8 the design and synthesis of a novel series of 3-hydroxy-3-(2-imino-3-methyl-5-oxoimidazolidin-4-yl)indolin-2-one analogs (3a-w) was undertaken. The resulting analogs were then evaluated for their cytotoxic activity against a panel of 57 human tumor cell lines.
Fig. 1.
Structures of potent cytotoxic isatin derivatives
The simple and N-alkyl substituted isatins (1a-j) were all prepared utilizing literature methods.9–11 A series of novel substituted 3-hydroxy-3-(2-imino-3-methyl-5-oxoimidazolidin-4-yl)indolin-2-one derivatives (3a-w) were synthesized by condensation of the appropriate substituted N-alkyl isatin with creatinine, in the presence of sodium acetate and acetic acid via both microwave irradiation and conventional heating methodologies (Scheme 1). Of these two methods, microwave irradiation was found to be advantageous over conventional heating, since the product yields were 83–94% for the former method, but only 70–83% for the latter method. In addition, the time course of the reaction was very fast using microwave irradiation (20–40 sec) compared to 6–8 h for conventional heating (Table 1). All the synthesized compounds were characterized by 1H-NMR and 13C-NMR spectrometry.16 The geometry of the hydroxyl position in the representative compounds 3a, 3b and 3t was established as trans to the 4′-methyne hydrogen from X-ray crystallographic data.12–14
Scheme 1.
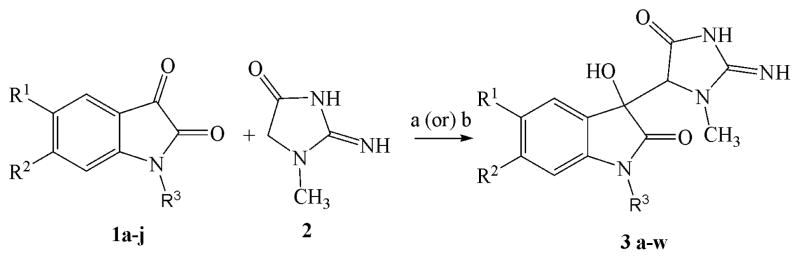
Reagents and conditions: (a) Method A: sodium acetate in acetic acid, microwave irradiation, 20–40 seconds, 83–94% yield; (b) Method-B: sodium acetate in acetic acid, 115–120 °C, 6–8 hours, 70–83% yield.
Table 1.
Reaction times and yields of 3-hydroxy-3-(2-imino-3-methyl-5-oxoimidazolidin-4-yl)indolin-2-ones (3a-w)
| Compd | R1 | R2 | R3 | Method-A | Method-B | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Yield (%) | Time (sec) | Yield (%) | Time (hr) | ||||
| 3a | H | H | H | 92 | 20 | 80 | 6 |
| 3b | F | H | H | 94 | 30 | 81 | 6 |
| 3c | Cl | H | H | 91 | 40 | 78 | 6 |
| 3d | Br | H | H | 87 | 40 | 75 | 7 |
| 3e | Br | Br | H | 89 | 40 | 79 | 8 |
| 3f | NO2 | H | H | 92 | 40 | 83 | 8 |
| 3g | H | H | -CH3 | 88 | 30 | 78 | 7 |
| 3h | F | H | -CH3 | 90 | 40 | 76 | 7 |
| 3i | Cl | H | -CH3 | 86 | 40 | 77 | 8 |
| 3j | Br | H | -CH3 | 85 | 40 | 80 | 8 |
| 3k | H | H | -Bz | 87 | 30 | 72 | 7 |
| 3l | Cl | H | -Bz | 91 | 40 | 76 | 7 |
| 3m | Br | H | -Bz | 88 | 40 | 75 | 8 |
| 3n | H | H | 4-OCH3 Bz | 84 | 30 | 70 | 6 |
| 3o | Cl | H | 4-OCH3 Bz | 85 | 30 | 74 | 8 |
| 3p | Br | H | 4-OCH3 Bz | 83 | 40 | 79 | 8 |
| 3q | H | H | 4-Cl Bz | 92 | 40 | 77 | 7 |
| 3r | H | H | 4-COOCH3Bz | 87 | 20 | 75 | 6 |
| 3s | H | H | 4-CN Bz | 86 | 40 | 79 | 8 |
| 3t | H | H | -C6H5 | 83 | 30 | 80 | 7 |
| 3u | H | H | -COCH3 | 87 | 30 | 73 | 7 |
| 3v | Cl | H | -COCH3 | 85 | 40 | 75 | 8 |
| 3w | H | H | -SO2C6H5 | 89 | 30 | 78 | 6 |
From the X-ray diffraction and 1H-NMR data, analogs 3a-3w were mixtures of RR and SS isomers. This is consistent with the mechanism of the aldol condensation reaction of 1 with 2, which proceeds via the formation of the E-enolate, as per the Zimmerman–Traxler model, which favors anti products, and is predicted to lead to the formation of equimolar RR and SS enantiomers. We also determined from the crystal structures of 3a, 3b and 3t that the 3-hydroxy group was trans to the 4′-methyne hydrogen, which may explain the inability of these analogs to undergo facile dehydration.
