Abstract
Vescular monoamine transporter-2 (VMAT2) is a viable target for development of pharmacotherapies for psychostimulant abuse. Lobeline (1) is a potent antagonist at α4β2* nicotinic acetylcholine receptors, has moderate affinity (Ki=5.46 μM) for VMAT2, and is being investigated currently as a clinical candidate for treatment of psychostimulant abuse. A series of carboxylic acid and sulfonic acid ester analogs 2–20 of lobeline were synthesized and evaluated for interaction with α4β2* and α7* neuronal nicotinic acetylcholine receptors (nAChRs), the dopamine transporter (DAT), serotonin transporter (SERT) and VMAT2. Both carboxylic acid and sulfonic acid esters had low affinity at α7* nAChRs. Similar to lobeline (Ki=4 nM), sulfonic acid esters had high affinity at α4β2* (Ki=5–17 nM). Aromatic carboxylic acid ester analogs of lobeline (2–4) were 100–1000-fold less potent than lobeline at α4β2* nAChRs, whereas aliphatic carboxylic acid ester analogs were 10–100-fold less potent than lobeline at α4β2*. Two representative lobeline esters, the 10-O-benzoate (2) and the 10-O-benzenesulfonate (10) were evaluated in the 36Rb+ efflux assay using rat thalamic synaptosomes, and were shown to be antagonists with IC50 values of 0.85 μM and 1.60 μM, respectively. Both carboxylic and sulfonic acid esters exhibited a range of potencies (equipotent to 13–45-fold greater potency compared to lobeline) for inhibiting DAT and SERT, respectively, and like lobeline, had moderate affinity (Ki=1.98–10.8 μM) for VMAT2. One of the more interesting analogs, p-methoxybenzoic acid ester 4, had low affinity at α4β2* nAChRs (Ki=19.3 μM) and was equipotent with lobeline, at VMAT2 (Ki=2.98 μM), exhibiting a 6.5-fold selectivity for VMAT2 over α4β2 nAChRs. Thus, esterification of the lobeline molecule may be a useful structural modification for the development of lobeline analogs with improved selectivity at VMAT2.
Keywords: Lobeline, Dopamine, Nicotinic receptors, Neurotransmitter transporters, Structure-activity relationships
1. Introduction
Methamphetamine is a Schedule II stimulant and has a high potential for abuse. The abuse of methamphetamine is escalating and has become a major public health problem world-wide, due to its abuse liability and potential neurotoxic effects. However, there are currently no medications that counteract the effects of methamphetamine or reduce methamphetamine abuse.1 Methamphetamine abuse leads to devastating medical, psychological, and social consequences, including memory loss, aggression, psychotic behavior, heart damage, malnutrition, and severe dental problems. Methamphetamine abuse also contributes to increased transmission of infectious diseases, such as hepatitis and HIV/AIDS.2
Lobeline (1, Scheme 1), the principal alkaloid of Lobelia inflata3 which was previously a candidate of pharmacotherapy for smoking cessation.4 More-recent findings suggest that lobeline may be a pharmacotherapy for methamphetamine abuse and its associated neurotoxicity5–8, and is currently in clinical trials.9 Lobeline has high affinity for α4β2* and α7* nicotinic acetylcholine receptors (nAChRs) and inhibits nicotine-evoked 86Rb+ efflux from rat thalamic synaptosomes.10–14 Lobeline inhibits nicotine-evoked [3H]dopamine (DA) overflow from rat striatal slices and interacts nonselectively with monoamine transporters, including the dopamine transporter (DAT), serotonin transporter (SERT) and vesicular monoamine transporter-2 (VMAT2)].5,10,12,15,16 In behavioral pharmacology studies, systemic administration of lobeline attenuates the locomotor activating effects of repeated nicotine injections, consistent with lobeline inhibition of nAChRs, and neurotransmitter transporters and/or alteration of presynaptic dopamine storage and release. 12,13,15–17
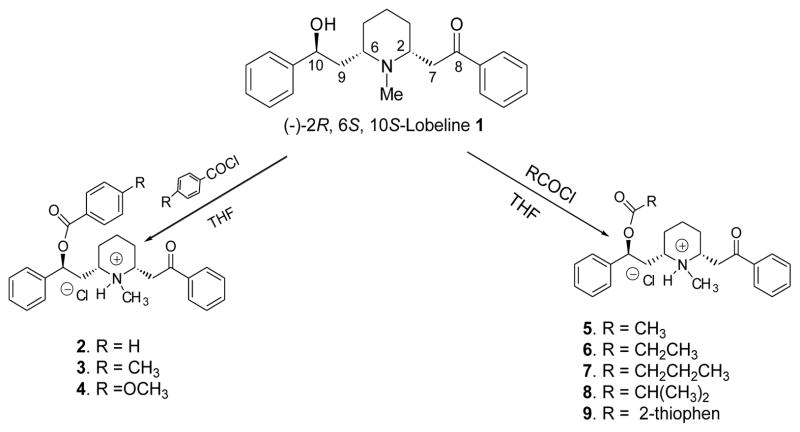
Scheme 1.
Preparation of 10-O-carboxylic acid ester analogs of lobeline.
The potential of lobeline as a treatment for methamphetamine abuse was suggested by promising preclinical results showing that lobeline decreases methamphetamine psychostimulant effects and self administration in rats; furthermore, lobeline does not reduce methamphetamine self-administration by acting as a substitute reinforcer, but rather appears to reduce the reward associated with methamphetamine self-administration.18,19 Lobeline blocked the discriminative-stimulus properties of both methamphetamine and cocaine.20,21 The underlying pharmacological mechanism is thought to involve alteration of vesicular storage and release of DA through an interaction with VMAT2. Lobeline has also been shown to attenuate amphetamine-evoked DA release from rat striatal slices20 as well as have protective effects against methamphetamine-induced neurotoxicity.6 Together, these studies suggested that lobeline lacks abuse liability while decreasing the stimulant, rewarding and neurotoxic effects of methamphetamine. Recent studies also suggest that lobeline functions as an μ opioid receptor antagonist and that the interaction of lobeline with μ opioid receptors may contribute to its known efficacy to diminish the reinforcing and rewarding properties of psychostimulants.22
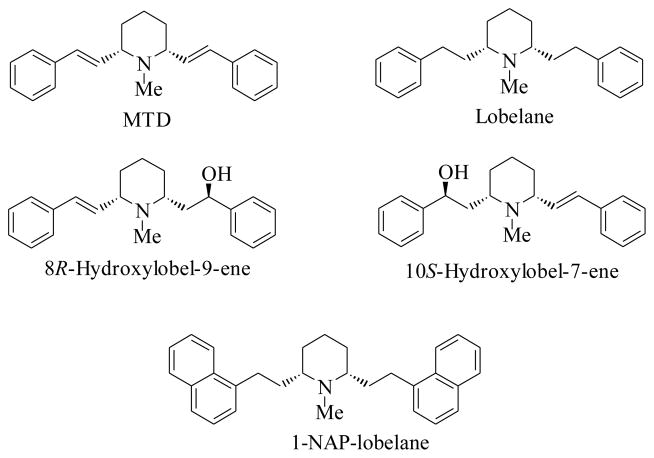
Recent studies have focused on the effect of structural modifications of the lobeline molecule at both nAChRs and monoamine transporters. The lobeline molecule is chirally unstable;23,24 and structural changes to the lobeline molecule, such as removal of both the oxygen functionalities, provided the more stable meso–transdiene [MTD] and lobelane (Fig. 1), which resulted in a dramatic decrease in the ligand binding sites on α4β2* and α7* nAChRs, while maintaining affinity at VMAT2 that was comparable with lobeline (Ki=2.76 μM). Also, lobelane was more selective and 3-fold more potent (Ki=0.97 μM) than lobeline at VMAT2. Replacing the phenyl groups of lobelane with 1-naphthyl moieties afforded N-methyl-2,6-cis-bis(naphthalene-1-ethyl)- piperidine (1-NAP-lobelane) (Fig. 1) which was VMAT2-selective and more potent (Ki=0.630 μM) than lobelane.10,11,25 Further studies on the modification of the lobelane molecule suggested that the entire lobelane scaffold was critical for high affinity binding at VMAT2.26,27 On the other hand, the des-keto analogs of lobeline, 8R-hydroxylobel-9-ene and 10S-hydroxylobel-7-ene (Fig. 1), had moderate affinity for VMAT2 (Ki=5.16 μM and 6.06 μM, respectively, but also exhibited affinity for SERT and DAT. The 8R-enantiomer had high potency and selectivity for both SERT and DAT (Ki=44 nM and 860 nM respectively).28
Fig. 1.
Structures of MTD, lobelane, 8R-hydroxylobel-9-ene, 10S-hydroxylobel-7-ene and N-methyl-2,6-cis-di-(naphthylene-1-ethyl)piperidine (1-NAP-lobelane)
As part of a strategy to improve the oral bioavailability of lobeline, we initially investigated the tosylate ester of lobeline (11), which was synthesized from 10-O-esterification of lobeline with tosyl chloride. Surprisingly, lobeline tosylate was found to be equipotent (Ki=5 nM) with lobeline in inhibiting [3H]nicotine binding to rat brain membranes, and was nearly 70-fold more potent (IC50=2 nM) than lobeline in inhibiting nicotine-evoked 86Rb+ efflux from rat thalamic synaptosomes, demonstrating potent antagonism of α4β2* nAChRs.10 Importantly, lobeline tosylate exhibited affinity for VMAT2 not different from that for lobeline.10 The present study was conducted to examine the structure-activity relationships (SARs) of 10-O-esters of lobeline to determine the structural requirements for such analogs to bind to nAChRs and neurotransmitter transporter sites, and to determine if 10-O-ester lobeline analogs could be identified with greater selectivity for VMAT2. Hence, various lobeline carboxylic acid and sulfonic acid ester analogs (2–20) were synthesized and evaluated for activity at α4β2* and α7* nAChRs, as well as at DAT, SERT and VMAT2.
