Abstract
This study characterized the within- and between-child variability in dimethylthiophosphate (DMTP) levels in the urine of 44 children living in an agricultural community in central Washington State in December 1997 and 1999. The goal of this analysis was to investigate these variability components during periods when organophosphate pesticides were and were not actively applied to orchards in that community. Each child provided between 10 and 26 biweekly urine samples over a 21-month period, and these samples were analyzed for six dialkylphosphate (DAP) metabolites common to organophosphate pesticides, including DMTP. Previous analysis of this dataset found that DAP concentrations were elevated during months when organophosphate pesticides were applied to orchards in this region. The current analysis demonstrates that the within-child component of day-to-day variability was much greater than the between-child component of variability by a factor of 3–7 across the DAP metabolites that were analyzed. Therefore, organophosphate pesticide exposure appeared to vary more than 3 times from day-to-day than from child-to-child. This finding has important implications for epidemiologic and exposure pathways research, since accounting for within-child variability may increase the power of a study and allow for the detection of differences that would not otherwise be possible without an analysis that separates out the within-child variability.
Keywords: Pesticides, Agriculture, Children, Variability, Biological monitoring
1. Introduction
Individuals experience different levels of exposure to environmental contaminants from day-to-day, and therefore it is recommended that repeated measurements on the same individual be taken routinely in order to effectively evaluate exposure (Boleij et al., 1995). Variations, both within individuals over time and between individuals, are important elements of consideration in exposure analysis (Kromhout et al., 1993). Repeated sampling allows for analysis of this variation and identification of its unique components and can be crucial in correctly characterizing exposure (Heederik et al., 1991). Longitudinal sampling with repeated measures is a method that has long been used in studies of occupational exposures (Kromhout et al., 1993; Symanski et al., 1996).
A number of studies have employed biological monitoring of children to examine pesticide exposure pathways (Adgate et al., 2001; Bradman et al., 2005; Coronado et al., 2006, 2009; Curl et al., 2002; Lu et al., 2000; Royster et al., 2002; Shalat et al., 2003; Thompson et al., 2003, 2008). Implicit in these studies is the notion that a single (or in some cases two) biologic sample is a representative measure of exposure that can be used as a dependent variable to test various independent variables thought to be predictors of exposure. This approach makes sense if between-child variability is relatively high compared to within-child variability. However, if the day-to-day variability in children’s biological sample measurements is high, this approach may result in a misclassification of children as to whether their exposures were high or low. Studies that have longitudinal measurements can provide estimates of the within- and between-child variability.
In a study of organophosphate pesticide exposures (Koch et al., 2002), biweekly urine samples were collected from a group of pre-school children over the course of 21 months covering two agricultural spray seasons for dimethyl organophosphate pesticides and two spray seasons for diethyl organophosphate pesticides. These samples were analyzed for the five dialkylphosphate (DAP) compounds that are common metabolic products of many organophosphate pesticides. Children enrolled in this study were aged 2–5 years and lived in an agricultural community in central Washington with orchard crops. Geometric mean concentrations of both dimethyl and diethyl DAPs in the urine samples collected during the spray months were found to be significantly higher than those collected during the non-spray months. The longitudinal measurements in that study permit characterization of the exposure variability and allow the estimation of the within- and between-child components of this variability. This is particularly relevant given the temporal variation found in the pesticide exposures of these children. In the current study, we compute these variability components and assess their significance.
2. Methods
Participants in this study included 44 children living in an agricultural community. Study participants were recruited from a Central Washington Women, Infant and Children’s Clinic, which provided health services to parents with young children (Koch et al., 2002). Participants willing to participate in a study, which included collection of biweekly urine samples over a period of one year were recruited and consented regardless of their residential location or occupation. Initial recruitment took place in December 1997 and because of attrition additional families were recruited in April 1998. Each family completed a questionnaire and was interviewed four times: once during enrollment, twice during an interim period, and once during an exit interview. Information collected included date of birth of children, weight, parental occupations, household pesticide use, and children’s activities. Koch et al. (2002) analyzes this data in detail. All procedures were reviewed and approved by the University of Washington Division of Human Subjects before we started data collection (UW IRB application number 12,542) (Koch et al., 2002). Urine samples were analyzed for five DAP metabolites: dimethylphosphate (DMP), dimethylthiophosphate (DMTP), dimethyldithiophosphate (DMDTP), diethylphosphate (DEP), and diethylthiophosphate (DETP). A global positioning system unit (Entertech, Mountain View, CA) was used to record the location of each child’s residence. Global positioning system data were plotted on 1998 digital aerial photographs of the area (US Forest Service), and the distance from each residence to the nearest orchard was determined using ArcView 3.2 (ESRI, Redlands, CA), an analysis software application that links databases of geographic and feature information. In the analysis, distance was categorized as close to (<60 m) or far from (>60 m) agricultural fields.
