Abstract
We have previously reported that selective dopamine (DA) D3 receptor antagonists are effective in a number of animal models of drug addiction, but not in intravenous drug self-administration, suggesting a limited ability to modify drug reward. In the present study, we evaluated the actions of S33138, a novel partially selective D3 receptor antagonist, in animal models relevant to drug addiction. S33138, at doses of 0.156 or 0.625 mg/kg (i.p.), attenuated cocaine-enhanced brain-stimulation reward (BSR), and the highest dose tested (2.5 mg/kg) produced a significant aversive-like rightward shift in BSR rate-frequency reward functions. Further, S33138 produced biphasic effects on cocaine self-administration, i.e., a moderate dose (2.5 mg/kg, p.o.) increased, while a higher dose (5 mg/kg, p.o.) inhibited, cocaine self-administration. The increase in cocaine self-administration likely reflects a compensatory response to a partial reduction in drug reward after S33138. In addition, S33138 (0.156–2.5 mg/kg, p.o.) also dose-dependently inhibited cocaine-induced reinstatement of drug-seeking behavior. The reduction in cocaine-enhanced BSR and cocaine-triggered reinstatement produced by lower effective doses (e.g., 0.156 or 0.625 mg/kg) of S33138 is unlikely due to impaired locomotion, as lower effective doses of S33138 decreased neither Ymax levels in the BSR paradigm, rotarod performance, nor locomotion. However, the higher doses (2.5 or 5 mg/kg) of S33138 also significantly inhibited sucrose self-administration and rotarod performance, suggesting non-D3 receptor-mediated effects on non-drug reward and locomotion. These data suggest that lower doses of S33138 interacting essentially with D3 receptors have pharmacotherapeutic potential in treatment of cocaine addiction, while higher doses occupying D2 receptors may influence locomotion and non-drug reward.
Keywords: S33138, Cocaine, Dopamine, Self-administration, Brain reward, Reinstatement
1. Introduction
Cocaine addiction is a serious health problem, yet no effective medications are available for its treatment at the human level. The rewarding effects of cocaine are thought to be primarily mediated by inhibition of dopamine (DA) re-uptake, thereby increasing mesocorticolimbic DA transmission (Wise, 2005). The mesocorticolimbic DA system originates in DA neurons in the ventral tegmental area (VTA) which project predominantly to the nucleus accumbens (NAc) and the prefrontal cortex (Wise, 2005). Based on the DA hypothesis, development of new medications for the treatment of cocaine addiction has largely focused on various classes of DA receptor antagonists (Platt et al., 2002; Heidbreder et al., 2005). However, blockade of D2-like receptors usually inhibits cocaine self-administration at doses that also inhibit food-taking behavior and/or locomotor activity in experimental animals (Glowa and Wojnicki, 1996; Kita et al., 1999). Further, clinical trials with D1-or D2-like antagonists for the treatment of cocaine addiction have failed, due either to ineffectiveness and/or to unfavorable side-effects such as dysphoria or extrapyramidal movements (for reviews see Rothman and Glowa, 1995; Platt et al., 2002; Gorelick et al., 2004).
Given the fact that D3 receptors are located predominantly on mesolimbic DA neurons (Diaz et al., 2000; Stanwood et al., 2000), it has been postulated that drugs targeted toward the D3 receptor may be of use in the treatment of cocaine addiction without producing significant neurological side-effects (Le Foll et al., 2005; Heidbreder et al., 2005; Xi and Gardner, 2007). This hypothesis is supported by recent findings that the D3 receptor antagonists SB-277011A and NGB-2904 significantly inhibit cocaine-, nicotine- or alcohol-seeking behaviors in multiple reinstatement models of relapse, and cocaine-taking behavior under progressive-ratio (PR) or high fixed-ratio (FR10) reinforcement conditions (see reviews by Le Foll et al., 2005; Heidbreder et al., 2005; Xi and Gardner, 2007). However, these D3 receptor antagonists appear to have little effect on cocaine (or nicotine and alcohol) self-administration under low FR (e.g., FR1, FR2) reinforcement (Vorel et al., 2002; Gál and Gyertyán, 2003; Xi et al., 2006; Andreoli et al., 2003; Heidbreder et al., 2005), suggesting a limited role in attenuating acute drug reward per se under low-effort high-payoff reinforcement conditions.
Consequently, partially selective D3 versus D2 receptor antagonism has been proposed as a promising strategy for treating drug addiction and other psychiatric diseases (Joyce and Millan, 2005). This is based upon evidence that simultaneous blockade of both D3 and D2 receptors may produce additive effects in antagonizing drug reward, but produce fewer locomotor side-effects due to opposite locomotor effects produced by D3 antagonism (locomotor facilitation) versus D2 antagonism (locomotor suppression) (Millan et al., 2000; Silverdale et al., 2004; Joyce and Millan, 2005). We have recently described a novel benzopyranopyrrolidine derivative, S33138 (N-[4-[2-[(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1]benzo pyrano[3,4-c]pyrrol-2(3H)-yl)-ethyl]phenyl acetamide), that behaves as a partially selective D3 versus D2 receptor antagonist at cloned, human, and native brain DA receptors (Millan et al.,2008b;Millan and Brocco, 2008), displaying 25-fold selectivity for human (h) D3 over hD2 (short and long isoform) receptors (pKi, 8.7 versus 7.1 and 7.3). In mice, S33138 appears more potent at increasing c-fos mRNA expression in the D3 receptor-rich nucleus accumbens than in the D2 receptor-rich caudate-putamen (Millan et al., 2008c). In rats, sub-chronic administration of S33138 is also more potent at reducing the number of spontaneously active DA neurons in the VTA than in the substantia nigra (Millan et al., 2008c). These data suggest partially selective D3 versus D2 receptor binding properties for S33138 in vivo.
Therefore, in the present study, we investigated the effects of S33138 on cocaine-enhanced brain-stimulation reward (BSR), intravenous cocaine self-administration and cocaine-triggered reinstatement of drug-seeking behavior in rats. In addition, we also observed the effects of S33138 on natural reward (oral sucrose self-administration) and on operant locomotor performance (rotorad test).
2. Materials and methods
2.1. Animals
Male Long-Evans rats (Charles River Laboratories, Raleigh, NC, USA) initially weighing 250–300 g were used for all experiments. They were housed individually in a climate-controlled animal colony room on a reversed light–dark cycle (lights on at 7:00 PM, lights off at 7:00 AM) with free access to food and water. The animals were maintained in a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, Washington, DC: National Academy Press, 1996) and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse of the U.S. National Institutes of Health.