2. Results and Discussion
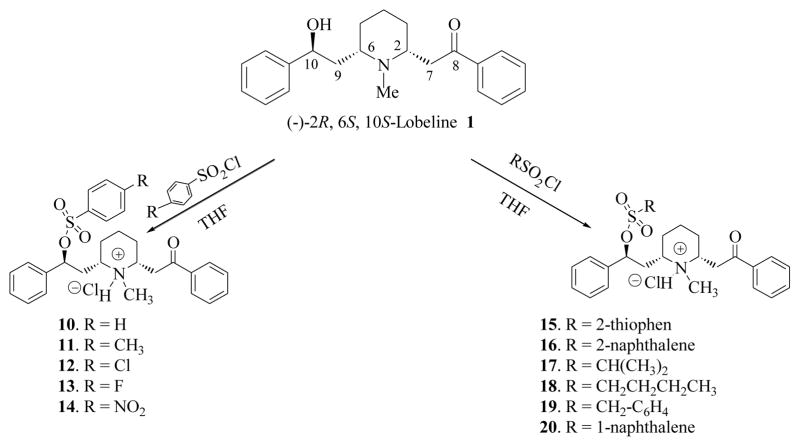
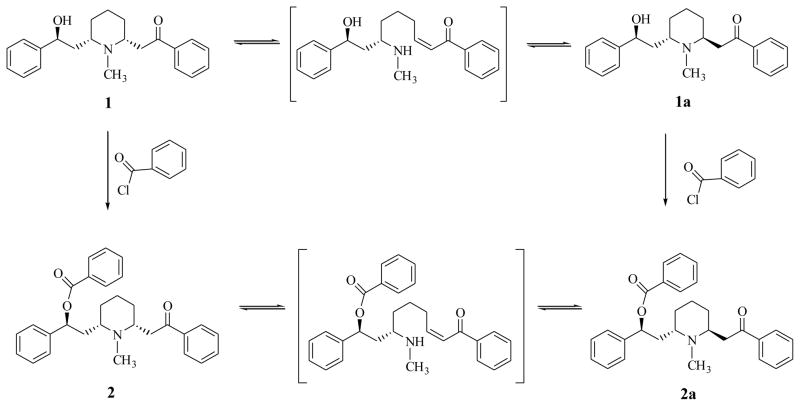
Carboxylic acid and sulfonic acid esters of lobeline (2–20) were synthesized via esterification of the 10-O-hydroxy group of lobeline (1) with various acyl and sulfonyl chlorides (see Schemes 1 and 2). In initial studies, when lobeline free base was treated with benzoyl chloride in the presence of an organic base such as pyridine or triethylamine in various organic solvents, the resulting 10-O-benzoyl ester was obtained as a mixture of the C2 epimers (2 and 2a, see Scheme 3). This epimerization was observed with other acyl and sulfonyl chlorides and is likely due to C2 epimerization of lobeline to afford a mixture of cis- and trans-lobelines,23,24 which can each be esterified under these basic conditions. Epimerization of lobeline is believed to be the result of a self (base) catalyzed equilibration via a transient retroconjugate addition reaction intermediate.24 This epimerization problem was overcome by carrying out the acylation and sulfonylation reactions in tetrahydrofuran in the absence of organic base, which afforded the desired (−)-2R, 6S, 10S-lobeline esters. The structures of all the 10-O-esters products were confirmed by 1H-NMR and 13C-NMR spectroscopy, and by mass spectral and elemental analyses (see Experimental Section).
Scheme 2.
Preparation of 10-O-sulfonic acid ester analogs of lobeline.
Scheme 3.
Epimerization of lobeline (1) and the 10-O-benzoyl ester of lobeline (2)
The above lobeline analogs (2–20) were synthesized and were evaluated as inhibitors of [3H]nicotine ([3H]NIC) binding and [3H]methyllycaconitine ([3H]MLA) binding to rat brain membranes, as inhibitors of [3H]DA uptake into rat striatal synaptosomes to assess DAT function, as inhibitors of [3H]serotonin ([3H]5-HT) uptake into rat hippocampal synaptosomes to assess SERT function, and as inhibitors of [3H]DTBZ (or [3H]MTBZ) binding to rat synaptic vesicle membranes to assess interaction with VMAT2.
Lobeline (1) exhibited low affinity (Ki = 6.26 μM; Table 1) at the [3H]MLA binding site probing α7* nAChRs, consistent with previous reports.10,11 Compared with lobeline, the carboxylic acid and sulfonic acid ester analogs of lobeline (2–20) showed generally lower affinity at the [3H]MLA binding site, (Table 1).
Table 1.
Lobeline (1) and 10-O-carboxylic acid ester and sulfonic acid ester analogs (2–20) inhibition of [3H]NIC and [3H]MLA, [3H]MTBZ binding and [3H]DA and [3H]5-HT uptake into rat striatal and hippocampal synaptosomes, respectively.
| Compound | Ki μM (M ± SEM)a
|
||||
|---|---|---|---|---|---|
| [3H]MLA (α7*) | [3H]NIC (α4β2*) | [3H]DA (DAT) | [3H]5-HT (SERT) | [3H]MTBZ/DTBZ (VMAT2) | |
| Nicotine | 0.33 ±0.061 | 0.0014 ±0.00017 | – | – | – |
|
| |||||
| GBR 12909 | – | – | 0.018 ±0.0001c | – | – |
|
| |||||
| Fluoxetine | – | – | – | 0.041 ±0.0001c | – |
|
| |||||
| Tetrabenazine | – | – | – | – | 0.013 ±0.0001 |
|
| |||||
| Lobeline 1 | 6.26 ± 1.30 | 0.004 ± 0.001 | 28.2 ± 6.73 | 46.8 ± 3.70 | 5.46 ± 1.30b |
| 2 | > 100 | 2.87 ± 0.52 | 2.31 ±0.34 | 2.07 ± 0.51 | 1.53 ± 0.38 |
| 3 | > 100 | 1.62 ± 0.30 | 2.06 ± 0.12 | 1.51 ± 0.31 | 1.76 ± 0.54 |
| 4 | 18.4 ±0.42 | 19.3 ± 8.80 | 3.80 ± 0.35 | 2.04 ± 0.36 | 2.98 ± 0.21 |
| 5 | > 100 | 0.07± 0.01 | 4.64 ± 1.10 | 31.4 ± 17.4 | 8.05 ± 1.06 |
| 6 | 8.86 ± 2.36 | 0.15 ± 0.04 | 6.88 ± 1.20 | 4.87 ± 0.96 | 4.75 ± 0.92 |
| 7 | > 100 | 0.50 ± 0.08 | 4.82 ± 0.88 | 12.2 ± 3.10 | 3.38 ± 0.98 |
| 8 | > 100 | 0.06 ± 0.01 | 2.38 ± 0.20 | 1.06 ± 0.15 | 2.61 ± 0.52 |
| 9 | 36.2 ± 6.03 | 0.86 ± 0.26 | 3.02 ± 0.50 | 9.70 ± 1.50 | 6.37± 1.16b |
| 10 | 34.8 ± 1.13 | 0.008 ± 0.001 | 10.7 ± 1.30 | 37.3 ± 8.60 | 3.50 ± 0.80 |
| 11 | 18.0 ± 4.56 | 0.005 ± 0.001 | 29.1 ± 1.23 | 16.2 ± 3.40 | 10.8 ± 4.64 b |
| 12 | >100 | 0.016 ± 0.002 | 10.8 ± 1.80 | 4.38 ± 0.64 | 3.24 ± 0.28 |
| 13 | 24.2 ± 1.59 | 0.012 ± 0.002 | 17.9 ± 0.50 | 4.68 ± 0.67 | 3.00 ± 0.48 |
| 14 | 53.8 ± 10.4 | 0.013 ± 0.002 | 19.9 ± 2.80 | 18.1 ± 3.80 | 4.23 ± 1.08 |
| 15 | 53.8 ± 10.4 | 0.013 ± 0.002 | 19.9 ± 2.80 | 18.1 ± 3.80 | 4.23 ± 1.08 |
| 16 | 18.5 ± 3.06 | 0.010 ± 0.001 | 13.4 ± 0.30 | 5.06 ± 1.40 | 2.48 ± 0.33 |
| 17 | 47.7 ± 7.03 | 0.015 ± 0.002 | 4.60 ± 0.58 | 16.10 ± 1.40 | 2.09 ± 0.30 |
| 18 | 41.0 ± 15.4 | 0.011 ± 0.001 | 11.8 ± 0.50 | 5.16 ± 0.50 | 1.98 ± 0.65 |
| 19 | 46.6 ± 5.22 | 0.013 ± 0.001 | 14.7 ± 1.20 | 4.52 ± 0.37 | 3.98 ± 0.74 |
| 20 | >100 | 0.017 ± 0.002 | 4.14 ± 0.30 | 34.5 ± 3.70 | 4.12 ± 1.15 |
GBR-12909 (a specific DAT inhibitor), fluoxetine (a specific SERT inhibitor) and tetrabenazine (a specific VMAT2 inhibitor) were used as standards for comparison.
Ki values represent data from at least four independent experiments, each performed in duplicate.
Assays determining the Ki for lobeline using [3H]MTBZ and [3H]DTBZ as ligands revealed no significant difference (5.46 ± 1.3 vs 2.76 ± 0.64 μM, respectively). [3H]MTBZ was utilized as the ligand for analogs 2–9 and [3H]DTBZ was utilized as the ligand for analogs 10–20, since [3H]MTBZ was no longer available.
Lobeline (1) potently inhibited [3H]nicotine binding probing α4β2* nAChRs to rat striatal membranes (Ki = 4 nM), which was comparable to the inhibition produced by nicotine (Ki = 1 nM) in this assay (Table 1). O-Esterification of the lobeline molecule with a variety of carboxylic acids led to carboxylic acid esters 2–9, which had a range of potencies for inhibiting [3H]nicotine binding. Interestingly, the aromatic carboxylic acid esters 2 (benzoyl ester), 3 (toluyl ester), 4 (anisoyl ester) and 9 (thiophenoyl) were 215–4800-fold less potent than lobeline in inhibiting [3H]nicotine binding; whereas the aliphatic carboxylic acid esters 5 (acetyl), 6 (propionyl), 7 (n-butanoyl) and 8 (isobutanoyl) were only 15 to 125-fold less potent than lobeline in inhibiting [3H]nicotine binding. The best analogs (5 and 8) were aliphatic esters and exhibited ~15-fold lower potency at the α4β2* nAChRs than lobeline. Thus, the α4β2* nAChRs does not appear to accommodate aromatic carboxylic acid esters (2, 3, 4 and 9) as well as it does aliphatic carboxylic acid esters (5, 6, 7 and 8) of lobeline.