A component of variance model was used to estimate the mean urinary concentrations of DAPs in each time period and the within- and between-child components of variance. This model had a component describing how children varied between each other and a component describing how much a single child could vary from day-to-day. These two components are summarized by a standard deviation for how much children vary between each other across the population, and a standard deviation describing the day-to-day variation within a child. Each child has one mean value and the between-child standard deviation describes how the children’s mean values vary across the population. The within-child standard deviation describes for each child how they vary from day-to-day about their mean value. A single within-child standard deviation describes the within-child variability in the population. In addition to the two components of variance we estimate seasonal trends as a mean value for each time period to describe how the average values change across time (Cox and Solomon, 2002; Pinheiro and Bates, 2000).
A five-dimensional multivariate normal model was used to describe the three dimethyl metabolites, DMP, DMTP, and DMDTP, and the two diethyl metabolites, DEP and DETP. A multivariate normal distribution was used because correlations between the metabolites inform us about the censored values below a limit of detection (Egeghy et al., 2005; Hughes, 1999; Lyles et al., 2001; Schisterman et al., 2003; Thiebaut and Jacqmin-Gadda, 2004; Vigoren et al., Unpublished results). In a single urine sample a value of any of the metabolites above the limit of detection alters the probability distribution of other metabolites below the limit of detection due to correlations between metabolites. For example, if DMTP is above its limit of detection and DMP and DMDTP are below their limits of detection then the particular value observed for DMTP informs us about the distribution of possible values for DMP and DMDTP through their correlations with DMTP. Correlations among the five metabolites are significant in this dataset. Estimation of the parameters of the components of variance multivariate normal model is complicated by the fact that many of the metabolite values are below the limit of detection. A common method for dealing with observations below the limit of detection is to substitute a constant value such as half of the limit of detection for the value—these methods assume that it is known where the censored values should be placed. In this analysis we chose to deal with the values below the limit of detection as censored values, that is, in this analysis the distribution of these values was constrained to be below the limit of detection and was conditional upon the observed values of the other metabolites in the same sample. This method provides more reliable statistical estimation and inference than do conventional methods. The components of variance model can be expressed as
| (1) |
where MVN5 is a five-dimensional multivariate normal distribution of the three dimethyl metabolites, DMP, DMTP, DMDTP, and two diethyl metabolites, DEP, DETP, Xjkl is the vector of the measured DMP, DMTP, DMDTP, DEP, DETP concentrations of the jth child in the kth exposure period on the lth collection day, μj is the vector of the means of the 5 metabolites for the jth child, θk is the vector of the means of the 5 metabolites for kth exposure period, Σw is the within-child variance–covariance matrix (5 × 5) for metabolites, Σb is the between-child variance–covariance matrix (5 × 5) for metabolites, and njk is the number of urine sample collected for the jth child during the kth exposure period.
For observations below the limit of detection for a particular metabolite, the value of the limit of detection is used to describe the upper limit of the censored lower left hand tail of the probability distribution described above. The components of variance model sets up the conceptual framework for the analysis of the data. Because of the large number of censored values, distributions of the model parameters were estimated using a Bayesian Markov chain Monte Carlo method known as Gibbs sampling. Bayesian methods treat parameters as random variables and thus provide posterior estimates of the distribution of parameters based upon the data and prior distributions of the parameters. In our analysis to minimize the effects of the prior distributions on the final estimates, we used uninformative priors that provide little preference for any particular value of the parameter. In our calculations we used a flat prior for the mean values and a Wishart distribution for the precision (inverse variance) of the multivariate normal distributions. We utilized the WinBUGS 1.4.3 software program available at www.mrc-bsu.cam.ac.uk/bugs to perform all calculations (Lunn et al., 2000). Further considerations can be found in the Technical appendix of the Supplementary material.
We used a multivariate normal distribution to model the logarithms of the metabolites to simultaneously analyze all of the metabolites. The correlations between the metabolites are used to provide more precise and representative estimates of the means and variances. This is particularly important in this dataset where we have over 50% of the metabolites below the limit of detection for DMP, DMDTP, DEP, and DETP during at least one sampling season. The method used to estimate the parameters of the components of variance model in (1) use the observed values and correlations among the metabolite measurements above the limit of detection and assume that the same relationship holds for those values below the limit of detection. Thus, the estimates of the parameters in (1) are based on the values above the limit of detection in conjunction with the number of values falling below the limit of detection for each metabolite. As the fraction of values below the limit of detection for a metabolite increases, the uncertainty of the parameter estimate for the metabolite increases.
