Abstract
Diabetes and coronary heart disease (CHD) are two of the most prevalent medical illnesses in the US population and comorbid depression occurs in up to 20% of these patients. Guidelines for management of diabetes and CHD overlap for healthy lifestyle and disease-control recommendations. However, the majority of patients with these medical illnesses have been shown to have inadequate control of key risk factors such as blood pressure, LDL cholesterol, or blood sugar. Comorbid depression has been shown to adversely affect self-care of diabetes and CHD, and is associated with an increased risk of complications and mortality. Interventions that have improved quality and outcomes of depression care alone in patients with diabetes and CHD have not demonstrated benefits in self-care, improved disease control or morbidity and mortality. This paper describes the design and development of a new biopsychosocial intervention (TEAMcare) aimed at improving both medical disease control and depression in patients with poor control of diabetes and/or CHD who met the criteria for comorbid depression. A team approach is used with a nurse interventionist who receives weekly psychiatric and primary care physician caseload supervision in order to enhance treatment by the primary care physician. This intervention is being tested in an NIMH-funded randomized controlled trial in a large integrated health plan.
Keywords: Diabetes, Depression, Coronary heart disease, TEAMcare study
1. Introduction
Complex patients are characterized by multiple, poorly controlled chronic diseases complicated by psychological and behavioral impairments including depression, unhealthy life-styles, and poor adherence to medication regimens [1]. Complex patients account for a disproportionate share of U.S. health care costs [2]. How to improve care for complex patients is one of the major challenges facing medicine today [3]. Recent evaluations of case-management services for patients with complex chronic disease have not yielded anticipated improvements in disease control or cost reductions [4]. While disease management interventions for single conditions, including congestive heart failure [5], diabetes,[6] and depression [7], have been shown to improve control of these chronic conditions, it remains unclear how to improve outcomes in complex patients who have multiple poorly controlled physical and psychological conditions [4].
Among patients with coronary heart disease (CHD) and/or diabetes with comorbid depressive illness, recent research has assessed whether overall health outcomes can be improved by effectively treating depression [8–11]. The rationale for this approach is that in patients with diabetes and CHD, there is a high prevalence of co-existing depression [12,13] and this comorbidity is associated with increased medical symptom burden [14,15], additive functional impairment [16], poor self-care (adherence to diet, exercise, cessation of smoking or taking disease-control medications as prescribed) [17], higher medical utilization, costs [16,18], macrovascular and microvascular complication rates, and mortality [19–21]. However, three trials of collaborative depression care versus usual primary care among patients with diabetes and comorbid depression have shown that improving quality of depression care and depressive outcomes has not resulted in improvements in diabetes selfcare or HbA1c levels [8–10]. Similarly, the largest depression effectiveness trial in patients with CHD and comorbid depression has shown that improving depressive outcomes was not associated with decreased cardiac events or mortality [11]. One possible interpretation of these results is that management of multi-condition patients with comorbid physical and psychosocial impairments requires an integrated biopsychosocial approach that simultaneously addresses their physical and psychological problems. Optimal care of complex patients may also need to target behavioral risk factors such as exercise and medication adherence.
Despite evidence that team approaches (such as collaborative care) integrated with primary care improve quality of care and disease outcomes of single chronic conditions such as depression [20], CHF [5] and diabetes [6] most systems of care are struggling with how to improve quality and reduce costs of care for complex patients with multiple chronic diseases and psychological impairments [4]. An integrated medical and psychological care management model that improved quality and outcomes of care for these complex patients might be a more cost-effective approach to organizing health care as it could be implemented for a broad range of patients, as opposed to disease management programs that target patients with particular chronic diseases. Since over 90% of Medicare beneficiaries have more than one chronic condition and 71% of Medicare beneficiaries with depression have 4 or more chronic conditions [2], the potential significance of an effective approach to caring for these complex patients is compelling.
This paper reports the development of a TEAMcare intervention to assist primary care management of complex patients with comorbid depression and poorly controlled diabetes and/or CHD that is currently being tested in a randomized controlled trial. There is considerable overlap in guidelines for management of diabetes [22] and CHD [23], and major depression is found in up to 20% of patients with these diseases [24]. Therefore, a collaborative care intervention for multiple illnesses would target a meaningful and commonly occurring cluster of chronic conditions. This paper also considers design issues encountered in developing an experimental evaluation of an integrated intervention for poorly controlled diabetes and/or heart disease patients who also had major depression and/or dysthymia.
2. Methods
The TEAMcare study was developed by a multidisciplinary team from the University of Washington and the Group Health (GH) Research Institute, and was implemented in GH primary care clinics. GH is a non-profit mixed model health care organization with 30 primary care clinics in Western Washington State. Fourteen GH primary care clinics in a 90-mile geographic region of Western Washington State were included in this study.
The study was funded by the National Institute of Mental Health (NIMH) Division of Intervention and Health Services Research. The randomized controlled trial proposed to test a primary care based multimodal intervention aimed at improving quality of care for complex patients with poorly controlled diabetes, CHD and depression with usual primary care. The care management intervention was provided by nurses who had previous training in enhancing quality of care for patients with diabetes. The nurse care manager role was designed to support management of complex patients by the primary care physician (PCP) and to strengthen the support of primary care management by relevant consultative specialists. Other goals for the care manager were: to work in partnership with the patient to develop a shared definition of significant problems, provide patient education and support, agree on specific targets/goals and an individualized action plan, offer support and problem-solving to optimize self-management, closely monitor adherence and outcomes, and facilitate return appointments to the PCP or specialist for patients with adverse outcomes or side-effects. The study protocol was reviewed and approved by the GH Institutional Review Board and a Data Monitoring Safety Board reviewed methods prior to initiation and outcomes every 6 months thereafter.
2.1. Sample recruitment
GH electronic medical records data were screened for patients with diabetes mellitus (all ICD-9 250 codes) or with CHD (all ICD-9 diagnosis codes 410–414 or ICD-9 procedure codes 00.66, and all 36 coronary artery procedure codes (CPT) 33510 to 33523, and 33533 to 33536, 33572, 92973 to 92975, 92977, 92980 to 92982, 92984, 92995 and 92996). The electronic medical record was then used to identify potentially eligible patients with one or more indicators of poor disease control within the previous 12 months: blood pressure >140/90 (based on 2 blood pressure recordings at two separate visits within 12 months), LDL >130 mg LDL, and HbA1c ≥8.5. The goal of recruitment was to screen approximately 10,000 patients with diabetes and/or CHD with evidence of poor disease control for depression with a brief depression screening scale in order to identify approximately 350 patients with major depression and/or dysthymia on the Patient Health Questionnaire-9 (PHQ-9) [25]. Patients were recruited between May 2007 and October 2009.
