Abstract
Recent studies have shown that the novel dopamine (DA) D3 receptor antagonists SB-277011A and NGB 2904 inhibit cocaine- and/or stress-induced reinstatement of drug-seeking behavior. The present study sought to determine if SB-277011A, NGB 2904, or BP-897 (a mixed D3 agonist/antagonist) similarly inhibit cocaine-associated cue-induced reinstatement of drug-seeking behavior. Long-Evans rats were allowed to self-administer cocaine. Each cocaine infusion was paired with discrete conditioned cue-light and tone. Subsequently, drug-seeking (i.e., lever-pressing) behavior was extinguished in the absence of cocaine and cocaine-associated cues. Rats were then tested for cue-induced reinstatement of drug-seeking. We found that cocaine-associated cues evoked robust reinstatement of lever-pressing. Acute intraperitoneal (i.p.) administration of SB-277011A (6, 12, or 24 mg/kg) produced a dose-dependent inhibition of cue-induced reinstatement of drug-seeking behavior by 35, 65, and 85%, respectively, compared to vehicle-treated animals. Acute i.p. administration of NGB 2904 (0.1, 1.0, or 5.0 mg/kg) produced a 45, 30, and 70% inhibition of cue-induced reinstatement, respectively, compared to vehicle-treated animals. Acute i.p. administration of either 0.1 or 1 mg/kg of BP 897 did not produce a significant effect on cue-induced reinstatement, whereas a dose of 3 mg/kg produced a 70% inhibition of cue-induced reinstatement. These findings, combined with previous data, suggest that DA D3 receptor antagonism may underlie the inhibitory effects of SB-277011A and NGB 2904 on cocaine cue-induced reinstatement, while the effects of BP 897 may involve D3 and non-D3 receptor mechanisms.
Keywords: addiction, BP 897, cocaine, cue, dopamine, D3 receptor, drug-seeking, NGB 2904, reinstatement, relapse, SB-277011A
INTRODUCTION
Drug craving is an important factor leading to drug-seeking and relapse. In human addicts, re-exposure to the environmental stimuli (cues) previously paired with drug taking provokes drug craving (“cue-induced craving”) and relapse to drug taking after prolonged abstinence (Rohsenow et al., 1990; Ehrman et al., 1992; Childress et al., 1993; O’Brien, 1997). In experimental animals, re-exposure to cocaine-associated stimuli also provokes drug-seeking behavior (Fuchs et al., 1998; Alleweireldt et al., 2001; Shalev et al., 2002; Di Ciano and Everitt, 2002, 2003).
Mesolimbic dopamine (DA) transmission from the midbrain ventral tegmental area (VTA) to the fore-brain nucleus accumbens (NAc) and the basolateral amygdala (BLA) appears to be critically involved in drug cue-induced craving and relapse (See, 2002; Everitt and Wolf, 2002; Di Ciano and Everitt, 2004a,b). Several lines of evidence support this hypothesis. Neuroimaging studies in humans have shown that cocaine cue-induced craving is associated with activation of DA-rich forebrain regions such as the BLA and NAc (Grant et al., 1996; Breiter et al., 1997; Childress et al., 1999; Garavan et al., 2000). Also, cocaine-associated stimuli reinstate extinguished drug-seeking behavior and activate the BLA and the dorsomedial prefrontal cortex (PFC) in rats after prolonged (4 months) cocaine abstinence (Ciccocioppo et al., 2001; Weiss et al., 2000). In contrast, selective lesion or functional deactivation of the BLA, the core of the NAc, or the dorsomedial PFC blocks cocaine cue-induced reinstatement of drug-seeking behavior (Whitelaw et al., 1996; Meil and See, 1997; See, 2002; Fuchs et al., 2004, 2005; Di Ciano and Everitt, 2004a,b). Further, presentation of non-contingent cocaine-conditioned cues significantly increases extracellular DA in the BLA and the NAc core (Fontana et al, 1993; Di Ciano et al., 1998; Ito et al., 2000; Weiss et al., 2000, 2001; Phillips et al., 2003), although such a DA response becomes undetectable during maintenance of drug-seeking induced by response-contingent presentation of conditioned cues (Neisewander et al., 1996; Ito et al., 2000; Di Ciano et al., 2001a). Consistent with this notion, intracranial infusion of non-selective DA receptor antagonists into the BLA or the core of the NAc prevents cocaine cue-induced reinstatement of drug-seeking (See et al., 2001; Di Ciano et al., 2001b; Yun et al., 2004). Together, these findings suggest that enhanced DA transmission in the BLA and/or the core of the NAc may be critically involved in at least the initial stages of drug-seeking triggered by drug-paired environmental stimuli.
Accumulating evidence suggests that DA D3 receptors may play a central role in DA transmission related to brain reward and relapse to drug-seeking behavior: (1) DA D3 receptors are highly expressed in brain reward-related regions, such as the VTA, NAc, and amygdala (Bouthenet et al., 1991; Lévesque et al., 1992; Murray et al., 1994; Levant, 1997; Diaz et al., 2000); (2) DA D3 receptors have the highest binding affinity to endogenous DA (Sokoloff et al., 1990, 1992), suggesting an important role of D3 receptors in DA transmission; (3) DA D3 receptors are up-regulated in the NAcc of cocaine overdose victims (Staley and Mash, 1996; Mash, 1997; Segal et al., 1997), and also in rodents after cocaine self-administration (Neisewander et al., 2004) or behavioral sensitization to cocaine-associated cues (Le Foll et al., 2002); (4) selective D3 receptor blockade by SB-277011A or NGB 2904, two novel highly-potent and highly-selective D3 receptor antagonists (Reavill et al., 2000; Yuan et al., 1998; Newman et al., 2003), attenuates cocaine’s rewarding effects as assessed by electrical brain-stimulation reward, conditioned place preference, and drug self-administration (Vorel et al., 2002; Xi et al., 2003); and (5) selective D3 receptor blockade attenuates cocaine- or stress-triggered reinstatement of drug-seeking behavior (Vorel et al., 2002; Xi et al., 2004). In addition, the selective D3 antagonist SB-277011A inhibits cocaine cue-induced drug-seeking under a second-order schedule for cocaine self-administration (Di Ciano et al., 2003). Also, BP 897, a mixed D3 agonist-antagonist whose D3 antagonist properties may predominate (Wood et al., 2000; Wick and Garcia-Ladona, 2001), inhibits cocaine cue-induced drug-seeking under second-order reinforcement (Pilla et al., 1999) and inhibits drug-seeking caused by presentation of cocaine-predictive stimuli (Cervo et al., 2003).
However, to date, there have been no published reports describing the effect of D3 receptor antagonists on reinstatement of drug-seeking following re-exposure to cues previously paired with cocaine self-administration. Therefore, in the present study, we examined and compared the effects of SB-277011A, NGB 2904 and BP 897 on cocaine cue-induced reinstatement (relapse) of extinguished drug-seeking behavior in rats.
MATERIALS AND METHODS
Animals
Male Long-Evans rats (Charles River, Raleigh, NC) were used, weighing 250–300 g at the beginning of the experiments. They were housed in a fully accredited animal facility and were maintained on a reversed 12-h light/dark cycle (lights on at 7:00 P.M., lights off at 7:00 A.M.) with food and water available in the home cage. The experimental procedures followed the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, 1996) and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse.
