Abstract
In rats, acute administration of SB-277011A, a highly selective dopamine (DA) D3 receptor antagonist, blocks cocaine-enhanced brain stimulation reward, cocaine-seeking behaviour and reinstatement of cocaine-seeking behaviour. Here, we investigated whether SB-277011A attenuates cocaine reinforcement as assessed by cocaine self-administration under variable-cost–variable-payoff fixed-ratio (FR) and progressive-ratio (PR) reinforcement schedules. Acute i.p. administration of SB-277011A (3–24 mg/kg) did not significantly alter cocaine (0.75 mg/kg/infusion) self-administration reinforced under FR1 (one lever press for one cocaine infusion) conditions. However, acute administration of SB-277011A (24 mg/kg, i.p.) progressively attenuated cocaine self-administration when: (a) the unit dose of self-administered cocaine was lowered from 0.75 to 0.125–0.5 mg/kg, and (b) the work demand for cocaine reinforcement was increased from FR1 to FR10. Under PR (increasing number of lever presses for each successive cocaine infusion) cocaine reinforcement, acute administration of SB-277011A (6–24 mg/kg i.p.) lowered the PR break point for cocaine self-administration in a dose-dependent manner. The reduction in the cocaine (0.25–1.0 mg/kg) dose–response break-point curve produced by 24 mg/kg SB-277011A is consistent with a reduction in cocaine’s reinforcing efficacy. When substituted for cocaine, SB-277011A alone did not sustain self-administration behaviour. In contrast with the mixed DA D2/D3 receptor antagonist haloperidol (1 mg/kg), SB-277011A (3, 12 or 24 mg/kg) failed to impede locomotor activity, failed to impair rearing behaviour, failed to produce catalepsy and failed to impair rotarod performance. These results show that SB-277011A significantly inhibits acute cocaine-induced reinforcement except at high cocaine doses and low work requirement for cocaine. If these results extrapolate to humans, SB-277011A or similar selective DA D3 receptor antagonists may be useful in the treatment of cocaine addiction.
Keywords: dopamine, D3 receptor, drug addiction, meso-accumbens, reward
Introduction
The dopamine (DA) D3 receptor is relatively restricted, neuroanatomically, to the mesolimbic DA system (Murray et al., 1994; Levant, 1997; Stanwood et al., 2000), a neural system critical to drug reinforcement and relapse (Wise, 1996a; Shalev et al., 2002; Wise & Gardner, 2002). For this and other reasons, the D3 receptor has been suggested as a therapeutic target for anti-addiction medications (Caine & Koob, 1993, 1995; Caine et al., 1997; Levant, 1997). Unfortunately, investigation of this suggestion has been hampered by lack of selective D3 compounds (for review, see Heidbreder et al., 2005). Recently, however, a high-potency high-selectivity competitive D3 receptor antagonist has been developed: trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolinecarboxamide (SB-277011A) (Stemp et al., 2000). This compound has 80- to 100-fold selectivity over other DA receptors, high affinity for the human (pKi 7.95) and rat (pKi 7.97) cloned DA D3 receptor, in vitro D3/D2 affinity ratios for human and rat of 120 and 80, respectively, and 100-fold selectivity over 66 other receptors, enzymes, ion channels and transporters in the central nervous system (Reavill et al., 2000; Stemp et al., 2000; Heidbreder et al., 2005). SB-277011A also readily passes the blood–brain barrier, is orally bioavailable and has shown promising results in animal models relating to addiction. For example, SB-277011A inhibits cocaine-seeking behaviour as measured by second-order reinforcement (Di Ciano et al., 2003), cocaine- or stress-triggered reinstatement of cocaine-seeking behaviour (Vorel et al., 2002; Xi et al., 2004) and nicotine-triggered reinstatement of nicotine-seeking behaviour (Andreoli et al., 2003b).
In contrast to its seemingly unambiguous effects on drug-seeking behaviour, it remains unclear whether SB-277011A also inhibits acute drug-induced reinforcement. On one hand, SB-277011A dose-dependently inhibits cocaine- or nicotine-enhanced brain stimulation reward (Vorel et al., 2002; Campos et al., 2003) and cocaine- or heroin-induced conditioned place preference (Vorel et al., 2002; Ashby et al., 2003), suggesting a D3 antagonist-induced reduction in cocaine-, heroin-, and nicotine-induced reinforcement. On the other hand, SB-277011A appears to have no effect on cocaine or nicotine self-administration under continuous reinforcement conditions (Andreoli et al., 2003a; Di Ciano et al., 2003; Gál & Gyertyán, 2003), one of the most widely used animal models to assess drug reinforcement.
Therefore, we here tested whether D3 receptor blockade induced by SB-277011A significantly alters cocaine reinforcement, and whether SB-277011A itself has any reinforcing properties, using variants of the intravenous drug self-administration animal model which have heretofore not been used to study D3 receptor blockade and cocaine-induced reinforcement. Specifically, we tested whether altering the fixed ratio (FR; number of lever presses required to obtain the reward) reinforcement level for cocaine self-administration, or alternatively the amount of cocaine delivered per reinforcement, alters the effects of SB-277011A on cocaine self-administration. We also tested whether SB-277011A alters the break point for cocaine self-administration under progressive-ratio (PR; increasing number of lever presses for successive rewards) reinforcement. Finally, we examined SB-277011A’s effects on locomotor activity and motor coordination in the same dose range (3–24 mg/kg i.p.) used in the experiments with FR and PR reinforcement schedules. This was done to determine whether SB-277011A, at doses used in the present study, exhibits D2 antagonist actions.
Materials and methods
Subjects
For all FR and PR experiments carried out under experimental procedures 1–4 (see below), male Long-Evans rats (Charles River Laboratories, Raleigh, NC, USA), experimentally naive at the start of the experiments and initially weighing 250–300 g, were used. They were housed individually in a climate-controlled animal colony room on a reversed light–dark cycle (lights on at 19.00 h, lights off at 07.00 h) and had access ad libitum to food and water. The animals were maintained in a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and all experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, 1996) and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse of the U.S. National Institutes of Health. For the motor activity and motor coordination experiments carried out under experimental procedure 5 (see below), male Wistar rats (Charles River Deutschland, Sulzfeld, Germany) experimentally naive at the start of experiments were used. They were housed individually in a climate-controlled environment with lights on from 06.00 to 18.00 h. Water was available ad libitum and animals were maintained at a constant body weight of 240–260 g. All experiments carried out under experimental procedure 5 were performed under a Project License obtained according to Italian law regulating animal experimentation (Article 7, Legislative Decree 116, 27 January 1992), which acknowledges European Directive 86/609/EEC for the protection of animals used for experimental and other scientific purposes.
Surgery
Animals for experimental procedures 1–4 were prepared for experimentation by surgical catheterization of the right external jugular vein. The venous catheters were constructed of microrenathane (Braintree Scientific Inc., Braintree, MA, USA). The surgical catheterization was carried out under sodium pentobarbital anaesthesia (65 mg/kg, i.p.), using aseptic surgical technique. Supplemental anaesthesia (20 mg/kg pentobarbital, i.p.) was given if needed during surgery. The right jugular vein was exposed by blunt dissection and a catheter was inserted into the vein and sutured into place. The distal portion of the catheter was passed subcutaneously to the top of the skull, where it exited into a connector (a modified 24-gauge cannula; Plastics One, Roanoke, VA, USA) mounted on the skull with jeweler’s screws and dental acrylic. After the connector was securely skull-mounted and the acrylic dry, the incision was closed with sutures. An obturator and cannula cap were placed over the opening of the skull-mounted connector during the postsurgical recovery period and at all other times when the rats were not in a self-administration session. During experimental sessions, the catheter was connected to the infusion pump via tubing encased in a protective metal spring, from the head-mounted connector to the top of the experimental chamber. To prevent clogging, the catheters were flushed daily with a gentamicin–heparin–saline solution (30 IU/mL heparin; ICN Biochemicals, Cleveland, OH, USA).
Apparatus
The experiments carried out under experimental procedures 1–4 were conducted in operant response test chambers (32 × 25 × 33 cm), each equipped with a house light, ventilator fan, drug infusion pump (3.33 r.p.m. motor, 10 mL syringe) and liquid swivel with counterbalance arm. Each test chamber had two levers located 6.5 cm above the floor, one active and one inactive. Depression of the active lever activated the infusion pump; depression of the inactive lever was counted but had no other consequence. A cue light and a speaker were located 12 cm above the active lever. At the start of each 3-h test session, the house light was turned on. When the animal made a lever-pressing response that resulted in a drug infusion, the cue light was illuminated and a cue sound (tone) was turned on for the duration of the infusion (i.e. drug-paired environmental cues). The equipment was obtained from MED Associates (Georgia, VT, USA). Scheduling of experimental events and data collection was accomplished using MED Associates software. For the locomotor activity experiments carried out under experimental procedure 5, animals were tested in infrared locomotor activity chambers that monitored the animals’ horizontal and vertical movements. Each such infrared activometer (TSE Systems, Bad Homburg, Germany) was 46 × 46 × 26 cm in size and was equipped with two infrared frames (4.5 and 13.5 cm height, respectively) with 16 beams per axis giving spatial resolution of 1.4 cm and temporal resolution of 20 ms. For the other motor activity and coordination experiments carried out under experimental procedure 5, animals were tested using: (i) a behavioural observation arena 23 × 35 × 20 cm in size; (ii) a wooden ‘log’ (8 × 8 × 12 cm) for testing ‘paws on log’ catalepsy (see below under ‘Experimental procedure 5: effects of SB-277011A on locomotor activity and motor coordination’); and (iii) a ‘rotarod’ rotating cylinder device for testing motor coordination on a moving surface (see below under ‘Experimental procedure 5: effects of SB-277011A on locomotor activity and motor coordination’).
