Abstract
Cell adhesion to the extracellular matrix (ECM) proteins occurs through interactions with integrins that bind to Arg-Gly-Asp (RGD) tripeptides, and syndecan-4, which recognizes the heparin-binding domain (HBD) of other proteins. Both receptors trigger signaling pathways, including those that activate RhoGTPases such as RhoA and Rac1. This sequence of events modulates cell adhesion to the ECM and cell migration. Using a neuron-astrocyte model, we have reported that the neuronal protein Thy-1 engages αVβ3 integrin and syndecan-4 to induce RhoA activation and strong astrocyte adhesion to their underlying substrate. Thus, because cell-cell interactions and strong cell attachment to the matrix are considered antagonistic to cell migration, we hypothesized that Thy-1 stimulation of astrocytes should preclude cell migration. Here, we studied the effect of Thy-1 expressing neurons on astrocyte polarization and migration using a wound-healing assay and immunofluorescence analysis. Signaling molecules involved were studied by affinity precipitations, western blots and the usage of specific antibodies. Intriguingly, Thy-1 interaction with its two receptors was found to increase astrocyte polarization and migration. The latter events required interactions of these receptors with both the RGD-like sequence and the HBD of Thy-1. Additionally, prolonged Thy-1-receptor interactions inhibited RhoA activation while activating FAK, PI3K and Rac1. Therefore, sustained engagement of integrin and syndecan-4 with the neuronal surface protein Thy-1 induces astrocyte migration. Interestingly we identify here, a cell-cell interaction that although initially induces strong cell attachment, upon persistant stimulation favors cell migration by engaging the same signaling receptors and molecules as those utilized by ECM proteins to stimulate cell movement.
Keywords: Thy-1, integrins, syndecan-4, neuron-astrocyte interaction, cell migration
1. INTRODUCTION
The Thy-1-integrin interaction, initially described to mediate neuron-astrocyte communication [1], has set the bases for various reports showing interactions in Trans of Thy-1 with integrin receptors containing β2 or β3 subunits [2]. Indeed, Thy-1 interacts with αXβ2 integrin, an integrin highly expressed in the surface of dendritic cells [3] and with αVβ3 in melanoma cells mediating their adhesion to activated endothelium [4]. In astrocytes, αVβ3 integrin directly binds to the tripeptide RLD present in Thy-1 [5]. In a neuron-astrocyte interaction, Thy-1-integrin binding recruits Focal Adhesion Kinase (FAK) to focal contacts formed by astrocytes and activates FAK and RhoA, thereby promoting the formation of robust focal adhesions and stress fibers in less than 20 minutes of stimulation [1, 5–7]. These events, together with Thy-1-syndecan-4 interaction via the Thy-1 heparin-binding domain (HBD), contribute to the activation of PKCα [8]. Altogether, these observations, in conjunction with other reports, indicate that Thy-1 plays an important role in stimulating cell adhesion and actin cytoskeleton changes [9–12]. In our neuron-astrocyte model and in view of the reported time frame of formation and maturation of focal adhesions [1, 5–8], the neuronal surface protein Thy-1 induces a rapid and strong astrocyte adhesion to the substratum, via a Trans-induced signaling.
Cell migration is a multistep cycle of protrusion, adhesion to a substrate, polarization to establish a leading edge and relocation of signaling molecules that reorganizes the cytoskeleton and organelles in the direction of subsequent migration, followed by cell body movement and detachment of the rear of the cell [13, 14]. Filopodia, lamellipodia and focal contacts at the leading edge, mature focal adhesions and stress fibers in the lateral proximity of front protrusions provide the contraction force required to move cells. Disassembly of focal adhesions and retraction of the rear allow complete displacement of the cells [15, 16]. Reportedly, cells with large and elongated focal adhesions are less motile, whereas those rapidly moving cells present small focal contacts that are highly dynamic [17]. Thus, we hypothesized that Thy-1-enhanced cell adhesion negatively controls migration of astrocytes.