Surgery
Animals were prepared for experimentation by surgical catheterization of the right external jugular vein. The venous catheters were consutructed of micro-renathane (Braintree Scientific Inc., Braintree, MA). Surgical catheterization was carried out under intra-peritoneal (i.p.) sodium pentobarbital anaesthesia (65 mg/kg), using aseptic surgical technique. Supplemental anaesthesia (20 mg/kg pentobarbital, i.p.) was given if needed during surgery. The jugular vein was exposed by blunt dissection and the catheter was inserted into the vein and sutured into place. The distal portion of the catheter was then passed subcutaneously to the top of the skull, where it exited into a connector (a modified 24-gauge cannula; Plastics One, Roanoke, VA) mounted to the skull with jeweler’s screws and dental acrylic. After the connector was securely skull-mounted, the incision was closed with sutures. An obturator and cannula cap were placed over the opening of the skull-mounted connector during post-surgical recovery and at all other times when the rats were not in a self-administration session. During experimental sessions, the catheter was connected to the injection pump via tubing encased in a protective metal spring, from the head-mounted connector to the top of the experimental chamber. To prevent clogging, catheters were flushed daily with a gentamicin-heparin-saline solution (30 IU/ml heparin; ICN Biochemicals, Cleveland, OH).
Apparatus
The experiments were conducted in operant response test chambers (32 × 25 × 33 cm), each equipped with a house light, ventilator fan, drug infusion pump (3.33-rpm motor, 10-ml syringe), and liquid swivel with a counterbalanced arm. Each test chamber had two levers located 6.5 cm above the floor, one “active” and one “inactive.” Depression of the active lever activated the infusion pump; depression of the inactive lever was counted but had no consequence. A cue light and a speaker were located 12 cm above the active lever. At the start of each 3-h test session, the house-light was turned on. When the animal made a lever-pressing response that resulted in a drug infusion, the cue-light was illuminated and a cue-sound (tone, ~20 dB above background) was turned on for the duration of the infusion (i.e., cocaine-associated environmental cues). All equipment was obtained from Med-Associates (Georgia, VT). Scheduling of experimental events and data collection were accomplished using Med-Associates software.
Self-administration procedure
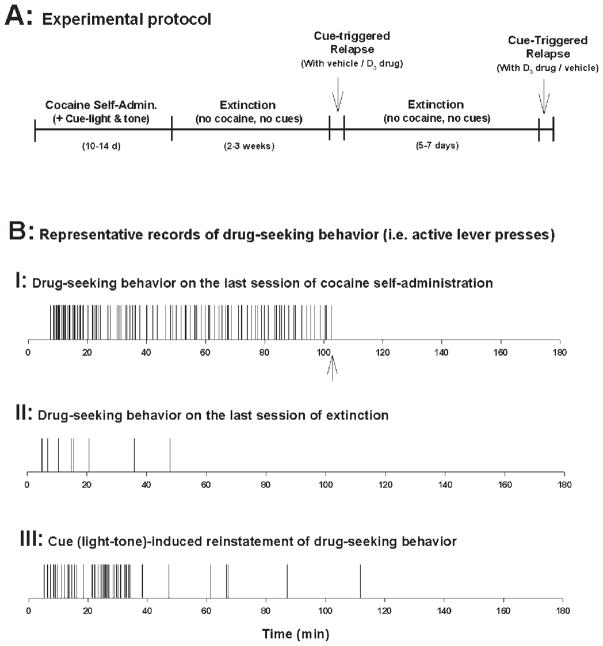
Figure 1A shows the experimental protocol, indicating the different phases of the present experiment. After recovery from surgery, all rats were placed into the test chambers and allowed to lever-press on a fixed-ratio (FR1) schedule of reinforcement for intravenous (i.v.) cocaine (1.0 mg/kg/infusion) delivered in 0.08 ml over 4.6 sec. Each session lasted for 3 h. This schedule (FR1) was used for 3–5 days until regular self-administration behavior was established. Rats were then changed to an FR2 schedule for a lower dose of cocaine (0.5 mg/kg) until the following criteria for stable cocaine-maintained responding were met: a minimum of 20 presses on the active lever per test session and stability criterion of less than 10% variability in inter-response interval, less than 10% variability in number of infusions taken, and less than 10% variability in number of presses on the active lever for at least 3 consecutive days. The maximum number of cocaine infusions was limited to 50 per session, to avoid accidental overdose. Responses on the active lever made during actual infusions were recorded, but did not lead to additional infusions. The dose of cocaine was chosen on the basis of previous studies conducted in our laboratory indicating that rats trained with 0.5 mg/kg/infusion display rapid and reliable acquisition of self-administration behavior (Xi et al., 2004). Total self-administration training lasted for 10–14 days. At the end of each daily 3-h session, animals were returned to the colony room.
Fig. 1.
A: The diagrammatic sequence of experimental phases. Each animal received two reinstatement tests after extinction, once with vehicle and once with one dose of a test drug in a counterbalanced manner. B: Typical representative event records of drug-seeking behavior during each phase. Each vertical line represents one lever press. Regular and stable drug-seeking behavior was maintained during cocaine self-administration training until the maximal 50 infusions were achieved (marked by an arrow at the 103 min time point on event record I). Such drug-seeking behavior is shown extinguished (<10 active lever presses) on the last session of extinction (event record II). Re-introduction of cocaine-associated cues (light and tone) triggered robust reinstatement of extinguished drug-seeking behavior in the presence of vehicle (1 ml 25% β-cyclodextrin) (event record III).
Extinction procedure
After meeting the above self-administration criteria, the animals were placed in the same operant chambers for self-administration under extinction conditions, during which cocaine was replaced by saline and the previous cocaine-associated cue-light and cue-tone were turned off. Responses on the previously active lever resulted in activation of the pump, but had no other programmed consequences; responses on the inactive lever were also recorded, but had no programmed consequences. Once daily 3-h extinction sessions were conducted until an extinction criterion of ≤10 responses on the active lever per session over 3 consecutive days was met.
Cue-induced reinstatement of drug-seeking behavior
Reinstatement testing began 24 h after rats met the above extinction criterion. During the testing, 1–2 non-contingent presentations of the cocaine-associated light and tone were given at the onset of the test session because toward the end of extinction training, most rats did not approach the lever. Subsequent lever presses then led to response-contingent deliveries of the same conditioned light-tone cues. On the test day, the animals were divided into 3 groups for testing the 3 different D3 compounds, respectively. Each animal randomly received either one systemic dose of a test D3 drug (below) or the vehicle [25% (2-hydroxypropyl)-β-cyclodextrin] (see Fig. 1A). SB-277011A (6.0, 12.0, 24.0 mg/kg) was administered systemically (i.p.) 60 min prior to testing for reinstatement, while NGB 2904 (0.1, 1.0, 5.0 mg/kg) or BP 897 (0.1, 1.0, 3.0 mg/kg) was administered systemically (i.p.) 30 min prior to testing for reinstatement. The doses of the drugs and the pretreatment times were chosen based upon the previously published pharmacokinetic and pharmacodynamic properties of the three D3 compounds (Yuan et al., 1998; Pilla et al., 1999; Austin et al., 2001; Beardsley et al., 2001; Robarge et al., 2001; Aujla et al., 2002; Di Ciano et al., 2003; Duarte et al., 2003; Garcia-Ladona and Cox, 2003; Heidbreder et al., 2005), our own previous studies (Vorel et al., 2002; Ashby et al., 2003; Campos et al., 2003, 2004; Gilbert et al., 2003; Lacroix et al., 2003; Xi et al., 2003, 2004), and pilot studies carried out in preliminary phases of the present investigation.