The trial was planned for a sample of 145 patients each in the intervention and usual care groups, which was projected to provide 80% power to detect a 0.165 (SD 0.5) difference in mean SCL-20 depression scores and a 15% difference in the proportion of patients achieving optimal disease control on all 3 measures (HbA1c <7.0% or ≥0.5 decrease, systolic blood pressure <130 mm Hg or ≥10-point decrease, or LDL <100 mg/dl or ≥15% decrease), assuming 15% attrition.
2.2. Screening and recruitment
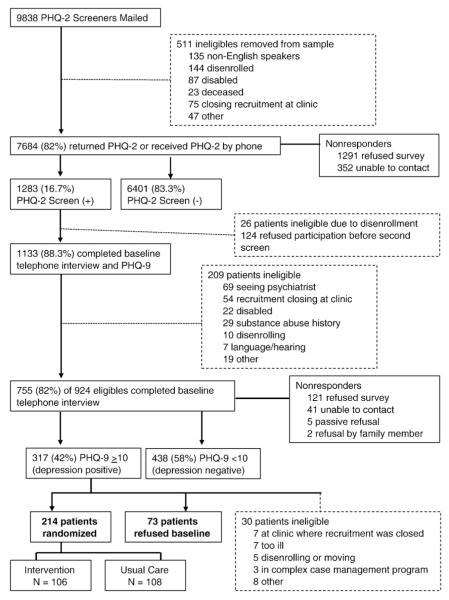
We used a brief one-page mail screener that included the Patient Health Questionnaire-2 (PHQ-2) [26] to identify depressed patients, accompanied by an invitation letter from the GH Clinical Improvement Medical Director. A $2 bill was included with the mailing to encourage response. The telephone survey team called patients who did not return the mailed questionnaire to attempt completing the brief screen by phone. A total of 82% of patients returned the questionnaire or were reached by survey. Fig. 1 describes the recruitment and reasons for ineligibility and referral at each stage of the study.
Fig. 1.
Recruitment flow diagram.
The PHQ-9 was chosen as the second stage screen because it is also brief, easy to score and provides a probable DSM-IV diagnosis of major depression and depression severity score [25]. The PHQ-9 score of ≥10 has been found in primary care and aging populations to have high sensitivity and specificity to the diagnosis of major depression based on structured psychiatric interview [25]. In our prior Pathways Study in patients with diabetes and depression we randomized patients to an intervention versus usual care based on the PHQ-9 score of ≥10 and found significant intervention versus usual care effects on outcomes of depression [10].
To recruit a representative sample of primary care patients, we had few medical or psychiatric exclusions. We included patients with diabetes and/or CHD who were already receiving antidepressant medications or psychotherapy from nonpsychiatrist clinicians, but who still had PHQ-9 depression scores ≥10. This decision was based on prior findings that many primary care patients are exposed to antidepressant medications at lower than guideline recommended dosage and duration, and few receive an adequate number of sessions of an evidence-based psychotherapy [27]. Prior studies such as IMPACT [28] and Pathways [10] have found that being treated with an antidepressant medicine prior to randomization did not modify depression outcomes. Eligible patients were ambulatory, English speaking, with adequate hearing to complete a telephone interview and planned to be enrolled in GHC over the next year. Medical exclusions were terminal illness, residence in a long-term care facility, planning to have bariatric surgery within 3 months and pregnancy or nursing; psychiatric exclusions were: care by a psychiatrist; a diagnosis based on GH automated data of bipolar disorder or schizophrenia; use of antipsychotic or mood stabilizer medication based on GH automated pharmacy in the prior year; and mental confusion in the interview suggesting dementia.
2.3. Randomization
Patients who screened positive on the initial screen (PHQ-2 score of ≥3) were asked to complete a telephone interview, including the PHQ-9. If they had a PHQ-9 score of ≥10, they were then invited to complete a baseline interview during the same call or at a later date and asked to give a verbal consent to order laboratory tests prior to an in-person visit. Following the baseline interview, patients were scheduled for an in-person visit to obtain informed consent, collect height, weight, waist, and blood pressure measurements as well as onsite lab testing (for HbA1c, LDL, HDL, triglycerides, microalbumin, and creatinine ratio).
Patients were then randomly assigned by a centralized randomization process to usual care or to the TEAMcare intervention group. Patients assigned to usual care received a letter stating they had been assigned to the control group. A nurse care manager or study staff member called patients randomized to the TEAMcare intervention to set an appointment to initiate the intervention.
2.4. TEAMcare intervention design
The TEAMcare intervention employed a “treat-to-target” approach [29] that integrated effective elements of disease management interventions for diabetes and CHD with collaborative care for depression [22,23,30]. These elements included: developing an individualized care plan; systematic monitoring of patient progress on disease-control parameters (PHQ-9, HbA1c, blood pressure, lipid levels), and relentless treatment adjustment (treat-to-target) and support of patient self-care to help patient achieve treatment goals. A nurse care manager was added to the primary care team for management of intervention patients. This nurse interventionist was supervised weekly by a psychiatrist (WK or PC) and either an internist (BY) or family physician (EHBL) with ready access to diabetes and cardiology consultants.
2.5. What type of professional should be trained to deliver the TEAMcare intervention?
Given the need for the nurse to be proficient in both management of complex patients with diabetes and/or CHD and depression, the intervention was delivered by highly experienced medical nurses. The interventionists were nurses with extensive experience in diabetes management already working in the GH system because of their skills in glycemic, lipid and blood pressure management. They were then trained in collaborative depression case management. The fact that these nurses were accredited in using the GH electronic medical records systems and were members of the GH nursing staff also increased the integration of the TEAMcare intervention with the delivery of primary health care in the participating clinics. Facilitating dissemination in the GH delivery system if the trial improved patient outcomes was also a consideration in using experienced nurses from the study setting.
Three half-time diabetes nurses provided the interventions with caseloads of approximately 35 to 50 patients. Each nurse covered four to five clinics that were geographically as far as 30 miles apart.