2.2. Experiment 1: Intracranial electrical brain-stimulation reward (BSR)
2.2.1. Surgery and general procedures for electrical BSR
Surgery and general procedures for electrical BSR were as we have reported previously (Xi et al., 2006; Spiller et al., 2008). Briefly, the surgery was performed under sodium pentobarbital anesthesia (65 mg/kg i.p.) with standard aseptic surgical and stereotaxic technique. A unilateral monopolar stainless-steel stimulating electrode (Plastics One, Roanoke, VA, USA) was placed into the medial fore-brain bundle at the anterior–posterior level of the lateral hypothalamus (AP −2.56, ML −1.9, and DV −8.6). After 7 days of recovery from surgery, rats were allowed to self-train (autoshape) to lever press for rewarding BSR. Each press on the lever resulted in a 500-ms train of 0.1-ms rectangular cathodal pulses through the electrode, followed by a 500 ms “timeout” in which further presses did not produce brain stimulation. Following establishment of lever-pressing for BSR, animals were presented with a series of 16 different pulse frequencies, ranging from 141 to 25 Hz in descending order. At each pulse frequency, animals responded for two 30-s time periods (“bins”), after which the pulse frequency was decreased by 0.05 log units. The response rate for each frequency was defined as the mean number of lever responses during the two 30-s bins. Following each 30-s bin, the lever retracted for 5 s. Throughout the experiment, animals were run for 3 sessions a day. Since lever-pressing behavior was variable during the first session (the “warm up” session), but was stable during the second and third sessions, the data from the first session were discarded, and the data from the second and third sessions were designated as the baseline session data and test session data, respectively.
The BSR threshold (θ0) was defined as the minimum frequency at which the animal responded for rewarding stimulation. The Ymax was defined as the maximal rate of response (number of lever presses for rewarding brain stimulation per unit of time). The BSR threshold (θ0) and Ymax were mathematically derived for each ‘baseline’ run and each ‘drug’ run by analyzing each rate-frequency BSR function generated by a given animal over a given descending series of pulse frequencies using ‘best-fit’ mathematical algorithms. Specifically, each rate-frequency BSR function was mathematically fitted, by iterative computer programs derived from the Gauss–Newton algorithm for non-linear regression, to three different sigmoid curve-fitting mathematical growth models that appear to accurately fit rate-frequency brain-stimulation reward functions (Coulombe and Miliaressis, 1987) – the Gompertz model (Y′ = ae− e(b−cX)), the logistic model (Y′ = a/[1 + e(b−cX)]), and the Weibull function (Y′ = a[1 − e−(bX)c]); where Y′ is the rate of response (number of lever presses for rewarding brain stimulation per unit of time), X is the pulse frequency, and a, b, and c are parameters approximated from each empirical rate-frequency data curve (a representing the asymptotic response rate value, b relating to the intercept of the rate-frequency curve with the y-axis, and c representing the rate at which Y increments). From each curve-fitting model, a solution for θ0 and a solution for Ymax were obtained. Thus, for each rate-frequency BSR function generated by a given animal over a given descending series of pulse frequencies, three solutions for θ0 and three solutions for Ymax were obtained. The three solutions for θ0 were averaged, to produce a mean θ0 for each rate-frequency BSR function generated by a given animal over a given descending series of pulse frequencies. Similarly, the three solutions for Ymax were averaged, to produce a mean Ymax for each rate-frequency BSR function generated by a given animal over a given descending series of pulse frequencies. The mean θ0 values and mean Ymax values were expressed as means ± SEM. Data analyses were performed on percent changes from baseline levels.
2.2.2. Testing the effects of cocaine and/or S33138 on BSR
Once baseline θ0 and Ymax values were achieved (<10% variation over 5 continuous days), the effects of cocaine and/or S33138 on BSR were assessed. On test days, animals randomly received vehicle or one of 3 different doses of cocaine (1, 2, 10 mg/kg, i.p.), or one of 3 different doses of S33138 (0.156, 0.625, 2.5 mg/kg, i.p.) 30 min prior to a cocaine injection (2 mg/kg, i.p.). After each test, animals received an additional 5–7 days of BSR re-stabilization until new baseline θ0 and Ymax values were established. The effect of S33138 on cocaine-enhanced BSR was evaluated by comparing cocaine-induced alterations in θ0 and Ymax values in the presence or absence of each dose of S33138 or vehicle pretreatment.
2.3. Experiment 2: Cocaine self-administration
2.3.1. Surgery, apparatus and general procedure
Surgery, apparatus and general procedure for cocaine self-administration were the same as reported previously (Xi et al., 2005, 2006). Briefly, intravenous (i.v.) catheterization was performed under sodium pentobarbital anesthesia (65 mg/kg, i.p.) with standard aseptic surgical techniques. After recovery from surgery, each rat was placed into a standard operant test chamber (32 × 25 × 33 cm) (Med Associates, Saint Albans, VT, USA) for i.v. cocaine self-administration. Each test chamber had two levers located 6.5 cm above the floor, one active and one inactive. Depression of the active lever activated the infusion pump; depression of the inactive lever was counted but had no other consequence. A cue-light and a speaker were located 12 cm above the active lever. At the start of each 3 h test session, the house-light was turned on. When the animal made a lever-pressing response that resulted in cocaine infusion (0.1 ml, 4.6 s), a cue-light (4 W) was illuminated and a cue-sound (tone, 30 Hz, 15 dB) was turned on for the duration of the infusion. Bar presses during the 4.6-s were counted, but did not lead to further infusions. There was no timeout after the completion of each infusion. FR1 reinforcement was used for 3–5 days until stable cocaine self-administration was established. The initial cocaine dose of 1 mg/kg/ infusion was chosen on the basis of our previous experience that this dose produces rapid and facile acquisition of cocaine self-administration behavior.
2.3.2. Cocaine self-administration under FR2 reinforcement
After transition from FR1 reinforcement, subjects were allowed to continue cocaine (0.5 mg/kg/infusion) self-administration under FR2 reinforcement until the following criteria for stable cocaine-maintained responding were met: less than 10% variability in inter-response interval and less than 10% variability in number of presses on the active lever for at least 3 consecutive days. The dose of cocaine was chosen based on previous findings that rats self-administering cocaine at 0.5 mg/kg/ infusion display highly stable self-administration behavior. Furthermore, we chose 0.5 mg/kg, rather than 1 mg/kg, of cocaine in order to modestly increase the work demand (i.e., lever presses) on the animals for the same amount of drug intake. In our experience, this approach increases the sensitivity of measuring changes in drug-taking or drug-seeking behavior. To avoid cocaine overdose during the self-administration period, each animal was limited to a maximum of 50 cocaine infusions per 3 h session. After stable rates of responding were established, each subject randomly received 1 of 4 doses of S33138 (0.156, 0.625, 2.5, 5.0 mg/kg, p.o.) or vehicle (distilled water) 30 min prior to the test session. Animals then received an additional 3–4 days of self-administration of cocaine alone until baseline response rate was re-established prior to testing the next dose of drug. The order of testing for the various doses of S33138 was counterbalanced.
2.3.3. Sucrose self-administration under FR2 reinforcement
The procedures for oral sucrose self-administration were identical to the procedures for cocaine self-administration except for the following: 1) no surgery was performed on the animals in the sucrose experiment and 2) active lever presses led to delivery of 0.1 ml of 5% sucrose solution into a liquid food tray on the operant chamber wall.
2.4. Experiment 3: Cocaine-induced reinstatement of drug-seeking behavior
Surgery and general procedures for cocaine self-administration prior to behavioral extinction were the same as described above.