Drugs
Cocaine HCl (Sigma Chemical Co., Saint Louis, MO) was dissolved in physiological saline. SB-277011A (trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolinecarboxamide) was provided by GlaxoSmithKline Pharmaceuticals (Verona, Italy, and Harlow, Essex, UK). NGB 2904 (N-(4-[4-{2,3-dichlorophenyl}-1-piperazinyl]butyl)-3-fluorenylcarboxamide) was synthesized as reported (Yuan et al., 1998) in the Medicinal Chemistry Section, Intramural Research Program, National Institute on Drug Abuse (Baltimore, MD). BP 897 (1-(4-(2-naphthoylamino)butyl)-4-(2-methoxyphenyl)-1A-piperazine) was purchased from Sigma Chemical Co. (Saint Louis, MO). All three compounds (SB-277011A, NGB 2904, BP 897) were dissolved in 25% 2-hydroxypropyl-β-cyclodextrin vehicle solution (Sigma/RBI, Saint Louis, MO).
Data analyses
All data are presented as means (±S.E.M.), and standard multivariate statistical procedures (Kirk, 1982) were used for all data analyses. One-way analysis of variance (ANOVA) was used to analyze the effects of each D3 compound on cocaine cue-triggered reinstatement of drug-seeking behavior. Individual group comparisons were carried out using Dunnett’s statistical method (Kirk, 1982). Student’s t-test was used to determine the statistical significance of cue-triggered reinstatement under vehicle conditions (i.e., in the absence of any of the tested D3 compounds).
RESULTS
Cocaine self-administration across different experimental groups
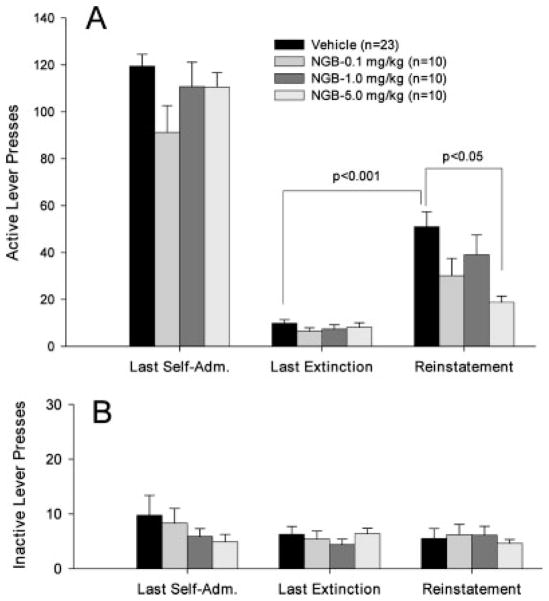
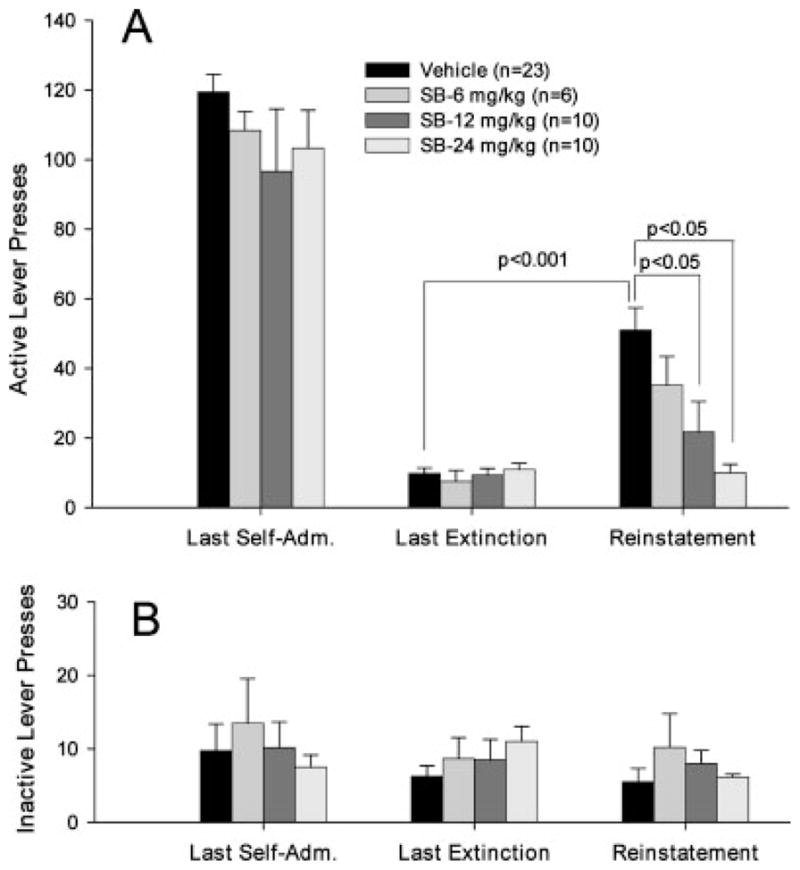
Figure 1 shows the general experiment protocol indicating the different phases of the experiment (Fig. 1A) and typical drug-seeking behavior from a representative single subject during each phase (Fig. 1B). Regular and stable drug-seeking behavior (i.e., active lever presses) was observed during the last session of cocaine self-administration under FR2 conditions until the maximal 50 cocaine infusions were achieved (marked by an arrow on event record I, Fig. 1B). Rats in all three groups exhibited stable responding on the active lever during the last 5–7 self-administration days with a within-subject variability of <10% in daily cocaine infusions. There was no difference in the mean numbers of cocaine infusions or the mean numbers of active lever presses between different groups of rats during the last three cocaine self-administration sessions, and one-way ANOVA revealed no significant difference between different groups in the numbers of active lever presses on the last session of cocaine self-administration (Fig. 2A, left panel: F3,45 = 1.32, P = 0.28; Fig. 3A, left panel: F3,49 = 0.92, P = 0.44; Fig. 4A, left panel: F3,49 = 1.23, P = 0.41). Responding on the inactive lever was minimal in all groups of rats. There was no significant difference in responding on the inactive lever between different groups during self-administration (Figs. 2B–4B, left panels).
Fig. 2.
Effects of the D3 receptor antagonist SB-277011A on cocaine-associated cue-induced reinstatement of drug-seeking behavior. Pretreatment with SB-277011A dose-dependently inhibited cocaine cue-induced reinstatement of drug-seeking behavior (A (right): F3,45 = 7.44, P < 0.01). Between-group comparisons with the Dunnett statistic revealed a statistically significant reduction in cocaine cue-induced drug-seeking after 12 and 24 mg/kg SB-277011A, but not 6 mg/kg SB-277011A administration, when compared with the vehicle pretreatment group (see Results for statistical analysis results in detail). In contrast, SB-277011A had no significant effect on inactive lever presses (B: right). Last Self-Adm.: last session of cocaine self-administration; Last Extinction: last session of extinction.
Fig. 3.
Effects of the D3 receptor antagonist NGB 2904 on cocaine cue-induced reinstatement of drug-seeking behavior. Pre-treatment with NGB 2904 significantly inhibited cocaine cue-induced reinstatement of drug-seeking behavior (A (right): F3,49 = 4.28, P = 0.009). Between-group comparisons with the Dunnett statistic revealed a statistically significant reduction in cocaine cue-induced drug-seeking after 5.0 mg/kg, but not 0.1 or 1.0 mg/kg NGB 2904, when compared with the vehicle pretreatment group. In contrast, NGB 2904 had no significant effect on inactive lever presses during reinstatement testing (B: right).
Fig. 4.
