Abstract
A series of azaaromatic quaternary ammonium analogs has been discovered as potent and selective α9α10 nicotinic acetylcholine receptor (nAChR) antagonists. The preliminary structure-activity relationships of these analogs suggest that increased rigidity in the linker units results in higher potency in inhibition of α9α10 nAChRs and greater selectivity over α7 nAChRs. These analogs represent a new class of analgesic for the treatment of neuropathic and tonic inflammatory pain.
Neuronal nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channel complexes, composed of five (α and/or β) subunits, each spanning the membrane four times and aligning around the central ion channel.1–3 Nine α (α1–7, α9, α10) and four β (β1–β4) subunits in mammals have been identified.4 Homomeric or heteromeric combinations of the α and β subunits produce a variety of nAChR subtypes with distinct pharmacological properties.5
The α7 and α9 subunits are the only known nAChR subunits that can assemble into a functional homomeric subtype.6 α9 nAChRs exist as both homomeric α9 and heteromeric α9α10 receptors; the latter is suggested to be the major subtype.7–8 Mutagenesis studies indicate that the stoichiometry of the major form is α9(2)α10(3).9 The α10 subunit has only been co-expressed functionally with an α9 subunit.7 In Xenopus oocytes, the co-injection of α9 and α10 subunits boosts functional nAChR expression 100-fold or more compared to injection of α9 subunits alone.7–8 The pharmacological profiles of the homomeric and heteromeric α9 nAChRs are essentially indistinguishable, and closely resemble those reported for endogenous cholinergic receptors found in vertebrate hair cells.7 A number of pharmacologically unique features of α9α10 nAChRs have been observed:10 i) α9α10 nAChRs can be activated not only by acetylcholine (ACh), but also by the classical muscarinic agonist oxotremorine, indicating a mixed nicotinic/muscarinic pharmacology; ii) α9α10 nAChRs are antagonized by most classical nAChR agonists, such as nicotinic, cytisine, and epibatidine; iii) the classical muscarinic agonist, muscarine, and the classical antagonist, atropine, both block α9α10 nAChRs; iv) α-bungarotoxin (α-BTX) and methyllycaconitine (MLA) are antagonists of α9α10 nAChRs; and v) a number of non-cholinergic antagonists, including strychnine (glycine receptor antagonist), bicuculline (GABA-antagonist) and ICS-205,930 (5HT3 receptor antagonist), also potently block α9α10 nAChRs. Thus, α9α10nAChRs have a pharmacological profile different from any other nicotinic or muscarinic cholinergic receptor subtype.
The α9 nAChR has been identified in a rich variety, yet restricted set of critical tissues, including lymphocytes and developing thymocytes, bronchial epithelium and airway fibroblasts, sensory organs, nervous tissue (dorsal root ganglia), and sperm, but are not present in brain.11 To date, with the exception of innervation of the sensory hair cells in the inner ear,7 the function of α9α10 receptors is still obscure, and research has been hampered by the paucity of selective ligands for probing receptor function. In this regard, chronic constriction of the sciatic nerve increases the number of nAChR-producing lymphocytes at the site of nerve injury,12 and data suggests that locally released ACh from the lymphocytes stimulates α9α10 nAChRs to maintain the immune response and behavioral hypersensitivity.12 Thus, the lymphocytic cholinergic system may be regulating local immune response associated with nerve injury.
