Abstract
Aim/Hypothesis
The experimental aim of this study was to determine whether ET-1-mediated vasoconstrictor tone is elevated in adult humans with impaired fasting blood glucose concentrations, independent of other cardiovascular risk factors.
Methods
Forearm blood flow (FBF: plethysmography) responses to intra-arterial infusion of selective ETA receptor blockade (BQ-123: 100 nmol/min for 60 min) and non-selective ETA/B blockade (BQ-123 + BQ-788: 50 nmol/min for 60 min) were determined in 28 middle-aged, sedentary adults (17 M/11 F): 14 with normal fasting blood glucose (age: 57±2 yr; 6F/8M; BMI: 29.2±0.9 kg/m2; glucose: 4.9±0.1 mmol/L) and 14 impaired fasting blood glucose (58±1 yr; 5F/9M; 29.6±1.1 kg/m2; 5.8±0.1 mmol/L) concentrations.
Results
Selective ETA receptor blockade elicited a significantly greater (~20%) increase in FBF in the impaired fasting glucose adults compared with the normoglycemia controls. ETA/B blockade resulted in a further 2-fold increase (P<0.05) in FBF above that elicited by ETA receptor antagonism in the impaired fasting glucose but not normal fasting glucose adults. There was a positive correlation between fasting blood glucose levels and the peak vascular responses to ETA (r=0.44; P<0.05) and ETA/B (r=0.62; P<0.05) blockade. No other anthropometric, hemodynamic or metabolic variable was correlated with the blood flow responses to ET-1 receptor blockade.
Conclusions/Interpretation
ET-1-mediated vasoconstrictor tone is elevated in adults with impaired fasting blood glucose concentrations, independent of other cardiometabolic risk factors. Enhanced ET-1 system activity may underlie endothelial vasomotor dysfunction and increased cardiovascular risk in adults with impaired fasting blood glucose concentrations.
Keywords: endothelin-1, glucose, vasoconstriction
Introduction
Approximately 80 million adults in the United States have impaired fasting blood glucose concentrations1, defined as fasting plasma glucose between 5.6–6.9 mmol/L2. It has recently been reported that middle-aged adults without diabetes, but with elevated fasting plasma glucose, are at an increased risk for coronary heart disease (4). For example, Alexander et al3 demonstrated that adults with impaired fasting glucose are at a 50% higher risk of developing cardiovascular disease compared with adults with normal fasting glucose. The mechanisms responsible for this apparent increase in vascular risk are not fully understood. Glucose has been shown to adversely affect endothelial cell function, which may propagate the atherosclerotic process4. Several clinical studies have shown that impaired fasting glucose is associated with endothelial dependent vasodilator dysfunction5, 6, a central feature of atherogenesis7.
Endothelin-1 (ET-1) is a potent vasoconstrictor peptide released by the endothelium that contributes to the regulation of vascular tone and has been implicated in the etiology of atherosclerotic vascular disease8. Interestingly, in vitro, a high glucose environment results in an elevation in endothelin-1 converting enzyme, suggesting a link between glucose and the ET-1 system9. Currently, it is unknown whether ET-1 system activity is altered in adult humans with impaired fasting plasma glucose. If so, this may contribute mechanistically to impaired endothelial vasomotor function and increased cardiovascular risk in this population. Thus, the aim of this study was to determine whether ET-1-mediated vasoconstrictor tone is elevated in adult humans with impaired fasting blood glucose concentrations, independent of other cardiovascular risk factors.
Methods
Subjects
Twenty-eight sedentary adults participated in this study: 14 (6F/8M) with normal plasma glucose (<5.6 mmol/L); and 14 (5F/9M) with impaired fasting plasma glucose (5.6–6.9 mmol/L) concentrations. Groups were stratified according to American Diabetes Association criteria2. Subjects were non-smokers and free of overt cardiovascular disease. Fasting plasma lipid, lipoprotein, glucose and insulin concentrations were determined using standard techniques. HOMA-IR was calculated as previously described10. All women were at least 1 year postmenopausal and not taking hormone replacement therapy. Written informed consent was obtained according to the guidelines of the University of Colorado at Boulder.