Interestingly, the sulfonic acid ester analogs (10–20) potently inhibited (Ki = 5–17 nM) [3H]nicotine binding at α4β2* nAChRs, with lobeline tosylate (11) being the most potent in this series, and equipotent with lobeline. These results suggest that esterification of the 10-hydroxy group of lobeline with aliphatic carboxylic acids can maintain their interaction with α4β2* nAChRs, whereas substitution with an aromatic carboxylic acid leads to a decrease in affinity for this site. However, when the 10-hydroxy group is esterified with either an aliphatic or aromatic carboxylic acids, these analogs have high affinity for this site comparable with lobeline. Thus, the nature of the ester linkage is also an important structural feature for interaction with the α4β2* nAChR.
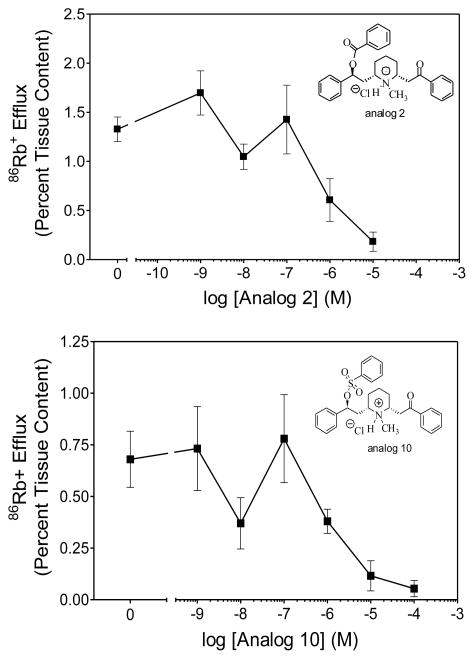
86Rb+ efflux from preloaded rat thalamic synaptosomes has been used as a functional assay for α4β2* nAChRs to determine whether compounds with affinity for this receptor subtype act as agonists or antagonists at this site.32 Lobeline (1) and lobeline tosylate (11) have been previously shown to act as potent nAChRs antagonists in the 86Rb+ efflux assay.10 A representative aromatic carboxylic acid ester, the O-benzoyl ester of lobeline (2) and a representative aromatic sulfonic acid ester, the O-benzoyl sulfonic acid ester of lobeline (10) were both assessed in this assay for inhibition of nicotine-evoked 86Rb+ efflux from rat thalamic synaptosomes. Analogs 2 and 10 both significantly inhibited nicotine-evoked 86Rb+ efflux with IC50 values of 0.85 and 1.6 μM, respectively (Fig. 2), which are comparable to the IC50 value for lobeline (IC50 = 0.73 μM) in this assay.10 These two analogs are both unsubstituted phenyl-based esters that vary only in the structure of the ester linkage between the phenyl ring and O-6 of the lobeline molecule, and were utilized to determine if any difference in nicotine function might be observed between carboxylate and sulfonate lobeline esters. Both compounds completely inhibited nicotine-evoked 86Rb+ efflux. Although analog 2 was 360-fold less potent than analog 10 in the [3H]nicotine binding assay, only a 2-fold difference in potency in their ability to inhibit nicotine-evoked 86Rb+ efflux was observed. This apparent inconsistency may be due to the fact that the [3H]nicotine binding assay assesses interactions at the desensitized α4β2* receptor, whereas the 86Rb+ efflux assay assesses interactions with the activatable form of the receptor.
Fig. 2.
Analog 2 (a representative aromatic carboxylic acid ester) and analog 10 (a representative aromatic sulfonic acid ester) inhibit nicotine-evoked 86Rb+ efflux from rat thalamic synaptosomes. Rat thalamic synaptosomes were preloaded with 86RbCl, superfused with buffer containing analog 2 or 10 (1 nM-10 μM) for 8 min and subsequently superfused for 3 min in the presence of nicotine (1 μM). The zero concentration point represents nicotine alone with no analog present. Data are presented as mean ± SEM percent 86Rb+ tissue content.
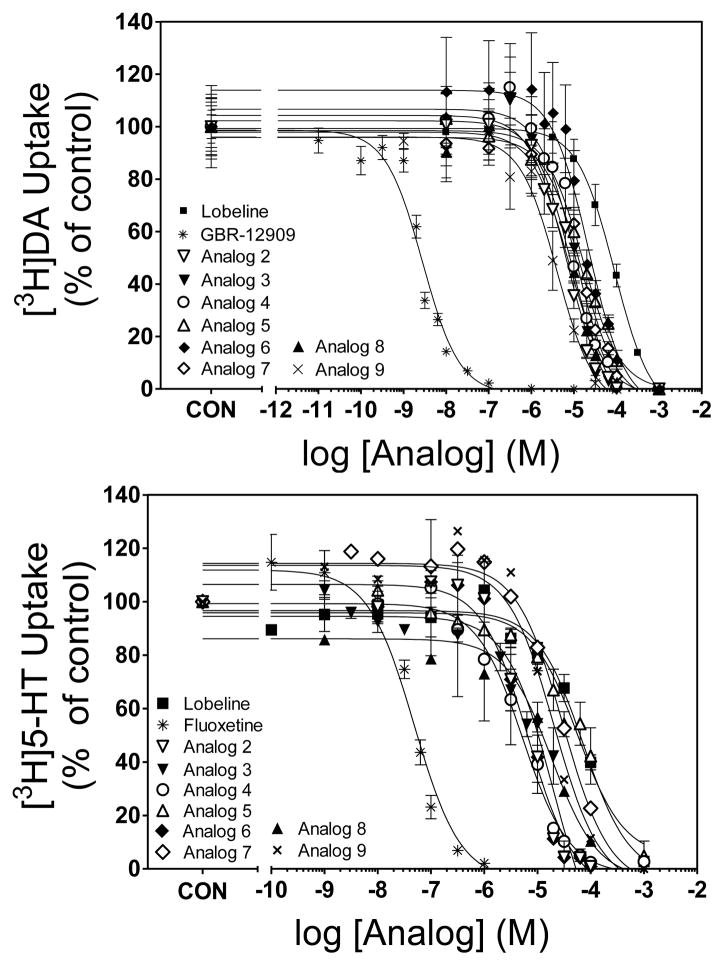
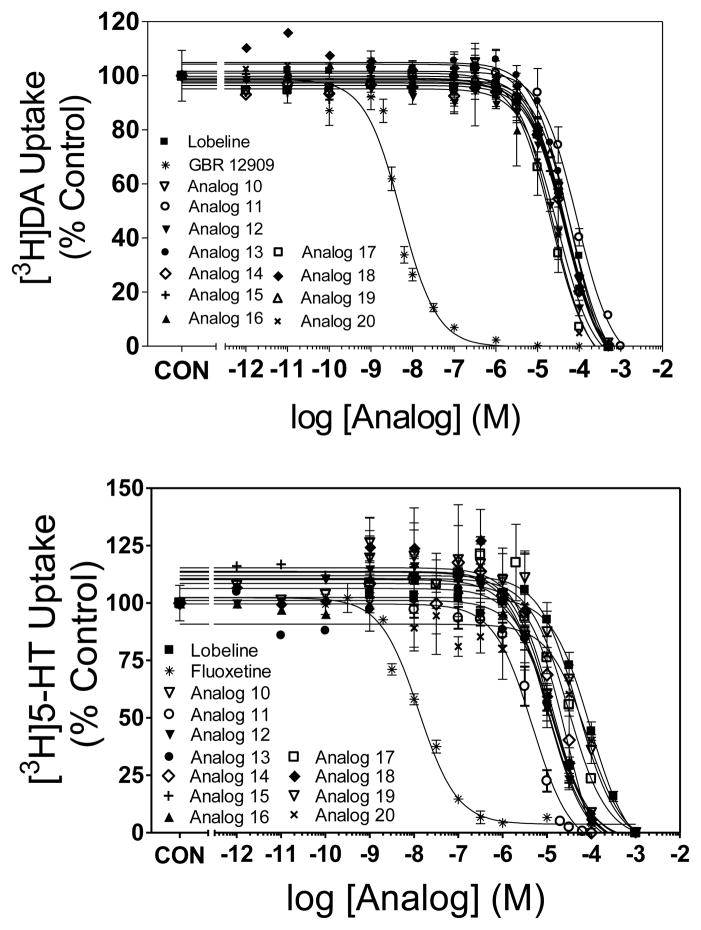
Lobeline (1) and the ester analogs (2–20) were evaluated as inhibitors of DAT, SERT and VMAT2 (Table 1, Figs. 3 and 4). Analog-induced inhibition was compared with that induced by lobeline and the selective DAT, SERT and VMAT2 transporter inhibitors, GBR-12909, fluoxetine and tetrabenazine, respectively.29–31 Lobeline exhibited moderate selectivity for VMAT2 (Ki = 5.46 μM) over DAT (Ki = 28.2 μM) and SERT (Ki = 46.8 μM), having relatively low affinity for the latter two transporters. The acetyl ester of lobeline (analog 5) was 6-fold more potent than lobeline at DAT, but equipotent with lobeline at SERT and VMAT2. The O-benzenesulfonic acid ester (10) was 3-fold more potent than lobeline at DAT but was equipotent with lobeline at SERT and VMAT2. Lobeline tosylate (11), p-fluorobenzene sulfonic acid ester (13) and p-nitrobenzene sulfonic acid ester (14), had similar potency (18–29 μM) to lobeline at DAT. The remaining lobeline carboxylic and sulfonic acid ester analogs (2–4, 6–9, 12, 15–20) were 2–13 fold more potent than lobeline at DAT. The lobeline acetyl ester (5), benzene sulfonic acid ester (10) and 1-naphthalene sulfonic acid ester (20), were equipotent (31–37 μM) with lobeline at SERT. The remaining lobeline carboxylic and sulfonic acid ester analogs (2–4, 6–9, 11–19) were from 3 to 45-fold more potent than lobeline at SERT. Thus, carboxylic acid esterification of the 10-hydroxy group of lobeline did not appear to alter affinity at VMAT2, but consistently enhanced affinity for DAT, whereas a more variable result was noted with respect to affinity for SERT. Generally, these analogs were more potent than lobeline at SERT with the exception of analog 5, which had affinity not different from lobeline. As with carboxylic acid esterification, sulfonic acid esterification generally did not alter affinity at VMAT2. Further, the sulfonic acids generally were not different from lobeline at DAT, with the exceptions of analogs 17 and 20, aliphatic and aromatic analogs, respectively, which exhibited enhanced affinity for DAT. No apparent SAR trends can be gleaned from the affinity of these sulfonic acid esters of lobeline at SERT.