2.4.1. Extinction
After stable cocaine self-administration was established, animals were exposed to extinction conditions, during which cocaine was replaced by saline, and the cocaine-associated cue-light and tone were turned off. Active lever-pressing led only to saline infusion. Daily 3 h extinction sessions for each rat continued until that rat lever-pressed less than 10 times per 3 h session for at least 3 consecutive days. After the animals met the established extinction criteria, they were divided into 5 groups (8–10 rats per group) for reinstatement testing.
2.4.2. Reinstatement test
On the reinstatement test day, each group of rats received either vehicle (distilled water) or 1 dose of S33138 (0.156, 0.625, 2.5 mg/kg, p.o.). 30 min after S33138 administration, all rats were given a priming injection of cocaine (10 mg/kg, i.p.) immediately before the initiation of reinstatement testing. During the reinstatement test, the conditions were identical to those in extinction sessions. Cocaine-induced active lever presses (reinstatement) were recorded, although these did not lead to either cocaine infusions or presentation of the conditioned cue-light and tone. Reinstatement test sessions lasted 3 h.
2.5. Experiment 4: Locomotor behavior
An additional group of animals was used to assess the effect of S33138 on locomotor behavior. These animals were first trained for cocaine self-administration. Once the self-administration rate was stable for at least 3 continuous days, the effect of S33138 on locomotion was assessed. Before S33138 administration, each animal was placed in a locomotor detection chamber (Accuscan, Columbus, OH, USA) for habituation (1 h/per day for 3 days). On test days, animals randomly received 1 of 3 different doses of S33138 (0.625, 2.5 or 5 mg/kg, p.o.) or vehicle. Immediately thereafter, rats were placed in the locomotor detection chambers for 3 h of data recording. After locomotor testing, animals received 3 additional days of re-stabilization on cocaine self-administration before being re-tested in the locomotor test paradigm. The order of testing for various doses of S33138 or vehicle was counterbalanced according to a Latin square design. Total distance counts were used to evaluate the effect of S33138 on locomotion.
2.6. Experiment 5: Rotarod performance
Performance on an accelerating rotarod was assessed using a four-station rat rotarod (AccuScan Instruments Inc., Columbia, Ohio, USA). We used two sets of parameters, i.e., “fast-run” (20 rpm) and “slow-run” (8 rpm) rotarod tests, to mimic “high-rate” operant responding in electrical BSR and “low-rate” operant responding in cocaine self-administration, respectively (Xi et al., 2005). The speed of rotation of the rotarod was increased from 2 to 8 or 20 rpm over 2 min. If the rat stayed on the cylinder for 2 min, the rotarod test was stopped. The mean time (s) of two trials at each time point on the cylinder was taken as final result. After 5–7 days of habituation and training on the rotarod device, vehicle or one dose of S33138 was given randomly before the rotarod test began. Given that S33138 was given i.p. in the “high-rate” operant BSR or p.o. in the “low-rate” operant self-administration and reinstatement paradigms, we observed the effects of i.p. S33138 (0.156, 0.625, 2.5, 5.0 mg/kg) on “fast-run” and p.o. administration of S33138 (0.626, 2.5, 5.0 mg/kg) on “slow-run” rotarod performance, respectively.
2.7. Drugs and chemicals
Cocaine HCl (Sigma Chemical Co., Saint Louis, MO, USA) was dissolved in physiological saline. S33138 was synthesized at the Institut de Recherches Servier (Croissy-sur-Seine, France), and dissolved in saline for i.p. injections in Experiment 1 (BSR), or in distilled water for p.o. administration in all other experiments. We chose i.p. administration of S33138 in Experiment 1 because p.o. gavage by itself appeared to affect BSR operant behavior. Sucrose (Sigma Chemical Co., Saint Louis, MO, USA) was dissolved in distilled water (5% solution) for oral self-administration.
2.8. Data analyses
All data are presented as means (±S.E.M.). One-way analysis of variance (ANOVA) was used to analyze the effects of S33138 on cocaine or sucrose self-administration, cocaine-triggered reinstatement, and on rotarod performance. Post-ANOVA individual group comparisons were carried out using pre-planned Bonferroni t-tests.
3. Results
3.1. S33138 inhibits cocaine-enhanced BSR
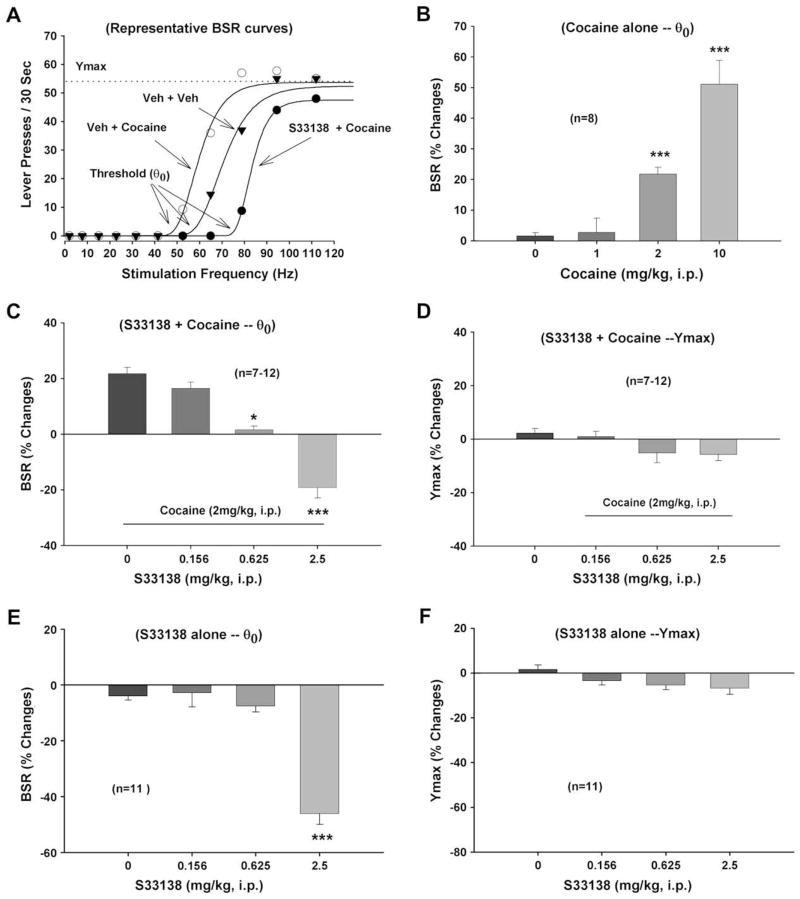
Fig. 1A illustrates representative rate-frequency function curves for BSR, indicating the BSR threshold (θ0, Hz) and Ymax (maximal lever presses/30 s), and the effects of cocaine and S33138 on BSR. Cocaine, at 2 mg/kg, produced a significant enhancement in BSR, as indicated by the leftward shift in the rate-frequency function curve, reflecting lowered BSR threshold (θ0) values. This cocaine-enhanced BSR was substantially attenuated in dose-dependent fashion by the partially selective D3 receptor antagonist S33138 (0.156–2.5 mg/kg). Cocaine did not significantly alter Ymax levels. Fig. 1B illustrates the dose-dependent enhancement of BSR by cocaine (F3,34 = 20.98, p < 0.001). Fig. 1C illustrates the averaged effects of S33138 on cocaine-enhanced BSR, indicating that 2 mg/kg cocaine-enhanced BSR was dose-dependently attenuated by S33138. One-way ANOVA for repeated measures revealed a statistically significant overall main effect (F3,30 = 45.69, p < 0.001) of the 3 doses of S33138. Individual group comparisons revealed statistically significant reductions in cocaine-enhanced BSR after 0.625 mg/kg (t = 4.68, p < 0.05) or 2.5 mg/kg S33138 (t = 10.69, p < 0.001), but not after 0.156 mg/kg (t = 0.57, p = NS) S33138 administration. Fig. 1D illustrates that the combination of S33138 and cocaine did not significantly alter Ymax levels (F3,27 = 2.25, p = NS). Fig. 1E illustrates the effects of S33138 alone on BSR, indicating that the low doses (0.156, 0.625 mg/kg) of S33138 had no effect, but that the moderate dose of S33138 (2.5 mg/kg) significantly shifted BSR curves to the right, producing an aversive-like effect. One-way ANOVA for repeated measures revealed a statistically significant overall treatment main effect of S33138 by itself (F3,40 = 34.37, p < 0.001). Individual group comparisons revealed a statistically significant inhibition of BSR after 2.5 mg/kg (t = 8.41, p < 0.001), but not after 0.156 mg/kg (t = 0.18, p = NS) or 0.625 mg/kg (t = 0.61, p = NS) S33138, when compared to vehicle. Fig. 1F shows that S33138 alone failed to alter Ymax levels (F3,40 = 1.32, p = NS).