The multivariate normal distribution of the logarithms of the metabolites can be easily related to other forms of analysis such as a regression analysis of the values of one of the metabolites to the other metabolites. There are simple relationships between the slope and intercept of a regression line and the means, standard deviation and correlations estimated in (1) (Neter and Wasserman, 1974). We choose to use the multivariate normal distribution in (1) to describe the analysis since it more clearly expresses the observational nature of the study, in that none of the variables are under control of the investigator and that each of the metabolites provides important aspects for understanding the exposure of children to organophosphate pesticides.
3. Results
A total of 44 children (one child per family) were enrolled in this study. Koch et al. (2002) reported demographic information such as age, gender, and ethnicity. Each of the 44 children provided between 10 and 26 urine samples; all samples were collected over a 21-month period between December 1997 and August 1999. Table 1 shows the calendar time periods when urine samples were collected categorized by 12 time periods spanning the 21-month interval during which they were collected. When children had more than one observation in a given time period all were included in the analysis. Dimethyl organophosphate pesticides (e.g. azinphos-methyl and phosmet) were applied to fields in central Washington State in May, June, and July of 1998 and June, July, and August of 1999 for crop protection.
Table 1.
Time periods used to categorize collection intervals showing times during which dimethyl and diethyl organophosphate pesticide (OP) spraying occurred.
| Time period | Dates | Dimethyl OP | Diethyl OP |
|---|---|---|---|
| 1 | December 15, 1997 to February 28, 1998 | ||
| 2 | March 1, 1998 to April 30, 1998 | Spraying | |
| 3 | May 1, 1998 to June 15, 1998 | Spraying | |
| 4 | June 16, 1998 to July 31, 1998 | Spraying | |
| 5 | August 1, 1998 to September 15, 1998 | ||
| 6 | September 16, 1998 to October 31, 1998 | ||
| 7 | November 1, 1998 to December 15, 1998 | ||
| 8 | December 16, 1998 to January 31, 1999 | ||
| 9 | February 1, 1999 to March 15, 1999 | Spraying | |
| 10 | March 16, 1999 to May 31, 1999 | ||
| 11 | June 1, 1999 to July 15, 1999 | Spraying | |
| 12 | July 16, 1999 to August 30, 1999 | Spraying |
A total of 972 samples were collected and analyzed for these five DAP compounds. Urine samples were analyzed for six DAP metabolites: dimethylphosphate (DMP), dimethylthiophosphate (DMTP), dimethyldithiophosphate (DMDTP), diethylphosphate (DEP), diethylthiophosphate (DETP), and diethyldithiophosphate (DEDTP), as described by Moate et al. (1999). Analysis of DEDTP was problematic due to an unstable analytic standard, and DEDTP concentration could not be standardized within several of the batch runs. Therefore, DEDTP was excluded from the statistical analysis. The limits of detection for the remaining compounds were 59 nmol/L for DMP, 7.8 nmol/L for DMTP, 45 nmol/L for DMDTP, 43 nmol/L for DEP, and 7.1 nmol/L for DETP.
Table 2 summarizes the results of the urinary analysis and lists the number of samples, which were detectable, non-detectable, and those for which there was a laboratory problem preventing analysis (“No value”). Four of the five metabolites were found in detectable concentrations in fewer than 50% of the samples during at least one of the sampling seasons (Table 2). DMTP, however, was found at detectable concentrations in 80% of the samples during the time of year when dimethyl pesticide agricultural spray was occurring and 69% of the samples during the time of the year when spray was not occurring. A summary of the sample sizes during the dimethyl spray and non-spray periods is presented in Table 3. Our analyses focus primarily on DMTP, since it was the only metabolite detected more than 50% of the time, and secondarily on the other metabolites.
Table 2.