General procedure
For experiments carried out under experimental procedures 1–4, animals were first allowed to recover from surgery. Then, each rat was placed into a test chamber and allowed to lever-press under an FR1 (one lever press per cocaine infusion) reinforcement schedule for intravenous cocaine (1.0 mg/kg/infusion) delivered in 0.08 mL over 4.6 s. During the 4.6-s infusion time, additional responses on the active lever were recorded but did not lead to additional infusions. Each session lasted 3 h. This schedule (FR1) was used for 3–5 days until regular patterns of cocaine self-administration were established. Then, subjects were randomly assigned to four different experimental groups, for testing under the four different experimental procedures 1–4. In all experiments, systemic SB-277011A was given 60 min prior to testing because its peak drug level in the rat brain is achieved ≈60 min after systemic administration (Austin et al., 2001). For the procedures used in experiments carried out under experimental procedure 5, see below under ‘Experimental procedure 5: effects of SB-277011A on locomotor activity and motor coordination’.
Experimental procedure 1: effects of SB-277011A on cocaine self-administration reinforced under an FR1 schedule
After the initial 3–5 days of cocaine self-administration, subjects (n = 7) were allowed to continue cocaine self-administration with a lower dose of cocaine (0.75 mg/kg/infusion) under the FR1 schedule until the following criteria for stable cocaine-maintained responding were met: < 10% variability in interresponse interval and < 10% variability in number of presses on the active lever for at least 3 consecutive days. To avoid cocaine overdose during self-administration, each animal was limited to a maximum of 50 cocaine infusions per session. After stable rates of responding were established, each subject randomly received one of four doses of SB-277011A (3, 6, 12 or 24 mg/kg, i.p.) or vehicle (1 mL of 25% 2-hydroxypropyl-β-cyclodextrin) 1 h prior to the test session. Then, animals received an additional 5–7 days of cocaine self-administration alone until the baseline response rate was re-established, prior to testing the next dose of SB-277011A. The order of testing for various doses of SB-277011A was counterbalanced according to a Latin square design. The effect of SB-277011A on cocaine self-administration was evaluated by comparing the number of cocaine infusions per test session.
Experimental procedure 2: effects of SB-277011A on cocaine self-administration reinforced under an FR10 schedule
After stable cocaine self-administration at 0.75 mg/kg/infusion was established, cocaine reinforcement was changed from FR1 to FR2 and the unit dose of cocaine per infusion was lowered to 0.5 mg/kg. These self-administration conditions were maintained for 3–5 days, and then cocaine self-administration was changed to an FR10 reinforcement schedule for one of three unit doses of cocaine (0.125, 0.25 and 0.5 mg/kg/infusion, n = 7). The order in which the animals received the three different unit cocaine doses was according to a Latin square rotation. After stable rates of responding (same as above) were established, rats received either vehicle or SB-277011A (24 mg/kg) pretreatment 1 h prior to the test session. The effect of SB-277011A (24 mg/kg, i.p.) on cocaine self-administration was assessed by comparing the numbers of cocaine infusions and responses on the active lever on vehicle days vs. SB-277011A days.
Experimental procedure 3: effects of SB-277011A on cocaine self-administration reinforced under a progressive ratio schedule
Initial cocaine self-administration under FR1 and FR2 reinforcement was the same as outlined above under experimental procedure 2. Then, subjects were assigned to six subgroups. Three of the six subgroups were used to determine the effects of a fixed dose of SB-277011A (24 mg/kg) on the PR break point for cocaine self-administration of three different doses of intravenous cocaine (0.25, 0.5 and 1.0 mg/kg/infusion; n = 6). The other three subgroups were used to determine the effects of different doses of SB-277011A (6, 12 and 24 mg/kg; n = 7) on PR break point for cocaine self-administration at a fixed dose of cocaine (0.5 mg/kg/infusion). For these PR breakpoint studies, the work requirement imposed upon the animal (lever presses) in order to receive a single i.v. cocaine infusion was progressively raised within each test session (see details in Richardson & Roberts, 1996) according to the following PR series: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492 and 603 until the break point was reached. The break point was defined as the number of completed lever presses prior to a 1-h period when no infusions were earned by the animal. Animals were allowed to continue daily sessions of cocaine self-administration under PR reinforcement until day-to-day variability in break point fell within 1–2 ratio increments for 3 consecutive days, with each daily test session lasting 3 h. Once stable break-point behaviour was established, each subject received one vehicle injection 3 days before SB-277011A administration. The effects of 24 mg/kg SB-277011A on cocaine self-administration break-point behaviour at the three different doses of i.v. cocaine (0.25, 0.5, 1.0 mg/kg/infusion) and the effects of different doses of SB-277011A (6, 12, 24 mg/kg) on cocaine self-administration break-point behaviour at the fixed dose of 0.5 mg/kg/infusion were then assessed in distinct subgroups of animals.
Experimental procedure 4: SB-277011A self-administration testing in rats formerly self-administering cocaine
After a stable pattern of daily cocaine (0.5 mg/kg/infusion) self-administration was established under FR2 reinforcement for at least 3 consecutive days, the animals were divided into three groups (n = 8 each). For the first group, cocaine (0.5 mg/kg/infusion) was available for self-administration the next day, for the usual 3-h test session. For the second group, cocaine was replaced by SB-277011A (1.25 mg/kg/infusion) the next day. For the third group, cocaine was replaced by saline (0.08 mL/infusion) the next day. The ability of SB-277011A to maintain self-administration behaviour was assessed by comparing the numbers of SB-277011A infusions to the levels established by the cocaine animals and by the saline animals, and the patterns of responding during daily test sessions. The dose of SB-277011A was chosen on two grounds. First, SB-277011A’s maximum solubility in the 25% 2-hydroxypropyl-β-cyclodextrin solution used as vehicle in these experiments is ≈12 mg/mL, making 1.25 mg/kg/infusion the maximal unit dose that could be used and still remain within the i.v. infusion unit volumes and infusion times used in these self-administration experiments. Second, as the self-infusions in this experiment were via the i.v. route, the chosen i.v. dose of 1.25 mg/kg/infusion is similar to ≈6–8 mg/kg via the i.p. route, which is within the range of SB-277011A doses (3–12 mg/kg i.p.) found to be behaviourally effective in previous testing on cocaine-enhanced brain-stimulation reward, cocaine-induced conditioned place preference and cocaine-triggered reinstatement of cocaine-seeking behaviour (Vorel et al., 2002), or in the present testing on cocaine self-administration under PR reinforcement (see Fig. 3D).
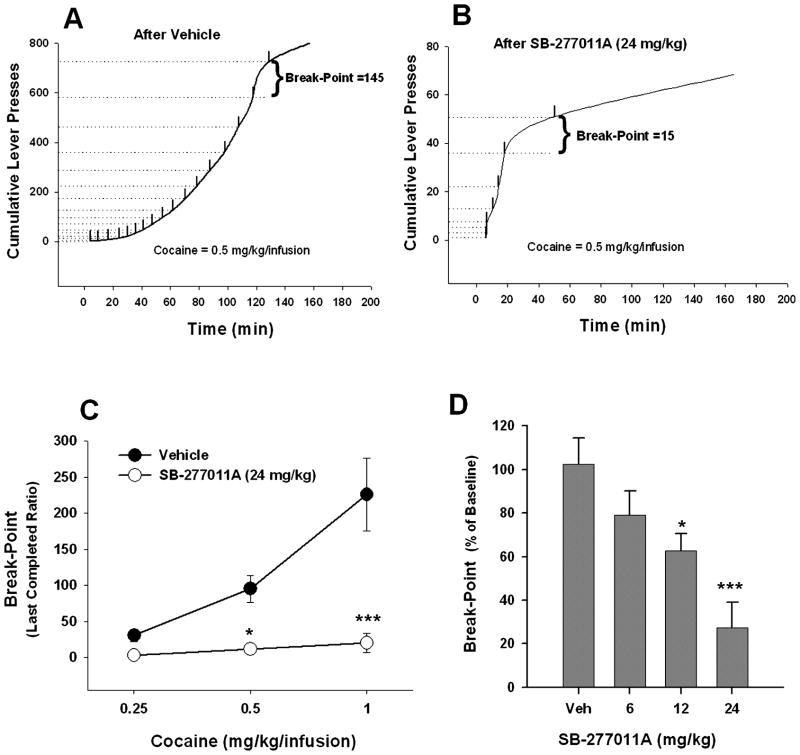
Fig. 3.
Effect of SB-277011A on cocaine self-administration under PR reinforcement. (A,B) Representative records of an individual animal, illustrating the effects of SB-277011A (24 mg/kg, i.p.) on the PR break point for cocaine self-administration. Each short upward mark on the cumulative lever-press records indicates one cocaine infusion. The PR break point was defined as the last completed ratio requirement (lever-presses) for a unit dose of infused cocaine. (C) Mean cocaine dose-dependent self-administration under PR reinforcement, illustrating the cocaine-dose-orderly reduction in PR break point produced by SB-277011A (24 mg/kg, i.p., n = 6 for each cocaine dose group). *P < 0.05, ***P < 0.001, individual group comparison between vehicle and SB-277011A, using the Tukey (a) statistic. (D) Percentage change in PR break point for cocaine self-administration (0.5 mg/kg/infusion) produced by 6, 12 or 24 mg/kg SB-277011A pretreatment on test day compared to PR break point for cocaine self-administration after vehicle pretreatment on test day. *P < 0.05, ***P < 0.001, individual group comparisons using the Tukey (a) statistic, when compared to the vehicle (Veh) pretreatment group.