Fig. 3.
Carboxylic acid esters inhibit specific [3H]DA (top panel) and [3H]5-HT (bottom panel) uptake into rat striatal and hippocampal synaptosomes, respectively. GBR 12909 and fluoxetine were used as a positive controls for [3H]DA and [3H]5-HT uptake assays, respectively . Nonspecific uptake was determined in the presence of nomifensine (10 μM) and fluoxetine (10 μM), respectively. Data are presented as mean ± SEM as a percent control (Control [3H]DA uptake values = 34.3 ± 1.53 pmol/min/mg; Control [3H]5-HT uptake values = 2.47 ± 0.15 pmol/min/mg).
Fig. 4.
Sulfonic acid esters inhibit specific [3H]DA (top panel) and [3H]5-HT (bottom panel) uptake into rat striatal and hippocampal synaptosomes, respectively. GBR 12909 and fluoxetine were used as a positive controls for [3H]DA and [3H]5-HT uptake assays, respectively . Nonspecific uptake was determined in the presence of nomifensine (10 μM) and fluoxetine (10 μM), respectively. Data are presented as mean ± SEM as a percent control (Control [3H]DA uptake values 25.6 ± 1.50; Control [3H]5-HT uptake values = 1.30 ± 0.10 pmol/min/mg).
With respect to selectivity for VMAT2, these results reveal that the carboxylic acid esters had diminished affinity for the two major nAChRs evaluated, whereas the affinity for VMAT2 was maintained. Thus, selectivity for VMAT2 increased for the carboxylic acid esters relative to lobeline. In contrast, for the sulfonic acid esters, affinities at both the nAChRs evaluated and at VMAT2 were not altered, and as such, selectivity for VMAT2 was not improved relative to lobeline. Thus, lobeline is 3-orders of magnitude more selective for α4β2* nAChRs over VMAT2, whereas one of the most interesting compounds, analog 4, had low affinity (Ki=19.3 μM) at α4β2 nAChRs and slightly improved affinity (Ki=2.98 μM) compared to lobeline at VMAT2, i.e 6.5-fold selectivity for VMAT2 over α4β2* nAChRs. Thus, esterification of the lobeline molecule with p-methoxybenzoic acid altered the relative interactions of lobeline at these neuronal proteins, which was not observed with the sulfonic acid esters of lobeline.
3. Summary
A series of carboxylic acid and sulfonic acid esters of lobeline, analogs 2–20, have been synthesized. Esterification of the 10-O-hydroxyl group of the lobeline molecule generally decreases affinity for α7* nAChRs compared to lobeline. The carboxylic acid ester analogs have also diminished affinity at α4β2* nAChRs compared to lobeline. Most of the analogs have similar potency to lobeline at VMAT2, and also have activity at DAT and SERT. However, there appears to be an interesting dichotomy between the carboxylic acid and sulfonic acid esters, in that only the latter series retained the high affinity for α4β2 nAChR observed with lobeline, and thus, the carboxylic esters exhibit enhanced selectivity at VMAT2 over α4β2 nAChRs. Thus, esterification may be a useful structural modification for the development of lobeline analogs that have improved selectivity at neuronal protein targets.
4. Experimental
Animals
Male Sprague-Dawley rats (200–250g upon arrival) were purchased from Harlan (Indianapolis, IN) and housed two per cage with ad libitum access to food and water in the Division of Laboratory Animal Resources at the College of Pharmacy at the University of Kentucky (Lexington, KY). Experimental protocols involving the animals were in accord with the 1996 NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Chemicals
Lobeline hemisulfate was obtained from Boehringer Ingleheim (Petersburg, VA). All other chemical reagents were obtained from either Aldrich Chemical Co. (Milwaukee, WI), Acros Organics (Somerville, NJ), or Lancaster Synthesis (Windham, NH), and were used without further purification. [3H]Nicotine (specific activity, 66.9 Ci/mmol), [3H]dopamine (DA; specific activity, 28.0 Ci/mmol), and [3H]5-hydroxytryptamine (5-HT; specific activity, 30.0 Ci/mmol) were purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). [3H]methyllcaconitine (MLA; specific activity, 100.0 Ci/mmol) and [3H]dihydrotetrabenazine (DTBZ; specific activity, 20.0 Ci/mmol) was obtained from American Radiolabled Chemicals, Inc. (St. Louis, MO). [3H]MTBZ (specific activity, 56.8 Ci/mmol) was a generous gift from Dr. Michael Kilbourn (Department of Radiology, University of Michigan Medical School, Ann Arbor, MI). Bovine serum albumin (BSA), catechol, cytisine, DA, Disodiumethylenediamine tetraacetate (EDTA), ethylene glycol tetraacetate (EGTA), fluoxetine HCl, 1-(2-(bis-(4-fluorophenyl)methoxy)ethyl)-4-(3-phenylpropyl)piperazine (GBR 12909), α–D-glucose, N-[2-hydroxyethyl] piperazine-N′-[2-ethanesulfonic acid] (HEPES), magnesium sulfate (MgSO4), S(−)-nicotine ditartrate (nicotine), nomifensine maleate, pargyline, polyethyleneimine (PEI), sucrose, tetrodotoxin (TTX), tris[hydroxymethyl]amino- methane hydrochloride (Trizma HCl), tris[hydroxymethyl]-aminomethane base (Trizma base), and L-(+) tartaric acid were purchased from Sigma-Aldrich (St. Louis, MO). L-ascorbic acid and sodium bicarbonate (NaHCO3) were obtained from Aldrich Chemical Co. (Milwaukee, WI). Calcium chloride (CaCl2), potassium chloride (KCl), potassium phosphate (K2PO4) and magnesium chloride (MgCl2), Sodium chloride (NaCl), and sodium phosphate (NaH2PO4), were purchased from Fisher Scientific Co. (Pittsburgh, PA). Tetrabenazine (TBZ) was a kind gift from Hoffman-LaRoche Inc. (Nutley NJ).
TLC analyses were carried out on glass plates precoated with silica gel 60 F254 from Analtech (Newark, DE), Melting points were determined on a Fisher-Johns melting point apparatus from Fisher Scientific (Pittsburgh, PA) and are uncorrected. 1H NMR and 13C NMR spectra were determined on a Varian (Palo Alto, CA) spectrometer (1H NMR at 300 MHz, 13C NMR at 75 MHz) in CDCl3 as solvent and utilizing tetramethylsilane (TMS) as an internal standard. High resolution electron impact ionization mass spectra (HRMS) and MALDI-TOF MS Mass spectra were recorded at 25eV on a JEOL JMS-700T MStation (Peabody, MA) at a resolution of greater than 10000, or on a Bruker Autoflex MALDI-TOF MS (Billerica, MA). Elemental analyses were performed by Atlantic Microlab, Inc. (Norcross, Georgia) on a COSTECH elemental combustion system and are within ± 0.4% of theoretical values.
(2S, 6S, 2R)-2-[6-(2-Benzoyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LBZ) (2)
Lobeline sulfate (398 mg) was dissolved in water, converted into the free base by addition of an excess of aqueous sodium bicarbonate solution, and the free base extracted with dichloromethane (20 ml). The combined organic layers were dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure to afford lobeline free base as a white powder. To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry tetrahydrofuran (THF, 3 ml) at 0 °C was added drop-wise benzoyl chloride (155 mg, 1.10 mmol) in dry THF (1ml). The mixture was stirred at 0 °C for 2 h under nitrogen, then poured into an ice-cold water (10 ml), and the mixture extracted with chloroform (2 x 10 ml). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude product was recrystallized from ethyl acetate to afford 2 (430 mg, 90%) as a white solid: mp 156–158 °C; 1H NMR (300 MHz, CDCl3) δ 1.50–1.95 (m, 6H), 2.05–2.30 (m, 1H), 2.68 (s, 3H, N-Me), 2.9–3.20 (m, 3H), 3.42–4.16 (m, 2H), 6.05 (dd, 1H, J = 3.6 Hz, J = 9.9 Hz), 7.31–7.58 (m, 11H), 7.95–8.13 (m, 4H) ppm; 13C NMR (75 MHz, CDCl3) δ 22.7, 37.6, 39.0, 39.6, 41.2, 55.1, 60.9, 72.8, 125.9, 126.1, 127.7, 128.1, 128.3, 128.4, 128.5, 128.7, 129.3, 129.6, 133.2, 133.6, 135.6, 138.1, 138.9, 165.9, 195.7 ppm; MS (MALDI) m/z 442 (M+1), 100), 322, 216. Anal. Calcd. for C29H32ClNO3: C, 72.86; H, 6.75; N, 2.93%. Found: C, 72.58; H, 6.69; N, 2.87%.