Chronic pain afflicts approximately 20% of the adult population in developed nations.13 Although there are numerous marketed analgesic drugs, they appear to act through a limited number of molecular mechanisms. Even when these medications are used in combination, substantial pain often persists. Therefore, it is highly desirable to develop new treatments that act through alternative mechanisms of action.14
A growing body of evidence indicates that α9α10 nAChR antagonism may represent a novel mechanism to produce analgesia.10, 15–16 α-Conotoxins Vc1.1 and RgIA are antagonists of α9α10 nAChRs.12 The subcutaneous or intramuscular administration of Vc1.1 and RgIA acutely alleviates pain resulting from traumatic, inflammatory, and metabolic neuronal injury.15 Intriguingly, analgesia appears to continue for days or weeks after administration of the conotoxin is discontinued, indicating that blockade of α9α10 nAChRs with these peptides may have disease-modifying effects.15 Thus, non-peptide, small molecule antagonists of α9α10 nAChRs on immune cell membranes may have the ability to mediate restorative effects rather than just symptomatic management of nerve injury, and thus, represent novel, potential therapeutics for modulating neuropathic and tonic inflammatorychronic pain. 10, 12, 15–16
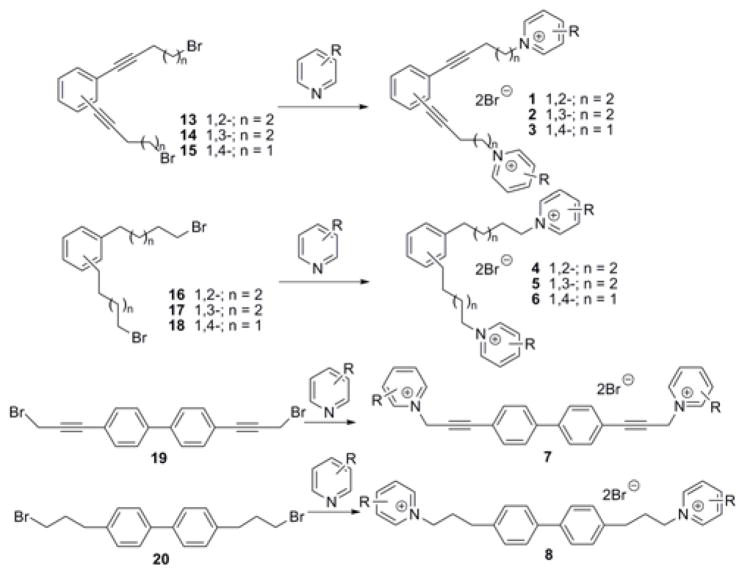
During the course of our search for potent and selective small molecule nAChR antagonists, we generated a diverse library of novel quaternary ammonium analogs.17–25 Recently, a number of these analogs were found to be highly potent α7 nAChR antagonists.26 Since the classic α7 antagonists α-BTX and MLA are also potent α9α10 antagonists,7 we hypothesized that these quaternary ammonium analogs might also be antagonists at α9α10 nAChRs. Thus, in the present work, we evaluated a family of bis-, tris-, and tetrakis-azaaromatic quaternary ammonium analogs (general structures 1–8, Fig. 1; 9 and 10, Fig. 2; and 11 and 12, Fig. 2, respectively) for their ability to inhibit α9α10nAChR -mediated responses in Xenopus oocytes. bis-Quaternary ammonium analogs of general structures 1–8 (Fig. 1 and Table 1) were prepared by coupling of the linker precursors 13–20 with the appropriate azaaromatic head-group precursors (Table 1, Fig. 1). Dibromide 13–18, linker precursors for the synthesis of analogs 1–6, have been synthesized previously.23 Dibromide 19 and 20, linker precursors for the synthesis of analogs 7 and 8, were prepared utilizing similar procedures as those used for the synthesis of 13–18, via Sonogashira coupling of 4,4′-dibromobiphenyl with propargyl alcohol. The synthesis of the tris- and tetrakis-quaternary ammonium analogs 9–12 has been described previously.24–25
Figure 1.
General synthesis and structures of the bis-azaaromatic quaternary ammonium analogs.
Figure 2.
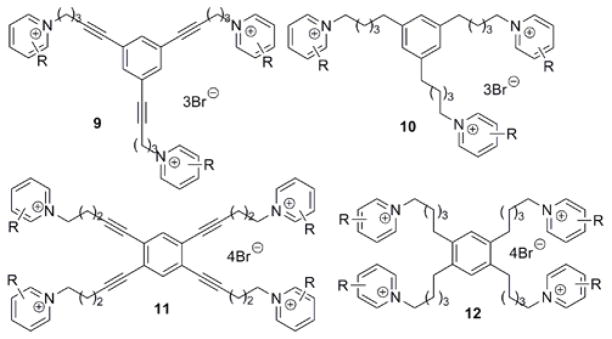
General structures of the tris- and tetrakis-azaaromatic quaternary ammonium analogs.
Table 1.