Fig. 1.
Effects of S33138 and cocaine on electrical BSR. Panel A shows representative rate-frequency function curves for BSR, indicating the BSR threshold (θ0) and Ymax (the maximal work output achieved by the animal). Cocaine (2 mg/kg, i.p.) shifted the rate-frequency function curve to the left, lowering the BSR threshold θ0 value and, thus, enhancing brain reward. Pretreatment with S33138 (2.5 mg/kg, i.p.) significantly blocked the cocaine-induced decrease in threshold (θ0), and (at this dose) elevated the BSR threshold by itself. Panel B shows dose-dependent enhancement by cocaine of BSR. Panel C shows that 2 mg/kg cocaine-enhanced BSR was dose-dependently attenuated by S33138 (0.156–2.5 mg/kg, i.p.). Panel D shows that S33138 pretreatment did not significantly alter Ymax levels. Panel E shows that S33138 alone, at 2.5 mg/kg, but not lower doses, produced an aversive-like inhibition of BSR. Panel F shows that S33138 alone did not alter Ymax levels. *p < 0.05, ***p < 0.001, when compared with vehicle (Panels B and E) or cocaine alone treatment group (Panel C).
3.2. S33138 inhibits intravenous cocaine self-administration
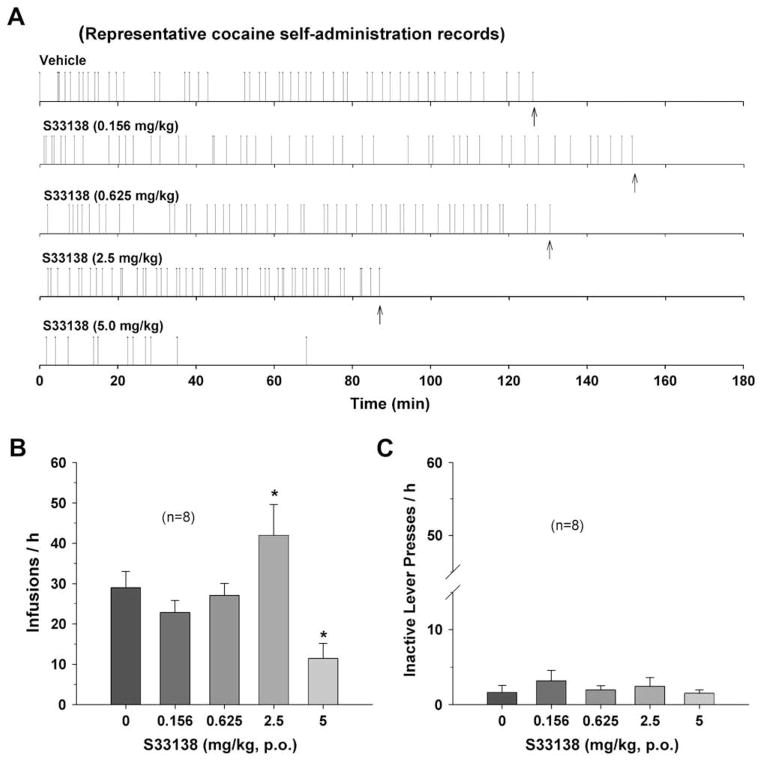
Fig. 2A illustrates representative individual records of cocaine self-administration, demonstrating a biphasic effect. Low doses (0.156, 0.625 mg/kg) of S33138 had no significant effect on cocaine self-administration, whereas a moderate dose (2.5 mg/kg) of S33138 increased cocaine self-administration (as indicated by more rapid self-administration per unit time), and a high dose (5 mg/kg) S33138 substantially inhibited cocaine self-administration behavior. Fig. 2B and C illustrates the changes in mean cocaine infusion rates and inactive lever presses after S33138 administration, respectively. One-way ANOVA for repeated measurements revealed a statistically significant treatment main effect of S33138 on cocaine infusion rate (Fig. 2B, F4,28 = 7.83, p < 0.001), but not on inactive lever presses (Fig. 2C, F4,28 = 0.77, p = NS). Individual group comparisons indicated a statistically significant increase in cocaine self-administration after 2.5 mg/kg (t = 2.99, p < 0.05), a statistically significant decrease in cocaine self-administration after 5 mg/kg S33138 (t = 3.15, p < 0.05), and no significant effect after 0.156 mg/kg (t = 1.10, p = NS) or 0.625 mg/kg (t = 0.33, p = NS) S33138.
Fig. 2.
The effects of S33138 on cocaine self-administration under FR2 reinforcement. Panel A shows representative cocaine self-administration records, illustrating that systemic administration of S33138 dose-dependently inhibited cocaine’s rewarding effects, as assessed by a compensatory increase in cocaine self-administration rates after moderate dose S33138 (2.5 mg/kg, p.o.) and cessation of cocaine self-administration after high dose S33138 (5 mg/kg, p.o.). Each vertical line represents a cocaine infusion (0.5 mg/kg/infusion). The arrows (↑) indicate the 50th cocaine infusion, after which cocaine was no longer available to the animal and the self-administration apparatus automatically terminated. Panel B shows mean cocaine self-administration (infusion) rates after different doses of S33138. Panel C shows mean inactive lever presses after S33138 administration. *p < 0.05, when compared with the vehicle control group.