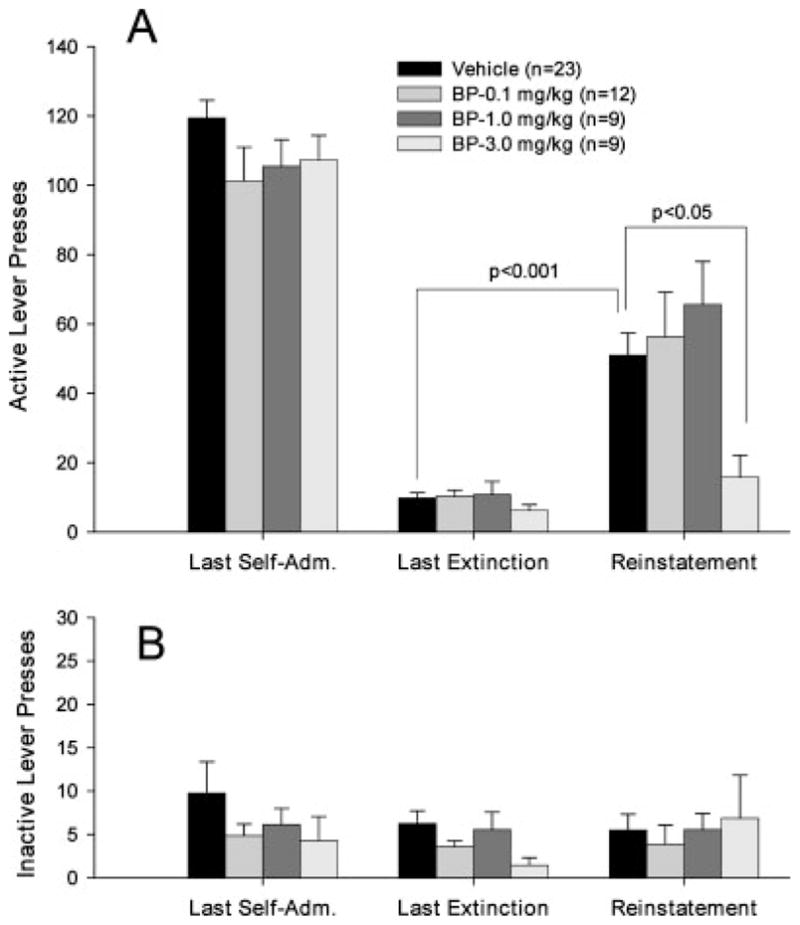
Effects of the mixed D3 agonist/antagonist BP 897 on cocaine cue-induced reinstatement of drug-seeking behavior. Pre-treatment with BP 897 significantly inhibited cocaine cue-induced reinstatement of drug-seeking behavior (A (right): F3,49 = 4.10, P < 0.05). Between-group comparisons with the Dunnett statistic revealed a statistically significant reduction in cocaine cue-induced drug-seeking after 3.0 mg/kg, but not 0.1 or 1.0 mg/kg BP 897 administration, when compared with the vehicle pretreatment group. In contrast, BP 897 had no significant effect on inactive lever presses during reinstatement testing (B: right).
Effect of extinction on drug seeking behavior in the absence of cocaine and cocaine-associated cues
In the absence of cocaine and cocaine-associated cues, the number of drug-seeking responses gradually decreased and met the extinction criterion (<10 lever presses per 3 h, see Fig. 1B, event record II) after 10– 14 daily sessions. Figures 2–4 (middle panels) show the mean numbers of responses for each group of rats on the last session of extinction. There was no difference in the number of extinction responses between the different groups on the last session of extinction immediately prior to reinstatement test (Fig. 2A: F3,45 = 1.11, P = 0.36; Fig. 3A: F3,49 = 0.54, P = 0.66; Fig. 4A: F3,49 = 1.52, P = 0.22). Similarly, there was no difference in responding on the inactive lever between self-administration and extinction responding or between different groups during self-administration or extinction (Figs. 2B–4B, middle panels).
Cocaine cues reinstate extinguished drug-seeking behavior
Figure 1B event record III illustrates representative cocaine-associated cue-induced responding on the active lever during reinstatement testing. The first non-contingent presentations of the cocaine-associated cues (light-tone) evoked immediate responding on the active lever, which led to response-contingent presentations of the cocaine conditioned cue-light and tone. Presentation of such cocaine-conditioned cues produced robust reinstatement of drug-seeking behavior in the vehicle control group (Student’s t-test, P < 0.001, when compared with the last preceding extinction session, Figs. 2A–4A). In contrast, presentation of cocaine-associated cues did not produce significant responding on the inactive lever (Figs. 2B–4B).
Effects of SB-277011A on cue-induced reinstatement of drug-seeking behavior
Figure 2 shows the effects of SB-277011A on cocaine cue-induced reinstatement of drug-seeking behavior. Pretreatment with SB-277011A (6, 12, 24 mg/kg i.p.) dose-dependently inhibited (by 35, 65, 85%, respectively) cocaine cue-induced reinstatement of drug-seeking behavior (F3,45=7.44, P < 0.01, one-way ANOVA, Fig. 2A, right panel). Individual planned group comparisons using Dunnett’s test revealed a statistically significant decrease in cocaine cue-induced responding after 12 mg/kg (D′ = 3.10, P < 0.05) and 24 mg/kg (D′ = 4.34, P < 0.05), but not 6 mg/kg (D′ = 1.38, P > 0.05) SB-277011A administration.
Effects of NGB 2904 on cue-induced reinstatement of drug-seeking behavior
Figure 3 shows data demonstrating cocaine cue-induced reinstatement of drug-seeking in the presence of vehicle or different doses of NGB 2904. NGB 2904, at 0.1, 1, 5 mg/kg i.p., significantly attenuated (by 45, 30, 70%, respectively) cocaine cue-induced reinstatement of drug-seeking in a dose-dependent manner (F3,49 = 4.28, P = 0.009, one-way ANOVA, Fig. 3A, right panel). Individual group comparisons using Dunnett’s test revealed statistically significant reduction in cocaine cue-induced reinstatement of drug-seeking after 5 mg/kg NGB 2904 administration (D′ = 3.43, P < 0.05), but not after 0.1 mg/kg (D′ = 2.01, P > 0.05) or 1 mg/kg (D′ = 1.27, P > 0.05) NGB 2904 administration.
Effects of BP 897 on cue-induced reinstatement of drug-seeking behavior
Figure 4 shows data demonstrating that BP-897 significantly inhibited cocaine-seeking triggered by cocaine-associated stimuli at 3 mg/kg (~70% inhibition), but not at 0.1 or 1 mg/kg i.p.. Multivariate statistical analysis revealed a significant overall reduction in cocaine cue-induced responding by BP 897 (F3,49 = 4.10, P < 0.05, one-way ANOVA, Fig. 3A, right panel). Individual group comparisons using Dunnett’s test revealed statistically significant reduction of cue-induced reinstatement after 3 mg/kg BP 897 (D′ = 2.74, P < 0.05), but not after 0.1 mg/kg (D′ = 0.45, P > 0.05) or 1 mg/kg (D′ = 1.14, P > 0.05) BP 897 administration.
Effects of SB-277011A, NGB 2904, or BP 897 on non-contingent behavioral responding
In contrast to the reduction in active lever responding produced by SB-277011A, NGB 2904, and BP 897 during reinstatement testing, none of these three compounds had any significant effect whatever on inactive lever responding during reinstatement testing.
DISCUSSION
The present experiments demonstrate that re-introduction of cocaine-associated cues (light-tone) reliably reinstates cocaine-seeking behavior in rats after 10–14 days of extinction from previous cocaine self-administration. This finding is consistent with past cue-induced reinstatement studies in rats (Grimm et al., 2001; See et al., 2001). The present experiments also demonstrate that pretreatment with the potent and selective D3 receptor antagonists SB-277011A and NGB 2904 or the mixed D3 agonist/antagonist BP 897 inhibit cocaine cue-induced reinstatement of drug-seeking behavior. These behavioral effects cannot be attributed to drug-induced disruption of behavior since responses on the inactive levers were not affected. In addition, it has previously been reported that all three of these compounds, within the dose ranges tested in the present experiments have no significant effect on either locomotor activity or food- or sucrose-taking behavior (Reavill et al., 2000; Cook et al., 2004; Xi et al., 2003; Grundt et al., 2004). Thus, the present data support the hypothesis that D3 receptors play an important role in mediating cocaine cue-induced reinstatement of drug-seeking behavior.