ACh-gated current responses at α9α10 nAChRs after coapplication of 100 nM of azaaromatic quaternary ammonium analogs.
| |||
|---|---|---|---|
| Compounda | α9α10 nAChRs (% response)b | Compounda | α9α10 nAChRs (% response)b |
| 1a | 31.3±5.8 | 4a | 72.0±6.9 |
| 1b | 67.6±16.9 | 4b | 124.4±10.2 |
| 4c | 10.9±1.4 | ||
| 1d | 28.03±3.87 | 4d | 31.15±8.65 |
|
| |||
| 2a | 76.9±11.9 | 5a | 62.7±7.7 |
| 2c | 41.6±6.7 | 5c | 42.6±9.5 |
| 2d | 39.7±11.4 | 5d | 37.5±6.6 |
| 2e | 23.2±3.8 | ||
|
| |||
| 3a | 10.6±4.6 | 6a | 36.7±11.3 |
| 3b | 76.2±8.3 | 6b | 97.1±9.9 |
|
| |||
| 7a | 8.1±6.1 | 8a | 89.3±10.8 |
| 8b | 113.8±4.7 | ||
|
| |||
| 9a | 61.12±6.95 | 10a | 62.74±7.85 |
| 9b | 66±12.61 | 10b | 79.77±9.71 |
| 9c | 10.46±3.48 | 10c | 15.9±3.5 |
| 10d | 6.9±4.71 | ||
| 9e | 5.5±2.6 | 10e | 3.84±1.95 |
| 9f | 36.15±7.23 | 10f | 16.1±9.3 |
|
| |||
| 11a | 47.4±8.2 | 12a | 11.0±3.1 |
| 11b | 0.3±0.07 | 12b | 0.6±0.4 |
| 11d | 11.6±3.1 | 12d | 17.9±3.3 |
| 11e | 0.3±0.09 | 12e | 6.5±2.9 |
The number in the compound designation corresponds to the type of linker as shown in Figure 1. The letter corresponds to the head groups.
Inhibition of α9α10-mediated responses to 10 μM ACh in the presence of 100 nM of analog, the data represent % of response; Each value represents data from 3–8 independent experiments.
The analogs were tested initially at a probe concentration of 100 nM on cloned α9α10 nAChRs heterologously expressed in Xenopus oocytes for their ability to block ACh-gated currents. Data represent percentile inhibitory responses during the co-application of ACh and analog, and are normalized to the responses to ACh alone (Table 1).27
The preliminary screen (Table 1) identified a number of analogs that potently inhibited α9α10 ACh-gated currents, from which tetrakis-analogs 11b, 11e, and 12b exhibited nearly complete inhibition at the probe concentration. In addition, analogs containing three or four quaternary ammonium head-groups were more effective in inhibiting α9α10 receptors at the probe concentration when compared with analogs within the bis-, tris-, and tetrakis-series. Furthermore, analogs bearing bulky, more hydrophobic cationic head-groups generally exhibited greater inhibition of α9α10nAChR s, with the exception of the tetrakis-analog series, in which the 3,4-lutidinium analogs 11b and 12b were more potent than the isoquinolinium analogs 11d and 12d.
Selected compounds, including 7a, 8a, 9e, 10c, 10d, 10e, 11e, and 12e,28 were evaluated further in full concentration-response studies for inhibition of ACh-gated currents (Table 2). Analog 11e, a tetrakis-quaternary ammonium compound with four 3-phenylpyridium head-groups, exhibited the highest potency in this series, with an IC50 value of 0.56 nM. Notably, analogs containing the more rigid triple bond linker units, i.e. 7a (IC50=16 nM), 9e (IC50=1.7 nM), and 11e (IC50=0.56 nM), were more potent than their corresponding alkyl linked analogs 8a (IC50=670 nM), 10e (IC50=3.7 nM), and 12e (IC50=5.4 nM), respectively, indicating that a conformationally rigid central core scaffold appears to be critical for optimal inhibitory activity at α9α10nAChR s.
Table 2.