Summary of urinary metabolite results during spray and non-spray seasons showing the number of samples for which the results of the chemical analysis were above or below the limit of detectiond or for which the reference standard was not reliable (percentages are in parentheses).
| Total number | Detectable | Non-detectable | No valuea | |
|---|---|---|---|---|
| DMPb | ||||
| Spray | 275 | 77 (28) | 198 (72) | 0 (0) |
| Non-spray | 697 | 181 (26) | 514 (74) | 2 (0.3) |
| DMTPb | ||||
| Spray | 275 | 219 (80) | 55 (20) | 1 (0.4) |
| Non-spray | 697 | 483 (69) | 211 (30) | 3 (0.4) |
| DMDTPb | ||||
| Spray | 275 | 97 (35) | 178 (65) | 0 (0) |
| Non-spray | 697 | 186 (27) | 510 (73) | 1 (0.1) |
| DEPc | ||||
| Spray | 119 | 92 (77) | 26 (22) | 1 (0.8) |
| Non-spray | 853 | 361 (42) | 491 (58) | 1 (0.1) |
| DETPc | ||||
| Spray | 119 | 98 (82) | 21 (18) | 0 (0) |
| Non-spray | 853 | 408 (48) | 444 (52) | 1 (0.1) |
“No value” indicates the number of samples for which there were laboratory difficulties in determining the concentration of the particular metabolite. Analysis of DEDTP was particularly problematic because the reference standard was unstable, and DEDTP could not be standardized within several of the batch runs. This led to high numbers of samples with No Value, and therefore DEDTP results are excluded from this analysis.
For the dimethyl metabolites DMP, DMTP, and DMDTP, spray season denotes samples collected during the dimethyl organophosphate pesticide application period of May–July of 1998 and June–August of 1999. Non-spray samples denote samples collected at all other times.
For the diethyl metabolites DEP and DETP, spray season denotes samples collected during the diethyl organophosphate pesticide application period of March April of 1998 and February–March of 1999. Non-spray samples denote samples collected at all other times.
Among the 972 urine samples there were only 28 (4%) samples for which all five metabolites were above the detection level, 104 (11%) samples with the three dimethyl metabolites, DMP, DMTP, DMDTP, above the detection limit, and 72 (7%) with the two diethyl metabolites DEP, DETP above the detection limit. Overall 54% of the analyses were below the limit of detection for the five metabolites.
Table 3.
Total, mean number, and range of urine samples collected from the 44 children during the dimethyl organophosphate pesticide spray and non-spray periods of the study.
| No. of samples | No. of samples per child
|
|||
|---|---|---|---|---|
| Mean | Min | Max | ||
| Total | 972 | 22 | 10 | 26 |
| Spray months | 275 | 6.3 | 2 | 11 |
| Non-spray months | 697 | 16 | 5 | 22 |
The estimated mean values for each metabolite for each time period, θk, k = 1, …,12, are shown in Fig. 1 with standard errors. These plots show the effects of spray seasons on the concentrations of metabolites excreted in urine. Fig. 1 shows that the dimethyl pesticide metabolites were observed to differ between the two non-spray periods (December 1997 through April 1998, and August 1998 through May 1999), and between the two spray periods (May through July of 1998 and June through August of 1999) (Koch et al., 2002). The trend is particularly evident with the dimethyl metabolites and specifically for DMTP. The effect of the spray season in this dataset has been shown previously for these data and that there was a lower level of metabolites in the second year (Koch et al., 2002).
Fig. 1.
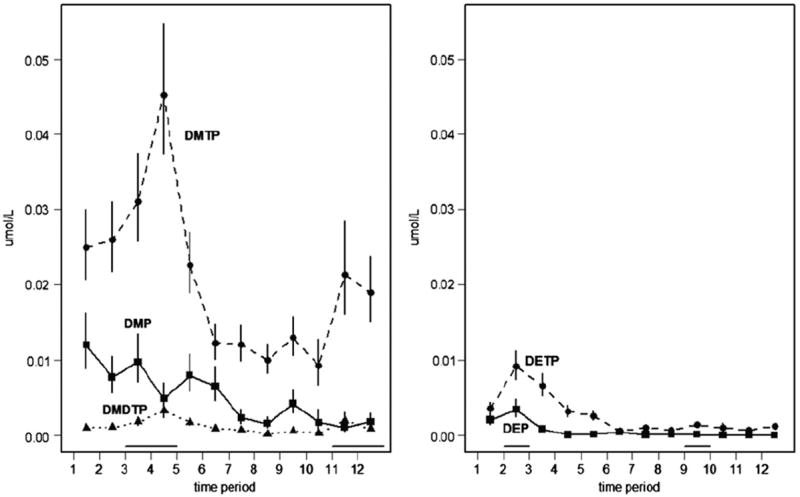
Estimated geometric mean concentration of metabolites for each of the 12 time periods used in this analysis. Error bars show standard errors. The lines immediately above the time period axis show the periods when spraying was occurring for dimethyl organophosphate pesticides and diethyl organophosphate pesticides.