Selective blockade of DA D3 receptors by SB-277011A
As mentioned above in the Introduction, non-selective D1-like or D2-like receptor antagonists inhibit cocaine cue-induced reinstatement of drug-seeking behavior (Weiss et al., 2000; Ciccocioppo et al., 2001; Cervo et al., 2003). Those findings raise the issue of whether the presently-observed inhibitory effects of D3-selective compounds in this relapse model might be attributable to D1 or D2 receptor-selective antagonism rather than D3 receptor-selective antagonism. However, an accumulating body of evidence does not support this assumption: (1) SB-277011A is a highly-potent and highly-selective D3 receptor antagonist with an 80- to 100-fold selectivity for D3 over other DA receptors; high affinity for the human (pKi 7.95) and rat (pKi 7.97) cloned DA D3 receptor; and a 100-fold selectivity over 66 other receptors, enzymes, ion channels, and transporters in the central nervous system (Reavill et al., 2000; Stemp et al., 2000); (2) the effects of SB-277011A in various animal models relating to addiction are significantly different from those produced by D2-preferring antagonists (Heidbreder et al., 2005); for example, D2antagonists elevate electrical brain-stimulation reward thresholds (Stein and Ray, 1960; Stein, 1962; Panagis and Spyraki, 1996), suggesting an aversion-like effect, while SB-277011A does not alter brain stimulation reward thresholds (Vorel et al., 2002; Campos et al., 2004); similarly, D2-preferring antagonists produce aversion in the conditioned place preference/aversion paradigm (Shippenberg and Herz, 1988), while SB-277011A produces neither reward nor aversion (Vorel et al., 2002; Ashby et al., 2003; Gyertyán and Gál, 2003); (3) SB-277011A does not significantly alter locomotor activity at doses approaching 100 mg/kg (much higher than the doses of SB-277011A used in the present study), whereas locomotor inhibition is a classic property of D2 antagonists (Reavill et al., 2000); and (4) at doses many-fold those used in the present experiments, SB-277011A does not block quinpirole-induced decreases in DA in the dorsal striatum where a high density of D2 receptors are located, and does not significantly alter prolactin levels (Reavill et al., 2000), whereas alteration of prolactin levels is a classic property of D2 antagonists. Also, SB-277011A significantly increases acetylcholine levels in the rat frontal cortex, but D2 antagonists fail to do so (Lacroix et al., 2003).
Since the acquisition of cue-triggered reinstatement presumably involves storage and encoding of cue-reward associations, and the expression of cue-triggered reinstatement presumably involves retrieval of memories of such cue-reward association, is it possible that SB-277011A-induced inhibition of cue-triggered reinstatement is mediated by interference with general aspects of memory encoding and retrieval? This seems unlikely, as SB-277011A does not appear to alter memory, as measured using a delayed non-matched position test (D. Jones and J.J. Hagan, personal communication). Furthermore, acute administration of SB-277011A significantly increases acetylcholine levels in the anterior cingulate cortex (Lacroix et al., 2003) and reverses scopolamine-induced memory deficits as assessed by the three choice point water labyrinth test (Laszy et al., 2003). Both of these effects would be expected to improve rather than to interfere with memory.
Selective blockade of DA D3 receptors by NGB 2904
Similar to SB-277011A, NGB 2904 is another highly-potent and highly-selective DA D3 receptor antagonist (Yuan et al., 1998). This compound has structural similarity to the mixed D3 agonist/antagonist BP 897, and demonstrates high binding affinity for cloned primate D3 receptors in CHO cells (Ki 1.4 nM) (Yuan et al., 1998; Robarge et al., 2001). NGB 2904 has 155-fold selectivity for primate D3 over D2 receptors and >800-fold selectivity for rat D3 versus D2 receptors in Sf9 cells (Yuan et al., 1998; Newman et al., 2003). In addition, NGB 2904 has >5,000-fold selectivity for D3 over D1, D4, and D5 receptors and 200- to 600-fold selectivity over other receptors (such as α1 and 5HT2) (Yuan et al., 1998). Consistent with its properties as a selective high-potency DA D3 antagonist and thus with its pharmacological similarity to SB-277011A, NGB 2904 in the present study also inhibited cocaine cue-induced reinstatement of drug-seeking behavior.
However, it must be noted that NGB 2904, at the highest dose tested in the present experiments, only inhibited cocaine cue-induced reinstatement by approximately 70% as compared to the 85% inhibition produced by the highest dose of SB-277011A tested. It is unclear if increasing the NGB 2904 dose would produce greater inhibition of cue-induced reinstatement. However, we have observed in previous experiments with NGB 2904 (Xi et al., 2003; Campos et al., 2004) that its D3 antagonist-like effects in various preclinical animal models related to addiction appear to wane at high doses, i.e., that a dose window seems to exist for NGB 2904’s putative anti-addiction effects. The present data clearly suggest that NGB 2904 strongly resembles SB-277011A in its ability to attenuate cocaine cue-triggered reinstatement of cocaine-seeking behavior and, thus, like SB-277011A, may have clinical utility for the treatment of addictive diseases.
BP 897’s actions may be mediated by D3 and/or other receptors
In contrast to SB-277011A and NGB 2904, BP 897 has been classified as a mixed D3 agonist/antagonist (Pilla et al., 1999; Wood et al., 2000; Wicke and Garcia-Ladona, 2001). It has lower (60–70-fold) selectivity for human D3 versus human D2 receptors than SB-277011A and NGB 2904, and similar (60–70-fold) selectivity over other receptors such as α1-, α2-adrenergic receptors, and 5-HT1A receptors (Pilla et al., 1999; Garcia-Ladona and Cox, 2003).
Most relevant for the present purposes, BP 897 also displays properties of a D2 receptor antagonist. For example, BP 897 (1–3 mg/kg) significantly increases brain stimulation reward thresholds (Campos et al., 2004), suggesting an aversive-like effect. In the conditioned place preference/aversion paradigm, BP 897 produces conditioned place aversion in rats (Duarte et al., 2003; Gyertyán and Gál, 2003). In addition, recent studies have indicated that BP 897-induced inhibition of L-DOPA-induced dyskinesias in MPTP-treated rats is likely to be mediated by BP 897-induced DA D2 receptor blockade (Visanji et al., 2004). Additional evidence for BP 897’s DA D2 receptor-blocking properties may be found in a recent extensive review by Heidbreder et al. (2005). Also, in an in vitro G-protein binding assay, BP-897 appears to serve as a partial 5-HT1A receptor agonist (Besong-Agbo et al., 2004). A recent study has shown that systemic administration of BP 897 and NGB 2904 in wild type mice produced a significant left-ward shift in the drug discrimination response curve to cocaine (O’Callaghan et al., 2005). However, in D3 knockout mice, NGB 2904 does not alter the drug discrimination response to cocaine whereas BP 897 produced a 1.5-fold leftward shift, suggesting that BP 897 has action at other receptors (O’Callaghan et al., 2005). Taken together, these data suggest that the inhibitory effect of 3 mg/kg of BP 897 on cue-induced reinstatement of drug-seeking behavior observed in the present study could be mediated by blocking D3 receptors, D2 receptors, and/or by acting on other receptors.