2.6. Training
Nurses received an initial 2-day training course on diagnosis and pharmacotherapy of depression and an introduction to behavioral strategies such as motivational interviewing, behavioral activation and problem-solving. Nurses also received updated training on glucose control, including insulin management, blood pressure management and treatment of high LDL and triglyceride levels. Recent treat-to-target guidelines developed by Kaiser Care Management Institute and used by GH for insulin and blood pressure management were included in the training [29,30]. These guidelines describe medication choices and recommended dose increments for patients with poor disease control. (See Appendix A).
A psychiatrist, family physician, internist–nephrologist, psychologist and lead diabetes nurse participated in the training. Core psychosocial skills that were taught included motivational interviewing [31], problem-solving [32], and behavioral activation [33,34]. The training included didactics, role playing and guided reading. The training was enhanced by development of a Nurse Training Manual and a Patient Manual (Tools for Managing Your Chronic Diseases).
Motivational interviewing (MI) is a collaborative, person-centered approach to elicit and strengthen motivation for change [31,35] Provider behaviors characteristic of MI include: a) seeking to understand the person’s frame of reference, particularly via reflective listening; b) expressing acceptance and affirmation; c) eliciting and selectively reinforcing the client’s own self-motivational statements and expressions of problem recognition, concern, desire and intention to change; d) monitoring the client’s degree of readiness to change and ensuring that resistance is not generated by jumping ahead of the client’s readiness to change; and e) affirming the client’s freedom of choice and self-direction.
Problem Solving Treatment (PST) is a brief, practical skill-building treatment designed for use in medical settings [36,37] This treatment has been shown to be successful for addressing depressive symptoms associated with conditions such as diabetes, as well as an approach to delivering self-management support to non-depressed individuals with chronic conditions [38]. The rationale for PST is that life problems associated with living with chronic illness can be precipitants of depressive and anxiety symptoms, and that once depressed, problems become more difficult to solve. PST teaches a structured procedure for addressing problems systematically.
Behavioral activation is a therapeutic process that emphasizes structured attempts to increase behaviors likely to expose patients to reinforcing environmental contingencies and produce corresponding improvements in thoughts, mood and overall quality of life [33,34]. Behavioral activation strategies are ideal for implementation in medical settings because they are brief, structured and uncomplicated compared to other psychological interventions for depression and can be delivered by health care providers other than mental health specialists [33,34]. Our research group has shown that this behavioral activation plan can be combined with antidepressant treatment and was associated with improved depressive outcomes compared to usual care [39].
2.7. TEAMcare intervention visits
The TEAMcare intervention included a 1 h initial visit followed by contacts by telephone and in person once or twice a month until the patient achieved his or her treatment goals. Half of these contacts were expected to be in-person and half by telephone. The first appointment included a biopsychosocial, semi-structured history (reviewing history and treatments for depression, diabetes and heart disease), patient education, development of the therapeutic alliance, understanding the patient’s explanatory model of illness, negotiation about starting depression treatment with antidepressant medication and/or behavioral activation, and developing an overall individualized care plan.
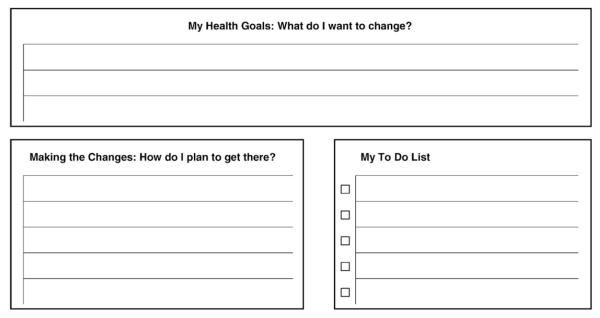
The nurse explained that treatment would occur in phases, starting with interventions to improve depression symptoms, then focusing on disease control of blood sugar, blood pressure or LDLs and, finally, an emphasis on increasing healthy eating, exercise or smoking cessation. Nurses also explained that they would be following the patient with this intervention over a 12-month period. A key task for the patient and nurse in the initial visit was to formulate an electronic version of My Better Health Care Plan (see Fig. 2), which would target specific goals for the nurse and patient to work toward together. Each nurse had weekly supervision with a team of a psychiatrist and PCP to review new cases and patient progress. Caseload supervision by specialists helps provide decision support to the PCPs when patients experience treatment resistance or side-effects to medications. In addition, nurses met with the psychologist on the project once a month initially to review difficult patient encounters in order to enhance motivational interviewing, problem-solving and behavioral activation skills. The nurse, psychiatrist and primary care supervisors reviewed patient adherence to medication and lifestyle recommendations, progress on PHQ-9, blood glucose, blood pressure and LDL targets in weekly supervision meetings. When the psychiatrist and primary care supervisor recommended medication changes, the nurse discussed or e-mailed these recommendations to the PCP, who ordered changes on all medications through the electronic record (EPIC Care).
Fig. 2.
My better health plan: next step.
Using an ACCESS database, a clinical monitoring system was developed in tandem with the GH electronic medical record to support the treat-to-target intervention (Fig. 3). The nurses entered tracking data after each contact, including primary care clinic, initial enrollment date, initial and most current PHQ score, HbA1c, blood pressure and LDL level. The ACCESS tracking data allowed nurses and supervisors to view all patients that were being followed by a 2-page printout (or 2 computer screens). The ACCESS tracking form included a flag for each disease-control measure that had not had a significant clinical improvement or reached guideline recommended levels over a 10-week period: cases that had not decreased 50% or more on the PHQ-9; not achieved a HbA1c decrease of ≥0.5 or had a HbA1c level of <7.0%; not achieved a systolic blood pressure of <130, or a decrease of ≥10 mm Hg, or had not had at least a 15% decrease in LDL or an LDL level of <100. Supervision started with new cases, progressed to flagged cases and then to cases in the initial stages of treatment. While it would have been substantially more efficient to use the EMR alone to support the TEAMcare intervention, developing modules that support complex case management has not been a priority for EPICARE or other developers of EMR software systems. In general, complex case management is carried out with stand-alone software not fully integrated with health plan EMR data.
Fig. 3.