Experimental procedure 5: effects of SB-277011A on locomotor activity and motor coordination
In a final series of experiments, the effects of SB-277011A on locomotor activity and motor coordination were assessed using the same doses (3–24 mg/kg i.p.) as in experimental procedures 1–4, and compared to the effects produced by the mixed DA D2/D3 receptor antagonist haloperidol (1 mg/kg i.p.). Locomotor activity was assessed in the infrared locomotor activity chambers described above under ‘Apparatus’. Rats were placed into these activity chambers and locomotor activity was measured for 30 min. Other aspects of motor activity and motor coordination were assessed by a battery of tests comprising: (i) behavioural observation by an experienced observer who rated each animal’s behaviour in the 23 × 35 × 20 observation arena according to a list of predefined behavioural patterns; the observer-rated behaviours included rearing (lifting forepaws off the floor) and catalepsy; (ii) a second catalepsy test during which each rat was placed with its forepaws on the wooden catalepsy test ‘log’ (8 × 8 × 12 cm) and tested for a maximum of three 30-s trials; if the rat failed to descend from the log within 30 s, catalepsy testing was stopped; and (iii) a rotarod test during which each rat was placed on a rotating cylinder (8 r.p.m) and had to move forward; if the rat stayed on the cylinder for 2 min, the rotarod test was stopped; each animal was tested for a maximum of three 2-min trials, and the maximum time on the cylinder from all trials was taken as the final result.
Drugs
Cocaine HCl (Sigma Chemical Co., Saint Louis, MO, USA) was dissolved in physiological saline. SB-277011A (trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinoli-necarboxamide) was provided by GlaxoSmithKline Pharmaceuticals (Verona, Italy, and Harlow, Essex, UK) and was placed into solution using 25% (2-hydroxypropyl)-β-cyclodextrin (Sigma/RBI, St Louis, MO, USA). The 25% (2-hydroxypropyl)-β-cyclodextrin alone was used as vehicle for systemic (i.p.) injections of SB-277011A. Haloperidol was obtained from Janssen-Cilag NV (Berchem, Belgium), was prepared in a 5 mg/mL solution and was administered i.p. in a volume of 1 mL/kg 30 min prior to the start of behavioural tests.
Data analyses
All behavioural data are presented as means (± SEM), and standard multivariate statistical procedures (Winer, 1962; Kirk, 1982) were used for all data analyses. For the experiments in which the effects of SB-277011A on FR1 cocaine self-administration (Fig. 1) or on PR cocaine self-administration (Fig. 3D) were assessed, one-way ANOVA for repeated measurements was used. For the experiments in which the effects of SB-277011A on FR10 cocaine self-administration (Fig. 2B) or on PR cocaine self-administration (Fig. 3C) were assessed, and for the experiments in which SB-277011A was tested for its ability to sustain self-administration behaviour (Fig. 4A), two-way ANOVA for repeated measurements was used. For the data summed-over-time reflecting the ability or inability of SB-277011A to sustain self-administration behaviour (Fig. 4B), one-way ANOVA was used to compare the summed data for cocaine self-administration vs. SB-277011A self-administration or saline self-administration. Post-ANOVA individual group comparisons were carried out using the Tukey (a) statistical procedure [also known as the Tukey Honestly Significant Difference (HSD) procedure]. The motor activity and motor coordination data were analysed using a two-step general linear model analysis for each parameter. First, a general linear model analysis was performed including the haloperidol (1 mg/kg) and vehicle groups. The effects of SB-277011A were analysed by means of a second general linear model analysis including the three doses (3, 12 and 24 mg/kg) of SB-277011A and the vehicle group (dose as linear factor, vehicle as zero-dose). Statistical significance was set at a probability level of P < 0.05 for all analyses.
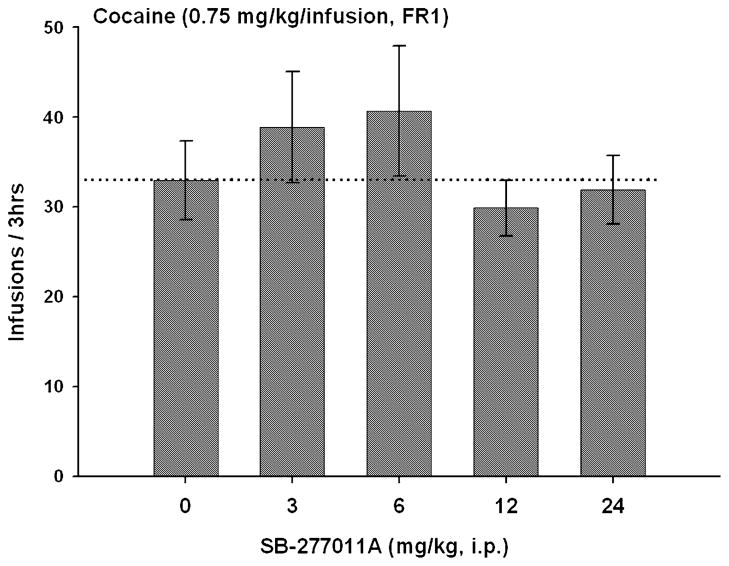
Fig. 1.
Effect of systemic administration of SB-277011A on cocaine self-administration (0.75 mg/kg/infusion, n = 7) under a continuous reinforcement (FR1) schedule. SB-277011A (3–24 mg/kg, i.p.) did not alter cocaine self-administration under FR1 cocaine reinforcement conditions. One-way ANOVA for repeated measurements over the SB-277011A dose range revealed no statistically significant effect of SB-277011A on cocaine self-administration (F4,24 = 1.87, P = 0.15). Each bar represents number of self-infusions (± SEM) of cocaine.
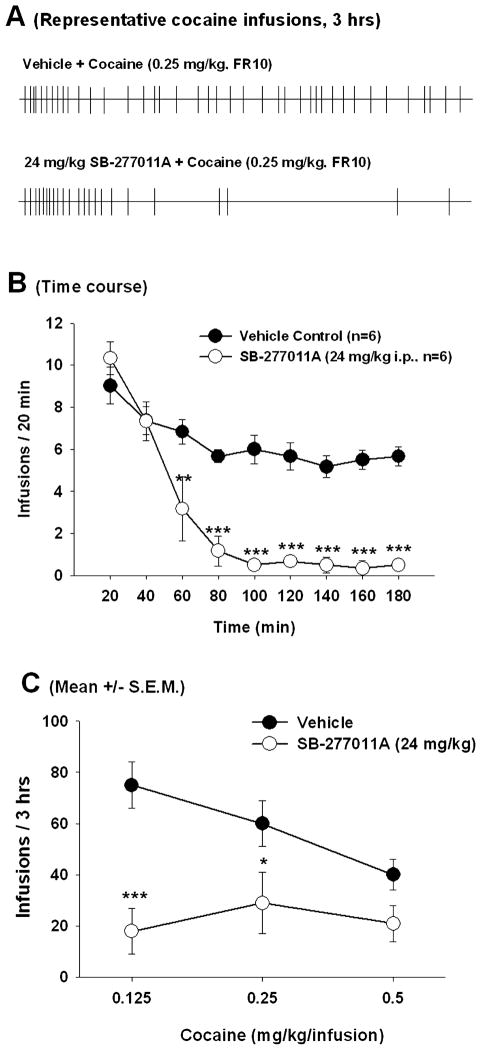
Fig. 2.
Effect of SB-277011A on cocaine self-administration under an FR10 reinforcement schedule for unit cocaine reinforcement infusions of 0.125, 0.25 or 0.5 mg/kg/infusion. (A) Representative event records of cocaine infusions (each vertical line represents an earned 0.5 mg/kg cocaine infusion under FR10 reinforcement conditions), illustrating a typical pattern of extinction responding after systemic SB-277011A (24 mg/kg, i.p.) administration. (B) Time courses of cocaine (0.25 mg/kg/infusion) self-administration after pretreatment with either the vehicle (25% 2-hydroxypropyl-β-cyclodextrin) or 24 mg/kg SB-277011A. (C) Mean number of infusions (± SEM, n = 7) during the 3-h session after administration of the vehicle or SB-277011A (24 mg/kg, i.p.) 1 h prior to the test session. *P < 0.05, **P < 0.01, ***P < 0.001; individual group comparisons using the Tukey (a) statistic, when compared with the vehicle treatment group (see more statistical details in the Results section).
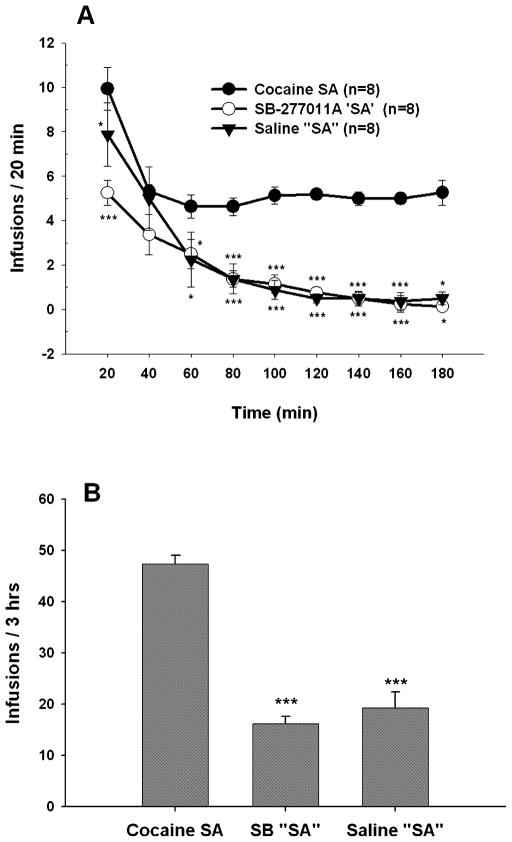
Fig. 4.