3.3. S33138 inhibits oral sucrose self-administration
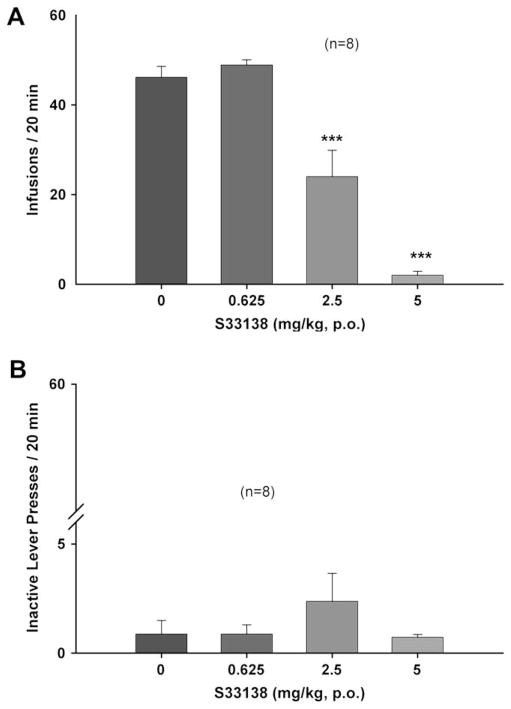
Fig. 3 illustrates mean sucrose infusion rates and inactive lever presses after S33138 administration, demonstrating that S33138, at 2.5–5.0 mg/kg, dose-dependently inhibited oral sucrose self-administration in rats. One-way ANOVA for repeated measurements revealed a statistically significant treatment main effect of S33138 on infusion rate (Fig. 3A: F3,21 = 35.55, p < 0.001), but not on inactive lever presses (Fig. 3B: F3,21 = 0.72, p = NS). Individual group comparisons indicated a statistically significant reduction in sucrose self-administration after 2.5 mg/kg (t = 4.98, p < 0.001) or 5 mg/kg (t = 8.37, p < 0.001), but not after 0.625 mg/kg (t = 1.25, p = NS) S33138, when compared with the vehicle group.
Fig. 3.
The effects of S33138 on oral sucrose self-administration under an FR2 reinforcement schedule. Panel A shows sucrose self-administration (infusion) rates after different doses of S33138 administration. Panel B shows inactive lever presses after different doses of S33138 administration. ***p < 0.001, when compared with the vehicle control group.
3.4. S33138 inhibits cocaine-triggered reinstatement of cocaine-seeking behavior
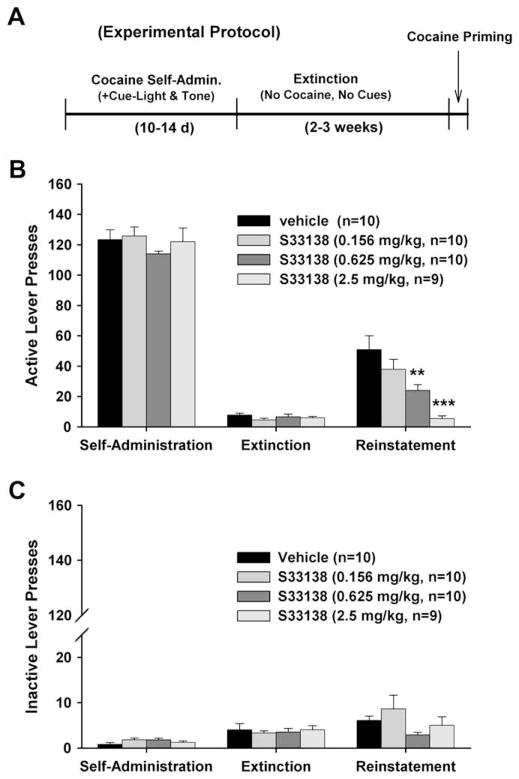
Fig. 4A depicts the general experimental protocol for cocaine-triggered reinstatement. Fig. 4B illustrates the total number of active lever presses observed during the last session of cocaine self-administration, the last session of extinction, and the reinstatement test session in the four different S33138 dose groups. A single, non-contingent cocaine priming dose (10 mg/kg, i.p.) produced robust reinstatement of extinguished operant responding (i.e., lever presses) in rats previously reinforced by i.v. cocaine infusions. One-way ANOVA for repeated measurements revealed a statistically significant overall main effect of S33138 on cocaine-triggered reinstatement (Fig. 4B, right panel, F3,35 = 9.82, p < 0.001). Individual group comparisons revealed a statistically significant reduction in cocaine-induced reinstatement after 0.625 mg/kg (t = 3.33, p < 0.01) or 2.5 mg/kg S33138 (t = 5.60, p < 0.001), but not after 0.156 mg/kg (t = 1.60, p = NS) S33138, when compared with the vehicle treatment group. Fig. 4C illustrates the total number of inactive lever presses observed during the last session of cocaine self-administration, the last session of extinction, and the reinstatement test session in the four different S33138 dose groups, indicating that S33138 had no effect on inactive lever presses (Fig. 4C, right panel, F3,35 = 1.70, p = NS) during reinstatement testing. There were also no statistically significant differences in inactive lever presses during the last session of cocaine self-administration (Fig. 4C, left panel, F3,35 = 1.52, p = NS) or the last session of extinction (Fig. 4C, middle panel, F3,35 = 0.14, p = NS) among the four S33138 dose groups.
Fig. 4.
The effects of S33138 on cocaine-triggered reinstatement. Panel A shows the general experimental protocol for cocaine-triggered reinstatement of drug-seeking behavior. Panel B shows the active lever presses during the last session of cocaine self-administration, the last session of extinction, and reinstatement testing after different doses of S33138. Panel C shows the inactive lever presses during the last session of cocaine self-administration, the last session of extinction, and reinstatement testing after different doses of S33138. Cocaine (10 mg/kg, i.p.) priming-induced reinstatement of drug-seeking behavior was dose-dependently inhibited by S33138 (0.156, 0.625, 2.5 mg/kg, p.o., 30 min prior to cocaine priming). **p < 0.01, ***p < 0.001, when compared with the vehicle control group.
3.5. S33138 has no effect on locomotor activity
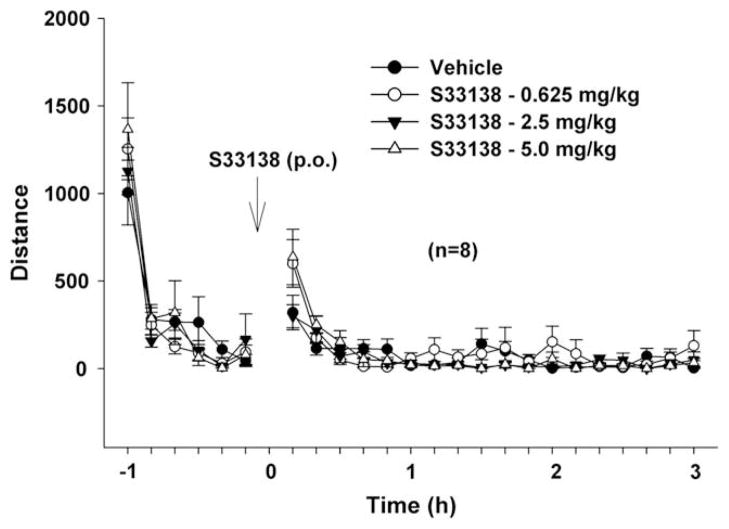
To determine whether the reduction in cocaine self-administration and reinstatement of drug-seeking behavior was due to a reduction in locomotor performance, we measured the effect of S33138 on locomotor activity in rats having cocaine self-administration experience. Fig. 5 illustrates that the same doses (0.625–5.0 mg/kg, p.o.) of S33138 did not inhibit locomotor activity. Although two-way ANOVA with repeated measurements over time revealed a statistically significant overall treatment main effect of S33138 (F3,28 = 3.89, p < 0.05), individual group comparisons revealed no statistically significant overall differences between the vehicle and any S33138 treatment group.