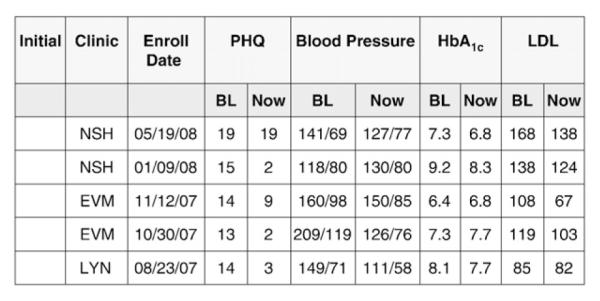
TEAMcare tracking system.
2.8. Stepped care treat-to-target approach
A stepped care treat-to-target approach was used in which management was guided by clinical response and outcomes [40]. Stepped care recognizes that patients have marked differences in response to medication and behavioral treatments [29,40]. Treat-to-target emphasizes persistent management to achieve guideline-levels of disease control on parameters that are poorly controlled at baseline [29]. In the TEAMcare study, all patients were offered both behavioral activation strategies and antidepressant medication to treat depression, lipid lowering and blood pressure medication for higher than guideline-level LDLs and blood pressure, and oral hypoglycemic medications and/or insulin for those with HbA1c levels >7.0%. For patients who had high PHQ-9, LDL, HbA1c or blood pressure levels who were not already treated with a medication, a medication was recommended. For those already on medication, the nurse initially carefully reviewed whether the patient was taking medications as prescribed to ensure that poor disease control was not due to poor adherence. Nurses also reviewed self-care activities, including dietary choices, exercise, smoking and whether the patient was monitoring blood sugar or blood pressure levels.
Patients were provided relevant educational materials. As seen in Table 1, patients were all provided with a book (The Depression Helpbook) [41] and a videotape to enhance understanding about depression. Patients with abnormal blood sugars were provided an American Diabetes Association (ADA) educational packet on glucose monitoring as well as the nurse ensuring that patients were proficient in using glucometers that were routinely provided by GH. For those with poorly controlled blood pressure, an educational packet on blood pressure adapted from the American Heart Association (AHA), was provided as well as an OMRON home monitoring blood pressure cuff. For those with high LDL levels, an AHA educational packet on lowering low-density lipids was provided. Motivational interviewing and problem-solving techniques were used for patients with poor adherence. Adherence was monitored based on patient self-report as well as nurse review of automated data on prescription refills. Behavioral activation focused on increasing pleasant activities, moderate exercise (such as walking) when possible and, for those interested, a pedometer was provided to reinforce taking an increased number of steps per day.
Table 1.
Self-care goals, behaviors and tools.
| Chronic disease | Self-care behaviors | Tools |
|---|---|---|
| Depression | Symptom | Videotape and The Depression |
| monitoring | Helpbook | |
| Antidepressant | PHQ-9, electronic monitoring | |
| medication | of outcomes | |
| Behavioral activation | Adherence packets/reminders | |
| Physical activity | Behavioral activation workbook |
|
| Social support | Pedometers, exercise log, groups |
|
| Blood sugar | Home glucose | ADA educational packet on |
| monitoring | glucose monitoring | |
| Medication adherence | Glucometer | |
| Physical activity | Adherence packets/reminders | |
| Healthy diet | Exercise log, classes, pedometer | |
| Foot checks | Daily weight | |
| Hypertension | Home blood pressure monitoring | AHA educational packet on BP |
| Medication | Blood pressure cuff (OMRON)/ | |
| adherence | home monitoring | |
| Physical activity | Adherence packets/reminders | |
| Low salt, healthy diet | Exercise log, classes, pedometer | |
| Hyperlipidemia | Low fat, low | AHA educational packet on |
| cholesterol diet | lowering LDLs | |
| Medication adherence | Adherence packets/reminders | |
| Physical activity | Exercise log, classes, pedometer |
About half of the patients in the intervention arm of the study had already been prescribed antidepressant medications prior to randomization. If adherence with antidepressant medications was adequate, then patients initially were treated by increasing doses of prescribed antidepressants until their PHQ-9 scores decreased by at least 50% or until they reached maximal dosage or side-effects limited titration. Similarly, patients not already on antidepressant had an initial antidepressant medication started and dose titrated until their PHQ-9 scores decreased by ≥50% or side-effects emerged.
For most patients not receiving antidepressant medicines at enrollment either citalopram was started (provided that they had not had two prior negative trials of SSRIs or side-effects on prior SSRI trials limiting dosage adjustment) or buproprion-SR was chosen as the initial antidepressant. These medications were inexpensive generic medications on the GH formulary. Citalopram was the SSRI chosen due to the relative lack of hepatic enzyme inhibition and potential drug–drug interaction with diabetes and/or heart disease medications. Buproprion-SR was chosen because of its lack of hepatic enzyme inhibition, lack of sexual side-effects (which can be particularly problematic in patients with diabetes due to preexisting autonomic dysfunction affecting sexual physiology) and evidence of weight loss associated with use of buproprion in trials in patients with depression and diabetes [42]. For those with a partial response to maximal doses of citalopram, buproprion-SR was often added to augment response based on the beneficial effect of this combination in the STAR-D trial [43]. Likewise, for those with a partial response to maximal doses of buproprion-SR, citalopram was often added. Those with poor or no response to either citalopram or buproprion-SR, or the combination of these two medications, were switched to an alternate medication, which in most cases was an SNRI such as venlafaxine-XR.
A stepped care approach also guided treatment for elevated blood pressure, lipid (low density lipoporotein (LDL)), and glycemic control. Algorithms were based on GH and Kaiser Care Management Institute treatment recommendations synthesized from national guidelines. Among patients who had not yet received pharmacotherapy, anti-hypertensives initiated included a diuretic (e.g. hydrochlorothiazide), an ACE inhibitor (e.g. lisinopril), while statins and metformin were the initial medicines of choice for hyperlipidemia and hyperglycemia, respectively, unless contraindicated. Among patients who were already prescribed relevant medications, adherence was evaluated and addressed by the nurse care managers as indicated. Medication adjustments as described in the medication guidelines were recommended for the PCP. (See treat-to-target medication guidelines in Appendix A). In the weekly case discussions of intervention patients, the clinical supervisors used the treat-to-target approach to focus on patient response and outcomes. Specific medical treatment adjustments, and self-care changes, were recommended for any patient who had not achieved their disease-control targets. The nurse care manager then followed up with both the PCP and the patients to help implement these recommendations.