Cell surface receptors and signaling pathways involved in cell migration are well described for cellular locomotion induced by extracelluar matrix (ECM) proteins, which generate intracellular signals via dual engagement of both integrins and syndecan-4 receptors. The former binds to the RGD tripeptide, while the latter engages the HBD of ECM proteins [18–22]. Key signaling events for migration, involved downstream of these receptors, are binding of PI3K (phosphatidylinositol 3-kinase) to phosphorylated FAK [23] and activation of the small GTPase Rac1 [24–26].
Here, we report that the strong cell adhesion induced by neuronal Thy-1 via its interaction with αVβ3 integrin and syndecan-4 on astrocytes, is decreased upon sustained stimulation with Thy-1, and thus, contrary to all predictions, astrocyte migration on a 2D surface is enhanced by Thy-1. In addition, Thy-1 utilizes the same two binding motives (RLD and HBD) involved in cell attachment to ligate its receptors and induce migration. Furthermore, prolonged Thy-1 binding to integrin and syndecan-4 inhibits RhoA GTPase and activates the FAK/PI3K/Rac1 signaling pathway, decreasing astrocyte adhesion while increasing cell polarization and migration over a substratum. Thus, a cell surface protein prevalent on neurons can trigger a cascade of signaling events that modulate adhesion and shape as well as the migratory capacity of astrocytes.
2. MATERIALS AND METHODS
2.1. Cell culture and transfections
The rat astrocytic cell line DI TNC1 (ATCC CRL-2005) was maintained in RPMI medium 1640 (GIBCO Life Technologies, Grand Island, NY) as previously described [5]. Astrocytes expressing a dominant negative form of Rac1 were obtained by nucleofecting the EGFP-N17Rac1-containing plasmid following manufacturer recommendations for astrocytes (Amaxa Biosystems, Lonza, Cologne, Germany). CAD cells, CAD shThy-1 and CAD shLuc were maintained in D-MEM/F12 medium (GIBCO Life Technologies, Grand Island, NY) as described [5]. CAD cells with decreased levels of Thy-1 were obtained by transducing the cells with lentivirus, containing the pure pLCO.1 vector, the shRNA sequence that targets Thy-1 and resistance to puromycin as previously reported [27].
2.2 Antibodies and reagents
The recombinant proteins Thy-1-Fc and its mutants, as well as TRAIL-R2-Fc were obtained as reported [1, 5, 8] and used coupled to protein A (Sigma Chemical Co., St. Louis, MO) for cell stimulation. The complexes were incubated in a 10:1 (recombinant fusion protein: Protein-A) ratio for 60 minutes at 4°C, shaking gently as reported [28]. Immunofluorescence reagents were rhodamine-conjugated phalloidin (Sigma Chemical Co., St. Louis, MO), DAPI (diamidino-2-phenylindole) (Sigma Chemical Co., St. Louis, MO) and the antibodies rabbit anti-Thy-1 (serum R287, donated by Dr. Claude Bron, [29]), mouse anti-vinculin mAb (Sigma Chemical Co., St. Louis, MO), rabbit anti-giantin (Covance Research Products, Denver, PA) and goat anti-mouse IgG fluorescein isothiocyanate (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA) or goat anti-mouse IgG conjugated to Alexa fluor 488 (Molecular Probes, Eugene, OR). Other antibodies used were anti-p-Akt (Ser 473) polyclonal Ab (Cell Signaling Danvers, MA), anti-Akt (Cell Signaling Danvers, MA), anti p-FAK (Tyr397) (Upstate Biotechnology, Lake Placid, NY), anti-Rac (BD Transduction Laboratories), anti β-actin (Sigma Chemical Co., St. Louis, MO) and horseradish peroxidase-conjugated goat anti-mouse IgG polyclonal antibody (Bio-Rad Laboratories, Inc., Hercules, CA) or goat anti-rabbit IgG-HRP pAb for immunoblot analysis. Mouse anti-αV integrin monoclonal antibody (BD Transduction Laboratories Franklin Lakes, NJ), mouse anti-rat β3 integrin mAb (clone F11, BD Transduction Laboratories Franklin Lakes, NJ) were used as blocking antibodies in the wound-healing assay. PI3K inhibitor used was LY294002 (Sigma Chemical Co., St. Louis, MO), a Rac1 inhibitor was NSC 23766 (Sta. Cruz Biotechnology, Santa Cruz, CA) and FAK inhibitor C14 that prevents FAK autophosphorylation at Tyr 397 (Tocris Bioscience, Ellisville MO). Other reagents used were Heparin (Sigma Chemical Co., St. Louis, MO), CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay (Promega Madison, WI).