(2S, 6S, 2R)-2-[6-(2-Toluyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LTO) (3)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise p-toluyl chloride (173 mg, 1.12 mmol) in dry THF (1 ml). The mixture was stirred at 0 °C for 1 h under nitrogen, and worked up utilizing the same procedure as described above for the preparation of compound 2. The crude product was recrystallized from diethyl ether to afford 3 (393 mg, 80%) as a white solid: mp 99–100 °C; 1H NMR (300 MHz, CDCl3). δ 1.55–2.40 (m, 8H), 2.41 (s, 3H, p-Me-Ph), 2.71 (s, 3H, N-Me), 3.0–3.60 (m, 2H), 3.7–4.3 (m, 2H), 6.01 (dd, 1H, J = 2.1 Hz, J = 9.9 Hz), 7.26–7.59 (m, 10H), 7.95–8.01 (m, 4H) ppm; 13C NMR (75 MHz, CDCl3) δ 20.7, 25.6, 32.0, 33.6, 37.0, 39.6, 41.2, 56.1, 60.8, 73.8, 125.7, 127.5, 128.3, 128.4, 128.6, 129.1, 129.6, 132.9, 137.4, 139.4, 142.0, 166.9, 196.6 ppm; MS (ESI) m/z 456 (M+1, 100), 453 (28), 336(16), 320 (22), 216 (23); HRMS (M+) calcd. for C30H33NO3: 455.2455, found 455.2458.
(2S, 6S, 2R)-2-[6-(2-Anisoyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LAN) (4)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise p-anisoyl chloride (188 mg, 1.10 mmol) in dry THF (1 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and worked up utilizing the same procedure as described above for the preparation of compound 2. The crude product was recrystallized from acetone to afford 4 (448 mg, 88%) as a white solid: mp 108–109 °C; 1H NMR (300 MHz, CDCl3) δ 1.55–2.40 (m, 8H), 2.71 (s, 3H, N-Me), 3.80 (s, 3H, p-MeO-Ph), 3.0–3.60 (m, 2H), 3.7–4.3 (m, 2H), 6.01 (dd, 1H, J = 2.1 Hz, J = 9.9 Hz), 7.26–7.59 (m, 10H), 7.95–8.01 (m, 4H) ppm; 13C NMR (75 MHz, CDCl3) δ 20.7, 25.6, 32.0, 33.6, 37.0, 39.6, 41.2, 56.1, 60.8, 73.8, 125.7, 127.5, 128.3, 128.4, 128.6, 129.1, 129.6, 132.9, 137.4, 139.4, 142.0, 166.9, 196.6 δ; MS (ESI) m/z 472 (M δ, 100), 352 (18), 320 (22), 216 (23); HRMS (M+) calcd. for C30H33NO4: 471.2404, found 471.2414.
(2S, 6S, 2R)-2-[6-(2-Acetyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LAC) (5)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise acetyl chloride (89 mg, 1.12 mmol) in dry THF (1 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and worked up utilizing the same procedure as described above for the preparation of compound 2. The crude product was recrystallized from ethyl acetate to afford 5 (387 mg, 93%) as a white solid: mp 76–77 °C; 1H NMR (300 MHz, CDCl3) δ 1.50–2.0 (m, 7H), 2.05 (s, 3H, O=C-CH3), 2.20–2.40 (m, 1H), 2.66 (s, 3H, N-Me), 2.80–3.60 (m, 2H), 3.72–4.35 (m, 2H), 5.75 (d, 1H, J = 3.6 Hz), 7.31–7.60 (m, 8H), 7.95–8.05 (m, 2H) ppm; 13C NMR (75 MHz, CDCl3) δ 17.6, 22.0, 37.6, 39.0, 39.6, 41.2, 55.1, 60.9, 72.8, 125.9, 126.8, 127.6, 128.3, 128.6, 133.1, 137.6, 139.6, 171.6, 196.7 ppm; MS (MALDI) m/z 381 (M+1, 100), 318, 260, 218. Anal. Calcd. for C24H30ClNO3.0.33 H2O: C, 68.32; H, 7.33; N, 3.32%. Found: C, 68.32; H, 7.35; N, 3.27%.
(2S, 6S, 2R)-2-[6-(2-Propionyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LPR) (6)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise propionyl chloride (106 mg, 1.12 mmol) in dry THF (1 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and worked up utilizing the same procedure as described above for the preparation of compound 2. The crude product was recrystallized from diethyl ether to afford 6 (413 mg, 96%) as a white solid: mp 45–46 °C; 1H NMR (300 MHz, CDCl3) δ 1.1 (t, 3H), 1.50–2.43 (m, 9H), 2.20–2.40 (m, 1H), 2.65 (s, 3H, N-Me), 3.1–3.5 (m, 2H), 3.80–4.45 (m, 2H), 5.75 (dd, 1H, J = 3.6 Hz, J = 9.6 Hz ), 7.32–7.63 (m, 8H), 7.96–8.01 (m, 2H) ppm; 13C NMR (75 MHz, CDCl3) δ 10.6, 26.7, 31.6, 32.3, 38.5, 39.0, 39.6, 41.2, 57.1, 60.6, 73.8, 126.3, 126.5, 127.6, 128.3, 128.4, 132.6, 138.5, 140.6, 172.8, 195.9 ppm; MS (ESI) m/z 394 (M+1, 100), 320 (20), 274 (32), 216 (36). HRMS (M+) calcd. for C25H31NO3: 393.2299, found 393.2297.
(2S, 6S, 2R)-2-[6-(2-Butyryloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LBU) (7)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise butyryl chloride (118 mg, 1.10 mmol) in dry THF (1 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and worked up utilizing the same procedure as described above for the preparation of compound 2. The crude product was recrystallized from diethyl ether to afford 7 (400 mg, 90%) as a white solid: mp 66–67 °C; 1H NMR (300 MHz, CDCl3) δ 0.90 (t, 3H), 1.50–2.00 (m, 9H), 2.20–2.40 (m, 3H), 2.62 (s, 3H, N-Me), 3.10–3.40 (m, 2H), 3.75–4.20 (m, 2H), 5.76 (m, 1H), 7.28–7.60 (m, 8H), 7.90–8.05 (m, 2H) ppm; 13C NMR (75 MHz, CDCl3) δ 13.6, 18.3, 26.7, 32.0, 33.3, 37.5, 38.6, 39.6, 40.6, 58.1, 61.6, 75.8, 125.3, 126.5, 127.6, 128.3, 128.4, 132.6, 138.5, 140.6, 172.6, 196.6 δ; MS (MALDI) m/z 408 (M+1, 100), 350 (2), 338 (3), 318 (4), 288 (6). Anal. Calcd. for C26H34ClNO3.0.51H2O: C, 68.90; H, 7.83; N, 3.09%. Found: C, 68.92; H, 7.86; N, 3.06%.
(2S, 6S, 2R)-2-[6-(2-Isobutyryloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LIBU) (8)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise isobutyryl chloride (118 mg, 1.10 mmol) in dry THF (1 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and worked up utilizing the same procedure as described above for the preparation of compound 2. The crude product was recrystallized from diethyl ether to afford 8 (382 mg, 86%) as a white solid: mp 71–72 °C; 1H NMR (300 MHz, CDCl3) δ 1.15 (d, 6H, J = 7.2 Hz), 1.19–1.22 (m, 1H), 1.46–2.50 (m, 7H), 2.52–2.65 (m, 1H), 2.68 (s, 3H, N-Me), 2.82–4.36 (m, 4H), 5.72 (m, 1H), 7.33–7.62 (m, 8H), 7.93–8.02 (m, 2H) ppm; 13C NMR (75 MHz, CDCl3) δ 17.6, 26.7, 32.0, 33.3, 36.5, 37.5, 39.6, 40.6, 59.1, 60.5, 74.8, 125.3, 126.5, 127.6, 128.3, 128.4, 132.6, 138.5, 140.6, 173.6, 196.8 ppm; MS (MALDI) m/z 408 (M+1, 100), 338 (18), 318 (3), 288 (8). Anal. Calcd. for C26H34ClNO3.0.33H2O: C, 69.40; H, 7.76; N, 3.11%. Found: C, 69.42; H, 7.85; N, 3.07%.
(2S, 6S, 2R)-2-[6-(2-(2-Thiophen)-carboxyl-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LTH) (9)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise thiophene 2-carbonyl chloride (158 mg, 1.08 mmol) in dry THF (1 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and worked up utilizing the same procedure as described above for the preparation of compound 2. The crude product was recrystallized from diethyl ether to afford 9 (436 mg, 90%) as a white solid: mp 116–117 °C; 1H NMR (300 MHz, CDCl3) δ 1.50–2.25 (m, 8H), 2.70 (s, 3H, N-Me), 2.9–4.25 (m, 4H), 5.98 (dd, 1H, J = 3.3 Hz, J = 10.5 Hz ), 7.12–7.20 (m, 2H), 7.31–7.75 (m,10H), 7.90–8.05 (m, 2H) ppm; 13C NMR (75 MHz, CDCl3) δ 26.0, 32.6, 33.5, 37.6, 39.6, 40.2, 59.1, 60.3, 74.8, 125.8, 126.5, 127.6, 128.1, 128.3, 128.6, 132.9, 133.2, 133.8., 134.5, 137.6, 139.9, 141.6, 162.1, 196.8 ppm; MS (ESI) m/z 448 (M+1, 100), 328 (28), 320 (22), 216 (23). HRMS (M+) calcd for C27H29NO3S: 447.1863, found 447.1862.
(2S, 6S, 2R)-2-[6-(2-Benzenesulfonyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LBS) (10)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise benzene sulfonyl chloride (188 mg, 1.06 mmol) in dry THF (1 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and evaporated to dryness under reduced pressure. The residue obtained was washed with diethyl ether and recrystallized from acetone to afford 10 (462 mg, 90%) as a white solid: mp 136–138 °C; 1H NMR (300 MHz, CDCl3) δ 1.7–2.0 (m, 7H), 2.3–2.4 (m, 1H), 2.75 (s, 3H), 3.0–3.2 (m, 1H), 4.88 (d, 1H, J = 9.6 Hz), 3.9–4.1 (m, 3H), 7.22–7.50 (m, 10H), 7.58–8.01 (m, 5H) ppm; 13C NMR (75 MHz, CDCl3) δ 22.7, 23.8, 24.22, 27.61, 40.7, 41.3, 61.1, 63.9, 71.1, 126.5, 126.7, 127.4, 127.6, 128.0, 128.5, 128.6, 128.9, 133.9, 135.9, 144.1, 146.9, 195.4 ppm; MS (ESI) m/z 478 (M+1, 100); HRMS (M+) calcd. for C28H31NO4S: 477.2055, found 477.2058.