2.9. Maintenance treatment
Once the patient reached a maximum achievable level of improvement on all four disease parameters, the nurse and patient developed a maintenance plan. This plan included dosages of maintenance medication, behavioral goals (i.e. walking for one-half hour 4 times a week), identification of prodromal symptoms associated with poor disease control, and stress reduction techniques. Once this plan was completed, an electronic version was provided for the PCP and the nurse and patient each kept a copy. After completion of this plan, patients were followed every 4 to 6 weeks by telephone calls from the nurse to review adherence, lab test results and to complete a PHQ-9. If the patient showed worsening of depression, hyperlipidemia or blood pressure, she/he could be offered more intense follow-up care.
2.10. Enhanced usual care (UC)
PCPs at GH provide medical services for patients with diabetes, depression and CHD. Specialty consultation can be obtained by self or PCP referral. After randomization, UC patients were advised to consult with their PCP to receive care for depression, diabetes and/or CHD. With patient permission, PCPs were notified about depression and poor medical disease control. All study baseline, 6- and 12-month follow-up labs were drawn by GH laboratories and results entered into the electronic medical record.
2.11. Assessment
Table 2 describes the variables that will be collected at screening, baseline and follow-up interviews. Screening was completed by telephone and with use of automated data. The baseline interview was completed by phone. The initial measurements and all follow-up assessments of blood pressure, height, weight and waist circumference were completed in-person. Research assistants blind to intervention status measured blood pressure three times after 20 min of rest in a relaxed sitting position using the OMRON Intellisense blood pressure unit, with the mean of the second and third blood pressure reading used in analysis. All subsequent measurement of depression outcomes, health risk behaviors, functional impairment, satisfaction with care of depression and diabetes/CHD were completed by the telephone survey team blind to group status.
Table 2.
Study measures and time of data collection
| Study measure | Screen | Baseline | 6 mos | 12mos | 18mos | 24 mos |
|---|---|---|---|---|---|---|
| Eligibility measures | ||||||
| Age, gender, marital status, education, work status CAGE-AID 6-item cognitive screen Diabetes and/or CHD duration Diabetes and/or CHD age of onset Type 1: Was insulin the first Rx? |
X X X X X X |
|||||
| Depression measures | ||||||
| PHQ-9 | X | X | X | X | X | X |
| SCL-20 | X | X | X | X | X | |
| Dysthymia # Prior depressive episodes PGI |
X X |
X | X | X | X | X |
| Disease self-report measures | ||||||
| Previous myocardial infarction Hx | X | |||||
| Family Hx heart disease | X | |||||
| Health risk behaviors (diet, physical activity, smoking, checking blood glucose and blood pressure) | X | X | X | X | X | |
| Self-efficacy scale | X | X | X | X | X | |
| % on aspirin | X | X | X | X | X | |
| In-person exam measures | ||||||
| Height, weight, hip-to-waist ratio, waist circumference | X | X | X | |||
| Blood pressure | X | X | X | X | X | |
| Disability measures | ||||||
| WHO-DAS | X | X | X | X | X | |
| Sheehan | X | X | X | X | X | |
| Satisfaction with care | ||||||
| Satisfaction with care of depression | X | X | X | X | X | |
| Satisfaction w/ care of diabetes and/or heart disease | X | X | X | X | X | |
| Quality of care measures depression | X | |||||
| % reaching adequate dose and duration of antidepressants in each 6-month period | X | X | X | X | X | |
| % on antidepressant medication at each follow-up period | X | X | X | X | X | |
| % receiving >4 sessions of psychotherapy by MH professional | X | X | X | X | X | |
| Quality of care diabetes/CHD | ||||||
| % with blood pressure < 130/80 (or ≥ 10-point reduction in systolic, ≥5 in diastolic) | X | X | X | X | X | |
| % with LDL <100 (or ≥ 15% reduction) | X | X | X | X | X | |
| % with HbA1c <7.0% (or ≥0.5% reduction) | X | X | X | X | X | |
| Creatine, microalbuminuria | X | X | X | |||
| Mean HDL and triglyceride levels | X | X | X | X | X | |
| Chronic disease score (Rx risk) | Automated data sources continuous measurement | |||||
| Myocardial infarction Diabetes complication score Antihypertensive, lipid lowering and diabetic medication adherence |
||||||
Depression diagnoses were established with the PHQ-9 [25] and dysthymia items from the Diagnostic Interview Schedule [44]. The PHQ-9 was used because it is brief, is recommended to be used to both define probable major depression in “real-world” primary care settings and to gauge success of treatment. The SCL-20 depression scale was chosen to measure depression severity because it has been extensively used in primary care trials and has been found to be as sensitive to change as other commonly used depression scales [45]. The Patient Global Rating of Change for depression was assessed with a 7-point scale with options feeling worse, the same, a little better, somewhat better, moderately better, a lot better or completely better [46].
Because multiple primary outcomes included depression, systolic blood pressure, glucose control and lipid control, we examined the literature to ascertain whether a global measure of enhanced disease control had been developed for diabetes and/or CHD. Given that, in addition to depression, two medical conditions and three disease-control parameters (systolic blood pressure, LDL and HbA1c levels) were of interest, a statistical approach to assessing effects across multiple disease outcomes was needed. We developed a plan to measure whether the intervention was more effective than usual care over time in improving all four disease outcomes (see analysis plan below).
GH’s computerized pharmacy and utilization records were used to measure adherence to antidepressant, oral hypoglycemic, lipid lowering and antihypertensive medication. The computerized pharmacy records allow examination of refills of antidepressant medications and whether the patient received an adequate dosage based on evidence-based guideline standards for 90 days or more within each 6-month period of time. The Continuous Multiple Gaps therapy Measure (CMG) will be used to measure adherence to the three classes of medical disease-control medications [47]. The CMG is defined as the ratio of number of days the patient did not have medication available (based on refill data) divided by the number of days the patient should have been on medication [47,48]. The CMG of ≥20% evidence of poor adherence has been correlated with adverse medical outcomes [48]. We will also measure the degree of intensification of medication treatment for depression, glycemic, lipid and blood pressure control for patients out of guideline range based on automated data [49].
Computerized pharmacy records will be used to estimate a revised chronic disease score (RxRisk), a measure of medical comorbidity based on prescription drug use in the prior 6 months [50]. A diabetes complication measure was also used to measure the number and severity of diabetes complications based on ICD-9 and lab records in previous 12 months [51]. The RxRisk and diabetes complication score will be used to assess the comparability of intervention and usual care patients at baseline.