The within- and between-child variability are presented in Table 4 as geometric standard deviations and their 95% prediction intervals for the five metabolites. The within-child geometric standard deviations are much larger than the between-child geometric standard deviations and differ by a factor of 3–7 across the five metabolites. Also shown in Table 4 are the intra-class correlation coefficients. The intra-class correlation coefficient is commonly used to compare variance components and measures the fraction of total variability in a measurement (the sum of the within- and between-child variance on the log scale) accounted for by the between-child variance. In our analysis the intra-class correlation coefficient is relatively similar for the five metabolites, varying from 0.050 to 0.078.
Table 4.
Geometric Standard Deviations for within- and between-child variability for three dimethyl metabolites, DMP, DMTP, and DMDTP, and two diethyl metabolites, DEP and DETP, of organophosphate pesticides. The intra-class correlation coefficient measures the fraction of total variability in a measurement (the sum of the within- and between-child variance on the log scale). Values in parentheses are 95% confidence intervals.
| DMP | DMTP | DMDTP | DEP | DETP | |
|---|---|---|---|---|---|
| Geometric Standard Deviation | |||||
| Within-child | 8.86 (7.00,11.7) | 5.04 (4.60,5.57) | 11.2 (8.99,14.5) | 14.9 (10.6,21.4) | 6.19 (5.37,7.29) |
| Between-child | 1.89 (1.51,2.56) | 1.51 (1.34,1.76) | 1.88 (1.52,2.50) | 1.86 (1.43,2.75) | 1.70 (1.45,2.10) |
| Intra-class Correlation | |||||
| 0.078 (0.033,0.157) | 0.060 (0.031,0.111) | 0.064 (0.028,0.126) | 0.050 (0.016,0.126) | 0.078 (0.039,0.143) | |
Table 5 shows location and activity measurements for study participants. Two questionnaire items from the study were considered relevant to the between-child variability component: parents had been asked how many hours per day on average their child spent away from the residence, and how many hours per day on average their child spent outdoors while at the residence (Table 5). There was a high degree of variability in the responses to these questions. While some children spent no time away from the residence on an average day, some children spent as much as 12 h elsewhere. The average time spent away from the residence was 4.1±4.3 h. Additionally, parents reported that while some children spent as much as 8 h outdoors at home, others did not go outside at all on an average day. The mean time spent outdoors at home was reported to be 1.5±1.5 h.
Table 5.
Mean, standard deviation, minimum, and maximum values for location and activity information for study participants.
| Time spent away from residence (h/day) | Time spent outdoors while at residence (h/day) | Distance from the nearest orchard (m) | |
|---|---|---|---|
| Mean | 4.1 | 1.5 | 670 |
| Std dev | 4.3 | 1.5 | 430 |
| Minimum | 0 | 0 | 12 |
| Maximum | 12 | 8 | 1300 |
The distance from the residence to the nearest orchard was also considered a relevant characteristic that might lead to a significant between-child variability component during the active spray season. Table 5 presents a summary of the results of the analysis of the participants’ residential proximity to the nearest orchards measured with a global positioning system that had a 2–3 m outdoor resolution (Elgethun et al., 2003). Again, there was a large amount of variability in these results. The closest home was 12 m (40 ft) from an orchard, and the furthest was 1300 m (4400 ft). The mean distance to the nearest orchard was 670 m (2200 ft) with a standard deviation of 430 m (1400 ft).
4. Discussion
The use of repeated sampling in this study was a strength that enabled us to characterize the within- and between-person components of variability. The day-to-day within-child component of variability was found to be much larger than the between-child component, regardless of whether or not pesticides were being actively sprayed in the community when the samples were collected. These findings suggest that individual factors did contribute significantly to organophosphate pesticide exposures during the months when pesticides were being applied to nearby fields.
Other factors influencing variability might include the location of a child’s home relative to spray or spray drift or the frequency with which a particular child plays in nearby fields. However, no clear association was found between either child behavior patterns or residential proximity to orchards and urinary DAP metabolite levels (Koch et al., 2002), but this is not surprising, given that no data were available on the timing of the pesticide applications in the specific orchards near each of the homes, or which pesticides were applied during these applications. Further, no information was available on child location when children were not at home, or how many hours per day children spent outdoors at a location other than their residence. The high degree of variability present in the available child location and activity data suggests that these other factors may vary greatly by child as well.
