Abstract
Use of ionizing radiation is essential for the management of many human cancers, and therapeutic hyperthermia has been identified as a potent radiosensitizer. Radiation therapy combined with adjuvant hyperthermia represents a potential tool to provide outstanding local-regional control for refractory disease. (Z)-(±)-2-(N-Benzylindol-3-ylmethylene)quinuclidin-3-ol (2) and (Z)-(±)-2-(N-benzenesulfoylindol-3-ylmethylene)quinuclidin-3-ol (4) were initially identified as potent thermal sensitizers that could lower the threshold needed for thermal sensitivity to radiation treatment. To define the structural requirements of the molecule that are essential for thermal sensitization, we have synthesized and evaluated a series of (Z)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-one (9), and (Z)-(±)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-ol (10) analogs that incorporate a variety of substituents in both the indole and N-benzyl moieties. These systematic structure-activity relationship (SAR) studies were designed to further the development and optimization of potential clinically useful thermal sensitizing agents. The most potent analog was compound 10 (R1=H, R2=4-Cl), which potently inhibited (93% inhibition at 50 µM) the growth of HT-29 cells after a 41°C/2hr exposure.
Keywords: indolylquinuclidin-3-ones, indolylquinuclidin-3-ols, thermal sensitization
Hyperthermia is one of the most potent radiosensitizers identified to date1. Over the last 30 years extensive experimentation using cell and animal models has demonstrated that hyperthermia can be an extremely effective radiation sensitizer, increasing the effectiveness of the radiation by up to 5-fold.1,2 One important feature is that thermal radiosensitization does not exhibit cell type specificity nor is it limited by hypoxic conditions, suggesting that hyperthermia has the potential to overcome significant radiation resistance. These studies have been complemented by a recent meta analysis of 23 clinical trials involving 1,861 patients, which has shown that radiation therapy administered with adjuvant hyperthermia yields highly significant (p < 0.0001) local region control of aggressive tumors located in chest wall, cervical, bladder, and head and neck.3
Heat-mediated changes in protein conformation represent the underlying biophysical/biochemical mechanism responsible for heat-mediated cytotoxicity.4 Westra and Dewey5 were the first to hypothesize that cell death following hyperthermic treatment was a consequence of protein denaturation. Subsequent investigations have validated their hypothesis.6–8 Thermal radiosensitization is also a consequence of protein unfolding and aggregation,4 and under most conditions, is directly related to the degree of thermal sensitization.
However, for many cancers, suboptimal thermal dosing limits radiosensitization.6 While temperatures of 41°C can be achieved clinically, administration of higher temperatures can be challenging. In order to overcome this problem we have undertaken a chemistry-driven approach to identify pharmacological agents that enhance the degree of radiosensitization produced by a moderate heat shock. The immediate goal was to synthesize small molecules that could enhance a clinically achievable heat shock but would not exhibit cytotoxicity at physiological temperatures.