Fig. 5.
Effects of S33138 on locomotor activity. S33138 (0.625, 2.5, 5 mg/kg, p.o.) did not inhibit locomotor activity in cocaine self-administration rats.
3.6. S33138 inhibits rotarod performance at high doses
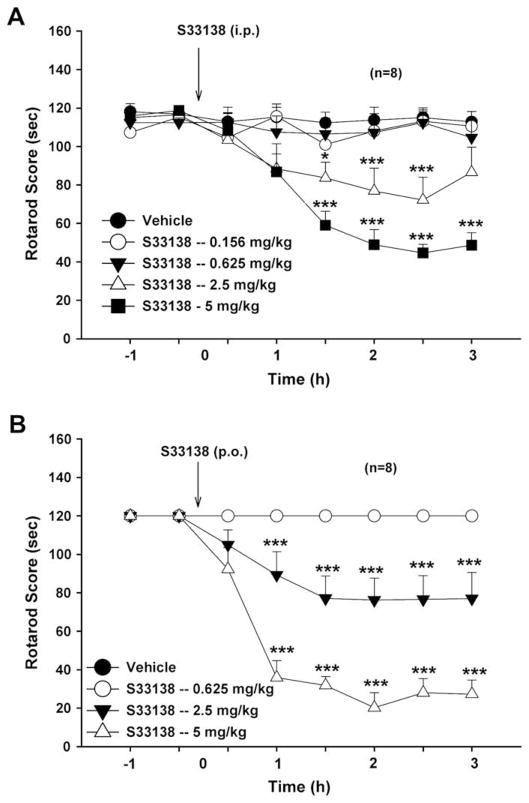
Fig. 6A illustrates that intraperitonial (i.p.) administration of S33138 dose-dependently inhibited locomotor performance on the “fast-run” (20 rpm) rotarod test. Two-way ANOVA for repeated measurements over time revealed a statistically significant treatment main effect (F4,28 = 13.44, p < 0.001), time main effect (F7,49 = 10.52, p < 0.001), and significant treatment × time interaction (F28,196 = 8.17, p < 0.001). Individual group comparisons revealed statistically significant reduction in rotarod performance after 5 mg/kg (t = 5.77, p < 0.001), but not after 2.5 mg/kg (t = 2.63, p = NS), 0.625 mg/kg (t = 0.22, p = NS) or 0.156 mg/kg (t = 0.04, p = NS) S33138, when compared with the vehicle treatment group. Fig. 6B illustrates that intragastric (p.o.) infusion of S33138 also dose-dependently inhibited locomotor performance on the “slow-run” (8 rpm) rotarod test. Two-way ANOVA for repeated measurements over time revealed a statistically significant treatment main effect (F3,21 =101.77, p < 0.001), time main effect (F7,49 = 42.67, p < 0.001), and treatment × time interaction (F21,147 = 20.74, p < 0.001). Individual group comparisons revealed a significant reduction in rotarod performance after 2.5 mg/kg (t = 6.17, p < 0.001), 5 mg/kg (t = 15.08, p < 0.001), but not after 0.625 mg/kg (t = 0.01, p = NS) S33138.
Fig. 6.
Effects of S33138 on rotarod performance in rats. Panel A shows effects of S33138 (0.156, 0.625, 2.5, 5.0 mg/kg, i.p.) on fast-running (20 rpm) rotarod performance. Panel B shows the effects of S33138 (0.625, 2.5, 5 mg/kg, p.o.) on slow-running (8 rpm) rotarod performance. S33138, at 2.5 or 5.0 mg/kg, inhibited rotarod performance, an effect lasted for about 3 h. *p < 0.05, ***p < 0.001, when compared with the baselines before S33138 administration in each treatment group.
4. Discussion
The present study, for the first time, demonstrates that a partially selective D3 receptor antagonist, S33138, significantly inhibits cocaine’s rewarding effects, as assessed by the BSR and cocaine self-administration paradigms, and cocaine-triggered reinstatement of drug-seeking behavior in laboratory rats. S33138, at a high dose, also inhibited BSR itself, sucrose self-administration and locomotor performance. These data suggest that lower doses of S33138 may have therapeutic potential in the treatment of cocaine addiction without significant side-effects, while high doses of S33138 may produce significant unwanted effects, including locomotion impairment, dysphoria or inhibition of natured reward.
4.1. S33138 inhibits cocaine-enhanced BSR
The electrical BSR paradigm is one of the most reliable and sensitive animal models for assessing the reward-relevant properties of addictive drugs (O’Brien and Gardner, 2005). The present data show that cocaine (1–10 mg/kg, i.p.) dose-dependently decreased BSR thresholds, reflecting summation or synergism between the reward induced by the electrical brain stimulation and that produced by cocaine. This cocaine-enhanced BSR was dose-dependently attenuated by S33138. Administered alone, S33138 had no effect on BSR at low doses (0.156, 0.625 mg/kg, i.p.), but significantly shifted the BSR curve to the right at the moderate dose (2.5 mg/kg, i.p.), suggesting that moderate-to-high doses of S33138 may produce aversive-like effects. This resembles the aversive-like BSR effects produced by haloperidol and raclopride, which block D2 and D3 receptors with similar affinities (Kita et al., 1999), but differs from the inactivity of selective D3 receptor antagonists on BSR (Vorel et al., 2002; Xi et al., 2006; Pak et al., 2006; Spiller et al., 2008). These data suggest that the aversive-like effects and/or locomotor impairment produced by moderate-to-high doses of S33138 are likely mediated by blockade of D2 rather than D3 receptors. This is consistent with the finding that S33138 exerts preferential (25-fold) interaction at D3 versus D2 receptors both in vitro and in vivo (Millan et al., 2008b,c).
4.2. S33138 inhibits cocaine self-administration
Intravenous drug self-administration is the most commonly used animal model for evaluating the reinforcing effects of drugs (O’Brien and Gardner, 2005). Under low FR reinforcement, the addictive drug is readily available to animals under low-effort and high reward conditions. In the present study, S33138 produced a dose-dependent, biphasic effect on cocaine self-administration. A moderate dose (2.5 mg/kg, p.o.) of S33138 increased, while a high dose (5 mg/kg, p.o.) of S33138 inhibited cocaine self-administration. Given that cocaine self-administration rate is, in part, a function of cocaine dose (or rewarding strength), i.e., a decrease in cocaine dose results in a compensatory increase in cocaine SA (Yokel and Wise, 1975), the moderate S33138 dose-induced increases in cocaine self-administration might be a compensatory response in drug-taking behavior to a partial reduction in cocaine reward after S33138 administration. At a high dose, S33138 (5 mg/kg, p.o.) appears to robustly inhibit cocaine reward, leading to cessation of drug-taking behavior. This effect is significantly different from the ineffectiveness of selective D3 receptor antagonists on cocaine self-administration under low FR reinforcement (Vorel et al., 2002; Xi et al., 2005, 2006). However, with increasing dose, S33138 also produces a significant inhibition of sucrose self-administration and rotarod performance (see more discussion below), effects similar to those produced by D2-preferring antagonists (Rothman and Glowa, 1995; Platt et al., 2002; Gorelick et al., 2004). These data suggest that moderate doses of S33138, which block both D3 and D2 (but predominantly D3) receptors, produce an additive suppression of cocaine self-administration. However, at high doses, D2 receptor blockade becomes predominant, therefore producing more D2-preferring antagonist-like effects.