Effect of substituting cocaine, SB-277011A or saline for cocaine in animals proficient at maintaining cocaine self-administration behaviour. (A) Time-courses of cocaine, SB-277011A or saline self-administration behaviour by 20-min intervals. Two-way ANOVA for repeated measures revealed a statistically significant difference between cocaine (0.5 mg/kg/infusion) self-administration behaviour and SB-277011A (1.25 mg/kg/infusion) self-administration behaviour, and between cocaine self-administration behaviour and saline (0.08 mL/infusion) self-administration behaviour. However, there were no significant differences between SB-277011A self-administration behaviour and saline self-administration behaviour at any time point tested. *P < 0.05, ***P < 0.001, individual group comparisons, when compared with cocaine self-administration group at each time point marked. (B) Mean numbers of drug infusions summed over the 3-h test session for cocaine (0.5 mg/kg/infusion), SB-277011A (1.25 mg/kg/infusion) or saline (0.08 mL/infusion) self-administration behaviour. Both the extinction-like pattern of responding (A) and the cumulative 3-h reduction in self-administration behaviour (B) after substitution of SB-277011A suggest that SB-277011A itself has no reinforcing effect at the test dose of 1.25 mg/kg/infusion. ***P < 0.001, individual group comparisons, when compared with cocaine self-administration group. SA, self-administration; SB, SB-277011A.
Results
Effects of SB-277011A on cocaine self-administration reinforced under an FR1 schedule
SB-277011A (3, 6, 12 or 24 mg/kg) had no significant effect on cocaine self-administration behaviour at a unit reinforcement dose of 0.75 mg/kg/infusion under FR1 reinforcement conditions (Fig. 1). One-way ANOVA for repeated measurements over the SB-277011A dose range revealed no statistically significant effect of SB-277011A on cocaine self-administration under these reinforcement conditions (F4,24 = 1.87, P = 0.15).
Effects of SB-277011A on cocaine self-administration reinforced under an FR10 schedule
In contrast to the results described immediately above (FR1 schedule of reinforcement and 0.75 mg/kg/infusion cocaine reinforcement unit dose), when the unit dose of cocaine was lowered from 0.75 mg/kg/infusion to a dose-range of 0.125–0.5 mg/kg/infusion and the work demand for cocaine self-administration was increased from the FR1 to FR10, SB-277011A (24 mg/kg) produced a significant reduction in both total number of active lever responses (data not shown) and total number of cocaine infusions (Fig. 2). Figure 2A shows representative event records while Fig. 2B shows the averaged time courses of cocaine (0.25 mg/kg/infusion) self-administration under FR10 conditions after vehicle or 24 mg/kg SB-277011A administration, demonstrating a typical extinction pattern of cocaine-seeking behaviour, i.e. an initial burst-like increase in responding followed by a progressive decrease in cocaine self-administration behaviour after SB-277011A administration. This pattern suggests a significant reduction or blockade of cocaine reinforcement by SB-277011A. Two-way ANOVA for repeated measurements performed on the data shown in Fig. 2B revealed a significant main effect of SB-277011A on cocaine self-administration behaviour (F1,5 = 358.87, P < 0.001), and a significant SB-277011A × time interaction (F1,8 = 5.65, P < 0.001). Figure 2C shows the mean number of infusions (± SEM, n = 7) during the 3-h session after administration of vehicle or SB-277011A (24 mg/kg, i.p.) 1 h prior to the test session. Two-way ANOVA for repeated measurements performed on the data shown in Fig. 2C revealed a significant main effect of SB-277011A on drug-seeking behaviour (F1,6 = 17.22, P = 0.006), and a significant SB-277011A × cocaine unit-infusion dose interaction (F2,12 = 6.46, P = 0.012). Individual Tukey (a) group comparisons revealed statistically significant SB-277011A-induced inhibition of cocaine self-administration behaviour at 0.125 mg/kg/infusion and 0.25 mg/kg/infusion cocaine, but not at 0.5 mg/kg/infusion cocaine (Fig. 2C): at 0.125 mg/kg/infusion cocaine, vehicle vs. SB-277011A q = 7.47, P < 0.001; at 0.25 mg/kg/infusion cocaine, vehicle vs. SB-277011A q = 3.85, P < 0.05; at 0.5 mg/kg/infusion cocaine, vehicle vs. SB-277011A q = 2.48, P = 0.10.
Effects of SB-277011A on cocaine self-administration reinforced under a progressive ratio schedule
SB-277011A significantly lowered the break point for cocaine self-administration behaviour reinforced on a PR schedule. Figure 3A and B shows representative individual cumulative response records for cocaine self-administration after vehicle or SB-277011A administration in the same animal, illustrating a typical PR break-point last-completed-ratio of 145 for cocaine self-administration under vehicle conditions (Fig. 3A) and a dramatically lower PR break-point last-completed-ratio of 15 for cocaine self-administration after administration of SB-277011A (24 mg/kg, i.p.; Fig. 3B). Figure 3C illustrates the observed SB-277011A-induced decrease in PR break point, defined as last-completed-ratio prior to a 1-h period during which no cocaine infusions were earned by the animal, for three different unit doses of cocaine reinforcement. At a dose of 24 mg/kg i.p., SB-277011A virtually abolished cocaine’s reinforcing value, as evidenced by the reduction to near-zero of the PR break point for cocaine self-administration at all amounts of cocaine reinforcement tested (0.25, 0.5 and 1 mg/kg/infusion; Fig. 3C). Two-way ANOVA for repeated measurements revealed a statistically significant SB-277011A vs. vehicle main effect (F1,5 = 28.09, P = 0.003), a statistically significant cocaine reinforcement dose main effect (F2,10 = 18.41, P < 0.001) and a statistically significant SB-277011A treatment × cocaine reinforcement dose interaction (F2,10 = 7.53, P < 0.025). Individual group comparisons using the Tukey (a) statistic revealed statistically significant SB-277011A-induced decreases in PR break point for cocaine self-administration at 1.0 mg/kg/infusion of cocaine (q = 8.65, P < 0.001) and at 0.5 mg/kg/infusion of cocaine (q = 3.52, P = 0.025), but not at 0.25 mg/kg/infusion of cocaine (q = 1.16, P = 0.43). Comparison of the effects of vehicle and SB-277011A (6, 12 or 24 mg/kg i.p.) on PR break point for cocaine self-administration revealed a dose-orderly inhibitory effect of SB-277011A on cocaine self-administration under PR reinforcement (Fig. 3D). One-way ANOVA performed on the data depicted in Fig. 3D revealed a significant SB-277011A-induced reduction in PR break point for cocaine self-administration (F3,24 = 10.45, P < 0.001). Individual group comparisons using the Tukey (a) statistical test revealed a statistically significant difference between PR break point for cocaine self-administration after vehicle vs. after 12 mg/kg SB-277011A (q = 4.17, P = 0.033), and between PR break point for cocaine self-administration after vehicle vs. after 24 mg/kg SB-277011A (q = 7.71, P < 0.001).
Efficacy of SB-277011A itself to sustain self-administration
To determine whether SB-277011A has reinforcing effects per se, cocaine was replaced by SB-277011A (1.25 mg/kg/infusion), saline (0.08 mL/infusion) or cocaine (0.5 mg/kg/infusion) in three separate groups of rats (n = 8 each) already experienced and behaviourally stable at cocaine self-administration under FR2 reinforcement. Figure 4 shows the results of such substitutions. SB-277011A, at this dose, did not sustain stable self-administration; rather, the self-administration behaviour underwent gradual extinction over the 3-h test period (Fig. 4A). The extinction pattern was essentially identical to that seen when saline was substituted for cocaine (Fig. 4A). In contrast, cocaine maintained self-administration behaviour, showing the typical loading phase of increased self-administration during the first 20 min of self-administration opportunity followed by stable cocaine self-administration thereafter (Fig. 4A). Two-way ANOVA for repeated measurements on the second factor revealed a significant main effect of substituting SB-277011A or saline for cocaine (F2,21 = 68.10, P < 0.001) and, importantly, a significant time × drug interaction (F16,168 = 2.14, P = 0.009). Individual group comparisons using the Tukey (a) test revealed statistically significant differences between drug-taking behaviour for cocaine and for SB-277011A at all but one 20-min point over the entire 3-h test period (q = 7.65, P < 0.001 at 20 min; q = 3.16, P = 0.065 at 40 min; q = 3.88, P = 0.017 at 60 min; q = 5.30, P < 0.001 at 80 min; q = 6.93, P < 0.001 at 100 min; q = 7.65, P < 0.001 at 120 min; q = 7.34, P < 0.001 at 140 min; q = 7.55, P < 0.001 at 160 min; and q = 3.98, P = 0.014 at 180 min; Fig. 4A). Additional individual group comparisons using the Tukey (a) test revealed statistically significant differences between drug-taking behaviour for cocaine and for saline at all but one 20-min point over the entire 3-h test period (q = 3.37, P = 0.046 at 20 min; q = 0.51, P = 0.931 at 40 min; q = 3.47, P = 0.038 at 60 min; q = 5.30, P < 0.001 at 80 min; q = 5.91, P < 0.001 at 100 min; q = 7.24, P < 0.001 at 120 min; q = 7.34, P < 0.001 at 140 min; q = 6.93, P < 0.001 at 160 min; and q = 3.37, P = 0.046 at 180 min; Fig. 4A). Further individual group comparisons using the Tukey (a) test revealed that there were no statistically significant differences between drug-taking behaviour for SB-277011A and drug-taking behaviour for saline at any 20-min test point over the entire 3-h test period (Fig. 4A). Figure 4B shows the total number of infusions during the 3-h test period for cocaine, saline and SB-277011A. One-way ANOVA revealed a statistically significant difference between the three drug groups (F2,21 = 68.12, P < 0.001). Individual group comparisons using the Tukey (a) test revealed statistically significant differences between total cocaine self-administration and total SB-277011A self-administration (q = 14.98, P < 0.001) and between total cocaine self-administration and total saline self-administration (q = 13.49, P < 0.001), but no differences between total SB-277011A self-administration and total saline self-administration (q = 1.50, P = 0.55).