The single dose in vitro cytotoxicity screening assays of the analogs were carried out in accordance with the procedures described in Rubinstein et al.15 The human tumor cell line panel includes leukemia, non-small cell lung, colon, CNS, melanoma, ovarian, renal, prostate, and breast cancer cell lines. From the preliminary results of the in vitro single dose screen, analogs containing an N-benzyl group in the isatin moiety exhibited good growth inhibition properties. The introduction of chloro group at C-5 of the isatin moiety also afforded analogs with good growth inhibition properties.
The two most active compounds (3n and 3o) from the preliminary 57 cell screen were subsequently evaluated in five dose-response studies for their in vitro cytotoxic effects on growth parameters against each of the 57 human tumor cell lines. Dose-response curves were created by plotting cytotoxic effect against the log10 of the drug concentration for each cell line. Cytotoxic effects of each compound were determined as GI50, TGI and LC50 values, which represent the molar drug concentration required to cause 50% growth inhibition, total growth inhibition, and the concentration that kills 50% of the cells, respectively. The results are presented in Table 2.
Table 2.
Antitumor activity (GI50/μM)a and toxicity (LC50/μM)b data of compounds selected for 5 dose studies for the NCI 57-cell lines screen
| Panel/cell line | Compound 3o | Compound 3n | ||
|---|---|---|---|---|
|
| ||||
| GI50 | LC50 | GI50 | LC50 | |
| Leukemia Cancer | ||||
| CCRF-CEM | 2.06 | >100 | 2.18 | >100 |
| HL-60(TB) | 3.22 | >100 | 4.91 | >100 |
| K-562 | 5.04 | >100 | 3.73 | >100 |
| MOLT-4 | 2.94 | >100 | 4.79 | >100 |
| RPMI-8226 | 2.62 | >100 | 2.87 | >100 |
| Non-Small Cell Lung Cancer | ||||
| A549/ATCC | 0.19 | 10.9 | 43.6 | >100 |
| EKVX | 28.5 | >100 | 71.7 | >100 |
| HOP-62 | 5.12 | >100 | 4.31 | >100 |
| NCI-H226 | 26.8 | >100 | 38.4 | >100 |
| NCI-H23 | 4.74 | >100 | 13.9 | >100 |
| NCI-H322M | 14.3 | >100 | 41.1 | >100 |
| NCI-H460 | 26.8 | >100 | 29.0 | >100 |
| NCI-H522 | 1.86 | 20.5 | 3.65 | >100 |
| Colon Cancer | ||||
| COLO 205 | 14.3 | >100 | 19.9 | >100 |
| HCC-2998 | 18.7 | 73.9 | 12.8 | 63.9 |
| HCT-116 | 3.00 | 34.5 | 2.40 | >100 |
| HCT-15 | 12.6 | >100 | 11.8 | >100 |
| HT29 | 1.38 | 44.1 | 4.06 | >100 |
| KM12 | 2.05 | 30.0 | 2.87 | 80.4 |
| SW-620 | 2.59 | 49.6 | 4.12 | >100 |
| CNS Cancer | ||||
| SF-268 | 3.31 | 64.9 | 5.00 | >100 |
| SF-295 | 3.71 | >100 | 26..0 | >100 |
| SF-539 | 3.12 | 79.1 | 3.73 | >100 |
| SNB-19 | 17.2 | >100 | 30.6 | >100 |
| SNB-75 | 3.39 | >100 | 28.8 | >100 |
| Melanoma Cancer | ||||
| LOX IMVI | 0.75 | 60.0 | 8.09 | >100 |
| MALME-3M | 29.6 | >100 | 27.4 | >100 |
| M14 | 3.59 | 61.4 | 4.93 | >100 |
| MDA-MB-435 | 6.12 | 93.1 | 10.6 | >100 |
| SK-MEL-2 | 10.4 | 96.0 | 95.2 | >100 |
| SK-MEL-28 | 50.3 | >100 | 36.9 | >100 |
| SK-MEL-5 | 22.6 | >100 | 37.3 | >100 |
| UACC-257 | 1.43 | 19.4 | 6.28 | >100 |
| UACC62 | 23.4 | >100 | 27.7 | >100 |
| Ovarian Cancer | ||||
| IGR-OV1 | 2.59 | >100 | 3.26 | >100 |
| OVCAR-3 | 2.08 | 11.5 | 2.29 | 30.1 |
| OVCAR-4 | 5.52 | >100 | 7.13 | >100 |
| OVCAR-5 | 34.9 | >100 | 42.1 | >100 |
| OVCAR-8 | 1.35 | 17.2 | 3.52 | >100 |
| NCI/ADR-RES | 1.88 | 36.5 | 1.99 | >100 |
| SK-OV-3 | 30.1 | >100 | 45.5 | >100 |