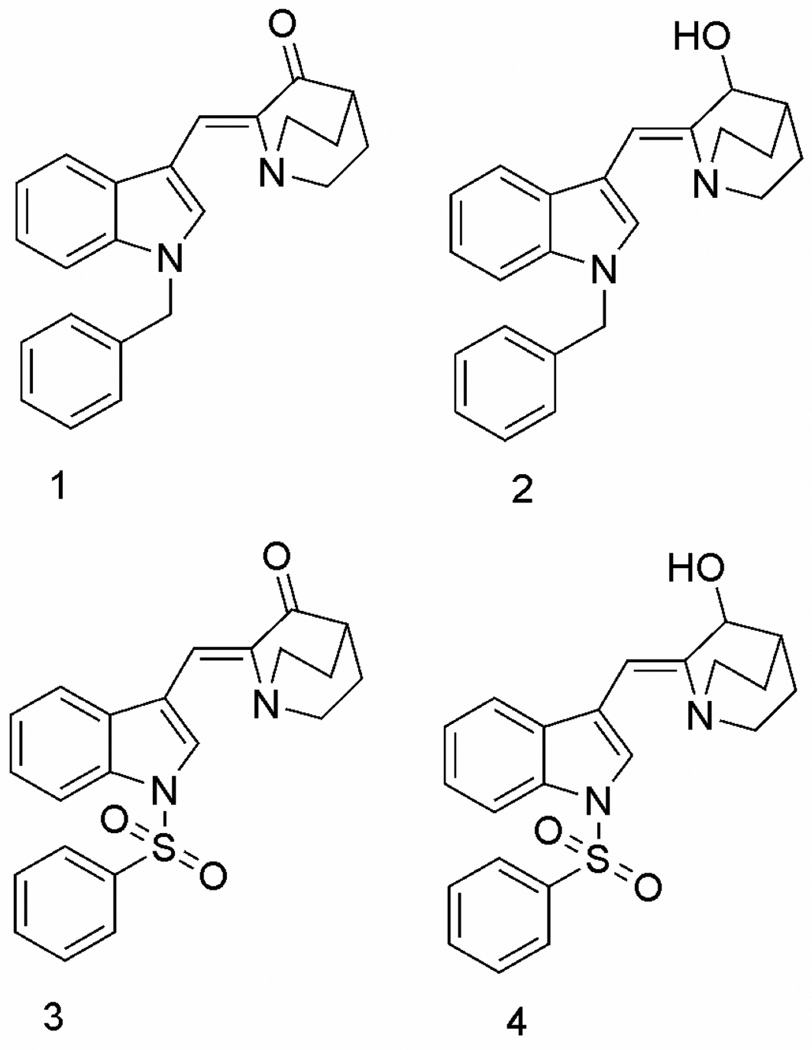
Previously, we have shown that structural mimics of indomethacin produced thermal sensitization and thermal radiosensitization of human HT-29 colon carcinoma cells, MCF7 breast adenocarcinoma cells, and H460 lung carcinoma cells at 41°C. Biochemically, this small molecule enhancement in thermal sensitization activity induces a loss of mitochondrial membrane potential, which is subsequently followed by mitotic catastrophe. The most potent small molecule enhancers identified were (Z)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-one, (Z)-(±)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-ol, (Z)-2-(N-benzenesulfonylindol-3-ylmethylene) quinuclidin-3-one and (Z)-(±)-2-(N-benzenesulfonylindol-3-ylmethylene)quinuclidin-3-ol (1, 2, 3 and 4, respectively)9 (Fig. 1). The indole nucleus played an important role in the activity of these compounds, as lack of an indole moiety produced inactive compounds. Absence of N-benzyl substitution also produced inactive or less active compounds. Also, N-benzenesulfonyl derivatives (3 and 4) were less potent than N-benzyl derivatives (2).
Figure 1.
Chemical structures of the potent thermal radiosensitizing agents 1–4.
These observations prompted us to undertake a more detailed investigation of the structure-activity relationships of various N-benzyl derivatives related to 1 & 2 (Fig. 1) that incorporate a variety of different substitutions in the phenyl ring of the N-benzyl moiety and in the indole moiety in these compounds. Thus, the present study focuses on the synthesis and evaluation of thermal sensitizing activity of various substituted (Z)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-ones (9a–9l) and (Z)-(±)-2-(N-benzylindol-3-ylmethylene) quinuclidin-3-ols (10a–10l).