Effects of SB-277011A on locomotor activity and motor coordination: a comparative profile vs. haloperidol
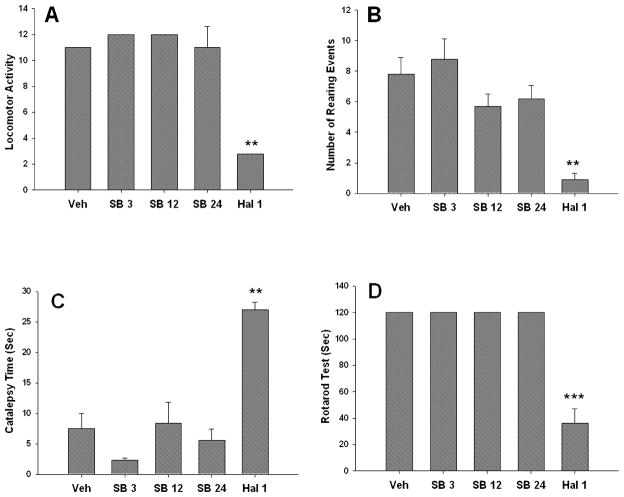
Haloperidol significantly reduced locomotor activity (F1,23 = 18.52, P < 0.001; Fig. 5A) and observer-rated rearing behaviour in the behavioural observation arena (F1,23 = 23.9, P < 0.001; Fig. 5B). Haloperidol also produced a significant increase in observer-rated cataleptic behaviour in the behavioural observation arena compared to the vehicle group (F1,23 = 15.4, P < 0.001; data not shown). In contrast, SB-277011A failed to alter any of these parameters [locomotor activity, F1,47 = 0.11, ns (Fig. 5A); observer-rated rearing: F1,47 = 1.51, ns (Fig. 5B); observer-rated catalepsy: F1,47 = 1.61, ns (data not shown)]. The second catalepsy test, in which time to descend from the wooden ‘log’ was measured, showed that the time (s) to descend was significantly increased by haloperidol (F1,23 = 39.9, P < 0.001; Fig. 5C). In contrast, SB-277011A produced no cataleptic behaviour in this ‘paws on log’ catalepsy test (F1,47 = 0.07, ns; Fig. 5C). The rotarod test showed that haloperidol significantly reduced the time (s) spent on the cylinder (F1,23 = 58.4, P < 0.0001; Fig. 5D). In contrast, SB-277011A did not affect the animals’ performance in the rotarod test under the same conditions (F1,47 = 0.06, ns; Fig. 5D).
Fig. 5.
Effect of haloperidol (1 mg/kg i.p.) and SB-277011A (0, 3, 12 or 24 mg/kg i.p.) on locomotor activity as assessed by behaviour in (A) an infrared activity monitoring chamber, (B) observer-rated rearing behaviour, (C) catalepsy time and (D) motor coordination performance in the rotarod test (time spent on the rotarod cylinder). Haloperidol significantly reduced locomotor activity (**P < 0.001) and rearing behaviour (**P < 0.001) compared to vehicle. Haloperidol treatment also significantly increased catalepsy time (**P < 0.001) and significantly impaired motor coordination in the rotarod test (***P < 0.0001) compared to vehicle. In contrast, SB-277011A failed to alter any of these in vivo behavioural parameters. Veh, vehicle; SB 3, SB-277011A 3 mg/kg i.p.; SB 12, SB-277011A 12 mg/kg i.p.; SB 24, SB-277011A 24 mg/kg i.p.; Hal 1, haloperidol 1 mg/kg i.p.
Discussion
The present findings show that the highly potent and highly selective DA D3 receptor antagonist SB-277011A attenuates cocaine reinforcement in a dose-orderly manner, as assessed by both PR and variable-cost–variable-payoff FR reinforcement schedules of cocaine self-administration.
A variety of preclinical behavioural paradigms have been developed to model various aspects of addictive behaviour (Wise & Gardner, 2004). Of these, the self-administration paradigm used in the present experiments offers the most obvious face validity to the human situation. At present levels of understanding, addiction is believed to be a disorder of DA-dependent habit-formation (Di Chiara, 1999; Everitt et al., 2001; Robbins & Everitt, 2002; Wise, 2004). The DA dependency seems to be crucial, as DA appears to be essential for the ‘stamping in’ of the response–reward and stimulus–reward associations that underlie the pathognomonic behavioural symptoms of addiction: the aberrantly strong motivational and reinforcing control over behaviour by drug-associated stimuli at the expense of other sources of reinforcement (Di Chiara, 1999; Everitt et al., 2001; Robbins & Everitt, 2002; Wise & Gardner, 2004; Wise, 2004). Viewed in this way, addiction is fundamentally a disorder of reinforcement (see also Koob et al., 1987; Wise, 1996a,b; Dackis & O’Brien, 2001; Wise & Gardner, 2002; Gardner, 2005).
In the present experiments, selective D3 receptor antagonism by acute administration of SB-277011A significantly inhibited cocaine self-administration only when the self-administration work-demand upon the animal was increased (either in terms of PR break point or in the shift from FR1 to FR10), or when the unit dose of reinforcing drug was lowered, thus lowering the reward value. The PR paradigm has been used extensively over the last decade to measure shifts in reinforcing efficacy and motivation to self-administer drugs (Richardson & Roberts, 1996; Arnold & Roberts, 1997; Stafford et al., 1998; Rowlett, 2000; Morgan & Roberts, 2004). Cocaine self-administration under PR conditions in rats has been shown to be dose-dependent (Richardson & Roberts, 1996; Arnold & Roberts, 1997; Stafford et al., 1998), to be extremely sensitive to manipulations affecting brain reward systems (Richardson & Roberts, 1996; Arnold & Roberts, 1997; Stafford et al., 1998), to yield dose–response functions that reflect addictive potential (Roberts & Bennett, 1993; French et al., 1995; Arnold & Roberts, 1997; Stafford et al., 1998) and to measure not only the reinforcing efficacy of cocaine but also cocaine-induced craving (Markou et al., 1993; Stafford et al., 1998; Rowlett, 2000).
As usually implemented, the FR paradigm of self-administration has been classically viewed as measuring the fact of reinforcement, but not the degree of reinforcing efficacy (Arnold & Roberts, 1997; Gardner, 2000, 2005; Wise & Gardner, 2004). However, as used in the present experiments, in which the amount of work-demand and amount of unit reinforcement were varied, the FR self-administration paradigm appears to model similar parameters to those modelled by the PR paradigm (Richardson & Roberts, 1996; Arnold & Roberts, 1997; Stafford et al., 1998; Rowlett, 2000; see also Ishiwari et al., 2004). Thus the fact that, in the present study, both the PR and FR experiments yielded the finding that high-potency high-selectivity DA D3 receptor blockade significantly diminished the reinforcing efficacy of cocaine may be taken, we believe, as an intermodel consistency serving to increase confidence in our conclusions.
Interpretation of the effects of drugs on cocaine self-administration under FR and PR schedules of reinforcement can be confounded by a number of factors. For example, the test agent may produce sedation, memory impairment, stimulation, motor dysfunctions, or rewarding or aversive actions by itself; and (as noted by such authorities as Richardson & Roberts, 1996; Arnold & Roberts, 1997) such nonspecific effects can lead to inappropriate conclusions. The presently observed SB-277011A-induced reduction in the reinforcing efficacy of cocaine under both FR and PR reinforcement seems unlikely to have resulted from SB-277011A having produced rewarding or aversive effects by itself, in view of the facts that SB-277011A: (i) does not by itself maintain self-administration (present study); (ii) produces neither conditioned place preference nor aversion (Vorel et al., 2002; Gyertyán & Gál, 2003); and (iii) does not alter electrophysiological brain-reward thresholds (Vorel et al., 2002). In addition, SB-277011A has no effect on responding for sucrose under second-order reinforcement (Di Ciano et al., 2003) and does not alter conditioned place preference for food (Vorel et al., 2002), suggesting that it does not alter the reinforcing action of natural rewards. Furthermore, SB-277011A reverses scopolamine-induced memory deficits (Laszy et al., 2005) and produces an increase in extracellular levels of acetylcholine in the anterior cingulate cortex (Lacroix et al., 2003). These latter effects would be expected to improve rather than interfere with memory. In addition, the present motor activity and motor coordination findings with SB-277011A strongly suggest that the presently observed SB-277011A-induced reduction in cocaine’s reinforcing efficacy is not an artefact of motoric stimulation or impairment.