| Renal Cancer | ||||
| 786-0 | 3.44 | 43.0 | 23.0 | >100 |
| A498 | 34.5 | >100 | >100 | >100 |
| ACHN | 20.4 | >100 | >100 | >100 |
| CAKI-1 | 1.98 | >100 | 53.9 | >100 |
| RXF 393 | 6.29 | >100 | 7.40 | >100 |
| SN12C | 5.94 | >100 | >100 | >100 |
| TK-10 | 4.11 | >100 | >100 | >100 |
| UO-31 | 4.15 | >100 | 83.2 | >100 |
| Prostate Cancer | ||||
| PC-3 | 4.27 | >100 | 46.9 | >100 |
| DU-145 | 3.76 | 74.6 | 21.4 | >100 |
| Breast Cancer | ||||
| MCF7 | 3.22 | >100 | 6.93 | >100 |
| MDA-MB-231/ATCC | 8.55 | >100 | 19.4 | >100 |
| HS 578T | 2.04 | >100 | 60.3 | >100 |
| BT-549 | 2.98 | 54.9 | 8.45 | 97.0 |
| T-47D | 13.5 | >100 | 32.0 | >100 |
| MDA-MB-468 | 2.29 | >100 | 11.6 | >100 |
NA: Not analyzed
GI50: 50% Growth inhibition, concentration of drug resulting in a 50% reduction in net protein increase compared with control cells.
LC50: Lethal concentration, concentration of drug lethal to 50% of cells.
Compound 3o exhibited growth inhibitory properties against 93% of all cancer cell lines in the panel, with GI50 values in the range of 0.19–30 μM (Table 2). Compound 3o exhibited good growth inhibitory activity against A549/ATCC non-small cell lung cancer cell lines (GI50=193 nM; TGI=1.96 μM, LC50=10.9 μM) and LOX IMVI melanoma cell lines (GI50= 750 nM; TGI= 11.8 μM; LC50=60.0 μM), and showed moderate growth inhibitory activity against NCI-H522 lung cancer (GI50= 1.86 μM), HT29 colon cancer (GI50= 1.38 μM), UACC-257 melanoma (GI50= 1.43 μM), and OVCAR-8 and NCI/ADR-RES ovarian cancer (GI50= 1.35 and 1.88 μM, respectively), and CAKI-1 renal cancer (GI50= 1.98 μM) cell lines.
Compound 3n exhibited growth inhibitory properties against 89% of all cancer cell lines in the panel, with GI50 values in the range of 2–60 μM. (Table 2). Moderate growth inhibitory activity was observed against CCRF-CEM leukemia (GI50=2.18 μM), HCT-116 colon (GI50=2.40 μM), and OVCAR-3 and NCI/ADR-RES ovarian (GI50=2.29 and 1.99 μM, respectively) cell lines.
Of particular significance was the observation that both 3o and 3n were effective in inhibiting the growth of all five cell lines in the sub-panel of leukemia cancer cells, with GI50 values in the narrow range of 2–5 μM.
In conclusion, a series of 3-hydroxy-3-(2-imino-3-methyl-5-oxoimidazolidin-4-yl) indolin-2-one analogs have been synthesized and evaluated for anticancer activity against a panel of 57 human tumor cell lines. Compounds 3n and 3o were identified as molecules of interest from a single dose assay, and were then evaluated for dose-dependent growth inhibition and cytotoxicity in all 57 human cancer cell lines. Compound 3o had GI50 values of 190 nM and 750 nM against A549/ATTC non-small cell lung cancer and LOX IMVI melanoma cell lines, respectively, and both 3n and 3o exhibited GI50 values ranging from 2–5 μM against CCRF-CEM, HL-60(TB), K-562, MOLT-4, and RPMI-8226 leukemia cell lines. These results indicate that N-4-methoxybenzyl-3-hydroxy-(2-imino-3-methyl-5-oxo-4-yl)indolin-2-one analogs may be useful leads for anticancer drug development.
Supplementary Material
Acknowledgments
We are grateful to the NCI/NIH for their financial support under grant number CA 140409 and to the NCI Developmental Therapeutic Program (DTP) for screening data.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi…..