Selective DA D3 receptor antagonists as potential anti-addiction medications
We and others have previously demonstrated that SB-277011A attenuates: (1) cocaine- and nicotine-enhanced electrical brain-stimulation reward (Vorel et al., 2002; Campos et al., 2003); (2) acquisition and expression of cocaine- or heroin-induced conditioned place preference (Vorel et al., 2002; Ashby et al., 2003); (3) cocaine-seeking behavior under second-order reinforcement conditions (Di Ciano et al., 2003); (4) cocaine self-administration under progressive ratio and variable-cost/variable-payoff fixed ratio reinforcement (Gilbert et al., 2003); (5) cocaine-, nicotine-, or stress-triggered relapse to cocaine-seeking behavior as assessed by the reinstatement model (Vorel et al., 2002; Andreoli et al., 2003b; Xi et al., 2004); (6) oral ethanol intake (Andreoli et al., 2003a; Rivera et al., 2003); and (7) relapse to ethanol-seeking behavior in animals extinguished from ethanol-taking behavior (Marcon et al., 2003). Consistent with those findings, the present study has shown that SB-277011A, within the same dose range as those previous experiments, inhibits cocaine cue-induced reinstatement of drug-seeking in a dose-dependent manner.
In addition, we have recently reported (Xi et al., 2003) that NGB 2904, within a dose range of 0.1–5 mg/kg, significantly inhibits: (1) the enhancement of brain stimulation reward produced by 2, but not 10 mg/kg i.p. of cocaine; (2) cocaine self-administration under a progressive ratio, but not under a fixed ratio reinforcement schedule; and (3) cocaine- but not sucrose-triggered reinstatement of drug-/food-seeking behaviors. It is noteworthy that NGB 2904 by itself within the dose range of 0.1–5 mg/kg has no effect on brain stimulation reward (Campos et al., 2004) or spontaneous locomotor activity (Grundt et al., 2004). It also does not replace cocaine to maintain self-administration behavior in rats (Xi et al., 2003). These data suggest that NGB 2904 produces similar pharmacological effects as SB-277011A in animal models of drug reward and relapse. Of importance is the fact that NGB 2904 itself has no rewarding or aversive property, which is significantly different from D2-preferring antagonists.
Similar to SB-277011A and NGB 2904, BP 897, at the dose range of 0.05–1.0 mg/kg, also dose-dependently inhibits: (1) cocaine-seeking behavior under a second-order schedule of reinforcement (Pilla et al., 1999); (2) cocaine-conditioned place preference (Duarte et al., 2003); (3) cocaine-induced hyperactivity (Aujla et al., 2002); (4) cocaine- or amphetamine-induced drug discrimination (Beardsley et al., 2001); and (5) cocaine-associated stimuli-induced reinstatement of drug-seeking (Cervo et al., 2003). However, in the present cue-induced relapse model, we did not see any inhibitory effect by 0.1–1 mg/kg i.p. BP 897, while a higher dose (3 mg/kg i.p.) of BP 897 produced a significant inhibitory effect on cue-induced reinstatement of drug-seeking behavior. It might, therefore, be argued that BP 897, at the highest dose presently tested (3 mg/kg i.p.), produced its inhibitory effect on cue-induced relapse to cocaine-seeking behavior by a similar mechanism to that of SB-277011A and NGB 2904. While nothing in the present experiments precludes that possibility, we believe that a careful overview of all of BP 897’s pharmacological and behavioral properties (see Heidbreder et al., 2005 for a comprehensive review) suggests instead that BP 897’s pharmacological profile, especially within the dose range found effective in the present experiments, is more congruent with that of a DA D2 antagonist than that of a pharmacologically selective D3 antagonist. Thus, as noted above, BP 897 increases brain stimulation reward thresholds (Campos et al., 2004) and produces conditioned place aversion (Duarte et al., 2003). Also, similar to administration of the DA D2 antagonist haloperidol (0.2 or 0.5 mg/kg), BP 897 (1 mg/kg i.p.) increases cocaine self-administration under FR1 reinforcement (Gál and Gyertyán, 2003). In addition, BP 897 shows properties of a DA D2 antagonist in several in vitro and ex vivo biochemical and physiological assay systems (see Heidbreder et al., 2005 for review). Finally, BP 897 has significant affinity for a number of other neurotransmitter receptors (for review, see Garcia-Ladona and Cox, 2003). Thus, BP 897 may have acted as a DA D3 antagonist in the present experiments, but it would appear imprudent to exclude the possibility that its actions may also have resulted from DA D2 antagonism or from interaction with other receptors.
In conclusion, the present study demonstrates that selective blockade of DA D3 receptors by SB-277011A and NGB 2904 inhibits cocaine cue-induced reinstatement of drug-seeking behavior. The partial agonist BP 897 also produced a similar inhibitory effect on cue-induced relapse but only at the highest dose tested in the present experiments (3 mg/kg i.p.). Based upon the established pharmacological profiles of SB-277011A and NGB 2904, we conclude that their anti-relapse effects are related to their selective DA D3 antagonist properties. While BP 897’s anti-relapse effects may similarly have resulted from DA D3 receptor antagonism, they may also have resulted from interaction with a number of other CNS receptors with which BP 897 has been shown to interact. As we noted above, both the BLA and the core of the NAc appear to be critically involved in cue-induced reinstatement of drug-seeking behavior, while the highest density of D3 receptors is located in the shell of the NAc. Thus, additional studies using the technique of intracranial microinjection are required to determine the precise loci of the D3 antagonism in the rat brain that contributes to attenuation of cue-triggered relapse to drug-seeking behavior. Overall, the present study suggests that DA D3 receptors play an important role in cue-induced craving and relapse, and that D3 receptor antagonists such as SB-277011A, NGB 2904, or other novel compounds merit further investigation as anti-relapse pharmacotherapies.
Footnotes
Preliminary data from these studies were presented in abstract form at the 2004 meetings of the Society for Neuroscience, San Diego, CA.