Intra-arterial Infusion Protocol
All studies were performed between 7 AM and 10 AM after a 12-hour overnight fast as previously described by our laboratory11. Briefly, following arterial catheterization, forearm blood flow (FBF: venous occlusion plethysmography) responses to BQ-123 (Clinalfa, AG), a selective ETA receptor antagonist, infused for 60 minutes with FBF measured every 10 minutes. Thereafter, FBF was assessed every 10 minutes for an additional 60 minutes with the coadministration of BQ-123 and BQ-788 (Clinalfa, AG), a specific antagonist of ETB receptors. Due to product availability, BQ-788 was infused in 7 of the 14 subjects in each group.
Statistical Analysis
Differences in subject characteristics were determined by between-group analysis of variance (ANOVA). Group differences in FBF responses to BQ-123 and BQ-123 + BQ-788 were determined by repeated-measures ANOVA. Relation between variables of interest was assessed by linear regression analysis. There were no significant main effects of gender on FBF responses to endothelin blockade of FBF × gender interactions, therefore the data were pooled and presented together. Data are expressed as means±SEM. Statistical significance was set at P<0.05.
Results
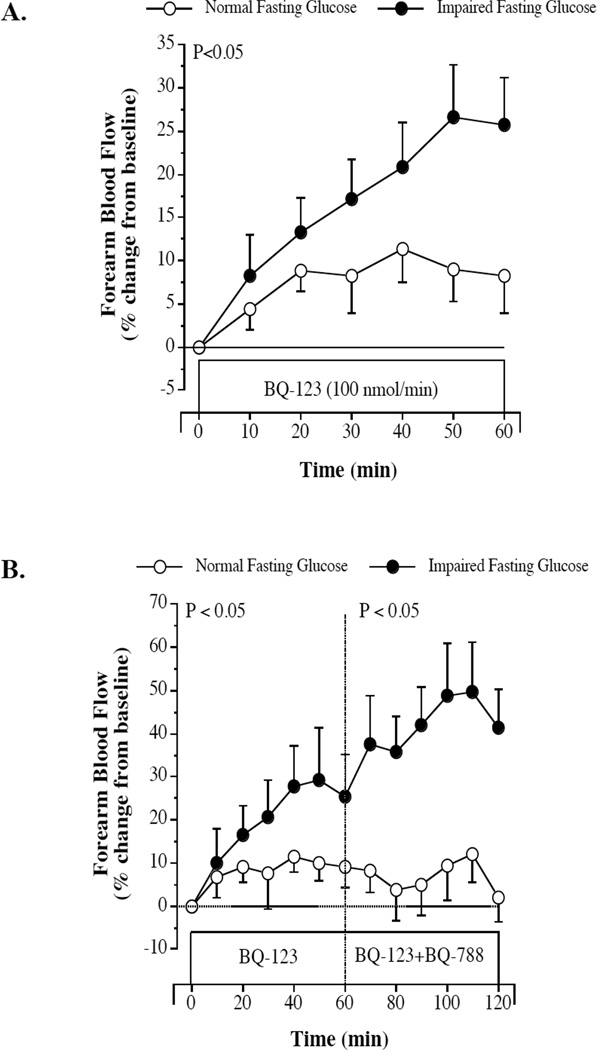
Subject characteristics are presented in the Table. There were no significant differences in baseline FBF between the normal (4.4±0.3 mL/100 mL of tissue/min) and impaired (4.0±0.3 mL/100 mL of tissue/min) fasting blood glucose groups. FBF responses to ET-receptor blockade are shown in the Figure. BQ-123 elicited a significantly greater (~20%) increase in FBF in the impaired fasting glucose than normal fasting glucose groups. Moreover, the addition of BQ-788 to BQ-123 resulted in a further 2-fold increase (P<0.05) in FBF in the impaired fasting glucose but not normal fasting glucose adults. In the overall study population, there was a strong and positive correlation between fasting blood glucose levels and the peak vascular responses to BQ-123 (r=0.44; P<0.05) and BQ-123 + BQ-788 (r=0.62; P<0.05). No other anthropometric, hemodynamic or metabolic variable was significantly correlated with the vascular responses to ET-1 receptor blockade.