These information gaps highlight an important limitation of this study, that we were not able to elucidate the potential causes of large day-to-day variability in children. If the pesticide application dates were known for the specific orchards adjacent to participating homes, the temporal distance between application and sample collection could be characterized by child. Additionally, if the specific locations and activities of the children were known, such as whether or not they played in or near treated fields either at their home or elsewhere, the relationship between-child location and metabolite concentration could be investigated. Other research has investigated child activity on a macro-scale by tracking child location over time using small, portable global positioning system units (Elgethun et al., 2003). This type of refined analysis might help to explain the significant between-child variability found during the active spray season.
Another limitation is the unknown amount of preformed DAP metabolites to which the children were exposed. Zhang et al. (2008) have shown that preformed DAP metabolites of organophosphate pesticides occur in the environment and in foods with a mean mole fraction of preformed DAPs/(DAPs+organophosphate pesticides) of 0.62. These could account for a portion of the excreted metabolites in this population. Our other research measuring organophosphate pesticides in orchard workers homes during spray seasons have shown, however, that levels of organophosphate pesticides are correlated with the DAP metabolites (Coronado et al., 2006, 2010; Curl et al., 2002). This suggests that the large relative differences between spray and non-spray seasons in DAP metabolites may be due to organophosphate pesticides in the home environment. Whether the parent works in pome or non-pome orchards affects the levels of DAP metabolites measured in the urine of children (Coronado et al., 2006, 2009).
Despite these limitations, the finding of high within-child variability in biologic pesticide exposure samples has important implications for epidemiologic and exposure pathways research. Mainly, if the within-child variability is not separated out from the total variability then there may not be sufficient power to detect differences between groups. The between-child variance is used to determine the differences between groups in an analysis. When the within-child variance is large compared to the between-child variance it would require much larger sample sizes to detect the differences (Curwin et al., 2007; Kromhout and Heederik, 2005; Liu et al., 1978; Preller et al., 1995a, 1995b).
Often, however, studies are not designed or analyzed to estimate within-child variability, and several related studies can be cited to illustrate this point. Curl et al. (2002) reported a weak association between pesticide concentrations in house dust and organophosphate pesticide metabolite concentrations for 211 children from urine samples composited from 2–3 urine voids. The confidence with which these associations could be made might have increased if non-composited voids from multiple days were available. Royster et al. (2002) collected spot urine samples four weeks apart from 15–17 children, and used each set of urine data separately to test for an association between residential proximity to treated fields and exposure. No significant association was found in either case, but estimation of within-child variance could have improved the analysis of the study. Shalat et al. (2003) collected a single spot urine sample from 41 children and reported a poor association between pesticide house dust concentrations and exposure. None of these studies discussed within-person variability in the biological measurements.
Because our study had a longitudinal design and took repeated samples per person, our analysis was able to characterize the within- and between-child variability in organophosphate pesticide exposure of children living in an agricultural area in central Washington State. Future work, including repeated biological sampling measurements, will be necessary to help explain the high day-to-day variability in organophosphate pesticide exposure to children. In addition, further studies combining data on child behavior and location, spray events, and longitudinal biomonitoring analysis could allow examination of the specific factors that contribute to increased between-child variability in urinary metabolite levels during agricultural spray seasons.
5. Conclusion
The use of repeated sampling enables researchers to characterize the within- and between-person components of variability. This type of analysis can elucidate the magnitude of day-to-day and person-to-person variations in exposures, and can determine whether or not groups of individuals are uniformly exposed. The current study describes the variation in organophosphate pesticide exposure over a 21-month period in a group of 44 children living in an agricultural community. The day-to-day within-child component of variability was found to be much larger than the between-child component, regardless of whether or not pesticides were being actively sprayed in the community when the samples were collected.
Supplementary Material
Abbreviations
- DAP
dialkylphosphate
- DEP
diethylphosphate
- DETP
diethylthiophosphate
- DEDTP
diethyldithiophosphate
- DMP
dimethylphosphate
- DMTP
dimethylthiophosphate
- DMDTP
dimethyldithiophosphate
Footnotes
Funding sources: The field collection was supported primarily by the EPA STAR program (R82517101). Additional analytical support was provided by cooperative agreement U07/CCU012926 (NIOSH Agricultural Centers Program), from the NIEHS (PO1 ES09601), and the EPA (R826886 and RD832733). Contents are solely the responsibility of the authors, and do not necessarily represent the official view of the US Environmental Protection Agency, the National Institute for Occupational Safety and Health, the Centers for Disease Control and Prevention or the National Institutes of Health.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.envres.2011.05.008.
Competing interest
None.
Human subjects approval
All procedures that involved adult or child participants were reviewed and approved by the University of Washington Division of Human Subjects before data collection began (UW IRB application number 12,542).