References
- Alleweireldt AT, Weber SM, Neisewander JL. Passive exposure to a contextual discriminative stimulus reinstates cocaine-seeking behavior in rats. Pharmacol Biochem Behav. 2001;69:555–560. doi: 10.1016/s0091-3057(01)00573-1. [DOI] [PubMed] [Google Scholar]
- Andreoli M, Marcon C, Hagan JJ, Heidbreder CA. Effect of selective antagonism of dopamine D3 receptor by SB-277011-A on oral alcohol self-administration in mice. Eur Neuropsychopharmacol. 2003a;13(Suppl 1):S17. [Google Scholar]
- Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology. 2003b;28:1272–1280. doi: 10.1038/sj.npp.1300183. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Paul M, Gardner EL, Heidbreder CA, Hagan JJ. Acute administration of the selective D3 receptor antagonist SB-277011-A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse. 2003;48:154–156. doi: 10.1002/syn.10188. [DOI] [PubMed] [Google Scholar]
- Aujla H, Sokoloff P, Beninger RJ. A dopamine D3 receptor partial agonist blocks the expression of conditioned activity. Neuroreport. 2002;13:173–176. doi: 10.1097/00001756-200201210-00039. [DOI] [PubMed] [Google Scholar]
- Austin NE, Baldwin SJ, Cutler L, Deeks N, Kelly PJ, Nash M, Shardlow CE, Stemp G, Thewlis K, Ayrton A, Jeffrey P. Pharmacokinetics of the novel, high-affinity and selective dopamine D3 receptor antagonist SB-277011 in rat, dog and monkey: in vitro/in vivo correlation and the role of aldehyde oxidase. Xenobiotica. 2001;31:677–686. doi: 10.1080/00498250110056531. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Sokoloff P, Balster RL, Schwartz J-C. The D3R partial agonist, BP 897, attenuates the discriminative stimulus effects of cocaine and D-amphetamine and is not self-administered. Behav Pharmacol. 2001;12:1–11. doi: 10.1097/00008877-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Besong-Agbo D, Merx R, Szabo L, Hahn A, Garcia-Ladona F. BP897 a potent blocker of cocaine seeking behavior acts as partial agonist at h5-HT1a receptors. 2004 Abstract Viewer/Itinerary Planner. Online; Abstracts of the 34th Annual Meeting of the Society for Neuroscience; 2004 November 23–27; San Diego, CA. Washington, DC: Society for Neuroscience; 2004. p. Abstract 394.9. [Google Scholar]
- Bouthenet M-L, Souil E, Martres M-P, Sokoloff P, Giros B, Schwartz J-C. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Campos AC, Xi Z-X, Gilbert J, Ashby CR Jr, Heidbreder CA, Gardner EL. The dopamine D3 receptor antagonist SB277011A antagonizes nicotine enhanced brain-stimulation reward in rat. 2003 Abstract Viewer/Itinerary Planner. Online; Abstracts of the 33rd Annual Meeting of the Society for Neuroscience; 2003 November 7–12; New Orleans, LA. Washington, DC: Society for Neuroscience; 2003. p. Abstract 322.8. [Google Scholar]
- Campos AC, Xi Z-X, Gilbert J, Ashby CR, Jr, Heidbreder CA, Newman AH, Gardner EL. Blockade of dopamine D3 receptors by SB-277011A, NGB 2904 or BP 897 attenuates nicotine-enhanced brain stimulation reward in rat. 2004 Abstract Viewer/Itinerary Planner. Online; Abstracts of the 34th Annual Meeting of the Society for Neuroscience; 2004 November 23–27; San Diego, CA. Washington, DC: Society for Neuroscience; 2004. p. Abstract 691.6. [Google Scholar]
- Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28:1150–1159. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgwin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D1 antagonists. Proc Natl Acad Sci USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CD, Newman JL, Winfree JC, Beardsley PM. Modulation of the locomotor activating effects of the noncompetitive NMDA receptor antagonist MK801 by dopamine D2/3 receptor agonists in mice. Pharmacol Biochem Behav. 2004;77:309–318. doi: 10.1016/j.pbb.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Reinstatement and spontaneous recovery of cocaine-seeking following extinction and different durations of withdrawal. Behav Pharmacol. 2002;13:397–405. doi: 10.1097/00008877-200209000-00013. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Differential control over drug-seeking behavior by drug-associated conditioned reinforcers and discriminative stimuli predictive of drug availability. Behav Neurosci. 2003;117:952–960. doi: 10.1037/0735-7044.117.5.952. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004a;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Contribution of the ventral tegmental area to cocaine-seeking maintained by a drug-paired conditioned stimulus in rats. Eur J Neurosci. 2004b;19:1661–1667. doi: 10.1111/j.1460-9568.2004.03232.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. The relation between dopamine oxidation currents in the nucleus accumbens and conditioned increases in motor activity in rats following repeated administration of d-amphetamine or cocaine. Eur J Neurosci. 1998;10:1113–1120. doi: 10.1046/j.1460-9568.1998.00124.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. Changes in dopamine efflux associated with extinction, CS-induced and d-amphetamine-induced reinstatement of drug-seeking behavior by rats. Behav Brain Res. 2001a;120:147–158. doi: 10.1016/s0166-4328(00)00373-9. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of Pavlovian approach behavior. J Neurosci. 2001b;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz J-C, Sokoloff P. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci. 2000;20:8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte C, Lefebvre C, Chaperon F, Hamon M, Thiébot M-H. Effects of a dopamine D3 receptor ligand, BP 897, on acquisition and expression of food-, morphine-, and cocaine-induced conditioned place preference, and food-seeking behavior in rats. Neuropsychopharmacology. 2003;28:1903–1915. doi: 10.1038/sj.npp.1300276. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana DJ, Post RM, Pert A. Conditioned increases in mesolimbic dopamine overflow by stimuli associated with cocaine. Brain Res. 1993;629:31–39. doi: 10.1016/0006-8993(93)90477-5. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Tran-Nguyen LTL, Specio SE, Groff RS, Neisewander JL. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology (Berl) 1998;135:151–160. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Gál K, Gyertyán I. Targeting the dopamine D3 receptor cannot influence continuous reinforcement cocaine self-administration in rats. Brain Res Bull. 2003;61:595–601. doi: 10.1016/s0361-9230(03)00217-x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho J-K, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Garcia-Ladona FJ, Cox BF. BP 897, a selective dopamine D3 receptor ligand with therapeutic potential for the treatment of cocaine-addiction. CNS Drug Rev. 2003;9:141–158. doi: 10.1111/j.1527-3458.2003.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J, Xi Z-X, Campos AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. The dopamine D3 receptor antagonist SB277011A inhibits cocaine reinforcement under fixed ratio and progressive ratio schedules. 2003 Abstract Viewer/Itinerary Planner. Online; Abstracts of the 33rd Annual Meeting of the Society for Neuroscience; 2003 Nov 7–12; New Orleans, LA. Washington, DC: Society for Neuroscience; 2003. p. Abstract 422.10. [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation: Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundt P, Cao J, Luedtke RR, McElveen E, Taylor M, Newman AH. Novel dopamine D3 receptor ligands as tools for in vivo evaluation. 2004 Abstract Viewer/Itinerary Planner. Online; Abstracts of the 34th Annual Meeting of the Society for Neuroscience; 2004 November 23–27; San Diego, CA. Washington, DC: Society for Neuroscience; 2004. p. Abstract 573.11. [Google Scholar]
- Gyertyán I, Gál K. Dopamine D3 receptor ligands show place conditioning effect but do not influence cocaine-induced place preference. Neuroreport. 2003;14:93–98. doi: 10.1097/00001756-200301200-00018. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi Z-X, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev. 2005 Feb 11; doi: 10.1016/j.brainresrev.2004.12.033. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk RE. Experimental design: procedures for the behavioral sciences. 2. Belmont, CA: Brooks/Cole; 1982. [Google Scholar]
- Lacroix LP, Hows MEP, Shah AJ, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors enhances monoaminergic and cholinergic neurotransmission in the rat anterior cingulate cortex. Neuropsychopharmacology. 2003;28:839–849. doi: 10.1038/sj.npp.1300114. [DOI] [PubMed] [Google Scholar]