4.3. S33138 inhibits cocaine-triggered reinstatement of drug-seeking behavior
In addition to attenuating cocaine reward as assessed in the BSR and self-administration paradigms, S33138 also dose-dependently inhibited cocaine-induced reinstatement (relapse) of drug-seeking behavior. That is, S33138, at the lower doses, partially inhibited, while higher doses completely inhibited reinstatement of drug-seeking behavior triggered by cocaine priming. Relapse to illicit drug use is a core feature of human drug addiction (Shalev et al., 2002). In humans, relapse can be triggered by administration of an addictive drug, by exposure to environmental stimuli previously associated with drug use, or by exposure to various stressors (Jaffe et al., 1989; O’Brien et al., 1992; Sinha, 2001). In experimental animals, reinstatement of (relapse to) previously extinguished drug-seeking behavior is evoked by the same triggers, making the reinstatement animal model the most commonly used animal homologue of human drug craving and relapse (Shalev et al., 2002). The present finding that S33138 dose-dependently inhibits cocaine-induced reinstatement suggests that partially selective D3 versus D2 receptor antagonists may be useful for attenuating drug craving and relapse in humans. This anti-relapse potential of S33138 is similar to that by D3-selective and D2-preferring receptor antagonists in experimental animals (Cervo et al., 2003; Self et al., 1996). This reduction in cocaine-seeking behavior appears unlikely due to a reduction in non-specific locomotion impairment, because: 1) the same doses (0.625–5 mg/kg) of S33138 have no effect on locomotor activity in rats having self-administration experience; 2) the lower effective dose (0.625 mg/kg) in both the BSR and reinstatement paradigms has no effect on rotarod performance; and 3) the moderate effective dose (2.5 mg/kg) of S33138 compensatorily enhances cocaine self-administration behavior although it also produces a reduction in motivated rotarod performance. Since the highest dose (5 mg/kg) of S33138 also produced significant inhibition of sucrose self-administration and rotarod locomotor performance, it is likely that non-specific locomotor impairment may contribute to the suppression of cocaine-seeking behavior at such a high dose.
It should be noted that the effective doses of S33138 for inhibiting cocaine self-administration (2.5–5.0 mg/kg) are 2–4 fold higher than those for inhibiting cocaine-triggered reinstatement (0.625–2.5 mg/kg). This difference in effective doses between the two paradigms could be related to the differences in total drug intake in cocaine self-administration (0.5 mg/kg/infusion × 50 infusions = 25 mg/kg, i.v.) and in reinstatement (10 mg/kg, i.p.) and/or in the roles of D2 and D3 receptors in mediating cocaine reward and cocaine-triggered reinstatement of drug-seeking behavior (Anderson and Pierce, 2005; Briand et al., 2008).
4.4. Potential side-effects of S33138
4.4.1. S33138 inhibits non-drug (sucrose) reward
S33138, at high doses, also inhibited oral sucrose self-administration, suggesting a possible influence on natural reward. This is further supported by the finding that S33138, at the highest tested dose (2.5 mg/kg), also inhibited BSR itself. Previous studies have shown that selective D3 receptor antagonism has no effect on food-reinforced place preference (Vorel et al., 2002), food-taking behavior under a PR schedule of reinforcement (Ross et al., 2007) or sucrose priming-induced reinstatement of drug-seeking behavior (Xi et al., 2006), suggesting a limited role of D3 receptor antagonism in natural reward. This contrasts to clear inhibitory effects on natural reward produced by D2-preferring antagonists (Rothman and Glowa, 1995; Platt et al., 2002; Gorelick et al., 2004). Thus, S33138’s inhibition of sucrose self-administration is likely mediated predominantly by blockade of D2 rather than D3 receptors.
4.4.2. S33138 inhibits locomotor performance
In addition, high doses of S33138 also significantly inhibited locomotor performance or locomotor coordination ability as assessed by the rotarod test, suggesting a possible side-effect of locomotor impairment. This is congruent with S33138’s pharmacological property of preferential (25-fold) D3 versus D2 receptor antagonism; thus, at high doses, S33138 may exert D2 antagonist effects (Millan et al., 2008a; Millan and Brocco, 2008).
4.4.3. S33138 binds to other non-DA receptors
In addition to its preference for D3 versus D2 sites, S33138 is also a modest α2C-adrenoceptor and serotonin 5-HT2A receptor antagonist (Millan et al., 2008a). Given that the α2-receptor antagonist yohimbine produces or potentiates reinstatement of cocaine-seeking behavior (Lee et al., 2004; Feltenstein and See, 2006), the present antagonism by S33138 of cocaine self-administration and reinstatement of drug-seeking behavior is unlikely to have been mediated by blockade of α2c receptors. The 5-HT2A receptor antagonist M100,907 fails to alter cocaine self-administration, but attenuates cocaine-induced hyperactivity and reinstatement of drug-seeking behavior (O’Neil et al., 1999; McMahon and Cunningham, 2001; Fletcher et al., 2002), suggesting that blockade of 5-HT2A receptors may have contributed to the presently observed antagonism by S33138 of cocaine-triggered reinstatement of drug-seeking behavior.
Finally, it seems unlikely that S33138 possesses addictive liability. We have shown that selective D3 receptor antagonists are not self-administered, do not enhance brain reward, and do not produce Conditioned Place Preference (Heidbreider et al., 2005; Xi and Gardner, 2007). In addition, drugs that have D2 antagonistic action have never been shown in any paradigm to be addicting (O’Brien and Gardner, 2005). In fact, animals will work to avoid receiving D2 receptor antagonists (Manzanedo et al., 2001). Thus, S33138 should be free of addictive liability.
In conclusion, reflecting blockade of D3 receptors, lower doses of S33138 attenuate cocaine’s rewarding effects in BSR and cocaine self-administration, and inhibit relapse to cocaine-seeking behavior, but exert no obvious rewarding or locomotor effects by themselves. These findings suggest that preferential D3-selective antagonists may be useful for treatment of cocaine addiction, and may show advantages over selective D2 and/or D3 receptor antagonists.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health (NIH), USA and Les Laboratoires Servier, France.
Footnotes
Disclosure/Conflict of Interest
Co-authors Peng, Spiller, Li, Gardner, and Xi declare that, except for income received from their primary employers, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. Co-author Ashby declares that, in addition to income received from his primary employer, he has served as a consultant to Les Laboratoires Servier for expert advice on Servier compounds. Coauthors Thomasson, Millan, Mocaër, and Muńoz declare that their primary employer is Les Laboratoires Servier.