2.3. Proliferation assays
Astrocytes DI TNC1 were seeded in 96-well plates at a density of 3 ×103 per well and incubated for 24 hours in the absence of serum. The cells were incubated with Thy-1-Fc-Protein-A (1 μg/0.1 μg) complexes for 16, 24, and 48 hours. As negative controls TRAIL-R2-Fc-Protein A (1 μg/0.1 μg) complexes were used while serum stimulated astrocytes were employed as positive controls.
Cell proliferation was evaluated by the MTS® assay (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium), according to the manufacturer’s (Promega, Madison, WI) instructions. Since the MTS assay measures both cell proliferation and viability, a trypan blue exclusion assay was also used to determine cell proliferation.
2.4. In vitro wound-healing assay
Astrocytes were seeded in 24-well plates for 18 hours at 70 – 80% confluency. Upon formation of a subconfluent monolayer, the wounds were created with a sterile pipette tip. After wounding, detached cells were washed twice with PBS, the medium replaced with serum-free medium RPMI, which was left for 30 minutes prior to the addition of Thy-1-Fc-Protein-A (4 μg/0.4 μg) complexes. As negative controls TRAIL-R2-Fc-Protein-A at the same concentration and non-stimulated astrocytes were used. Astrocytes stimulated with 3% fetal bovine serum in RPMI medium were used as a positive control.
Wound closure was monitored by time-lapse microscopy with a Carl Zeiss Axiovert-135 microscope coupled to Nikon Coolpix 995 digital camara. Images were analyzed for void area using NIH Image J software. When using the PI3K inhibitor, LY294002 (3 μM) or Rac1 inhibitor, NSC 23766 (5 μM), the inhibitors were added to the medium 30 minutes before addition of Thy-1-Fc/Protein-A complex. When using anti-αV and β3 integrin blocking antibodies astrocytes were pre-incubated for 10 minutes at 37°C before the scratch was made. In other experiments, Thy-1-Fc-Protein A was incubated with heparin (400 μg/ml) for 30 minutes at 4°C before the stimulation.
2.5. RhoA and Rac1 activity assays
Astrocytes were grown in 6 cm plates, and RhoA or Rac1 activities were measured using affinity precipitation assays. Briefly, RhoA affinity precipitation was performed using GST-RBD as previously described [6]. For Rac1 activity measurements, cells were serum starved for 16 hours and subsequently stimulated with 40 μg of Thy-1-Fc coupled to Protein A-Sepharose beads in 800 μl of 50% slurry for different times. Serum-stimulation (60 minutes) was used as a positive control in various experiments. Cells were harvested in 300 μl of lysis buffer [25 mM HEPES (pH 7.4), 100 mM NaCl, 1% NP40, 10 mM MgCl2, 5 mM, 10% glycerol, 1 mM DTT, 10 μg/ml leupeptin, 10 μg/ml aprotinin and 1 mM sodium orthovanadate]. Cell lysates were incubated with 30 μg of GST-PBD beads for 15 minutes at 4°C and mixed gently on a rocking shaker. Bound proteins were resolved by SDS-PAGE in 12% Bis-Tris gels (BioRad, Hercules, CA) and transferred to PVDF membranes (Millipore, Billerica MA). Active GTP-bound RhoA or Rac1 was determined by Western blot analysis. The fold-increase in RhoA or Rac1 activity was normalized to total protein.