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Vine KL, Locke JM, Ranson M, Benkendorff K, Pyne SG, Bremner JB. Bioorg Med Chem. 2007;15:931. doi: 10.1016/j.bmc.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 2.Vine KL, Locke JM, Ranson M, Pyne SG, Bremner JB. J Med Chem. 2007;50:5109. doi: 10.1021/jm0704189. [DOI] [PubMed] [Google Scholar]
- 3.Matesic L, Locke JM, Bremner JB, Pyne SG, Skropeta D, Ranson M, Vine KL. Bioorg Med Chem. 2008;16:3118. doi: 10.1016/j.bmc.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Hall MD, Salam NK, Hellawell JL, Fales HM, Kensler CB, Ludwig JA, Szakacs G, Hibbs DE, Gottesman MM. J Med Chem. 2009;52:3191. doi: 10.1021/jm800861c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrow KL, Ranson M, Locke M, Bremner JB, Pyne SG. WO 2008/074078 A1
- 6.Sonar VN, Thirupathi Reddy Y, Sekhar KR, Sasi S, Freeman ML, Crooks PA. Bioorg Med Chem Lett. 2007;17(24):6821. doi: 10.1016/j.bmcl.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narsimha Reddy P, Thirupathi Reddy Y, Crooks PA. Bioorg Med Chem Lett. 2010;20(2):591. [Google Scholar]
- 8.Thirupathi Reddy Y, Sekhar KR, Sasi N, Narsimha Reddy P, Freeman ML, Crooks PA. Bioorg Med Chem Lett. 2010;20(2):600. doi: 10.1016/j.bmcl.2009.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Nature (London, United Kingdom) 2007;445(7127):541. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs TL, Winstein S, Linden GB, Robson JH, Levy EF, Seymour D. Organic Synthesis. 1948;28:70. [Google Scholar]
- 11.Shindikar AV, Khan F, Viswanathan CL. Euro J Med Chem. 2006;41(6):786. doi: 10.1016/j.ejmech.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Narsimha Reddy P, Thirupathi Reddy Y, Parkin S, Crooks PA. Acta Crystallographica. 2009;E65(11):o2909. doi: 10.1107/S1600536809043797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narsimha Reddy P, Thirupathi Reddy Y, Parkin S, Crooks PA. Acta Crystallographica. 2009;E65(10):o2439–o2440. doi: 10.1107/S1600536809033881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narsimha Reddy P, Thirupathi Reddy Y, Parkin S, Crooks PA. Acta Crystallographica. 2009;E65(3):o552. doi: 10.1107/S1600536809004875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubinstein LV, Shoemaker RH, Paull KD, Simo RM, Tosini S, Skehan P, Scudiero PA, Monks A, Boyd MR. J Natl Cancer lnst. 1990;82:1113. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- 16.Analytical data for compound 3n. MF: C20H20N4O4, mp: 213–215 °C, 1H NMR (DMSO-d6): δ 3.14 (s, 3H, CH3), 3.71 (s, 3H, OCH3), 4.20 (s, 1H, CH), 4.65–4.84 (Abq, 2H, CH2), 6.56 (s, 1H, OH), 6.65–6.68 (d, J= 8.1 Hz, 1H, C7H), 6.85–6.94 (m, 4H, Ar-H), 7.10–7.16 (m, 2H, C5H, C6H), 7.33–7.40 (d, J=8.4 Hz, 1H, C4H), 7.57 (bs, 2H, NH2) ppm; 13C NMR (DMSO-d6): δ 32.64 (CH3), 42.30 (CH2), 54.97 (OCH3), 69.46 (CH), 75.99 (C-OH), 109.05, 111.00, 113.62, 121.79, 123.69, 127.43, 127.81, 128.55, 129.30, 137.74, 143.15, 158.20, 171.98 (C=N), 174.31 (isatin C=O), 182.29 (creatinine C=O) ppm.Analytical data for compound 3o. M.F: C20H19ClN4O4, mp: 188–190 °C, 1H NMR (DMSO-d6): δ 3.18 (s, 3H, CH3), 3.72 (s, 3H, OCH3), 4.21 (s, 1H, CH), 4.66–4.85 (Abq, 2H, CH2), 6.67 (s, 1H, OH), 6.70–6.72 (d, J= 5.1 Hz, 1H, C7H), 6.85–6.88 (d, J= 8.7 Hz, 2H, Ar-H), 7.06–7.07 (d, J= 2.4 Hz, 1H, C4H), 7.24–7.27 (dd, J= 8.1Hz, 2.4 Hz, 1H, C6H), 7.37–7.40 (d, J=8.4 Hz, 2H, Ar-H), 7.57 (bs, 1H, NH), 7.82 (bs, 1H, NH) ppm; 13C NMR (DMSO-d6): δ 32.94 (CH3), 42.42 (CH2), 55.03 (OCH3), 69.62 (CH), 76.05 (C-OH), 110.63, 113.72 (2C) 123.73, 125.88, 127.43, 128.62 (2C), 129.16, 129.45, 142.10, 158.31 (C-Cl), 172.21 (C=N), 174.00 (isatin C=O), 182.08 (creatinine C=O) ppm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.