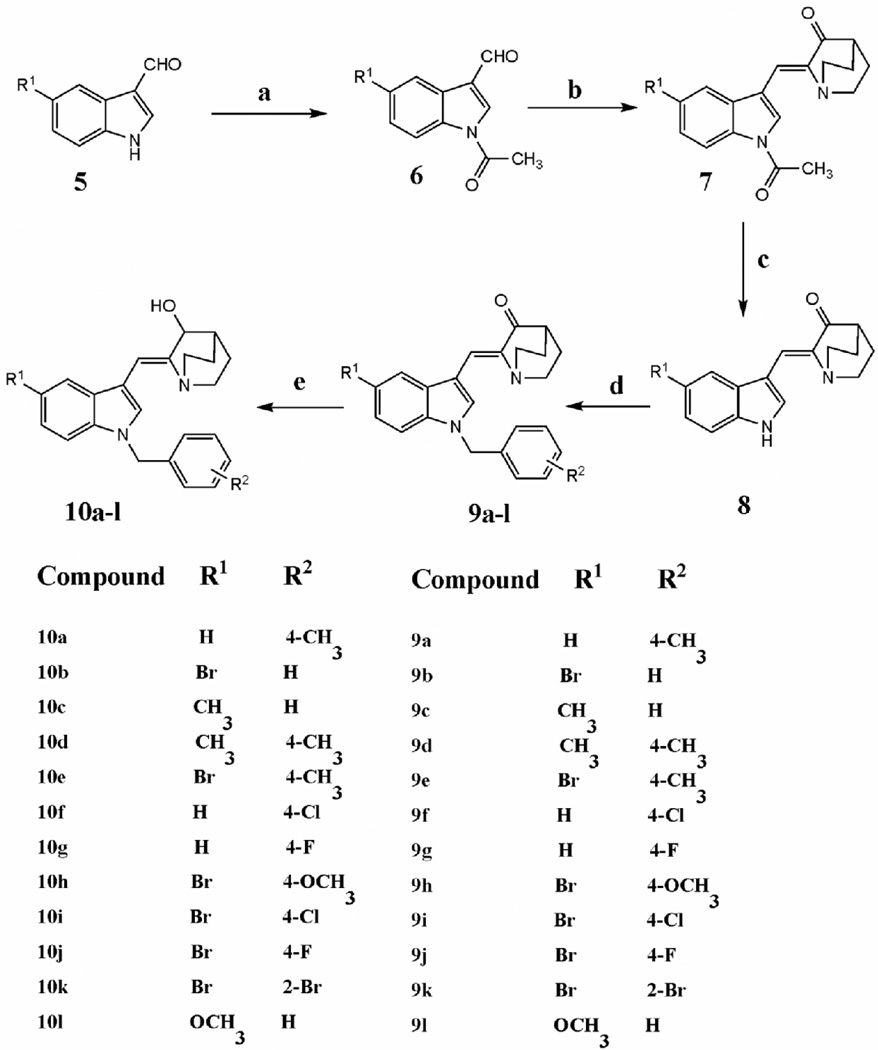
The synthetic routes to the substituted (Z)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-ones 9a–9l and the (Z)-(±)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-ols 10a–10l are illustrated in Scheme 1.
Scheme 1.
Reagents and conditions: (a) Ac2O, Et3N, DCM, reflux; (b) quinuclidin-3-one hydrochloride, THF, LDA, −78°C; (c) 1N NaOH aq, reflux; (d) substituted benzyl halide, 50% NaOH aq, triethylbenzylammonium chloride, DCM, RT; (e) NaBH4, methanol, RT.
The appropriate 1-acetylindole-3-carboxaldehyde 6 was prepared by N-acetylation of the corresponding indole-3-carboxaldehyde 5 with acetic anhydride in the presence of triethylamine in dichloromethane under reflux. Aldol condensation of 6 with quinuclidin-3-one hydrochloride in the presence of lithium diisopropylamide in tetrahydrofuran at −78°C afforded the (Z)-2-(N-acetylindol-3-ylmethylene)quinuclidin-3-one derivative, 7.11 Subsequent cleavage of the N-acetyl group by refluxing with 1N NaOH aqueous solution afforded the appropriate (Z)-2-(1H-indol-3-yl methylene)quinuclidin-3-one, 8. The substituted (Z)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-one derivatives 9a–9l were prepared in 85–90% yield by treating 8 with various substituted benzyl halides under phase-transfer catalytic (PTC) conditions using triethylbenzylammonium chloride and 50% w/v aqueous NaOH solution in dichloromethane. Reduction of the above substituted (Z)-2-(N-benzylindol -3-ylmethylene)quinuclidin-3-one derivatives 9a–9l with NaBH4 in methanol afforded the corresponding (Z)-(±)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-ol derivatives 10a–10l in 80–85% yield. All the synthesized compounds were characterized by 1H NMR and 13C NMR spectrometry and HRMS analysis.10 The geometry of the double bond in 3, 4 and 8 (R1=H) was established from x-ray crystallographic data.11, 12
Colony formation of tumor cells in culture was used to assess potency.9 HT-29 cells growing exponentially in T25 flasks (n=4) were exposed to DMSO/drug for 2 hrs with or without heat treatment (41°C). Drug was then washed off, and cells incubated in fresh medium for 14 days. Viable cells formed colonies (defined as greater than 50 cells) that were stained with crystal violet and counted. The plating efficiency was defined as the ratio of the number of colonies counted divided by the initial number of cells exposed to vehicle control (DMSO) for 2h at 37°C, and was typically ≥90%. The survival of cells exposed to vehicle alone for 2 hrs at 41°C was calculated from the ratio of the number of colonies counted divided by initial number of cells heated, corrected for plating efficiency. Survival following a 2hr, 41°C heat shock was not toxic: survival was not significantly different from plating efficiency (p > 0.05 Student's t test) and was arbitrarily set at 1.0. The surviving fraction of cells exposed to drug for 2 hrs at 41°C was calculated