At present, five DA receptor subtypes are known. Of these, the D1 and D2 have been much studied and considerable evidence indicates that both receptors play significant roles in drug-induced reward and reinforcement (de Wit & Wise, 1977; Ettenberg et al., 1982; Spyraki et al., 1987; Nakajima, 1989; Wise & Rompré, 1989; Nakajima et al., 1993; Ikemoto et al., 1997; Baker et al., 1998; Wise & Gardner, 2002; Gardner, 2005). There is much less evidence to link drug-induced reward to the D3 receptor subtype. This is due primarily to the fact that pharmacological agents with satisfactory selectivity for the D3 receptor have heretofore been nonexistent. However, the development of the high-affinity high-selectivity D3 antagonist SB-277011A has changed that situation, and a number of recent studies using SB-277011A strongly implicate the D3 receptor in cocaine-induced reward and reward-related functions. Thus, SB-277011A attenuates: (i) cocaine-enhanced electrical brain-stimulation reward (Vorel et al., 2002); (ii) acquisition of cocaine-induced conditioned place preference (Vorel et al., 2002); (iii) expression of cocaine-induced conditioned place preference (Vorel et al., 2002); (iv) cocaine-seeking behaviour as assessed by second-order reinforcement (Di Ciano et al., 2003); (v) cue-triggered relapse to cocaine-seeking as assessed by the reinstatement model (Cervo et al., 2003); and (vi) cocaine-triggered relapse to cocaine-seeking as assessed by reinstatement (Vorel et al., 2002).
In this context, it is also important to note the unique ways in which D3 antagonism differs functionally from D1 and D2 antagonism in animal models commonly used to assess drug-induced reward and reward-related processes. Using the electrical brain-stimulation reward paradigm, DA D1- and D2-preferring antagonists inhibit brain-reward functions, in a manner that appears diametrically opposite to the brain-reward enhancement produced by addictive drugs (e.g. Stein & Ray, 1960; Stein, 1962; Panagis & Spyraki, 1996; for reviews, see Wise, 1982; Gardner, 2005). In striking contrast, selective high-potency DA D3 receptor antagonism does not alter electrical brain-reward thresholds (Vorel et al., 2002; Campos et al., 2003; Xi et al., 2003). In the conditioned place preference or aversion paradigm, D1- and D2-preferring agonists produce reward while D1-preferring and mixed D1/D2 antagonists produce aversion (e.g. Shippenberg & Herz, 1987, 1988; Acquas & Di Chiara, 1994; for review, see Tzschentke, 1998). In contrast, selective high-potency DA D3 receptor antagonism does not alter basal reward state as assessed by the conditioned place preference or aversion paradigm (Vorel et al., 2002; Ashby et al., 2003; Gyertyán & Gál, 2003). In the self-administration paradigm, D1-and D2-preferring agonists are self-administered and therefore inferred to be rewarding (e.g. Davis & Smith, 1977; Yokel & Wise, 1978; Woolverton et al., 1984; Self & Stein, 1992; for reviews see Gardner, 2000, 2005). Conversely, D1- and D2-preferring antagonists are negative reinforcers (e.g. Hoffmeister & Wuttke, 1975; Kandel & Schuster, 1977), and are thus inferred to be aversive. In contrast, the highly selective high-potency D3 receptor antagonist SB-277011A does not support self-administration (present data) and thus, at the doses tested, appears devoid of reward efficacy as inferred from self-administration. To our knowledge, no highly selective DA D3 receptor antagonist has been tested in the negative reinforcement variant of the self-administration paradigm. In the PR break-point shift paradigm, in which cocaine is used as the compound to maintain basal self-administration, D1- and D2-preferring antagonists produce dose-dependent reductions in cocaine-maintained break points, i.e. reduced reward efficacy (e.g. Roberts & Vickers, 1987; Roberts et al., 1989; Hubner & Moreton, 1991; McGregor & Roberts, 1993; Richardson et al., 1994; for reviews, see Arnold & Roberts, 1997; Stafford et al., 1998). Conversely, nonselective DA agonists produce increased cocaine-maintained break points (i.e. increased reward efficacy: Caine & Koob, 1995; Roberts & Ranaldi, 1995).
The similarity of the decreased reward efficacy seen with DA D1- or D2-preferring antagonists in the PR break-point shift paradigm to that seen in the present experiments with SB-277011A raises the issue of whether or not the presently observed robust decreases in cocaine-induced reinforcement produced by SB-277011A might be attributable to DA D1 or D2 receptor-selective antagonism rather than the DA D3 receptor-selective antagonism that we believe to have been at work in the present experiments. This is unlikely, as the above-cited information indicates that SB-277011A is a highly selective D3 receptor antagonist that, at the doses used in this study, does not act as a functional antagonist at D1 or D2 receptors. Furthermore, with respect to the possibility that the effects observed in the present experiments might be mediated by action on D2 rather than D3 receptors, we note that SB-277011A appears to not produce functional antagonism at DA D2 receptors in laboratory rodents in vivo, as evidenced by the following: (i) in contrast to DA D2 antagonists, SB-277011A does not elicit catalepsy when given at doses in excess of three times the highest dose used in the present experiments (Reavill et al., 2000); (ii) in contrast to DA D2 antagonists, SB-277011A does not affect spontaneous or stimulant-induced locomotion (Reavill et al., 2000); (iii) in contrast to DA D2 antagonists, SB-277011A does not increase serum prolactin levels (Reavill et al., 2000); and (iv) in contrast to DA D2 antagonists, SB-277011A does not increase DA levels in the neostriatum (Reavill et al., 2000). In addition, SB-277011A reverses the decreased extracellular DA levels in the nucleus accumbens (D3 receptor-rich) produced by the DA D3-preferring agonist quinelorane (Reavill et al., 2000), but quinelorane’s effect on extracellular DA in the dorsal striatum (D3 receptor-poor; D2 receptor-rich) is not reversed by doses of SB-277011A up to four times the highest dose used in the present experiments. Finally, the results of the present study further confirm that the motor coordination and psychomotor activity profile of SB-277011A is significantly different from that of haloperidol, a mixed DA D2/D3 receptor antagonist. Thus, haloperidol administration significantly reduced locomotor activity, significantly reduced rearing behaviour, significantly increased catalepsy and significantly impaired performance in the rotarod test, all compared to vehicle treatment. These findings are consistent with other reports indicating that drugs with potent D2 receptor antagonist action impair locomotor activity and motor coordination. In contrast, i.p. administration of SB-277011A at doses up to 24 mg/kg, the highest dose used in the present FR and PR experiments, did not significantly alter any of the locomotor or behavioural coordination measures as compared to vehicle treatment. These in vivo behavioural data are congruent with in vitro data showing that SB-277011A is a highly selective DA D3 antagonist, and thus add strongly to our belief that the effects of SB-277011A on cocaine’s action in the FR and PR paradigms are unlikely to be related to any effects on D2 receptors in adult male rats.
It has previously been reported that SB-277011A-induced selective D3 receptor antagonism has no effect on intravenous cocaine self-administration under continuous reinforcement (FR1) conditions (Di Ciano et al., 2003; Gál & Gyertyán, 2003). The present experiments replicate and confirm those previous findings, and thus raise the issue of the proper interpretation to be placed upon the present findings of robustly decreased cocaine self-administration as measured by PR reinforcement and by variable-cost–variable-payoff FR reinforcement at the same time that we observed no change in cocaine self-administration under simple low-ratio FR reinforcement conditions. This requires careful consideration. As noted by Arnold & Roberts (1997), low-cost–high-payoff FR reinforcement drug self-administration procedures were not designed to estimate reinforcer magnitude, and attempts to use them for such purposes have yielded logical conundrums. For example, Yokel & Wise (1975, 1976) proposed that, because animals compensatorily increase their rate of drug self-administration (under low FR reinforcement conditions) following decreases in unit amount of self-administered drug, such increased rates of FR drug self-administration must reflect decreased reinforcer efficacy. However, experiments in which the meso-accumbens DA system has been depleted of DA by microinjections of the neurotoxin 6-hydroxydopamine (6-OHDA) into the nucleus accumbens (Roberts et al., 1980) confound that interpretation. In such experiments, partial depletion of nucleus accumbens DA produced partial inhibition of cocaine self-administration. If the initial DA depletion was < 80%, cocaine self-administration recovered over time and eventually regained baseline pre-lesion rates. As cocaine’s reinforcing efficacy is believed to be related to its enhancement of nucleus accumbens DA (de Wit & Wise, 1977; Ettenberg et al., 1982; Spyraki et al., 1987; Wise & Rompré, 1989; Gardner, 2000, 2005; Wise & Gardner, 2002), the decreased FR drug self-administration seen during recovery from the 6-OHDA-induced DA depletion has been interpreted as reflecting decreased reinforcer efficacy. As pointed out by Roberts & Zito (1987), the logical conundrum is obvious: how can the same alteration in cocaine’s reinforcing efficacy (i.e. decreased efficacy) manifest itself equally by opposite patterns of FR drug self-administration (i.e. by either increased or decreased FR drug-self-administration)? Furthermore, as noted by Arnold & Roberts (1997), the logical imperative is unmistakable: drug self-administration under low response-cost FR reinforcement conditions is an ambiguous measure of reinforcing efficacy at best, and possibly even an inaccurate measure that is insensitive to changes in reinforcing efficacy (Roberts, 1989; Arnold & Roberts, 1997).
In contrast, the PR break-point shift paradigm was specifically developed, and has been widely used and accepted, as a measure of reinforcing efficacy and of motivation to self-administer addictive drugs (Richardson & Roberts, 1996; Arnold & Roberts, 1997; Stafford et al., 1998; Rowlett, 2000; Morgan & Roberts, 2004). Cocaine self-administration under PR conditions has been shown to be dose-dependent and to yield dose–response functions that reflect addictive potential (Roberts & Bennett, 1993; French et al., 1995; Arnold & Roberts, 1997; Stafford et al., 1998). Although less well studied, drug self-administration under the type of variable-cost–variable-payoff FR reinforcement conditions used in the present study appears (as noted above) to emulate many of the properties of self-administration under PR reinforcement conditions. We therefore feel that the fact that we here found that SB-277011A did not alter cocaine self-administration under FR1 reinforcement conditions (thus replicating and agreeing with the prior findings of Di Ciano et al., 2003 and of Gál & Gyertyán, 2003), but robustly decreased the rewarding efficacy and incentive motivational properties of cocaine under both variable-cost–variable-payoff FR reinforcement and PR reinforcement conditions is internally consistent, especially as low response-cost FR reinforcement schedules and PR reinforcement schedules may well measure entirely different aspects of reinforcement (Arnold & Roberts, 1997; Morgan & Roberts, 2004).