Table.
Selected subject characteristics
| Variable | Normal Fasting Glucose (n=14) |
Impaired Fasting Glucose (n=14) |
|---|---|---|
| Age (yrs) | 57 ± 2 | 58 ± 1 |
| Gender, M/F | 8/6 | 9/5 |
| Body Mass (kg) | 84.9 ± 3.9 | 87.5 ± 3.9 |
| Body Mass Index (kg/m2) | 29.1 ± 0.9 | 29.7 ± 1.0 |
| Body Fat (%) | 36.1 ± 1.5 | 35.4 ± 1.6 |
| Waist Circumference (cm) | 94.3 ± 3.4 | 99.1 ± 2.8 |
| Systolic BP (mmHg) | 124 ± 2 | 127 ± 3 |
| Diastolic BP (mmHg) | 79 ± 2 | 79 ± 2 |
| Total Cholesterol (mmol/L) | 5.0 ± 0.2 | 5.3 ± 0.3 |
| LDL-Cholesterol (mmol/L) | 3.0 ± 0.2 | 3.4 ± 0.2 |
| HDL-Cholesterol (mmol/L) | 1.3 ± 0.1 | 1.2 ± 0.1 |
| Triglycerides (mmol/L) | 1.5 ± 0.2 | 1.5 ± 0.2 |
| Glucose (mmol/L) | 4.9 ± 0.1 | 5.8 ± 0.1* |
| Insulin (pm/L) | 49.8 ± 6.6 | 51.0 ± 6.6 |
| HOMA-IR | 1.9 ± 0.2 | 2.4 ± 0.32 |
BP = blood pressure; HDL=high-density lipoprotein; LDL=low-density lipoprotein; HOMA-IR=homeostasis model assessment of insulin resistance; values are mean ± SEM
P < 0.05 vs. normal fasting glucose
Figure.
Forearm blood flow responses to BQ-123 (100 nmol/min), a selective ETA receptor antagonist (panel A) and BQ-788 (50 nmol/min), a selective ETB receptor antagonist (panel B), in normal fasting glucose and impaired fasting glucose adults. Values are mean±SEM. The P value refers to the difference in the FBF response to ETA and ETA/B blockade in the normal vs. impaired fasting glucose groups.
Discussion
The key finding of the present study is that ET-1 mediated vasoconstrictor tone is elevated in adults with impaired fasting blood glucose concentrations independent of other cardiometabolic risk factors. Moreover, the enhancement in ET-1 vasoconstriction is facilitated by both the ETA and ETB receptors. To our knowledge, this is the first study to assess the influence of impaired fasting blood glucose concentrations on ET-1 system activity.
In a recent study, DeVan and colleagues6 reported that endothelial vasodilator function is impaired in middle-aged adults with impaired fasting blood glucose. Indeed, brachial artery flow-mediated dilation was ~30% lower in the adults with impaired fasting glucose compared with healthy controls of similar age. The results of the present study compliment and extend these findings by demonstrating that endothelial vasomotor dysfunction with impaired fasting glucose is not limited to vasodilation. Indeed, the seminal findings presented herein demonstrate that ET-1-mediated vasoconstrictor tone is markedly higher in middle-aged adults with impaired fasting glucose. FBF responses to both selective and non-selective ET-1 receptor blockade were markedly higher (20% and 40%, respectively) in the adults with impaired fasting glucose compared with their normal fasting plasma glucose counterparts. Of note, ETA/B receptor blockade resulted in a further increase in FBF above that observed with ETA blockade alone in the impaired fasting glucose adults only, demonstrating that the ETB receptor also contributes to the elevation in ET-1 vasoconstrictor tone with impaired fasting blood glucose. It should be noted that we assessed ET-1 system activity by pharmacologically blocking both ET-1 receptors (located on vascular smooth muscle cells and endothelium) instead of relying on circulating plasma concentrations of ET-1. The physiological relevance of plasma ET-1 levels is questionable as ET-1 is predominantly (>80%) released abluminally toward the vascular smooth muscle12. Thus, circulating levels provide little information on the vascular effects of the peptide at the level of the vessel wall.