Computerized health plan data will be used to identify all health plan services provided or paid for by GH during the two years after randomization (inpatient and outpatient services for mental health or general medical care). All outpatient and inpatient services provided by GH are assigned costs based on health plan accounting records (including actual personnel, supply and overhead costs). Services purchased by GH from external providers are assigned costs equal to the amount reimbursed by GH for that type of care.
2.11.1. Principal analysis
Statistical comparisons of outcomes for the two treatment groups across 6- and 12-month follow-up points will be based on regression models adjusting for baseline values, estimated using Generalized Estimating Equations (GEE) that account for correlation in outcomes. Our primary analysis will test for an overall effect of the intervention (as randomized) on 12-month outcomes (SCL-20, HbA1c, SBP, LDL) using a scaled marginal model [52]. This approach scales outcomes by their standard error (SE), so that intervention effects can be interpreted as effect sizes. This model will be estimated by iterating between estimation of the covariance associated with outcomes and GEE estimation of scaled outcomes. All observations will be used in the GEE step; but only observations with complete covariate and outcome data will be used to update the SE estimate. We will use a score test to assess the equality of the intervention effect across outcomes. This model will also be used to estimate the effect on medical outcomes (HbA1c, SBP, and LDL).
Additional analyses will describe the relationship between intervention status and PGI, clinical depression response (defined as ≥50% decrease in SCL-20), and satisfaction with care using logistic regression models across 6- and 12-month outcomes. Analyses describing the relationship between intervention status and quality of life, an ordinal measure, will be based on linear regression. Analyses across time points will be estimated using GEE and adjusted for baseline measures when these are available. Pearson’s chi-square test will be used to evaluate between-group differences in the proportion of patients with overall medical improvement (all 3 medical measures below guidelines or showing clinically significant change).
2.11.2. Cost-effectiveness
We will evaluate patient costs and clinical outcomes over a 2-year period after randomization. Previous cost-effectiveness analyses of patients with comorbid depression and diabetes have shown that the increased mental health costs of a depression collaborative care program were offset by savings in total ambulatory medical costs, most of which occurred in Year 2 [53,54]. Costs and effectiveness of the intervention will be compared with incremental cost-effectiveness ratios following guidelines developed by Gold et al. [55]. We will take a health plan perspective and, in the numerator, will estimate the two-year differences in total ambulatory costs. In the denominator we will use the method by Lave et al [56] to estimate differences in depression-free days between intervention and control patients over a 24-month period. Bootstrap resampling with 1000 draws using bias correction will be used to estimate confidence intervals for both incremental cost measures and depression-free days and the ratio of incremental costs to incremental depression-free days [57]. The bootstrap method will allow us to evaluate the probability of this intervention being in each of the four quadrants in Fig. 4. Most new interventions are in the upper left quadrant (costs more, but more effective), however, there is also a possibility that, if improved depression and disease control is associated with less diabetes symptoms and complications, there may be savings in medical costs that partially or completely make-up for increased costs of providing the TEAMcare intervention.
Fig. 4.
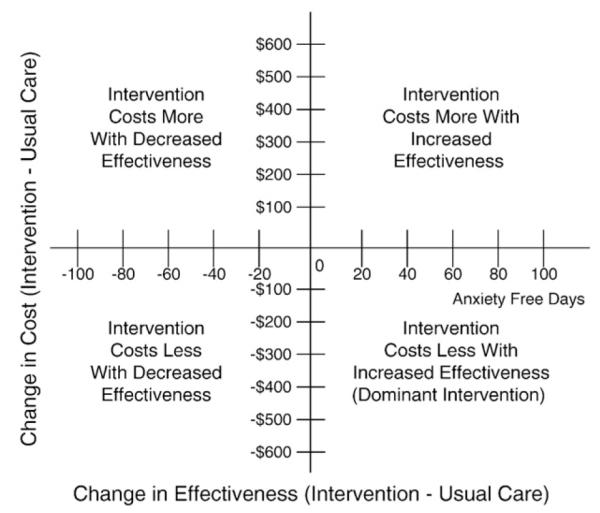
Incremental cost-effectiveness quandrant.
2.11.3. Baseline data
Over an 18-month period, 214 patients were randomized with 82 (38%) with HbA1c levels ≥8.5%, 58 (27%) with LDL levels >130, 115 (54%) with systolic blood pressure levels >140 and 30 (14%) with diastolic >90, and the mean PHQ-9 score was 14.3. A total of 183 (85.5%) had diabetes with or without CHD and 56 (26.2%) had CHD alone.
As shown in Table 3, the intervention and usual care groups were well balanced on sociodemographic and clinical variables. The mean age of the population was in the late 50 s, approximately half were female, over half had ≥1 years of college, about 20% to 25% were minorities and over half were still employed at least part-time. Western Washington populations have similar rates of diabetes and CHD as national estimates, but are more educated and have lower percentages of Hispanic and African American populations. Almost three quarters were depressed for ≥2 years based on the dysthymic questions from the Diagnostic Interview Schedule [44] and the PHQ-9 and SCL scores reflected moderate severity levels of depression. The mean HbA1c was approximately 8.0%, mean LDL levels were 106 to 109, mean systolic blood pressure levels were 132 to 136, and the mean BMI was 36 to 37.
Table 3.
Sociodemographic and clinical characteristics of patients with diabetes, CHD and depression
| Intervention N =108 |
Usual care N = 106 |
|
|---|---|---|
| Age, mean (SD) | 57.4 (10.5) | 56.3 (12.1) |
| % female | 48% | 56% |
| % ≥ some college | 61% | 56% |
| % White | 75% | 82% |
| % employed PT or fulltime | 52% | 59% |
| PHQ-9, mean (SD) | 14.7 (3.8) | 13.9 (3.1) |
| % ≥ 2years depression | 72% | 76% |
| SCL-20, mean (SD) | 1.7 (0.6) | 1.7 (0.6) |
| Duration of diabetes, mean (SD) | 8.5 (8.5) | 10.4 (9.6) |
| HbA1c, mean (SD) | 8.1 (2.0) | 8.0 (1.9) |
| LDL, mean (SD) | 106.5 (35.3) | 109.0 (36.5) |
| Systolic BP, mean (SD) | 136 (18.4) | 132 (17.2) |
| % diabetes with or without CHD | 89% | 82% |
| % CHD | 23% | 30% |
| BMI, mean (SD) | 36.9 (8.3) | 36.6 (8.5) |
3. Discussion
The research team has successfully recruited over 200 patients with depression and poorly controlled diabetes and/or CHD, developed a nurse treatment manual, trained diabetes nurses in the TEAMcare approach, developed an electronic disease register to track patient progress, developed electronic templates for initial visit, progress and relapse prevention notes, and utilized efficient one- to two-hour weekly physician caseload supervision sessions with nurses. The feasibility of recruitment, training and implementation of the TEAMcare intervention was established.