References
- Adgate JL, Barr DB, Clayton CA, Eberly LE, Freeman NC, Lioy PJ, Needham LL, Pellizzari ED, Quackenboss JJ, Roy A, Sexton K. Measurement of children’s exposure to pesticides: analysis of urinary metabolite levels in a probability-based sample. Environ Health Perspect. 2001;109:583–590. doi: 10.1289/ehp.01109583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boleij J, Buringh E, Heederik D, Kromhout H. Occupational Hygiene of Chemical and Biological Agents. Elsevier; Amsterdam: 1995. [Google Scholar]
- Bradman A, Eskenazi B, Barr DB, Bravo R, Castorina R, Chevrier J, Kogut K, Harnly ME, McKone TE. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Perspect. 2005;113:1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado G, Griffith W, Vigoren EM, Faustman EM, Thompson B. Where’s the dust? Characterizing locations of azinphos-methyl residues in house and vehicle dust among farmworkers with young children. J Occup Environ Hyg. 2010;7:663–671. doi: 10.1080/15459624.2010.521028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado GD, Igoren EM, Griffith WC, Faustman EM, Thompson B. Organophosphate pesticide exposure among pome and non-pome farmworkers: a subgroup analysis of a community randomized trial. J Occup Environ Med. 2009;51:500–509. doi: 10.1097/JOM.0b013e31819b9ce8. [DOI] [PubMed] [Google Scholar]
- Coronado GD, Vigoren EM, Thompson B, Griffith WC, Faustman EM. Organophosphate pesticide exposure and work in pome fruit: evidence for the take-home pesticide pathway. Environ Health Perspect. 2006;114:999–1006. doi: 10.1289/ehp.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR, Solomon PJ. Components of Variance. Chapman & Hall/CRC; Boca Raton, FL, London: 2002. [Google Scholar]
- Curl CL, Fenske RA, Kissel JC, Shirai JH, Moate TF, Griffith W, Coronado G, Thompson B. Evaluation of take-home organophosphorus pesticide exposure among agricultural workers and their children. Environ Health Perspect. 2002;110:A787–A792. doi: 10.1289/ehp.021100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curwin BD, Hein MJ, Sanderson WT, Striley C, Heederik D, Kromhout H, Reynolds SJ, Alavanja MC. Urinary pesticide concentrations among children, mothers and fathers living in farm and non-farm households in iowa. Ann Occup Hyg. 2007;51:53–65. doi: 10.1093/annhyg/mel062. [DOI] [PubMed] [Google Scholar]
- Egeghy PP, Quackenboss JJ, Catlin S, Ryan PB. Determinants of temporal variability in NHEXAS-Maryland environmental concentrations, exposures, and biomarkers. J Expo Anal Environ Epidemiol. 2005;15:388–397. doi: 10.1038/sj.jea.7500415. [DOI] [PubMed] [Google Scholar]
- Elgethun K, Fenske RA, Yost MG, Palcisko GJ. Time-location analysis for exposure assessment studies of children using a novel global positioning system instrument. Environ Health Perspect. 2003;111:115–122. doi: 10.1289/ehp.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heederik D, Boleij J, Kromhout H, Smid T. Use and analysis of exposure monitoring data in occupational epidemiology: an example of an epidemiological study in the Dutch animal food industry. Appl Occup Environ Hyg. 1991;6:458–464. [Google Scholar]
- Hughes JP. Mixed effects models with censored data with application to HIV RNA levels. Biometrics. 1999;55:625–629. doi: 10.1111/j.0006-341x.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- Koch D, Lu C, Fisker-Andersen J, Jolley L, Fenske RA. Temporal association of children’s pesticide exposure and agricultural spraying: report of a longitudinal biological monitoring study. Environ Health Perspect. 2002;110:829–833. doi: 10.1289/ehp.02110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromhout H, Heederik D. Effects of errors in the measurement of agricultural exposures. Scand J Work Environ Health. 2005;31(Suppl. 1):33–8. discussion 5–7. [PubMed] [Google Scholar]
- Kromhout H, Symanski E, Rappaport SM. A comprehensive evaluation of within- and between-worker components of occupational exposure to chemical agents. Ann Occup Hyg. 1993;37:253–270. doi: 10.1093/annhyg/37.3.253. [DOI] [PubMed] [Google Scholar]
- Liu K, Stamler J, Dyer A, McKeever J, McKeever P. Statistical methods to assess and minimize the role of intra-individual variability in obscuring the relationship between dietary lipids and serum cholesterol. J Chronic Dis. 1978;31:399–418. doi: 10.1016/0021-9681(78)90004-8. [DOI] [PubMed] [Google Scholar]