The mechanisms underlying greater ET-1 vasoconstrictor tone with impaired fasting glucose are not well understood. It is important to emphasize that there were no differences between our groups with respect to body composition, blood pressure or plasma lipid and lipoproteins, all factors that are independently associated with increased ET-1 system activity and often coexist with the impaired fasting glucose condition. For example, overweight and obesity, a common co-morbidity with impaired fasting glucose, has been shown to adversely influence endothelial vasomotor regulation via increased ET-1 system activity11. The subjects in the present study, however, were remarkable similar anthropometrically; discounting the influence of body composition on our findings. Notably, the only variable in the present study that correlated with the vascular responses to ET-1 receptor blockade was fasting plasma glucose. Peak blood flow responses to both selective ETA receptor (r=0.44) and non-selective ETA/B receptor (r=0.62) blockade were significantly and positively associated with plasma glucose concentrations. At the very least, these correlative data provide directional support that the observed group differences were indeed glucose-related. While not measured in the present study, it is possible that oxidative stress and inflammatory burden may underlie the impaired fasting glucose-related increase in ET-1-mediated vasoconstrictor tone. Both oxidative stress and inflammation have been shown to exacerbate ET-1 system activity13 and are prevalent with the impaired fasting glucose condition14. Future studies are needed to determine whether the increase in ET-1 vasoconstriction with impaired fasting glucose is due, at least in part, to oxidative and inflammatory processes.
There are two experimental considerations regarding the present study that deserve mention. Firstly, given our cross-sectional study design, we cannot discount the possibility that genetic and/or lifestyle behaviors may have influenced our results. To minimize the influence of lifestyle behaviors, we studied sedentary adults who were non-smokers and not currently taking any medication that could influence endothelial vasomotor function. Moreover, to isolate the primary influence of impaired fasting blood glucose concentrations, we studied adults of similar age who were free of other cardiometabolic abnormalities that are known to influence endothelial function, such as hypertension15 and dyslipidemia16. Secondly, the present study has a modest sample size and all of the subjects were Caucasian. Thus, any generalizations to larger, more diverse populations require further study.
In summary, the results of this study demonstrate that ET-1-mediated vasoconstrictor tone is elevated in adults with impaired fasting blood glucose concentrations, independent of other cardiometabolic risk factors. From a public health perspective, endothelial vasomotor dysfunction, characterized by ET-1 system hyperactivity, is a well-established vascular abnormality with type II diabetes that contributes to cardiovascular risk17. Our findings indicate that augmented ET-1 vasoconstrictor tone is already apparent in the impaired fasting glucose prediabetic state. This provides further support for early intervention in adults with impaired fasting blood glucose concentrations not only to reduce their risk of developing diabetes but cardiovascular abnormalities as well.