The TEAMcare intervention offers patients and clinicians the necessary resources to provide evidence-based depression, diabetes and CHD treatments as well as necessary support for patients to initiate changes in self-care. The nurse TEAMcare model exemplifies a system of care that both supports the primary care delivery system and provides patient-centered care.
A limitation of the study is that the trial is being conducted in 14 clinics of one large health maintenance organization in the Pacific Northwest, potentially limiting generalizability to other populations or other types of health care systems. However, research interventions that were initially developed at GH such as collaborative depression care [58] or the Wagner chronic illness model [59] have been adapted and successfully disseminated to a wide range of diverse clinic systems in multiple geographic regions of the United States and worldwide. Another limitation is that GH has been a national leader in achieving NCQA targets for diabetes care and patients in the UC arm had several enhancements to usual care.
This is the first study to test an intervention for a natural cluster of illnesses that are highly prevalent in primary care populations and that are associated with high medical costs [18] and adverse outcomes [19,20,60,61]. If the TEAMcare intervention proves effective in improving depression and medical disease-control outcomes, it may be relevant to efforts to improve chronic disease management among complex patients in diverse health care settings.
Supplementary Material
Acknowledgement
This research was supported by grants from the National Institute of Mental Health to Dr. Katon (MH41739 and K24 MH069741).
Footnotes
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.cct.2010.03.009.
References
- [1].Rundall TG, Shortell SM, Wang MC, et al. As good as it gets? Chronic care management in nine leading US physician organisations. BMJ. 2002;325:958–61. doi: 10.1136/bmj.325.7370.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Partnership for Solutions National Program Office . Chronic Conditions: Making the Case for Ongoing Care. Robert Wood Johnson Foundation; 2001. [Google Scholar]
- [3].Weiss KB. Managing complexity in chronic care: an overview of the VA state-of-the-art (SOTA) conference. J Gen Intern Med. 2007;22(Suppl 3):374–8. doi: 10.1007/s11606-007-0379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wolff JL, Boult C. Moving beyond round pegs and square holes: restructuring Medicare to improve chronic care. Ann Intern Med. 2005;143:439–45. doi: 10.7326/0003-4819-143-6-200509200-00008. [DOI] [PubMed] [Google Scholar]
- [5].Phillips CO, Wright SM, Kern DE, Singa RM. Shepperd S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta-analysis. JAMA. 2004;291:1358–67. doi: 10.1001/jama.291.11.1358. [DOI] [PubMed] [Google Scholar]
- [6].Renders CM, Valk GD, Griffin SJ, Wagner EH, Eijk Van JT, Assendelft WJ. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care. 2001;24:1821–33. doi: 10.2337/diacare.24.10.1821. [DOI] [PubMed] [Google Scholar]
- [7].Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166:2314–21. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- [8].Ell K, Katon W, Xie B, et al. Collaborative care management of major depression among low-income, predominantly Hispanics with diabetes: a randomized controlled trial. Diabetes Care. 2010;33:706–13. doi: 10.2337/dc09-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Williams JW, Jr, Katon W, Lin EH, et al. The effectiveness of depression care management on diabetes-related outcomes in older patients. Ann Intern Med. 2004;140:1015–24. doi: 10.7326/0003-4819-140-12-200406150-00012. [DOI] [PubMed] [Google Scholar]
- [10].Katon WJ, Von Korff M, Lin EH, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61:1042–9. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- [11].Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289:3106–16. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- [12].Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–78. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- [13].Simon GE. Evidence review: efficacy and effectiveness of antidepressant treatment in primary care. Gen Hosp Psychiatry. 2002;24:213–24. doi: 10.1016/s0163-8343(02)00198-6. [DOI] [PubMed] [Google Scholar]
- [14].Ludman EJ, Katon W, Russo J, et al. Depression and diabetes symptom burden. Gen Hosp Psychiatry. 2004;26:430–6. doi: 10.1016/j.genhosppsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- [15].Ciechanowski PS, Katon WJ, Russo JE, Hirsch IB. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. Gen Hosp Psychiatry. 2003;25:246–52. doi: 10.1016/s0163-8343(03)00055-0. [DOI] [PubMed] [Google Scholar]
- [16].Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278–85. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- [17].Lin EH, Katon W, Von Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27:2154–60. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- [18].Simon G, Katon W, Lin E, et al. Diabetes complications and depression as predictors of health care costs. Gen Hosp Psychiatry. 2005;27:344–51. doi: 10.1016/j.genhosppsych.2005.04.008. [DOI] [PubMed] [Google Scholar]
- [19].Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26:2822–8. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- [20].Katon WJ, Rutter C, Simon G, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 2005;28:2668–72. doi: 10.2337/diacare.28.11.2668. [DOI] [PubMed] [Google Scholar]
- [21].Lin EH, Heckbert SR, Rutter CM, et al. Depression and increased mortality in diabetes: unexpected causes of death. Ann Fam Med. 2009;7:414–21. doi: 10.1370/afm.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].American Diabetes Association Standards of medical care in diabetes. Diabetes Care. 2007;30:S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- [23].Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113:2363–72. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- [24].Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54:216–26. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- [25].Kroenke K, Spitzer R, Williams J. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- [27].Katon W, Von Korff M, Lin E, Simon G. Rethinking practitioner roles in chronic illness: the specialist, primary care physician, and the practice nurse. Gen Hosp Psychiatry. 2001;23:138–44. doi: 10.1016/s0163-8343(01)00136-0. [DOI] [PubMed] [Google Scholar]