(2S, 6S, 2R)-2-[6-(2-p-Toluenesulfonyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LTS) (11)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise p-toluene sulfonyl chloride (225 mg, 1.18 mmol) in dry THF (1 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and evaporated to dryness under reduced pressure. The residue obtained was washed with diethyl ether and recrystallized from ether/acetone to afford 11 (459 mg, 87%) as a white powder: mp 154–155 °C; 1H NMR (300 MHz, CDCl3) δ 1.46–2.16 (m, 8H), 2.25 (s, 3H), 2.73 (s, 3H, N-Me), 2.95–3.04 (m, 1H), 3.75–4.15 (m, 3H), 4.89 (d, 1H, J = 8.7 Hz), 6.98 (d, 2H, J = 7.8 Hz), 7.22–7.65 (m, 10H), 7.90 (d, 2H, J = 7.5 Hz) ppm; 13C NMR (75 MHz, CDCl3) δ 21.5, 22.7, 23.7, 27.7, 31.2, 40.6, 41.7, 61.1, 63.9, 71.1, 125.6, 125.8, 127.5, 128.6, 128.8, 129.0, 133.8, 135.9, 140.0, 142.1, 143.9, 144.3, 195.0 ppm; MS (MALDI) m/z 492 (M+1), 356, 338, 216; HRMS (M+) calcd. for C29H33NO4S: 491.2186, found 491.2190.
(2S,6S, 2R)-2-[6-(2-p-Chloro-benzenesulfonyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LCBS) (12)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise p-chlorobenzene sulfonyl chloride (228 mg, 1.08 mmol) in dry THF (2 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and evaporated to dryness under reduced pressure. The residue obtained was washed with diethyl ether and recrystallized from acetone to afford 12 (438 mg, 80%) as a white powder: mp 160–161 °C; 1H NMR (300 MHz, CDCl3) δ 1.50–2.00 (m, 7H), 2.20–2.40 (m, 1H), 2.79 (s, 3H, N-Me), 2.98–3.10 (m, 1H), 3.80–4.20 (m, 3H), 4.92 (d, 1H, J = 8.8 Hz), 7.13 (d, 2H, J = 8.7 Hz), 7.23–7.68 (m, 10H), 7.92 (d, 2H, J = 7.5 Hz) ppm; 13C NMR (75 MHz, CDCl3) δ 22.7, 23.9, 24.3, 27.7, 40.6, 41.3, 61.1, 64.3, 71.4, 125.6, 127.5, 127.6, 128.3, 128.6, 128.9, 134.0, 135.8, 135.9, 143.3, 144.3, 195.0 ppm; MS (ESI) m/z 512 (M+1); HRMS (M+) calcd. for C28H30ClNO4S: 511.1584, found 511.1589.
2S, 6S, 2R)-2-[6-(2-p-Fluoro-benzenesulfonyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LFBS) (13)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise p-fluorobenzene sulfonyl chloride (210 mg, 1.08 mmol) in dry THF (2 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and evaporated to dryness under reduced pressure. The residue obtained was washed with ethyl acetate and recrystallized from acetone to afford 13 (458 mg, 86%) as a white powder: mp 180–182 °C; 1H NMR (300 MHz, CDCl3) δ 1.40–2.10 (m, 7H), 2.20–2.40 (m, 1H), 2.78 (s, 3H, N-Me), 2.95–3.10 (m, 1H), 3.85–4.25 (m, 3H), 5.00 (d, 1H, J = 8.8 Hz), 6.8 (m, 2H), 7.25–7.80 (m, 10H), 7.90 (d, 2H, J = 7.5 Hz) ppm; 13C NMR (75 MHz, CDCl3) δ 22.7, 24.0, 24.3, 27.7, 40.6, 41.3, 61.1, 64.3, 71.4, 114.9, 115.2, 125.6, 127.8, 128.3, 128.6, 128.9, 134.0, 135.8, 140.8, 161.8, 165.1, 194.9 ppm. MS (ESI) m/z 458 (M+1); HRMS (M+) calcd. for C28H30FNO4S: 495.1880, found 495.1886.
(2S, 6S, 2R)-2-[6-(2-p-Nitro-benzenesulfonyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LNBS) (14)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise p-nitrobenzene sulfonyl chloride (236 mg, 1.04 mmol) in dry THF (2 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and evaporated to dryness under reduced pressure. The residue obtained was washed with diethyl ether and recrystallized from acetone to afford 14 (436 mg, 78%) as a white powder: mp 178–179 °C; 1H NMR (300 MHz, CDCl3) δ 1.60–2.00 (m, 7H), 2.10–2.30 (m, 1H), 2.81 (s, 3H, N-Me), 3.05–3.10 (m, 1H), 3.70–4.25 (m, 3H), 5.10 (d, 1H, J = 8.6 Hz), 7.25–7.60 (m, 10H), 7.82–7.99 (m, 4H) ppm; 13C NMR (75 MHz, CDCl3) δ 22.7, 24.0, 24.2, 27.7, 40.6, 41.2, 61.1, 64.3, 71.4, 125.6, 127.5, 127.6, 128.3, 128.5, 128.6, 128.9, 134.0, 135.8, 140.8, 195.1 ppm; MS (ESI) m/z 523 (M+1); HRMS (M+) calcd. for C28H30N2O6S: 522.1825, found 522.1828.
(2S, 6S,2R)-2-[6-(2-(2-Thiophene)-sulfonyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LTHS) (15)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise 2-thiophene sulfonyl chloride (196 mg, 1.03 mmol) in dry THF (2 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and evaporated to dryness under reduced pressure. The residue obtained was washed with diethyl ether and recrystallized from ether/acetone to afford 15 (458 mg, 88%) as a white powder: mp 166–167 °C; 1H NMR (300 MHz, CDCl3) δ 1.50–2.00 (m, 7H), 2.26–2.37 (m, 1H), 2.74 (s, 3H, N-Me), 3.00–3.15 (m, 1H), 3.90–4.20 (m, 3H), 4.87 (d, 1H, J = 10.8 Hz), 6.78–7.60 (m, 11H), 7.96 (d, 2H, J = 8.7 Hz) ppm; 13C NMR (75 MHz, CDCl3) δ 22.7, 23.8, 24.2, 27.6, 40.6, 41.4, 61.1, 63.9, 71.1, 125.6, 126.5, 127.4, 127.6, 128.0, 128.5, 128.6, 128.8, 133.9, 135.9, 144.17, 146.9, 194.8 ppm; MS (ESI) m/z 484 (M+1); HRMS (M+) calcd. for C26H29NO4S2: 483.1538, found 483.1536.
(2S, 6S,2R)-2-[6-(2-(2-Naphthalene)-sulfonyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LINPS) (16)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise 2-naphthalene sulfonyl chloride (236 mg, 1.03 mmol) in dry THF (2 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and evaporated to dryness under reduced pressure. The residue obtained was washed with ethyl acetate and recrystallized from acetone to afford 16 (508 mg, 90%) as a white powder: mp 172–173 °C; 1H NMR (300 MHz, CDCl3) δ 1.53–2.00 (m, 7H), 2.30–2.40 (m, 1H), 2.75 (s, 3H, N-Me), 2.95–3.10 (m, 1H), 3.80–4.20 (m, 3H), 4.89 (d, 1H, J = 10.2 Hz), 7.22–8.28 (m, 17H) ppm; 13C NMR (75 MHz, CDCl3) δ 22.6, 24.0, 24.3, 27.7, 40.6, 41.3, 61.1, 64.3, 71.4, 122.8, 124.6, 125.7, 126.9, 127.3, 127.7, 128.2, 128.4, 128.6, 128.8, 132.5, 132.8, 133.9, 137.6, 143.3, 195.1 ppm; MS (ESI) m/z 528 (M+1); HRMS (M+) calcd. for C32H33NO4S: 527.2130, found 527.2136.
(2S, 6S,2R)-2-[6-(2-Isopropylsulfonyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LIPS) (17)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise isopropyl sulfonyl chloride (154 mg, 1.08 mmol) in dry THF (1 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and evaporated to dryness under reduced pressure. The residue obtained was washed with ethyl acetate and recrystallized from acetone to afford 17 (346 mg, 72%) as a white powder: mp 136–138 °C; 1H NMR (300 MHz, CDCl3) δ 1.10 (d, 6H), 1.45–2.00 (m, 8H), 2.25–2.40 (m, 1H), 2.78 (s, 3H, N-Me), 2.98–3.15 (m, 1H), 3.85–4.25 (m, 3H), 4.86 (d, 1H, J = 10.2 Hz), 7.22–8.28 (m, 17H) ppm; 13C NMR (75 MHz, CDCl3) δ 15.2, 21.2, 22.5, 24.0, 25.0, 27.5, 40.6, 44.0, 59.6, 64.6, 73.4, 125.6, 127.0, 128.2, 128.6, 128.9, 133.6, 137.1, 145.5, 198.3 ppm; MS (ESI) m/z 444 (M+1); HRMS (M+) calcd. for C25H33NO4S: 443.2130, found 443.2135.
(2S, 6S,2R)-2-[6-(2-Butylsulfonyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LBUS) (18)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise n-butyl sulfonyl chloride (160 mg, 1.02 mmol) in dry THF (1 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and evaporated to dryness under reduced pressure. The residue obtained was washed with diethyl ether and recrystallized from acetone to afford 18 (405 mg, 82%) as a white powder: mp 138–139 °C; 1H NMR (300 MHz, CDCl3) δ 0.73 (t, 3H), 1.22–1.30 (m, 2H), 1.50–2.38 (m, 10H), 2.72–2.78 (m, 2H), 2.76 (s, 3H, N-Me), 3.00–3.15 (m, 1H), 3.90–4.25 (m, 3H), 4.87 (d, 1H, J = 10.5 Hz), 7.20–7.60 (m, 8H), 7.99 (d, 1H, J = 7.2 Hz) ppm; 13C NMR (75 MHz, CDCl3) δ 13.9, 22.1, 22.7, 23.9, 24.1, 27.2, 27.6, 40.6, 41.6, 51.6, 61.0, 63.7, 71.16, 125.6, 127.5, 128.4, 128.5, 128.8, 133.9, 136.0, 144.5, 196.2 ppm; MS (ESI) m/z 458 (M+1); HRMS (M+) calcd. for C26H35NO4S: 457.2287, found 457.2290.