from the ratio of the number of colonies counted divided by the initial number of cells treated, corrected for plating efficiency and for survival following a 2h exposure to drug at 37°C. Cell survival following a 2 hr/37°C exposure to 150 µM of compounds 9a–9l and compounds 10b, 10c, 10e, & 10h–10k was 70% or greater. However, survival was significantly less than 70% following a 2 hr/37°C exposure to 150 µM of 10a, 10d, 10f, 10g, and 10l. Consequently these compounds were evaluated at a concentration of 50 µM, a concentration in which survival was 70% or more following a 2 hr/37°C exposure (Table 3).
Table 3.
Relative survival of cultured HT-29 cells and the relative potency of the substituted (Z)-(±)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-ol analogs at 50 µM concentration.
| Compound | Relative survival* of HT-29 cells at 41°C |
Relative Potency** |
|---|---|---|
| 10a | 0.59 | 1.69 |
| 10d | 0.59 | 1.69 |
| 10f | 0.07 | 14.28 |
| 10g | 0.70 | 1.43 |
| 10l | 0.76 | 1.00 |
| 2 | 1.00 | 1.00 |
| Vehicle | 1.00 | 1.00 |
Relative survival was calculated by correcting for vehicle effects andeffects of drugs at 37° C.
Relative potency is equal to the reciprocal value of relative survival value, and is statistically significant from vehicle control (p < 0.05 Student's t test).
Substitutents in the indole moiety of (Z)-2-(Nbenzylindol-3-ylmethylene)quinuclidin-3-one analogs 9a–e and 9h–l did not increase thermal sensitivity compared to vehicle control (p > 0.05 Student's t test). However, introduction of a 4-chloro or 4-fluoro substituent in the N-benzyl moiety (compounds 9f and 9g) significantly increased thermal sensitivity compared to solvent control (p < 0.05 Student's t test).
With regard to the (Z)-(±)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-ol analogs, compounds 10b, 10h, 10j and 10k increased thermal sensitivity relative to vehicle control (p < 0.05 Student's t test) but were not as effective as compound 2 (p > 0.05 Student's t test) as thermal sensitizers. (Z)-(±)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-ol analogs 10a, 10d, 10f, 10g, and 10l were tested at a concentration of 50 µM and all but 10l increased thermal sensitivity (p < 0.05 Student's t test). Analogs 10a, 10d, 10f, and 10g were significantly more effective than compound 2 when tested at 50 µM (p < 0.05 Student's t test), 10f being the most potent.
Based on the data from the HT-29 cell survival studies, the following observation can be made. Substituted (Z)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-ones are less effective sensitizers than the unsubstituted lead compound, (Z)-(±)-2-(N-benzylindol-3-ylmethyl-ene)-quinuclidin-3-ol (2). Introducing a 4-chloro (but not a 4-fluoro) substituent in the N-benzyl group afforded compound 10f, which exhibited the same magnitude of sensitization as compound 2, but required only one third the concentration (50 µM vs 150 µM) to achieve this effect.