References
- Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology. 2003;28:1272–1280. doi: 10.1038/sj.npp.1300183. [DOI] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology. 2008;33:2969–2980. doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28:1150–1159. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- Coulombe D, Miliaressis E. Fitting intracranial self-stimulation data with growth models. Behav Neurosci. 1987;101:209–214. doi: 10.1037//0735-7044.101.2.209. [DOI] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, Sokoloff P. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci. 2000;20:8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT2A receptor antagonist M100, 907 and the 5-HT2C receptor antagonist SB242,084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Gál K, Gyertyán I. Targeting the dopamine D3 receptor cannot influence continuous reinforcement cocaine self-administration in rats. Brain Res Bull. 2003;61:595–601. doi: 10.1016/s0361-9230(03)00217-x. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Wojnicki FH. Effects of drugs on food- and cocaine-maintained responding, III: dopaminergic antagonists. Psychopharmacology. 1996;128:351–358. doi: 10.1007/s002130050144. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Gardner EL, Xi ZX. Agents in development for the management of cocaine abuse. Drugs. 2004;64:1547–1573. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology. 1989;110:76–84. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Millan MJ. Dopamine D3 receptor antagonists as therapeutic agents. Drug Discov Today. 2005;10:917–925. doi: 10.1016/S1359-6446(05)03491-4. [DOI] [PubMed] [Google Scholar]
- Kita K, Shiratani T, Takenouchi K, Fukuzako H, Takigawa M. Effects of D1 and D2 dopamine receptor antagonists on cocaine-induced self-stimulation and locomotor activity in rats. Eur Neuropsychopharmacol. 1999;9:1–7. doi: 10.1016/s0924-977x(97)00098-9. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of α2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Manzanedo C, Aguilar MA, Rodríguez-Arias M, Miñarro J. Effects of dopamine antagonists with different receptor blockade profiles on morphine-induced place preference in male mice. Behav Brain Res. 2001;121:189–197. doi: 10.1016/s0166-4328(01)00164-4. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Cunningham KA. Antagonism of 5-hydroxytryptamine2A receptors attenuates the behavioral effects of cocaine in rats. J Pharmacol Exp Ther. 2001;297:357–363. [PubMed] [Google Scholar]
- Millan MJ, Brocco M. Cognitive impairment in schizophrenia: a review of developmental and genetic models, and pro-cognitive profile of the optimised D3 > D2 antagonist, S33138. Thérapie. 2008;63:187–229. doi: 10.2515/therapie:2008041. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Dekeyne A, Rivet JM, Dubuffet T, Lavielle G, Brocco M. S33084, a novel, potent, selective, and competitive antagonist at dopamine D3-receptors: II. Functional and behavioral profile compared with GR218,231 and L741,626. J Pharmacol Exp Ther. 2000;293:1063–1073. [PubMed] [Google Scholar]
- Millan MJ, Loiseau F, Dekeyne A, Gobert A, Flik G, Cremers TI, Rivet JM, Sicard D, Billiras R, Brocco M. S33138, a preferential dopamine D3 versus D2 receptor antagonist and potent antipsychotic agent. III Actions in models of therapeutic activity and induction of side-effects. J Pharmacol Exp Ther. 2008a;324:1212–1226. doi: 10.1124/jpet.107.134536. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Mannoury la Cour C, Novi F, Maggio R, Audinot V, Newman-Tancredi A, Cussac D, Pasteau V, Boutin JA, Dubuffet T, Lavielle G. S33138, [N-[4-[2-[(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1]benzopyrano[3,4-c]pyrrol-2(3H)-yl)-ethyl]phenyl acetamide] a preferential dopamine D3 versus D2 receptor antagonist and potential antipsychotic agent. I Receptor-binding profile and functional actions at G-protein-coupled receptors. J Pharmacol Exp Ther. 2008b;324:587–599. doi: 10.1124/jpet.107.126706. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Svenningsson P, Ashby CR, Jr, Hill M, Egeland M, Dekeyne A, Brocco M, Di Cara B, Lejeune F, Thomasson N, Muńoz C, Mocaër E, Crossman A, Cistarelli L, Girardon S, Iob L, Veiga S, Gobert A. S33138, [N-[4-[2-[(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1]benzopyrano[3,4-c]pyrrol-2(3H)-yl)-ethyl]phenyl acetamide] a preferential dopamine D3 versus D2 receptor antagonist and potential antipsychotic agent. II A neurochemical, electrophysiological and behavioral characterization in vivo. J Pharmacol Exp Ther. 2008c;324:600–611. doi: 10.1124/jpet.107.132563. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- O’Neill MF, Heron-Maxwell CL, Shaw G. 5-HT2 receptor antagonism reduces hyperactivity induced by amphetamine, cocaine and MK-801 but not D1 agnoist C-APB. Pharmacol Biochem Behav. 1999;63:237–243. doi: 10.1016/s0091-3057(98)00240-8. [DOI] [PubMed] [Google Scholar]
- Pak AC, Ashby CR, Jr, Heidbreder CA, Pilla M, Gilbert J, Xi ZX, Gardner EL. The selective dopamine D3 receptor antagonist SB-277011A reduces nicotine-enhanced brain reward and nicotine-paired environmental cue functions. Int J Neuropsychopharmacol. 2006;9:585–602. doi: 10.1017/S1461145706006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology. 2002;163:265–282. doi: 10.1007/s00213-002-1137-8. [DOI] [PubMed] [Google Scholar]
- Ross JT, Corrigall WA, Heidbreder CA, LeSage MG. Effects of the selective dopamine D3 receptor antagonist SB-277011A on the reinforcing effects of nicotine as measured by a progressive-ratio schedule in rats. Eur J Pharmacol. 2007;559:173–179. doi: 10.1016/j.ejphar.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Glowa JR. A review of the effects of dopaminergic agents on humans, animals, and drug-seeking behavior, and its implications for medication development. Focus on GBR 12909. Mol Neurobiol. 1995;11:1–19. doi: 10.1007/BF02740680. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Silverdale MA, Nicholson SL, Ravenscroft P, Crossman AR, Millan MJ, Brotchie JM. Selective blockade of D3 dopamine receptors enhances the anti-parkinsonian properties of ropinirole and levodopa in the MPTP-lesioned primate. Exp Neurol. 2004;188:128–138. doi: 10.1016/j.expneurol.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Peng XQ, Newman AH, Ashby CR, Jr, Heidbreder C, Gaál J, Gardner EL. The selective dopamine D3 receptor antagonists SB-277011A and NGB 2904 and the putative partial D3 receptor agonist BP-897 attenuate methamphetamine-enhanced brain stimulation reward in rats. Psychopharmacology. 2008;196:533–542. doi: 10.1007/s00213-007-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD, Artymyshyn RP, Kung MP, Kung HF, Lucki I, McGonigle P. Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [125I]7-OH-PIPAT: evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J Pharmacol Exp Ther. 2000;295:1223–1231. [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. Pharmacological actions of NGB-2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev. 2007;13:240–259. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost–variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gitajn L, Gardner EL. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187:547–549. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]