- Laszy J, Gyertyán I, Laszlovszky I, Szombathely Z. Dopamine D3 receptor antagonists show cognitive enhancer activity. Proceedings of the Sixth IBRO World Congress of Neuroscience; 2003 July 10–15; Prague: International Brain Research Organization; 2003. p. 45.p. abstract 1126. [Google Scholar]
- Le Foll B, Frances H, Diaz J, Schwartz J-C, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- Lévesque D, Diaz J, Pilon C, Martres M-P, Giros B, Souil E, Schott D, Morgat J-L, Schwartz J-C, Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci USA. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcon C, Andreoli M, Pilla M, Tessari M, Heidbreder CA. A new model to assess drug and cue-induced relapse to ethanol self-administration in mice. Behav Pharmacol. 2003;14(Suppl 1):S66. [Google Scholar]
- Mash DC. D3 receptor binding in human brain during cocaine overdose. Mol Psychiatry. 1997;2:5–6. [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Murray AM, Ryoo HL, Gurevich E, Joyce JN. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci USA. 1994;91:11271–11275. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academy of Sciences (National Research Council, Commission on Life Sciences, Institute of Laboratory Animal Resources) Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Neisewander JL, O’Dell LE, Tran-Nguyen LTL, Castañeda E, Fuchs RA. Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology. 1996;15:506–514. doi: 10.1016/S0893-133X(96)00097-8. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RA, Tran-Nguyen LTL, Weber SM, Coffey GP, Joyce JN. Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1479–1487. doi: 10.1038/sj.npp.1300456. [DOI] [PubMed] [Google Scholar]
- Newman AH, Cao J, Bennett CJ, Robarge MJ, Freeman RA, Luedtke RR. N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl, butenyl and butynyl}arylcarboxamides as novel dopamine D3 receptor antagonists. Bioorg Med Chem Lett. 2003;13:2179–2183. doi: 10.1016/s0960-894x(03)00389-5. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- O’Callaghan MJ, Xu M, Katz JL. Effects of dopamine D2/3 ligands on cocaine discrimination in dopamine D3 receptor KO and WT mice. Abstract 311.5, Experimental Biology 2005 and XXXV International Congress of Physiological Sciences, American Society for Pharmacology and Experimental Therapeutics (ASPET) Program; 2005 April 2–6; San Diego, CA. 2005. [Google Scholar]
- Panagis G, Spyraki C. Neuropharmacological evidence for the role of dopamine in ventral pallidum self-stimulation. Psychopharmacology (Berl) 1996;123:280–288. doi: 10.1007/BF02246582. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Howland JG. Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior. Neurosci Biobehav Rev. 2003;27:543–554. doi: 10.1016/j.neubiorev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz J-C, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, Boyfield I, Branch CL, Cilia J, Coldwell MC, Hadley MS, Hunter AJ, Jeffrey P, Jewitt F, Johnson CN, Jones DNC, Medhurst AD, Middlemiss DN, Nash DJ, Riley GJ, Routledge C, Stemp G, Thewlis KM, Trail B, Vong AKK, Hagan JJ. Pharmacological actions of a novel, high-affinity, and selective human dopamine D3 receptor antagonist, SB-277011-A. J Pharmacol Exp Ther. 2000;294:1154–1165. [PubMed] [Google Scholar]
- Rivera SN, Katana J, Ashby CR, Jr, Piyis YS, Gardner EL, Pena LA, Heidbreder CA, Volkow ND, Thanos PK. A novel dopamine D3 receptor antagonist (SB-277011-A) attenuates ethanol consumption in ethanol preferring (P) and non-preferring (NP) rats. Abstract Viewer/Itinerary Planner. Online; Abstracts of the 33rd Annual Meeting of the Society for Neuroscience; 2003 November 2–12; New Orleans, LA. Washington, DC: Society for Neuroscience; 2003. p. Abstract 665.7. [Google Scholar]
- Robarge MJ, Husbands SM, Kieltyka A, Brodbeck R, Thurkauf A, Newman AH. Design and synthesis of [(2,3-dichlorophenyl)-piperazin-1-yl]alkylfluorenylcarboxamides as novel ligands selective for the dopamine D3 receptor subtype. J Med Chem. 2001;44:3175–3186. doi: 10.1021/jm010146o. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Niaura RS, Childress AR, Abrams DB, Monti PM. Cue reactivity in addictive behaviors: theoretical and treatment implications. Int J Addict. 1990;25:957–993. doi: 10.3109/10826089109071030. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- Segal DM, Moraes CT, Mash DC. Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Res Mol Brain Res. 1997;45:335–339. doi: 10.1016/s0169-328x(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Motivational effects of opioids: influence of D-1 versus D-2 receptor antagonists. Eur J Pharmacol. 1988;151:233–242. doi: 10.1016/0014-2999(88)90803-5. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres M-P, Bouthenet M-L, Schwartz J-C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Martres M-P, Giros B, Bouthenet M-L, Schwartz J-C. The third dopamine receptor (D3) as a novel target for anti-psychotics. Biochem Pharmacol. 1992;43:659–666. doi: 10.1016/0006-2952(92)90227-a. [DOI] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L. Effects and interactions of imipramine, chlorpromazine, reserpine, and amphetamine on self-stimulation: possible neurophysiological basis of depression. Recent Adv Biol Psychiatry. 1962;4:297–311. doi: 10.1007/978-1-4684-8306-2_27. [DOI] [PubMed] [Google Scholar]
- Stein L, Ray OS. Brain stimulation reward “thresholds” self-determined in rat. Psychopharmacologia (Berl) 1960;1:251–256. doi: 10.1007/BF00402746. [DOI] [PubMed] [Google Scholar]
- Stemp G, Ashmeade T, Branch CL, Hadley MS, Hunter AJ, Johnson CN, Nash DJ, Thewlis KM, Vong AKK, Austin NE, Jeffrey P, Avenell KY, Boyfield I, Hagan JJ, Middlemiss DN, Reavill C, Riley GJ, Routledge C, Wood M. Design and synthesis of trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quino-linecarboxamide (SB-277011): a potent and selective dopamine D3 receptor antagonist with high oral bioavailability and CNS penetration in the rat. J Med Chem. 2000;43:1878–1885. doi: 10.1021/jm000090i. [DOI] [PubMed] [Google Scholar]
- Visanji NP, Millan MJ, Brotchie JM. The “selective” dopamine D3 receptor partial agonist BP897 has actions in addition to attenuation of D3 transmission in modulating locomotion induced by L-DOPA in monoamine depleted rats. 2004 Abstract Viewer/Itinerary Planner. Online; 2004 Abstracts of the 34th Annual Meeting of the Society for Neuroscience; 2004 November 23–27; San Diego, CA. Washington, DC: Society for Neuroscience; 2004. p. Abstract 308.18. [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Whitelaw RB, Markou A, Robbins TW, Everitt BJ. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology (Berl) 1996;127:213–224. [PubMed] [Google Scholar]
- Wicke K, Garcia-Ladona J. The dopamine D3 receptor partial agonist, BP 897, is an antagonist at human dopamine D3 receptors and at rat somatodendritic dopamine D3 receptors. Eur J Pharmacol. 2001;424:85–90. doi: 10.1016/s0014-2999(01)01054-8. [DOI] [PubMed] [Google Scholar]
- Wood MD, Boyfield I, Nash DJ, Jewitt FR, Avenell KY, Riley GJ. Evidence for antagonist activity of the dopamine D3 receptor partial agonist, BP 897, at human dopamine D3 receptor. Eur J Pharmacol. 2000;407:47–51. doi: 10.1016/s0014-2999(00)00732-9. [DOI] [PubMed] [Google Scholar]
- Xi Z-X, Gilbert J, Campos AC, Ashby CR, Jr, Gardner EL, Newman AH. The dopamine D3 receptor antagonist NGB 2904 inhibits cocaine reward and cocaine-triggered reinstatement of cocaine-seeking behavior. 2003 Abstract Viewer/Itinerary Planner. Online; Abstracts of the 33rd Annual Meeting of the Society for Neuroscience; 2003 Nov 7–12; New Orleans, LA. Washington, DC: Society for Neuroscience; 2003. p. Abstract 422.9. [Google Scholar]
- Xi Z-X, Gilbert J, Campos AC, Kline N, Ashby CR, Jr, Hagan JJ, Heidbreder CA, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology (Berl) 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Chen X, Brodbeck R, Primus R, Braun J, Wasley JWF, Thurkauf A. NGB 2904 and NGB 2849: two highly selective dopamine D3 receptor antagonists. Bioorg Med Chem Lett. 1998;8:2715–2718. doi: 10.1016/s0960-894x(98)00469-7. [DOI] [PubMed] [Google Scholar]
- Yun IA, Nicola SM, Fields HL. Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. Eur J Neurosci. 2004;20:249–263. doi: 10.1111/j.1460-9568.2004.03476.x. [DOI] [PubMed] [Google Scholar]