In conclusion, a compound containing an N-benzylindole nucleus linked to a quinuclidin-3-ol moiety via a double bond with Z-geometry, and incorporating a lipophilic chloro substituent at the 4-position of the N-benzyl group, exhibits potent thermal sensitizing properties. The synthesis of such small molecules may afford promising tools of value in either clinical hyperthermia as radiation sensitizers for the treatment of recurrent tumors, or as enhancers of other cancer therapies that may be augmented by hyperthermia, such as chemotherapy and immunotherapy.
Table 1.
Relative survival of cultured HT-29 cells and the relative potency of the substituted (Z)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-one analogs at 150 µM concentration.
| Compound | Relative survival* of HT-29 cells at 41° C |
Relative potency** |
|---|---|---|
| 9a | 1.00 | 1.00 |
| 9b | 1.00 | 1.00 |
| 9c | 0.98 | 1.00 |
| 9d | 1.00 | 1.00 |
| 9e | 1.00 | 1.00 |
| 9f | 0.56 | 1.79 |
| 9g | 0.71 | 1.41 |
| 9h | 0.86 | 1.00 |
| 9i | 1.00 | 1.00 |
| 9j | 0.81 | 1.00 |
| 9k | 0.96 | 1.00 |
| 9l | 0.75 | 1.00 |
| 1 | 0.50 | 2.00 |
| Vehicle | 1.00 | 1.00 |
Relative survival was calculated by correcting for vehicle effects and effect of drugs at 37° C.
Relative potency is equal to the reciprocal value of relative survival value, and is statistically significant from vehicle control (p < 0.05 Student's t test).
Table 2.
Relative survival of cultured HT-29 cells and the relative potency of the substituted (Z)-(±)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-ol analogs at 150 µM concentration.
| Compound | Relative survival* of HT-29 cells at 41°C |
Relative Potency** |
|---|---|---|
| 10a | - | - |
| 10b | 0.59 | 1.69 |
| 10c | 1.00 | 1.00 |
| 10d | - | - |
| 10e | 0.78 | 1.00 |
| 10f | - | - |
| 10g | - | - |
| 10h | 0.61 | 1.64 |
| 10i | 0.90 | 1.00 |
| 10j | 0.52 | 1.92 |
| 10k | 0.58 | 1.72 |
| 10l | - | - |
| 2 | 0.06 | 16.70 |
| Vehicle | 1.00 | 1.00 |
Relative survival was calculated by correcting for vehicle effects and effects of drugs at 37° C.
Relative potency is equal to the reciprocal value of relative survival value, and is statistically significant from vehicle control (p < 0.05 Student's t test).
Acknowledgement
This research was supported by NIH/National Cancer Institute grant PO1 CA104457.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kampinga HH, Dikomey EH. Int.J. Radiat. Biol. 2001;77:399. doi: 10.1080/09553000010024687. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen OS, Horsman M, Overgaard J. Eur. J Cancer. 2001;37:1587. doi: 10.1016/s0959-8049(01)00193-9. [DOI] [PubMed] [Google Scholar]
- 3.Horsman M, Overgaard J. Clin Oncol (R Coll Radio) 2007;19:418. doi: 10.1016/j.clon.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Lepock JR. Inter. J Hyperthermia. 2005;21:681. doi: 10.1080/02656730500307298. [DOI] [PubMed] [Google Scholar]