- Lu C, Fenske RA, Simcox NJ, Kalman D. Pesticide exposure of children in an agricultural community: evidence of household proximity to farmland and take home exposure pathways. Environ Res. 2000;84:290–302. doi: 10.1006/enrs.2000.4076. [DOI] [PubMed] [Google Scholar]
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- Lyles RH, Fan D, Chuachoowong R. Correlation coefficient estimation involving a left censored laboratory assay variable. Stat Med. 2001;20:2921–2933. doi: 10.1002/sim.901. [DOI] [PubMed] [Google Scholar]
- Moate TF, Lu C, Fenske RA, Hahne RM, Kalman DA. Improved cleanup and determination of dialkyl phosphates in the urine of children exposed to organophosphorus insecticides. J Anal Toxicol. 1999;23:230–236. doi: 10.1093/jat/23.4.230. [DOI] [PubMed] [Google Scholar]
- Neter J, Wasserman W. Applied Linear Statistical Models. Chapter 12 Richard D Irwin, Inc.; Homewood, IL: 1974. [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. Springer; New York: 2000. [Google Scholar]
- Preller L, Heederik D, Kromhout H, Boleij JS, Tielen MJ. Determinants of dust and endotoxin exposure of pig farmers: development of a control strategy using empirical modelling. Ann Occup Hyg. 1995a;39:545–557. [PubMed] [Google Scholar]
- Preller L, Kromhout H, Heederik D, Tielen MJ. Modeling long-term average exposure in occupational exposure-response analysis. Scand J Work Environ Health. 1995b;21:504–512. doi: 10.5271/sjweh.67. [DOI] [PubMed] [Google Scholar]
- Royster MO, Hilborn ED, Barr D, Carty CL, Rhoney S, Walsh D. A pilot study of global positioning system/geographical information system measurement of residential proximity to agricultural fields and urinary organophosphate metabolite concentrations in toddlers. J Expo Anal Environ Epidemiol. 2002;12:433–440. doi: 10.1038/sj.jea.7500247. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Moysich KB, England LJ, Rao M. Estimation of the correlation coefficient using the Bayesian approach and its applications for epidemiologic research. BMC Med Res Methodol. 2003;3:5. doi: 10.1186/1471-2288-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalat SL, Donnelly KC, Freeman NC, Calvin JA, Ramesh S, Jimenez M, Black K, Coutinho C, Needham LL, Barr DB, Ramirez J. Nondietary ingestion of pesticides by children in an agricultural community on the US/Mexico border: preliminary results. J Expo Anal Environ Epidemiol. 2003;13:42–50. doi: 10.1038/sj.jea.7500249. [DOI] [PubMed] [Google Scholar]
- Symanski E, Kupper LL, Kromhout H, Rappaport SM. An investigation of systematic changes in occupational exposure. Am Ind Hyg Assoc J. 1996;57:724–735. doi: 10.1080/15428119691014585. [DOI] [PubMed] [Google Scholar]
- Thiebaut R, Jacqmin-Gadda H. Mixed models for longitudinal left-censored repeated measures. Comput Methods Prog Biomed. 2004;74:255–260. doi: 10.1016/j.cmpb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Thompson B, Coronado GD, Grossman JE, Puschel K, Solomon CC, Islas I, Curl CL, Shirai JH, Kissel JC, Fenske RA. Pesticide take-home pathway among children of agricultural workers: study design, methods, and baseline findings. J Occup Environ Med. 2003;45:42–53. doi: 10.1097/00043764-200301000-00012. [DOI] [PubMed] [Google Scholar]
- Thompson B, Coronado GD, Vigoren EM, Griffith WC, Fenske RA, Kissel JC, Shirai JH, Faustman EM. Para ninos saludables: a community intervention trial to reduce organophosphate pesticide exposure in children of farmworkers. Environ Health Perspect. 2008;116:687–694. doi: 10.1289/ehp.10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigoren E, Griffith W, Krogstad F, Faustman E. Using multivariate correlational structure methods to improve analysis of datasets with values below the limits of detection. Environmental Science and Technology. Unpublished results. [Google Scholar]
- Zhang X, Driver JH, Li Y, Ross JH, Krieger RI. Dialkylphosphates (DAPs) in fruits and vegetables may confound biomonitoring in organophosphorus insecticide exposure and risk assessment. J Agric Food Chem. 2008;56:10638–10645. doi: 10.1021/jf8018084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