(2S, 6S,2R)-2-[6-(2-Benzylsulfonyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LBNS) (19)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise benzyl sulfonyl chloride (202 mg, 1.06 mmol) in dry THF (1 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and evaporated to dryness under reduced pressure. The residue obtained was washed with diethyl ether and recrystallized from ether/acetone to afford 19 (475 mg, 90%) as a white powder: mp 128–129 °C; 1H NMR (300 MHz, CDCl3) δ 1.40–2.35 (m, 8H), 2.56 (s, 3H, N-Me), 2.90–3.10 (m, 1H), 3.51–3.95 (m, 3H), 4.03 (s, 2H), 4.66 (d, 1H, J = 10.2 Hz), 7.07–7.09 (m, 3H), 7.28–7.35 (m, 7H), 7.42–7.62 (m, 3H), 7.97 (d, 2H, J = 7.5 Hz) ppm; 13C NMR (75 MHz, CDCl3) δ 23.0, 24.0, 25.6, 27.2, 40.3, 40.8, 61.0, 62.5, 68.5, 74.6, 125.5, 125.7, 128.3, 128.4, 128.6, 129.2, 137.4, 139.4, 142.6, 193.8 ppm; MS (LC/MS) m/z 492 (M+1); HRMS (M+) calcd. for C29H33NO4S: 491.2130, found 491.2135.
(2S, 6S,2R)-2-[6-(2-(1-Naphthalene)-sulfonyloxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone hydrochloride (cis-LNPS) (20)
To a stirred solution of lobeline free base 1 (338 mg, 1.0 mmol) in dry THF (3 ml) at 0 °C was added drop-wise 1-naphthalene sulfonyl chloride (236 mg, 1.03 mmol) in dry THF (2 ml). The mixture was stirred at 0 °C for 2 h under nitrogen, and evaporated to dryness under reduced pressure. The residue obtained was washed with ethyl acetate and recrystallized from acetone to afford 20 (497 mg, 88%) as a white powder: mp 170–172 °C; 1H NMR (300 MHz, CDCl3) δ 1.53–2.00 (m, 7H), 2.30–2.40 (m, 1H), 2.75 (s, 3H, N-Me), 2.95–3.10 (m, 1H), 3.80–4.20 (m, 3H), 4.89 (d, 1H, J = 10.2 Hz), 7.22–8.28 (m, 17H) ppm; 13C NMR (75 MHz, CDCl3) δ 22.6, 24.0, 24.3, 27.7, 40.6, 41.3, 61.1, 64.3, 71.4, 122.8, 124.6, 125.7, 126.9, 127.3, 127.7, 128.2, 128.4, 128.6, 128.8, 132.5, 132.8, 133.9, 137.6, 143.3, 195.1 ppm; MS (ESI) m/z 528 (M+1); HRMS (M+) calcd. for C32H33NO4S: 527.2130, found 527.2136.
[3H]Nicotine and [3H]MLA binding assays
Whole brain excluding cortex and cerebellum was homogenized using a Tekmar polytron (Tekmar-Dohrmann, Mason, OH) in 20 vol of ice-cold modified Krebs-HEPES buffer, containing: 2 mM HEPES, 14.4 mM NaCl, 0.15 mM KCl, 0.2 mM CaCl2·2H2O and 0.1 mM MgSO4·7H2O, pH adjusted to 7.5. Homogenates were centrifuged at 31,000g for 17 min at 4 °C (Avanti J-301 centrifuge, Beckman Coulter, Fullerton, CA). Pellets were resuspended by sonication (Vibra Cell, Sonics& Materials Inc, Danbury, CT) in 20 vol of the Krebs-HEPES buffer and incubated at 37 °C for 10 min (Reciprocal Shaking Bath Model 50, Precision Scientific, Chicago, IL). Suspensions were centrifuged again using the above conditions. Resulting pellets were resuspended by sonication in 20 vol buffer and centrifuged at 31,000g for 17 min. Final pellets were stored in incubation buffer, containing: 20 mM HEPES, 144 mM NaCl, 1.5 mM KCl, 2.0 CaCl2·2H2O, and 1.0 MgSO4·7H2O, pH 7.5. Membrane suspensions (100–140 μg membrane protein/100 μL) were added to assay tubes containing analog (7–9 concentrations, 1 nM – 1 mM) and 3 nM [3H]nicotine or [3H]MLA for a final assay volume of 250 μL. Samples were incubated for 60 min at room temperature (22 ± 1 °C). Reactions were terminated by harvesting samples on a Unifilter-96 GF/B filter plate presoaked in 0.5% PEI using a Packard Filter Mate Harvester. Samples were washed 5 times with 350 μL of ice-cold buffer. Filter plates were dried for 60 min at 45 °C, bottom-sealed and each well filled with 40 μL Packard’s Microscint 20 cocktail. Bound radioactivity was determined via liquid scintillation spectroscopy using a Packard Windows NT based operating system. Nonspecific binding was determined in the presence of 10 μM cytisine and 10 μM nicotine for the [3H]nicotine and [3H]MLA assays, respectively. Specific [3H]nicotine and [3H]MLA binding were determined by subtracting nonspecific binding from total binding. Concentrations of inhibitor that produced 50% inhibition of specific binding (IC50 values) were determined from the concentration effect curves via an iterative curve-fitting program (Prism 3.0; GraphPad Software Inc., San Diego, CA). Inhibition constants (Ki values) were determined using the Cheng-Prusoff equation.33
86Rb+ efflux Assay
The ability of the representative analogs to evoke 86Rb+ efflux was determined using a previously published method.32 Thalamus preparations were homogenized and centrifuged at 1000×g for 10 min at 4°C. Supernatants were centrifuged at 12,000×g for 20 min at 4°C. Synaptosomes were incubated for 30 min in 35 μl of uptake buffer (140 μM NaCl, 1.5 μM KCl, 2.0 μM CaCl2, 1.0 μM MgSO4, 20 μM D-glucose; pH 7.5 containing 4 μCi of 86Rb+. 86Rb+ uptake was terminated by filtration of the synaptosomes onto glass fiber filters (6 mm; Type A/E, Gelman Sciences, Ann Arbor, MI) under gentle vacuum (0.2 atm), followed by three washes with superfusion buffer (0.5 ml each). Subsequently, each filter with 86Rb+-loaded synaptosomes (~40 μg of protein/25 μl) was placed on a 13 mm glass fiber filter (Type A/E) mounted on a polypropylene platform. Synaptosomes were perfused with 86Rb+ efflux assay buffer (125 μM NaCl, 5 μM CsCl, 1.5 μM KCl; 2 μM CaCl2, 1 μM MgSO4, 25 μM HEPES, 20 μM α-D-glucose, 0.1 μM TTX, 1.0 g/l bovine serum albumin; pH 7.5) at a rate of 2.5 ml/min. TTX and CsCl were included in the buffer to block voltage-gated Na+ and K+ channels, respectively, and to reduce the rate of basal 86Rb+ efflux.32 Experiments determined the ability of analog (1 nM–10 μM) to inhibit 86Rb+ efflux evoked by 1 μM nicotine. The concentration of nicotine was chosen based on the results of the previous experiment.32 After 8 min of perfusion, basal samples were collected for 5 min. Subsequently, synaptosomes were perfused for 3 min with one of five concentrations (1 nM–10 μM) of analog in the absence and presence of nicotine (1 μM), followed by superfusion for 3 min. Each aliquot part of thalamic synaptosomes was exposed to only one concentration of analog. In each experiment, one synaptosomal aliquot part was also exposed to nicotine (1 μM) in the absence of analog. In each experiment, one synaptosomal aliquot part was superfused in the absence analog or nicotine to determine basal 86Rb+ efflux during the entire course of the experiment. Samples were analyzed by liquid scintillation spectrometry (Packard model B1600 TR Scintillation Counter).