- 5.Westra A, Dewey WC. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1971;19:467. doi: 10.1080/09553007114550601. [DOI] [PubMed] [Google Scholar]
- 6.Lepock JR, Frey HE, Rodahl AM, Kruuv JJ. Cell Physiol. 1988;137:14. doi: 10.1002/jcp.1041370103. [DOI] [PubMed] [Google Scholar]
- 7.Massicotte-Nolan P, Glofcheski DJ, Kruuv J, Lepock JR. Radiat. Res. 1981;87:284. [PubMed] [Google Scholar]
- 8.Nguyen VT, Morange M, Bensaude OJ. Biol. Chem. 1989;264:10487. [PubMed] [Google Scholar]
- 9.Sekhar KR, Sonar VN, Venkatraj M, Soumya S, Laszlo A, Sawani J, Horikoshi N, Higashikubo R, Bristow RG, Borrelli MJ, Crooks PA, Lepock JR, Roti Roti JL, Freeman ML. Cancer Res. 2007;67:695. doi: 10.1158/0008-5472.CAN-06-3212. [DOI] [PubMed] [Google Scholar]
- 10.Analytical data and yields for four of the most active compounds: (9f): 1H NMR (300 MHz, CDCl3 δ): 2.01 (m, 4H), 2.62 (p, J=3.3 Hz, 1H), 2.98 (m, 2H), 3.18 (m, 2H), 5.35 (s, 2H), 7.03–7.29 (m, 7H), 7.46 (s, 1H), 7.87 (m, 1H), 8.36 (s, 1H); 13C NMR (75 MHz, CDCl3 δ): 26.5, 40.6, 47.9, 50.5, 110.3, 110.8, 118.1, 119.4, 121.8, 123.0, 128.2, 129.6, 129.8, 133.8, 134.4, 135.2, 136.0, 140.5, 205.6; HRMS (EI+): m/z found 376.1339, calcd C23H21ClN2O (EI+) 376.1337; Yield: 89%: (10a): 1H NMR (300 MHz, CDCl3 δ): 1.45 (m, 2H), 1.70 (m, 1H) 1.94 (m, 2H), 2.08 (m, 1H), 2.28 (s, 3H), 2.85 (m, 1H), 3.07 (m, 3H), 4.42 (bs, 1H), 5.30 (s, 2H), 6.59 (s, 1H), 6.98–7.21 (m, 7H), 7.70 (m, 1H), 8.10 (s, 1H); 13C NMR (75 MHz, CDCl3 δ): 19.0, 21.8, 24.6, 30.6, 47.5, 48.2, 50.4, 71.2, 109.8, 113.6, 118.4, 119.8, 121.9, 126.6, 127.5, 128.2, 128.7, 134.4, 135.6, 136.6; HRMS (EI+): m/z found 358.2038, calcd C24H26N2O (EI+) 358.2039; Yield: 82%: (10d): 1H NMR (300 MHz, CDCl3 δ): 1.43 (m, 2H), 1.68 (m, 1H) 1.88 (m, 2H), 2.07 (m, 1H), 2.28 (s, 3H), 2.43 (s, 3H), 2.83 (m, 1H), 3.03 (m, 3H), 4.40 (bs, 1H), 5.26 (s, 2H), 6.56 (s, 1H), 6.93–7.08 (m, 6H), 7.50 (m, 1H), 8.04 (s, 1H); 13C NMR (75 MHz, CDCl3 δ): 19.2, 21.3, 21.8, 25.3, 31.0, 47.3, 48.2, 50.3, 71.4, 109.7, 113.5, 118.3, 123.4, 126.5, 128.2, 129.0, 129.4, 130.0, 134.1, 134.7, 137.1; HRMS (EI+): m/z found 372.2193, calcd C25H28N2O (EI+) 372.2196; Yield: 84%: (10f): 1H NMR (300 MHz, CDCl3 δ): 1.48 (m, 2H), 1.73 (m, 1H) 1.98 (m, 2H), 2.13 (m, 1H), 2.91 (s, 3H), 3.12 (m, 3H), 4.46 (bs, 1H), 5.31 (s, 2H), 6.66 (s, 1H), 7.03–7.29 (m, 7H), 7.69 (m, 1H), 8.18 (s, 1H); 13C NMR (75 MHz, CDCl3 δ): 19.0, 21.8, 24.6, 30.6, 47.5, 48.2, 50.4, 71.2, 109.8, 113.6, 118.4, 119.8, 121.9, 126.6, 127.5, 128.2, 128.7, 134.4, 135.6, 136.6; HRMS (EI+): m/z found 378.1492, calcd C23H23ClN2O (EI+) 378.1493; Yield: 83%.
- 11.Sonar VN, Parkin S, Crooks PA. Acta. Cryst. 2004;C60:o6. doi: 10.1107/s0108270103026076. [DOI] [PubMed] [Google Scholar]
- 12.Sonar VN, Parkin S, Crooks PA. Acta. Cryst. 2004;C60:o659. doi: 10.1107/S0108270104015847. [DOI] [PubMed] [Google Scholar]