IC50 values for the azaaromatic quaternary ammonium analog inhibition of α9α10 and α7nAChRs.
| Compounds | α9α10 nAChR (IC50, nM) | α7 nAChR (IC50, nM) |
|---|---|---|
| 7a | 16 | 1190 |
| 8a | 670 | 6010 |
| 9e | 1.7 | 185 |
| 10c | 4.2 | 1510 |
| 10d | 4.8 | 170 |
| 10e | 3.7 | 91 |
| 11e | 0.56 | 8.8 |
| 12e | 5.4 | 99 |
The above quaternary ammonium analogs are expected to have very limited brain bioavailability due to the highly polar nature of their cationic head-groups, which makes such compounds more attractive as antagonists for α9α10nAChRs, due to the absence of this receptor in brain. On the other hand, in the design of antagonists of α9α10nAChRs for chronic pain, their selectivity over peripheral α7 nAChRs is critical, due to the fact that antagonism of these two peripheral nAChRs produces diametrically opposite pharmacodynamic effects.15 Thus, the lead analogs in Table 2 also were evaluated for their ability to block ACh-gated currents in α7nAChRs heterologously expressed in Xenopus oocytes (Table 2). Interestingly, the presence of a rigid central core scaffold appears to be important in affording selectivity for the α9α10 subtype over the α7 subtype. bis-Analogs 7a and 9e, which incorporate conformationally restricted linker units in their structures, were 75-fold and 109-fold more selective, respectively, for the α9α10 subtype vs. the α7 subtype. Their corresponding alkyl linker counterparts, 8a and 10e, were only 9- and 25-fold more selective, respectively, for the α9α10 vs. α7 subtypes. However, conformational restriction of the linker units in the tetrakis-series of analogs appears to have no significant effect on selectivity, since analogs 11e and 12e were equipotent, at α9α10 and α7 nAChRs. Interestingly, the nature of the head-group appears to have greater influence on selectivity than potency. For example, IC50 values of the tris-analogs 10c, 10d, and 10e at α9α10 are similar, whereas selectivity for the α9α10 subtype vs. the α7 subtype is 360-, 35-, and 25-fold, respectively, for these three analogs. Thus, analog 11e was the most potent α9α10 antagonist (IC50=560 pM), but was less selective vs α7 when compared to the slightly less potent analog 10c.
In preliminary proof-of-concept studies, the most potent α9α10 antagonist, compounds 7a, 10c, and 11e have recently been evaluated in in vivo rat models of pain.16, 29 These three analogs were found to be ineffective as an analgesic in the rat tail-flick test, but demonstrated an analgesic effect in the rat formalin model of tonic inflammatory pain (phase 2) and reversed mechanical hyperalgesia in a dose-related manner in the rat chronic constriction nerve injury (CCI) model of neuropathic pain. Furthermore, tolerance to the antihyperalgesic effect was not observed after repeated dosing (7 days) of drug treatment. In addition, efficacy was achieved at doses well below those that produced toxicity in the motor function rotarod test. The above analogs were 10 to 5000-fold less potent as antagonists at other nAChRs, including α1β1δε, α2β2, α2β4, α3β2, α3β4, α4β2, α4β4, α6/α3β2β3, and α6/α3β4 subtypes; The most potent analog 11e is a selective inhibitor of α9α10 nAChRs, having relatively poor inhibitory potency against α4β2 (IC50=3.8 μM), α3β4 (IC50=0.8 μM), and muscle (IC50=0.12 μM) nAChRs.16 We have also shown that analog 11e does not interact with GABAA or GABAB receptors, and has several orders of magnitude lower affinity for 5HT3 and κ-opioid receptors compared to α9α10 nAChRs (unpublished data). More recently, the bis-analog 7a, was shown to be effective in alleviating vincristin-induced neuropathic pain in the rat CCI pain model.30 These collective results provide support for the contention that α9α10 nAChRs are a new target for studying pain modulation, and suggests that further development of small, non-peptide α9α10 antagonists as treatments for chronic pain may lead to novel, clinically useful, non-opioid analgesics.
In conclusion, a series of azaaromatic quaternary ammonium analogs have been discovered as potent and selective antagonists of α9α10 nAChRs. These first-in-class compounds represent excellent leads for the subsequent development of highly potent and subtype-selective small molecule antagonists at α9α10nAChR s as treatments for chronic pain.