Inhibition of [3H]DA and [3H]5-HT Uptake into Rat Striatal and Hippocampal Synaptosomes, Respectively
Lobeline- and analog-induced inhibition of [3H]DA and [3H]5-HT uptake into rat striatal and hippocampal synaptosomes, respectively, was assessed using modifications of a previously described method.10 Analog-induced inhibition was compared with that induced by the selective DAT and SERT transporter inhibitors, GBR-12909 and fluoxetine, respectively.29,30 Brain regions were homogenized in 20 ml of ice-cold 0.32 M sucrose solution containing 5 mM NaHCO3 (pH 7.4) with 16 up-and-down strokes of a Teflon pestle homogenizer (clearance = 0.003). Homogenates were centrifuged at 2,000g for 10 min at 4°C and resulting supernatants centrifuged at 20,000g for 17 min at 4°C. Pellets were resuspended in 1.5 ml of Krebs buffer (125 μM NaCl, 5 μM KCl, 1.5 μM MgSO4, 1.25 μM CaCl2, 1.5 μM KH2PO4, 10 μM α-D-glucose, 25 μM HEPES, 0.1 μM EDTA, 0.1 μM pargyline, and 0.1 μM ascorbic acid saturated with 95% O2 /5% CO2, pH 7.4. Final protein concentrations were 400 μg/ml and were determined by protein-dye binding.34 Assays were performed in duplicate in a total volume of 500 μl. Aliquot parts of synaptosomal suspension (50 μl) were added to tubes containing 350 μl of Krebs buffer and 50 μl of buffer containing final concentrations of 1 nM to 1 μM drug, or 50 μl of buffer without drug. Tubes were incubated at 34°C for 10 min before the addition of 50 μl of [3H]DA (final concentration, 10 nM) or 50 μl of [3H]5-HT (final concentration, 10 nM). Accumulation proceeded for 10 min at 34°C. Reactions were terminated by the addition of 3 ml of ice-cold Krebs buffer. Nonspecific [3H]DA and [3H]5-HT uptake was determined in the presence of 10 μM nomifensine and 10 μM fluoxetine, respectively. Samples were rapidly filtered through a Whatman GF/B filter using a cell harvester (MP-43RS; Brandel Inc.), and filters were subsequently washed three times with 4 ml of ice-cold Krebs buffer containing catechol (1 μM). Radioactivity retained by the filters was determined by liquid scintillation spectrometry (B1600 TR scintillation counter; PerkinElmer Life and Analytical Sciences). Specific [3H]DA and [3H]5-HT uptake were determined by subtracting nonspecific uptake from total uptake. Concentrations of inhibitor that produced 50% inhibition specific binding (IC50 values) were determined from the concentration effect curves via an iterative curve-fitting program (Prism 3.0; GraphPad Software Inc., San Diego, CA). Inhibition constants (Ki values) were determined using the Cheng-Prusoff equation.33
Inhibition of [3H]MTBZ or [3H]DTBZ Binding to Vesicles Prepared from Rat Whole Brain
Analog-induced inhibition of [3H]MTBZ or [3H]DTBZ binding was determined using modifications of a previously described method.16 Nonspecific binding was determined in the presence of 20 μM tetrabenazine. Rat whole brain (excluding cerebellum) was homogenized in 20 ml of ice-cold 0.32 M sucrose solution with seven up-and-down strokes of a Teflon pestle homogenizer (clearance = 0.003). Homogenates were centrifuged at 1,000g for 12 min at 4°C and resulting supernatants were centrifuged at 22,000g for 10 min at 4°C. Resulting pellets were incubated in 18 ml of cold water for 5 min, and 2 ml of 25 mM HEPES and 100 mM potassium-tartrate solution was subsequently added. Samples were centrifuged (20,000g for 20 min at 4°C), and 1 mM MgSO4 solution was then added to the supernatants. Solutions were centrifuged at 100,000g for 45 min at 4°C and resuspended in cold assay buffer (25 μM HEPES, 100 mM potassium-tartrate, 5 mM MgSO4, 0.1 μM EDTA, and 0.05 mM EGTA, pH 7.5. The final protein concentration was 15 μg of protein/100 μl.34 Assays were performed in duplicate in 96-well plates. Aliquot parts of vesicular suspension (100 μl) were added to wells containing 50 μl of [3H]MTBZ or [3H]DTBZ (final concentration, 3 nM), 50 μl of lobeline or analog, and 50 μl of buffer. Nonspecific uptake was determined in the presence of 50 μl of 20 μM tetrabenazine. Reactions were terminated by filtration (Filtermate harvester; PerkinElmer Life and Analytical Sciences) onto Unifilter-96 GF/B filter plates (presoaked in 0.5% polyethylenimine). Filters were subsequently washed five times with 350 μl of ice-cold buffer (25 μM HEPES, 100 μM K2-tartrate, 5 μM MgSO4, and 10 μM NaCl, pH 7.5). Filter plates were dried and bottom-sealed, and each well was filled with 40 μl of scintillation cocktail (MicroScint 20; PerkinElmer Life and Analytical Sciences). Radioactivity in filters was determined by liquid scintillation spectrometry (TopCount NXT scintillation counter; PerkinElmer Life and Analytical Sciences).
Acknowledgments
This research was supported by grants from the National Institute of Health (R01DA13519, T32DA16716, T32DA 07304, F32DA06043). The authors also thank Dr. Jack Goodman of the Mass Spectrometry Facility, University of Kentucky for performing the mass spectra analyses. For purposes of full disclosure, the University of Kentucky holds patent on lobeline which have been licensed by Yaupon Therapeutics Inc. (Lexington, KY). A potential royalty stream to L.P.D. and P.A.C. may occur consistent with University of Kentucky policy. Both L.P.D. and P.A.C. are founders of, and have financial interest in Yaupon Therapeutics, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Institute on Drug Abuse (NIDA) Research Report Series. 2006 Sep; http://www.drugabuse.gov.
- 2.United Nations Office on Drugs and Crimes (UNODC) 2007 World Drug Report. http://www.unodc.org.
- 3.Millspaugh CF. American medicinal plants: an illustrated and descriptive guide to plants indigenous to and naturalized in the United States which are used in medicine. Dover; New York: 1974. Lobelia inflata; p. 385. [Google Scholar]
- 4.(a) Nunn-Thompson CL, Simon PA. Clin Pharm. 1989;8:710. [PubMed] [Google Scholar]; (b) Thompson MA. US 2006204598 US Pat Appl Publ. 2006:6.; (c) Kalyuzhnyy VV. J Neural Psychiatry. 1968;68:1864. [Google Scholar]; (d) Davison GC, Rosen RC. Psychol Rep. 1972;31:443. doi: 10.2466/pr0.1972.31.2.443. [DOI] [PubMed] [Google Scholar]
- 5.Dwoskin LP, Crooks PA. Biochem Pharmacol. 2002;63:89. doi: 10.1016/s0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- 6.Eyerman DJ, Yamamoto BK. J Pharmacol Exp Ther. 2005;312:160. doi: 10.1124/jpet.104.072264. [DOI] [PubMed] [Google Scholar]
- 7.Miller DK. New Research on Methamphetamine Abuse. Nova Science Publishers; Hauppauge, NY: 2006. [Google Scholar]
- 8.Tatsuta T, Kitanaka N, Kitanaka J, Morita Y, Takemura M. Neurochem Res. 2006;31:1359. doi: 10.1007/s11064-006-9180-1. [DOI] [PubMed] [Google Scholar]
- 9.National Institute on Drug Abuse (NIDA) Clinical Trials. http://clinicaltrials.gov/ct2/show/NCT00439504?term=lobeline&rank=7.
- 10.Miller DK, Crooks PA, Zheng G, Grinevich VP, Norrholm S, Dwoskin LP. J Pharmacol Exp Ther. 2004;310:1035. doi: 10.1124/jpet.104.068098. [DOI] [PubMed] [Google Scholar]
- 11.Flammia D, Malgorzata D, Damaj MI, Martin B, Glennon RA. J Med Chem. 1999;42:3726. doi: 10.1021/jm990286m. [DOI] [PubMed] [Google Scholar]
- 12.Miller DK, Crooks PA, Dwoskin LP. Neuropharmacology. 2000;39:2654. doi: 10.1016/s0028-3908(00)00140-4. [DOI] [PubMed] [Google Scholar]
- 13.Gallardo KA, Leslie FM. J Neurochem. 1998;70:663. doi: 10.1046/j.1471-4159.1998.70020663.x. [DOI] [PubMed] [Google Scholar]
- 14.(a) Damaj MI, Patrick GS. J Pharmacol Exp Ther. 1997;282:410. [PubMed] [Google Scholar]; (b) Briggs CA, McKenna DG. Mol Pharmacol. 1998;37:1095. doi: 10.1016/s0028-3908(98)00110-5. [DOI] [PubMed] [Google Scholar]
- 15.Teng L, Crooks PA, Sonsalla PK, Dwoskin LPJ. Pharmacol Exp Ther. 1997;280:1432. [PubMed] [Google Scholar]
- 16.Teng L, Crooks PA, Dwoskin LP. J Neurochem. 1998;71:258. doi: 10.1046/j.1471-4159.1998.71010258.x. [DOI] [PubMed] [Google Scholar]
- 17.Miller DK, Harrod SB, Green TA, Wong MY, Bardo MT, Dwoskin LP. Pharmacol Biochem Behav. 2003;74:279. doi: 10.1016/s0091-3057(02)00996-6. [DOI] [PubMed] [Google Scholar]
- 18.Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, Bardo MT. J Pharmacol Exp Ther. 2001;298:172. [PubMed] [Google Scholar]
- 19.Harrod SB, Dwoskin LP, Green TA, Gehrke BJ, Bardo MT. Psychopharmacology. 2003;165:397. doi: 10.1007/s00213-002-1289-6. [DOI] [PubMed] [Google Scholar]
- 20.Miller DK, Crooks PA, Teng LH, Witkin JM, Munzar P, Goldberg SR, Dwoskin LP. J Pharmacol Exp Ther. 2001;296:1023. [PubMed] [Google Scholar]
- 21.Cunningham CS, Polston JE, Jany JR, Segert IL, Miller DK. Drug Alcohol Depend. 2006;84:211. doi: 10.1016/j.drugalcdep.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Miller DK, Lever JR, Rodvelt KR, Baskett JA, Will MJ, Kracke GR. Drug and Alcohol Dependence. 2007;89:282. doi: 10.1016/j.drugalcdep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Compe’re D, Marazano C, Das BC. J Org Chem. 1999;64:4528–4532. [Google Scholar]
- 24.Zheng G, Dwoskin LP, Crooks PA. J Org Chem. 2004;69:8514. doi: 10.1021/jo048848j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng G, Dwoskin LP, Deaciuc AG, Norrholm SD, Crooks PA. J Med Chem. 2005;48:5551. doi: 10.1021/jm0501228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng G, Dwoskin LP, Deaciuc AG, Norholm SD, Jones MD, Crooks PA. Bioorg Med Chem. 2005;13:3899. doi: 10.1016/j.bmc.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng G, Dwoskin LP, Crooks PA. AAPS J. 2006;8:E682. doi: 10.1208/aapsj080478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng G, Horton D, Deaciuc AG, Dwoskin LP, Crooks PA. Bioorg Med Chem Lett. 2006;16:5018. doi: 10.1016/j.bmcl.2006.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carroll FI, Lewin AH, Marscarella SW. In: Neurotransmitter Transporters: Structure, Function and Regulation. Reith ME, editor. Humana Press; Totowa, NJ: 2002. p. 381. [Google Scholar]
- 30.Fuller RW, Wong DT, Robertson DW. Med Res Rev. 1991;11:17. doi: 10.1002/med.2610110103. [DOI] [PubMed] [Google Scholar]
- 31.Lee LC, Vander Borght T, Sherman PS, Frey KA, Kilbourn MR. J Med Chem. 1996;39:191. doi: 10.1021/jm950117b. [DOI] [PubMed] [Google Scholar]
- 32.Wilkins LH, Jr, Miller DK, Ayers JT, Crooks PA, Dwoskin LP. AAPS J. 2006;7:E922. doi: 10.1208/aapsj070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng YC, Prusoff WH. Biochem Pharmacol. 1973;22:3099. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 34.Bradford MM. Anal Biochem. 1976;72:248. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]