The statistically significant cocaine dose × SB–277011A treatment interaction effect found for the data illustrated in Fig. 2C raises the possibility that the observed decreases in cocaine self-administration following SB-277011A may not be fully explained solely in terms of an attenuation of cocaine’s reinforcing effects. This significant interaction suggests the existence of a ‘floor’ effect, limiting the SB-277011A-induced decrease in cocaine self-administration. Indeed, Fig. 2B shows precisely such a floor effect, illustrating what seems to be an SB-277011A-induced extinction effect.
It should also be noted that SB-277011A significantly decreases the expression of cocaine-induced conditioned place preference and both cue- and stress-induced reinstatement (Vorel et al., 2002; Gilbert et al., 2004; Xi et al., 2004), behaviours that are drug-free and that therefore do not measure reinforcement. Such findings suggest that SB-277011A, in addition to diminishing the reinforcing action of cocaine, also significantly attenuates incentive motivational or drug-seeking behaviour elicited by cocaine.
Addiction at the human level includes, by definition, an increase in motivation to self-administer drug(s) despite adverse consequences. The present findings, then, that SB-277011A reduces such motivation and reduces cocaine’s reinforcing efficacy adds to an accumulating body of evidence that highly selective D3 receptor antagonists may be clinically useful in the treatment of addiction. Noteworthily, this potential anti-addiction utility does not seem restricted to cocaine or psychostimulants. We and others have reported that SB-277011A attenuates: (i) nicotine-enhanced electrical brain-stimulation reward (Campos et al., 2003); (ii) nicotine-triggered relapse to nicotine-seeking behaviour as assessed by the reinstatement model (Andreoli et al., 2003b); (iii) acquisition of heroin-induced conditioned place preference (Ashby et al., 2003); (iv) expression of heroin-induced conditioned place preference (Ashby et al., 2003); (v) ethanol self-administration (Andreoli et al., 2003a; Rivera et al., 2003) and (vi) relapse to ethanol-seeking behaviour (Marcon et al., 2003). Thus, highly selective DA D3 receptor antagonism appears to warrant further investigation in the search for clinically useful anti-addiction medications.
In summary, the present findings show that the selective DA D3 receptor antagonist SB-277011A significantly attenuates the reinforcing and incentive motivational properties of cocaine in laboratory rats, and add yet additional evidence that D3 receptors play an important role in these processes. The present findings further suggest that potent selective DA D3 receptor antagonists may hold promise as anti-addiction pharmacotherapeutic agents.
Acknowledgments
The authors thank Roy A. Wise for insights into ways in which the animal models used or cited in the present work differ from each other. This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, US Department of Health and Human Services, and by the Centres of Excellence for Drug Discovery, GlaxoSmithKline Pharmaceuticals, Harlow, Essex, UK, and Verona, Italy. Preliminary reports on this research were presented at the 2002, 2003 and 2004 Annual Meetings of the College on Problems of Drug Dependence, and at the 2003 Annual Meeting of the Society for Neuroscience.
Abbreviations
- DA
dopamine
- FR
fixed ratio
- PR
progressive ratio
- SB-277011A
Trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinoli-necarboxamide
References
- Acquas E, Di Chiara G. D1 receptor blockade stereo-specifically impairs the acquisition of drug-conditioned place preference and place aversion. Behav Pharmacol. 1994;5:555–569. doi: 10.1097/00008877-199410000-00001. [DOI] [PubMed] [Google Scholar]
- Andreoli M, Marcon C, Hagan JJ, Heidbreder CA. Effect of selective antagonism at dopamine D3 receptor by SB-277011-A on oral alcohol self-administration in mice. Eur Neuropsychopharmacol. 2003a;13 (Suppl 1):S17. [Google Scholar]
- Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology. 2003b;28:1272–1280. doi: 10.1038/sj.npp.1300183. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Roberts DCS. A critique of fixed ratio and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Paul M, Gardner EL, Heidbreder CA, Hagan JJ. Acute administration of the selective D3 receptor antagonist SB-277011-A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse. 2003;48:154–156. doi: 10.1002/syn.10188. [DOI] [PubMed] [Google Scholar]
- Austin NE, Baldwin SJ, Cutler L, Deeks N, Kelly PJ, Nash M, Shardlow CE, Stemp G, Thewlis K, Ayrton A, Jeffrey P. Pharmacokinetics of the novel, high-affinity and selective dopamine D3 receptor antagonist SB-277011 in rat, dog and monkey: in vitro/in vivo correlation and the role of aldehyde oxidase. Xenobiotica. 2001;31:677–686. doi: 10.1080/00498250110056531. [DOI] [PubMed] [Google Scholar]
- Baker DA, Fuchs RA, Specio SE, Khroyan TV, Neisewander JL. Effects of intraaccumbens administration of SCH-23390 on cocaine-induced locomotion and conditioned place preference. Synapse. 1998;30:181–193. doi: 10.1002/(SICI)1098-2396(199810)30:2<181::AID-SYN8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Pretreatment with the dopamine agonist 7-OH-DPAT shifts the cocaine self-administration dose-effect function to the left under different schedules in the rat. Behav Pharmacol. 1995;6:333–347. [PubMed] [Google Scholar]
- Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz JC, Sokoloff P. D3 receptor test in vitro predicts decreased cocaine self-administration in rats. Neuroreport. 1997;8:2373–2377. doi: 10.1097/00001756-199707070-00054. [DOI] [PubMed] [Google Scholar]
- Campos AC, Xi Z-X, Gilbert J, Ashby CR, Jr, Heidbreder CA, Gardner EL. The dopamine D3 receptor antagonist SB277011A antagonizes nicotine-enhanced brain-stimulation reward in rat. Soc Neurosci Abstr. 2003;322.8 [Abstracts of the 33rd Annual Meeting of the Society for Neuroscience; 2003 November 7–12; New Orleans, LA, 2003 Abstract Viewer/Itinerary Planner. Online. Society for Neuroscience, Washington, DC.] [Google Scholar]
- Cervo L, Cocco A, Petrella C, Heidbreder CA, Bendotti C, Mennini T. SB-277011-A, a selective dopamine D3 receptor antagonist, reduces cocaine-seeking behavior in response to drug-associated stimuli in rats. Soc Neurosci Abstr. 2003:420.3. [Abstracts of the 33rd Annual Meeting of the Society for Neuroscience; 2003 November 7–12; New Orleans, LA, 2003 Abstract Viewer/Itinerary Planner. Online. Society for Neuroscience, Washington, DC.] [Google Scholar]
- Dackis CA, O’Brien CP. Cocaine dependence: a disease of the brain’s reward centers. J Subst Abuse Treat. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Davis WM, Smith SG. Catecholaminergic mechanisms of reinforcement: direct assessment by drug self-administration. Life Sci. 1977;20:483–492. doi: 10.1016/0024-3205(77)90391-5. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- French ED, Lopez M, Peper S, Kamenka JM, Roberts DCS. A comparison of the reinforcing efficacy of PCP, the PCP derivatives TCP and BTCP, and cocaine using a progressive ratio schedule in the rat. Behav Pharmacol. 1995;6:223–228. [PubMed] [Google Scholar]
- Gál K, Gyertyán I. Targeting the dopamine D3 receptor cannot influence continuous reinforcement cocaine self-administration in rats. Brain Res Bull. 2003;61:595–601. doi: 10.1016/s0361-9230(03)00217-x. [DOI] [PubMed] [Google Scholar]
- Gardner EL. What we have learned about addiction from animal models of drug self-administration. Am J Addict. 2000;9:285–313. doi: 10.1080/105504900750047355. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Brain reward mechanisms. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, editors. Substance Abuse: a Comprehensive Textbook. 4. Lippincott, Williams & Wilkins; Philadelphia: 2005. pp. 48–97. [Google Scholar]
- Gilbert JG, Xi Z-X, Campos AC, Peng X-Q, Ashby CR, Jr, Heidbreder CA, Newman AH, Gardner EL. The dopamine D3 receptor antagonists SB277011A and NGB2904 inhibit cocaine-associated cue-induced reinstatement of drug-seeking behavior in rat. Soc Neurosci Abstr. 2004:691.7. [Abstracts of the 34th Annual Meeting of the Society for Neuroscience; 2004 October 23–27; San Diego, CA, 2004 Abstract Viewer/Itinerary Planner. Online. Society for Neuroscience, Washington, DC.] [Google Scholar]
- Gyertyán I, Gál K. Dopamine D3 receptor ligands show place conditioning effect but do not influence cocaine-induced place preference. Neuroreport. 2003;14:93–98. doi: 10.1097/00001756-200301200-00018. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi Z-X, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR. The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Rev. 2005 doi: 10.1016/j.brainresrev.2004.12.033. [12 February 2005, Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister F, Wuttke W. Psychotropic drugs as negative reinforcers. Pharmacol Rev. 1975;27:419–428. [PubMed] [Google Scholar]
- Hubner CB, Moreton JE. Effects of selective D1 and D2 dopamine antagonists on cocaine self-administration in the rat. Psychopharmacology. 1991;105:151–156. doi: 10.1007/BF02244301. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997;17:8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwari K, Weber SM, Mingote S, Correa M, Salamone JD. Accumbens dopamine and the regulation of effort in food-seeking behavior: modulation of work output by different ratio or force requirements. Behav Brain Res. 2004;151:83–91. doi: 10.1016/j.bbr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Kandel DA, Schuster CR. An investigation of nalorphine and perphenazine as negative reinforcers in an escape paradigm. Pharmacol Biochem Behav. 1977;6:61–71. doi: 10.1016/0091-3057(77)90160-5. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. 2. Brooks/Cole; Belmont, CA: 1982. [Google Scholar]