Acknowledgments
This work was supported by NIH(Grant U19DA017548 to LPD and PAC, MH53631 and GM48677 to JMM) and a seed grant from the University of Utah Research Foundation to JMM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Le Novere N, Changeux JP. J Mol Evol. 1995;40:155. doi: 10.1007/BF00167110. [DOI] [PubMed] [Google Scholar]
- 2.Ortells MO, Lunt GG. Trends Neurosci. 1995;18:121. doi: 10.1016/0166-2236(95)93887-4. [DOI] [PubMed] [Google Scholar]
- 3.Changeux J, Edelstein SJ. Curr Opin Neurobiol. 2001;11:369. doi: 10.1016/s0959-4388(00)00221-x. [DOI] [PubMed] [Google Scholar]
- 4.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Physiol Rev. 2009;89:73. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nashmi R, Lester HA. J Mol Neurosci. 2006;30:181. doi: 10.1385/JMN:30:1:181. [DOI] [PubMed] [Google Scholar]
- 6.Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. Cell. 1994;79:705. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 7.Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. Proc Natl Acad Sci U S A. 2001;98:3501. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sgard F, Charpantier E, Bertrand S, Walker N, Caput D, Graham D, Bertrand D, Besnard F. Mol Pharmacol. 2002;61:150. doi: 10.1124/mol.61.1.150. [DOI] [PubMed] [Google Scholar]
- 9.Plazas PV, Katz E, Gomez-Casati ME, Bouzat C, Elgoyhen AB. J Neurosci. 2005;25:10905. doi: 10.1523/JNEUROSCI.3805-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIntosh JM, Absalom N, Chebib M, Elgoyhen AB, Vincler M. Biochem Pharmacol. 2009;78:693. doi: 10.1016/j.bcp.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIntosh JM, Plazas PV, Watkins M, Gomez-Casati ME, Olivera BM, Elgoyhen AB. J Biol Chem. 2005;280:30107. doi: 10.1074/jbc.M504102200. [DOI] [PubMed] [Google Scholar]
- 12.Vincler M, Wittenauer S, Parker R, Ellison M, Olivera BM, McIntosh JM. Proc Natl Acad Sci U S A. 2006;103:17880. doi: 10.1073/pnas.0608715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett DS, Simon S, Brennan M, Shoemaker SA. J Opioid Manag. 2007;3:101. doi: 10.5055/jom.2007.0046. [DOI] [PubMed] [Google Scholar]
- 14.Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Rheumatology (Oxford) 2008;47:670. doi: 10.1093/rheumatology/ken021. [DOI] [PubMed] [Google Scholar]
- 15.Vincler M, McIntosh JM. Expert Opin Ther Targets. 2007;11:891. doi: 10.1517/14728222.11.7.891. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh JM, Dwoskin LP, Crooks PA, Holtman JR. Biochem Pharmacol Suppl. 2009;78:921. [Google Scholar]
- 17.Dwoskin LP, Wilkins LH, Pauly JR, Crooks PA. Ann N Y Acad Sci. 1999;868:617. doi: 10.1111/j.1749-6632.1999.tb11334.x. [DOI] [PubMed] [Google Scholar]
- 18.Wilkins LH, Jr, Haubner A, Ayers JT, Crooks PA, Dwoskin LP. J Pharmacol Exp Ther. 2002;301:1088. doi: 10.1124/jpet.301.3.1088. [DOI] [PubMed] [Google Scholar]