- [28].Unützer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–45. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- [29].Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–6. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- [30].Dudl RJ, Wang MC, Wong M, Bellows J. Preventing myocardial infarction and stroke with a simplified bundle of cardioprotective medications. Am J Manag Care. 2009;15:e88–94. [PubMed] [Google Scholar]
- [31].Rollnick S, Miller W. What is motivational interviewing? Behav Cogn Psychother. 1995;23:325–34. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- [32].Mynors-Wallis LM, Gath DH, Lloyd-Thomas AR, Tomlinson D. Randomised controlled trial comparing problem solving treatment with amitriptyline and placebo for major depression in primary care. BMJ. 1995;310:441–5. doi: 10.1136/bmj.310.6977.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lewinsohn PM, Graf M. Pleasant activities and depression. J Consult Clin Psychol. 1973;41:261–8. doi: 10.1037/h0035142. [DOI] [PubMed] [Google Scholar]
- [34].Martell C, Addis M, Jacobson N. Depression in context: strategies for guided action. N.W. Norton; New York: 2001. [Google Scholar]
- [35].Miller WR, Rollnick S. Ten things that motivational interviewing is not. Behav Cogn Psychother. 2009;37:129–40. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- [36].Harpole LH, Williams JW, Jr, Olsen MK, et al. Improving depression outcomes in older adults with comorbid medical illness. Gen Hosp Psychiatry. 2005;27:4–12. doi: 10.1016/j.genhosppsych.2004.09.004. [DOI] [PubMed] [Google Scholar]
- [37].Arean P, Hegel M, Vannoy S, Fan MY, Unuzter J. Effectiveness of problem-solving therapy for older, primary care patients with depression: results from the IMPACT project. Gerontologist. 2008;48:311–23. doi: 10.1093/geront/48.3.311. [DOI] [PubMed] [Google Scholar]
- [38].Hill-Briggs F, Gemmell L. Problem solving in diabetes self-management and control: a systematic review of the literature. Diabetes Educ. 2007;33:1032–50. doi: 10.1177/0145721707308412. discussion 51-2. [DOI] [PubMed] [Google Scholar]
- [39].Katon W, Robinson P, Von Korff M, et al. A multifaceted intervention to improve treatment of depression in primary care. Arch Gen Psychiatry. 1996;53:924–32. doi: 10.1001/archpsyc.1996.01830100072009. [DOI] [PubMed] [Google Scholar]
- [40].Von Korff M, Tiemens B. Individualized stepped care of chronic illness. West J Med. 2000;172:133–7. doi: 10.1136/ewjm.172.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Katon W, Ludman E, Simon G. The depression helpbook. Bull Publishing Company; Boulder, CO: 2003. [Google Scholar]
- [42].Lustman PJ, Williams MM, Sayuk GS, Nix BD, Clouse RE. Factors influencing glycemic control in type 2 diabetes during acute- and maintenance-phase treatment of major depressive disorder with bupropion. Diabetes Care. 2007;30:459–66. doi: 10.2337/dc06-1769. [DOI] [PubMed] [Google Scholar]
- [43].Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- [44].Robins L, Helzer J. Diagnostic Interview Schedule (DIS) Version III-A. Washington University School of Medicine; St. Louis, MO: 1985. [Google Scholar]
- [45].Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL). A measure of primary symptom dimensions. Mod Probl Pharmacopsychiatry. 1974;7:79–110. doi: 10.1159/000395070. [DOI] [PubMed] [Google Scholar]
- [46].Guy W. ECDEU Assessment Manual for Psyhcopharmacology. U. S. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; Rockville, MD: 1976. [Google Scholar]
- [47].Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- [48].Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27:2800–5. doi: 10.2337/diacare.27.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Schmittdiel JA, Uratsu CS, Karter AJ, et al. Why don’t diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med. 2008;23:588–94. doi: 10.1007/s11606-008-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Fishman PA, Goodman MJ, Hornbrook MC, Meenan RT, Bachman DJ, O’Keeffe Rosetti MC. Risk adjustment using automated ambulatory pharmacy data: the RxRisk model. Med Care. 2003;41:84–99. doi: 10.1097/00005650-200301000-00011. [DOI] [PubMed] [Google Scholar]
- [51].Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14:15–23. [PMC free article] [PubMed] [Google Scholar]
- [52].Roy J, Lin X, Ryan LM. Scaled marginal models for multiple continuous outcomes. Biostatistics. 2003;4:371–83. doi: 10.1093/biostatistics/4.3.371. [DOI] [PubMed] [Google Scholar]
- [53].Simon GE, Katon WJ, Lin EH, et al. Cost-effectiveness of systematic depression treatment among people with diabetes mellitus. Arch Gen Psychiatry. 2007;64:65–72. doi: 10.1001/archpsyc.64.1.65. [DOI] [PubMed] [Google Scholar]
- [54].Katon W, Unutzer J, Fan MY, et al. Cost-effectiveness and net benefit of enhanced treatment of depression for older adults with diabetes and depression. Diabetes Care. 2006;29:265–70. doi: 10.2337/diacare.29.02.06.dc05-1572. [DOI] [PubMed] [Google Scholar]
- [55].Gold M, Siegel J, Russel L, Weinstein M. Cost-effectiveness in health and medicine. Oxford University Press; New York, NY: 1996. [Google Scholar]
- [56].Lave JR, Frank RG, Schulberg HC, Kamlet MS. Cost-effectiveness of treatments for major depression in primary care practice. Arch Gen Psychiatry. 1998;55:645–51. doi: 10.1001/archpsyc.55.7.645. [DOI] [PubMed] [Google Scholar]
- [57].O’Brien BJ, Drummond MF, Labelle RJ, Willan A. In search of power and significance: issues in the design and analysis of stochastic cost-effectiveness studies in health care. Med Care. 1994;32:150–63. doi: 10.1097/00005650-199402000-00006. [DOI] [PubMed] [Google Scholar]
- [58].Katon WJ, Unutzer J. Pebbles in a pond: NIMH grants stimulate improvements in primary care treatment of depression. Gen Hosp Psychiatry. 2006;28:185–8. doi: 10.1016/j.genhosppsych.2006.01.004. [DOI] [PubMed] [Google Scholar]
- [59].Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288:1909–14. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- [60].Lin E, Heckbert S, Rutter C, Katon W, Ludman E, Ciechanowski P. Depression and increased mortality in diabetes: unexpected causes of death. Ann Fam Med. 2009;7:414–21. doi: 10.1370/afm.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270:1819–25. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.