- Koob GF, Vaccarino F, Amalric M, Bloom FE. Positive reinforcement properties of drugs: search for neural substrates. In: Engel J, Oreland L, editors. Brain Reward Systems and Abuse. Raven Press; New York: 1987. pp. 35–50. [Google Scholar]
- Lacroix LP, Hows MEP, Shah AJ, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors enhances monoaminergic and cholinergic neurotransmission in the rat anterior cingulate cortex. Neuropsychopharmacology. 2003;28:839–849. doi: 10.1038/sj.npp.1300114. [DOI] [PubMed] [Google Scholar]
- Laszy J, Laszlovszky I, Gyertyán I. Dopamine D3 receptor antagonists improve the learning performance in memory-impaired rats. Psychopharmacology. 2005;179:567–575. doi: 10.1007/s00213-004-2096-z. [DOI] [PubMed] [Google Scholar]
- Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- Marcon C, Andreoli M, Pilla M, Tessari M, Heidbreder CA. A new model to assess drug and cue-induced relapse to ethanol self-administration in mice. Behav Pharmacol. 2003;14 (Suppl 1):S66. [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- McGregor A, Roberts DCS. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive-ratio schedules of reinforcement. Brain Res. 1993;624:245–252. doi: 10.1016/0006-8993(93)90084-z. [DOI] [PubMed] [Google Scholar]
- Morgan D, Roberts DCS. Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neurosci Biobehav Rev. 2004;27:803–812. doi: 10.1016/j.neubiorev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Murray AM, Ryoo HL, Gurevich E, Joyce JN. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci USA. 1994;91:11271–11275. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S. Subtypes of dopamine receptors involved in the mechanism of reinforcement. Neurosci Biobehav Rev. 1989;13:123–128. doi: 10.1016/s0149-7634(89)80020-x. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Liu X, Lau CL. Synergistic interaction of D1 and D2 dopamine receptors in the modulation of the reinforcing effect of brain stimulation. Behav Neurosci. 1993;107:161–165. doi: 10.1037//0735-7044.107.1.161. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences (National Research Council, Commission on Life Sciences, Institute of Laboratory Animal Resources) Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Panagis G, Spyraki C. Neuropharmacological evidence for the role of dopamine in ventral pallidum self-stimulation. Psychopharmacology. 1996;123:280–288. doi: 10.1007/BF02246582. [DOI] [PubMed] [Google Scholar]
- Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, Boyfield I, Branch CL, Cilia J, Coldwell MC, Hadley MS, Hunter AJ, Jeffrey P, Jewitt F, Johnson CN, Jones DNC, Medhurst AD, Middlemiss DN, Nash DJ, Riley GJ, Routledge C, Stemp G, Thewlis KM, Trail B, Vong AKK, Hagan JJ. Pharmacological actions of a novel, high-affinity, and selective human dopamine D3 receptor antagonist, SB-277011-A. J Pharmacol Exp Ther. 2000;294:1154–1165. [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Meth. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Smith AM, Roberts DCS. A single injection of either flupenthixol decanoate or haloperidol decanoate produces long-term changes in cocaine self-administration in rats. Drug Alcohol Depend. 1994;36:23–35. doi: 10.1016/0376-8716(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Rivera SN, Katana J, Ashby CR, Jr, Piyis YS, Gardner EL, Pena LA, Heidbreder C, Volkow ND, Thanos PK. A novel dopamine D3 receptor antagonist (SB-277011-A) attenuates ethanol consumption in ethanol preferring (P) and non-preferring (NP) rats. Soc Neurosci Abstr. 2003:665.7. doi: 10.1016/j.pbb.2005.03.013. [Abstracts of the 33rd Annual Meeting of the Society for Neuroscience; 2003 November 7–12; New Orleans, LA, 2003 Abstract Viewer/Itinerary Planner. Online. Society for Neuroscience, Washington, DC.] [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Roberts DCS. Breaking points on a progressive ratio schedule reinforced by intravenous apomorphine increase daily following 6-hydroxy-dopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1989;32:43–47. doi: 10.1016/0091-3057(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Bennett SAL. Heroin self-administration in rats under a progressive ratio schedule of reinforcement. Psychopharmacology. 1993;111:215–218. doi: 10.1007/BF02245526. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Loh EA, Vickers G. Self-administration of cocaine on a progressive-ratio schedule in rats: dose–response relationship and effect of haloperidol pretreatment. Psychopharmacology. 1989;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Ranaldi R. Effect of dopaminergic drugs on cocaine reinforcement. Clin Neuropharmacol. 1995;18 (Suppl 1):S84–S95. [Google Scholar]
- Roberts DCS, Vickers GJ. The effect of haloperidol on cocaine self-administration is augmented with repeated administrations. Psychopharmacology. 1987;93:526–528. doi: 10.1007/BF00207247. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Zito KA. Interpretation of lesion effects on stimulant self-administration. In: Bozarth MA, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer-Verlag; New York: 1987. pp. 87–103. [Google Scholar]
- Rowlett JK. A labor-supply analysis of cocaine self-administration under progressive-ratio schedules: antecedents, methodologies, and perspectives. Psychopharmacology. 2000;153:1–16. doi: 10.1007/s002130000610. [DOI] [PubMed] [Google Scholar]
- Self DW, Stein L. The D1 agonists SKF 82958 and SKF 77434 are self-administered by rats. Brain Res. 1992;582:349–352. doi: 10.1016/0006-8993(92)90155-3. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of μ- and κ-opioid agonists. Brain Res. 1987;436:169–172. doi: 10.1016/0006-8993(87)91571-x. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Motivational effects of opioids: influence of D-1 versus D-2 receptor antagonists. Eur J Pharmacol. 1988;151:233–242. doi: 10.1016/0014-2999(88)90803-5. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Nomikos GG, Varonos DD. Intravenous cocaine-induced place preference: attenuation by haloperidol. Behav Brain Res. 1987;26:57–62. doi: 10.1016/0166-4328(87)90016-7. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Artymyshyn RP, Kung MP, Kung HF, Lucki I, McGonigle P. Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [125I]7-OH-PIPAT: evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J Pharmacol Exp Ther. 2000;295:1223–1231. [PubMed] [Google Scholar]
- Stein L. Effects and interactions of imipramine, chlorpromazine, reserpine, and amphetamine on self-stimulation: possible neurophysiological basis of depression. Recent Adv Biol Psychiatry. 1962;4:297–311. doi: 10.1007/978-1-4684-8306-2_27. [DOI] [PubMed] [Google Scholar]
- Stein L, Ray OS. Brain stimulation reward ‘thresholds’ self-determined in rat. Psychopharmacologia. 1960;1:251–256. doi: 10.1007/BF00402746. [DOI] [PubMed] [Google Scholar]
- Stemp G, Ashmeade T, Branch CL, Hadley MS, Hunter AJ, Johnson CN, Nash DJ, Thewlis KM, Vong AKK, Austin NE, Jeffrey P, Avenell KY, Boyfield I, Hagan JJ, Middlemiss DN, Reavill C, Riley GJ, Routledge C, Wood M. Design and synthesis of trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl) ethyl]cyclohexyl]-4-quinolinecarboxamide (SB-277011): a potent and selective dopamine D3 receptor antagonist with high oral bioavailability and CNS penetration in the rat. J Med Chem. 2000;43:1878–1885. doi: 10.1021/jm000090i. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. McGraw-Hill; New York: 1962. [Google Scholar]
- Wise RA. Neuroleptics and operant behavior: the anhedonia hypothesis. Behav Brain Sci. 1982;5:39–53. [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996a;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996b;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:1–12. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA, Gardner EL. Functional anatomy of substance-related disorders. In: D’haenen H, Den Boer JA, Willner P, editors. Biological Psychiatry. Wiley; New York: 2002. pp. 509–522. [Google Scholar]
- Wise RA, Gardner EL. Animal models of addiction. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. 2. Oxford University Press; London: 2004. pp. 683–697. [Google Scholar]
- Wise RA, Rompré PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- de Wit H, Wise RA. Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Can J Psychol. 1977;31:195–203. doi: 10.1037/h0081662. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Goldberg LI, Ginos JZ. Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. J Pharmacol Exp Ther. 1984;230:678–683. [PubMed] [Google Scholar]
- Xi Z-X, Gilbert J, Campos AC, Ashby CR, Jr, Gardner EL, Newman AH. The dopamine D3 receptor antagonist NGB 2904 inhibits cocaine reward and cocaine-triggered reinstatement of cocaine-seeking behavior. Soc Neurosci Abstr. 2003:422.9. [Abstracts of the 33rd Annual Meeting of the Society for Neuroscience; 2003 November 7–12; New Orleans, LA, 2003 Abstract Viewer/Itinerary Planner. Online. Society for Neuroscience, Washington, DC. [Google Scholar]
- Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR, Jr, Hagan JJ, Heidbreder CA, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology. 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implication for a dopamine theory of reward. Science. 1975;187:547–549. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Attenuation of intravenous amphetamine reinforcement by central dopamine blockade in rats. Psychopharmacology. 1976;48:311–318. doi: 10.1007/BF00496868. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Amphetamine-type reinforcement by dopaminergic agonists in the rat. Psychopharmacology. 1978;58:289–296. doi: 10.1007/BF00427393. [DOI] [PubMed] [Google Scholar]