- 19.Ayers JT, Dwoskin LP, Deaciuc AG, Grinevich VP, Zhu J, Crooks PA. Bioorg Med Chem Lett. 2002;12:3067. doi: 10.1016/s0960-894x(02)00687-x. [DOI] [PubMed] [Google Scholar]
- 20.Wilkins LH, Jr, Grinevich VP, Ayers JT, Crooks PA, Dwoskin LP. J Pharmacol Exp Ther. 2003;304:400. doi: 10.1124/jpet.102.043349. [DOI] [PubMed] [Google Scholar]
- 21.Grinevich VP, Crooks PA, Sumithran SP, Haubner AJ, Ayers JT, Dwoskin LP. J Pharmacol Exp Ther. 2003;306:1011. doi: 10.1124/jpet.103.051789. [DOI] [PubMed] [Google Scholar]
- 22.Sumithran SP, Crooks PA, Xu R, Zhu J, Deaciuc AG, Wilkins LH, Dwoskin LP. Aaps J. 2005;7:E201. doi: 10.1208/aapsj070119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng GR, Zhang ZF, Pivavarchyk M, Deaciuc AG, Dwoskin LP, Crooks PA. Bioorg Med Chem Lett. 2007;17:6734. doi: 10.1016/j.bmcl.2007.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng GR, Sumithran SP, Deaciuc AG, Dwoskin LP, Crooks PA. Bioorg Med Chem Lett. 2007;17:6701. doi: 10.1016/j.bmcl.2007.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang ZF, Zheng GR, Pivavarchyk M, Deaciuc AG, Dwoskin LP, Crooks PA. Bioorg Med Chem Lett. 2008;18:5753. doi: 10.1016/j.bmcl.2008.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Hernandez GY, Thinschmidt JS, Zheng G, Zhang Z, Crooks PA, Dwoskin LP, Papke RL. Mol Pharmacol. 2009;76:652. doi: 10.1124/mol.109.056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.In the voltage clamp electrophysiology assays (Tables 1 and 2), Xenopus oocytes were voltage-clamped and exposed to ACh and test compounds as previously described.11 Briefly, the oocyte chamber consisting of a cylindrical well (~30 μL in volume) was gravity perfused at a rate of ~2 mL/min with ND96 containing 0.01% (wt/vol) BSA and 1 μM atropine to block potential contaminating signal from endogenous muscarinic receptors. For experiments involving α7 and α9α10, atropine was excluded from the perfusion solution because it has been shown to block these receptor subtypes. Oocytes were exposed once a minute to 1 sec pulses of ACh. ACh concentrations used were 200 μM for α7, 10 μM for α1β1δε and α9α10 and 100 μM for all other subtypes. Compounds were applied at the beginning of a five min static bath incubation. The % block was calculated as a % of ND96 control (no compound) response. Concentration-response data were fit to the equation Y = 100/(1 + 10^((LogIC50 – Log[Toxin])*Hill Slope)) by nonlinear regression analysis using GraphPad Prism (GraphPad Software, San Diego, CA). Data points in the concentration-response represent the mean ± SEM from at least 3 oocytes.