Acknowledgements
We would like to thank all the subjects who participated in this study. This study was supported by National Institutes of Health awards HL077450, HL076434, and UL1 TR000154, American Heart Association awards 0840167N and 09PRE2230382.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association, Clinical Practice Recommendations 2012. Diabetes Care. 2012;35:S1–S110. [PubMed] [Google Scholar]
- 3.Alexander CM, Landsman PB, Teutsch SM. Diabetes mellitus, impaired fasting glucose, atherosclerotic risk factors, and prevalence of coronary heart disease. Am J Cardiol. 2000;86:897–902. doi: 10.1016/s0002-9149(00)01118-8. [DOI] [PubMed] [Google Scholar]
- 4.Azcutia V, Abu-Taha M, Romacho T, Vazquez-Bella M, Matesanz N, Luscinskas FW, Rodriguez-Manas L, Sanz MJ, Sanchez-Ferrer CF, Peiro C. Inflammation determines the pro-adhesive properties of high extracellular d-glucose in human endothelial cells in vitro and rat microvessels in vivo. PLoS One. 2010;5:e10091. doi: 10.1371/journal.pone.0010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiang GD, Wang YL. Regular aerobic exercise training improves endothelium-dependent arterial dilation in patients with impaired fasting glucose. Diabetes Care. 2004;27:801–802. doi: 10.2337/diacare.27.3.801. [DOI] [PubMed] [Google Scholar]
- 6.Devan AE, Eskurza I, Pierce GL, Walker AE, Jablonski KL, Kaplon RE, Seals DR. Regular aerobic exercise protects against impaired fasting plasma glucose-associated vascular endothelial dysfunction with aging. Clin Sci (Lond) 2013;124:325–331. doi: 10.1042/CS20120291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross R. The pathogenesis of atherosclerosis: a prospective for the 1990's. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 8.Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annual Reviews of Physiology. 1999;61:391–415. doi: 10.1146/annurev.physiol.61.1.391. [DOI] [PubMed] [Google Scholar]
- 9.Keynan S, Khamaisi M, Dahan R, Barnes K, Jackson CD, Turner AJ, Raz I. Increased expression of endothelin-converting enzyme-1c isoform in response to high glucose levels in endothelial cells. J Vasc Res. 2004;41:131–140. doi: 10.1159/000077132. [DOI] [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Weil BR, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Enhanced endothelin-1 system activity with overweight and obesity. Am J Physiol Heart Circ Physiol. 2011;301:H689–H695. doi: 10.1152/ajpheart.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner OF, Christ G, Wojta J, Vierhapper H, Parzer S, Nowotny PJ, Schneider B, Waldhausl W, Binder BR. Polar secretion of endothelin-1 by cultured endothelial cells. J Biol Chem. 1992;267:16066–16068. [PubMed] [Google Scholar]
- 13.Patel JN, Jager A, Schalkwijk C, Corder R, Douthwaite JA, Yudkin JS, Coppack SW, Stehouwer CD. Effects of tumour necrosis factor-alpha in the human forearm: blood flow and endothelin-1 release. Clin Sci (Lond) 2002;103:409–415. doi: 10.1042/cs1030409. [DOI] [PubMed] [Google Scholar]
- 14.Su Y, Liu XM, Sun YM, Jin HB, Fu R, Wang YY, Wu Y, Luan Y. The relationship between endothelial dysfunction and oxidative stress in diabetes and prediabetes. Int J Clin Pract. 2008;62:877–882. doi: 10.1111/j.1742-1241.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 15.Cardillo C, Kilcoyne C, Waclawiw M, Cannon R, Panza J. Role of endothelin in the increased vascular tone of patients with essential hypertension. Hypertension. 1999;33:753–758. doi: 10.1161/01.hyp.33.2.753. [DOI] [PubMed] [Google Scholar]
- 16.Cardillo C, Kilcoyne C, Cannon R, Panza J. Increased activity of endougenous endothelin in patients with hypercholesterolemia. Journal of American College of Cardiology. 2000;36:1483–1488. doi: 10.1016/s0735-1097(00)00910-4. [DOI] [PubMed] [Google Scholar]
- 17.Cardillo C, Campia U, Bryant MB, Panza JA. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation. 2002;106:1783–1787. doi: 10.1161/01.cir.0000032260.01569.64. [DOI] [PubMed] [Google Scholar]