- 28.Spectra data of selective compounds: 7a, 1H NMR (300MHz, CDCl3) δ 9.01 (s, 2H), 8.97 (d, J=6.3 Hz, 2H), 7.99 (d, J=6.3 Hz, 2H), 7.76 (dd, J=6.6, 2.1 Hz, 4H), 7.61 (dd, J=6.6, 2.1 Hz, 4H), 5.81 (s, 4H), 2.52 (s, 6H), 2.40 (s, 6H) ppm; 13C NMR (75 MHz, CDCl3) δ 15 9.6, 143.1, 142.0, 140.4, 138.7, 133.1, 128.9, 127.7, 120.9, 88.8, 83.1, 50.4, 20.7, 17.2 ppm; 8a, 1H NMR (300MHz, CDCl3) δ 8.82 (s, 2H), 8.73 (d, J=6.3 Hz, 2H), 7.78 (d, J=6.3 Hz, 2H), 7.46 (d, J=8.4 Hz, 4H), 7.29 (d, J=8.4 Hz, 4H), 4.64 (t, J=7.2 Hz, 4H), 2.79 (t, J=7.2 Hz, 4H), 2.47 (s, 6H), 2.39 (s, 6H), 2.32–2.42 (m, 4H) ppm; 13C NMR (75 MHz, CDCl3) δ 158.4, 143.0, 141.4, 139.6, 138.5, 138.3, 129.0, 128.3, 126.6, 60.6, 32.6, 32.0, 19.4, 16.1 ppm; 9e, 1H NMR (300 MHz, CD3OD) δ 9.50 (s, 3H), 9.09 (d, J=6.0 Hz, 3H), 8.78 (d, J=8.1 Hz, 3H), 8.16 (dd, J=8.1, 6.0 Hz, 3H), 7.75–7.87 (m, 6H), 7.42–7.65 (m, 9H), 7.17 (s, 3H), 4.96 (t, J=6.9 Hz, 6H), 2.69 (t, J=6.3 Hz, 6H), 2.42 (m, 6H) ppm; 13C NMR (75 MHz, CD3OD) δ 144.4, 144.3, 144.2, 142.6, 134.9, 134.5, 131.5, 130.8, 129.5, 128.7, 125.1, 90.0, 81.5, 62.6, 30.8, 17.4 ppm; 10c, 1H NMR (300 MHz, CD3OD) δ 9.46 (dd, J=6.0, 1.5 Hz, 3H), 9.22 (d, J=8.4 Hz, 3H), 8.57 (d, J=9.0 Hz, 3H), 8.45 (dd, J=8.4, 1.5 Hz, 3H), 8.30 (m, 3H), 8.02–8.14 (m, 6H), 6.80 (s, 3H), 5.11 (t, J=7.5 Hz, 6H), 2.56 (t, J=7.5 Hz, 6H), 2.14 (m, 6H), 1.69 (m, 6H), 1.52 (m, 6H) ppm; 13C NMR (75 MHz, CD3OD) δ 150.3, 148.9, 143.4, 139.4, 137.3, 132.2, 131.8, 131.4, 127.2, 123.1, 119.9, 59.4, 36.7, 32.3, 31.1, 27.3 ppm; 10d, 1H NMR (300 MHz, CD3OD) δ 9.99 (s, 3H), 8.69 (dd, J=6.9, 1.5 Hz, 3H), 8.47–8.54 (m, 6H), 8.22–8.36 (m, 6H), 8.07 (m, 3H), 6.78 (s, 3H), 4.78 (t, J=7.5 Hz, 6H), 2.53 (t, J=7.5 Hz, 6H), 2.15 (m, 6H), 1.67 (m, 6H), 1.44 (m, 6H) ppm; 13C NMR (75 MHz, CD3OD) δ 150.8, 143.2, 138.8, 138.2, 135.8, 132.5, 131.5, 129.0, 128.5, 127.5, 127.1, 62.8, 36.5, 32.3, 32.0, 26.8 ppm; 10e, 1H NMR (300 MHz, CD3OD) δ 9.39 (s, 3H), 8.98 (d, J=6.0 Hz, 3H), 8.85 (ddd, J=6.0, 1.8, 1.2 Hz, 3H), 8.15 (dd, J=8.1, 6.0 Hz, 3H), 7.78–7.90 (m, 6H), 7.50–7.65 (m, 9H), 6.82 (s, 3H), 4.74 (t, J=7.8 Hz, 6H), 2.55 (t, J=7.5 Hz, 6H), 2.11 (m, 6H), 1.69 (m, 6H), 1.45 (m, 6H) ppm; 13C NMR (75 MHz, CD3OD) δ 144.2, 143.9, 143.4, 142.8, 134.6, 131.5, 130.8, 129.4, 128.7, 127.2, 63.3, 36.7, 32.7, 32.2, 27.0 ppm; 11e, 1H NMR (300 MHz, CD3OD) δ 9.50 (s, 4H), 9.09 (d, J=6.0 Hz, 4H), 8.74 (dd, J=8.4, 1.2 Hz, 4H), 8.14 (dd, J=8.1, 6.0 Hz, 4H), 7.70–7.85 (m, 8H), 7.45–7.62 (m, 12H), 7.23 (s, 2H), 4.95 (t, J=6.9 Hz, 8H), 2.71 (t, J=6.6 Hz, 8H), 2.30–2.47 (m, 8H) ppm; 13C NMR (75 MHz, CD3OD) δ 144.2, 142.6, 136.2, 134.5, 131.5, 130.8, 129.6, 128.7, 126.1, 94.8, 81.0, 62.6, 31.1, 17.7 ppm; 12e, 1H NMR (300 MHz, CD3OD) δ 9.48 (s, 4H), 9.05 (d, J=6.0 Hz, 4H), 8.83 (d, J=8.4 Hz, 4H), 8.14 (dd, J=7.8, 6.3 Hz, 4H), 7.89 (dd, J=7.8, 1.2 Hz, 8H), 7.40–7.65 (m, 12H), 6.85 (s, 2H) 4.80 (t, J=7.5 Hz, 8H), 2.52 (t, J=6.9 Hz, 8H), 2.11 (t, J=6.6 Hz, 8H), 1.37–1.69 (m, 16H) ppm; 13C NMR (75 MHz, CD3OD) δ 142.9, 142.8, 142.6, 141.2, 137.3, 133.3, 130.3, 130.1, 129.6, 128.4, 127.6, 62.1, 32.0, 31.7, 31.0, 26.2 ppm.
- 29.Holtman JR, Dwoskin LP, Wala EP, Crooks PA, McIntosh JM. J Pain, Suppl. 2010;11:S33. [Google Scholar]
- 30.Wala EP, Crooks PA, McIntosh JM, Elliot M, Walter J, Holtman JR. American Pain Society Annual Meeting. Austin; Texas: 2011. [Google Scholar]