2.6. Indirect immunofluorescence assays
Astrocytes DI TNC1 cells were grown on sterile coverslips in 24-well plates for 18 hours in complete medium. The cells were incubated for 30 minutes in serum-free medium and subsequently stimulated with Thy-1-Fc-Protein A (4 μg/0.4 μg). TRAIL-R2-Fc-Protein A was used as a negative control and 3% FBS, as a positive control. Astrocytes were fixed for 20 minutes with 4% paraformaldehyde in 100 mM PIPES buffer, pH 6.8, containing 0.04 M KOH, 2 mM EGTA, and 2 mM MgCl2 after 1 hour of stimulation to observe formation of typical migratory structures, such as filopodia and lamellipodia, or after 7 hours to follow cell polarization. Afterwards, they were washed three times with buffer 50 mM Tris-HCl (pH 7.6), 0.15 N NaCl and 0.1% sodium azide (Universal buffer). Cells were permeabilized with 0.1% Triton X-100 in Universal buffer for 10 minutes, washed twice with the same buffer, and then blocked with 2% bovine serum albumin.
To evaluate filopodia and lamellipodia formation, cells were stained for focal adhesions and stress fibers, as previously described [1]. To monitor cell polarization, the cells were stained with anti-giantin followed by secondary antibodies coupled to FITC. Filamentous actin was stained with phalloidin conjugated to Rhodamine (1:1000) and the nucleus was stained with DAPI (0.0125 μg/ml). Cells with the Golgi located within a segment of 120° facing the wound were scored as positive. To evaluate focal adhesion formation, astrocytes (15.000 cells) were stimulated with CAD WT, CAD shThy-1 or CAD shLuc cells as reported [5]. Stimulated astrocytes were fixed for 20 minutes and focal adhesions were identified with the mouse anti-vinculin mAb, actin stress fibers with rhodamine-conjugated phalloidin and DAPI was used to stain nuclei. Fluorophores were visualized by Olympus IX81 DSU microscopy, objective PLAPON 60X with the camara XM10. The number of focal adhesions per cell and the average area per focal adhesion was quantified using the ImageJ software program from National Institutes of Health public domain as reported [6].
Cell morphology and Thy-1 expression in CAD WT, CAD shLuc and CAD shThy-1 cells were evaluated using Rhodamine-conjugated phalloidin and the anti-Thy-1 polyclonal antibody (1:200) followed by secondary antibodies coupled to FITC, respectively.
2.7. Astrocytes treatment and immunoblot analysis
Astrocytes were seeded in 24-well plates and grown for 18 hours to 70–80% confluency. Then, cells were stimulated with Thy-1-Fc-Protein A (4 μg/0.4 μg) complexes or Thy-1 (RLE)-Fc-Protein A at different time points (0, 10, 20, 30, 40 and 60 minutes) using the same positive and negative controls previously indicated. For immunoblot analysis, cells were washed with ice-cold PBS and lyzed with Laemmli sample buffer [2% SDS, 10% Glycerol, 62.5 mM Tris-HCl (pH 6.8), 5% β-mercaptoethanol and 0.01% bromophenol blue] containing 1 mM sodium orthovanadate, 2 μg/ml antipain, 1 μg/ml leupeptin, 10 μg/ml benzamidine, 1 mM PMSF and 50 μg/ml Calyculin-A. Cell lysates were boiled for 5 minutes, subjected to 10% SDS-PAGE and transferred to nitrocellulose membrane (BioRad, Hercules, CA). The membranes were blocked with 5% gelatin in TBS + 0.1% Tween 20 (TBS-T) or TBS-T 5% milk. Immunoblots were performed by incubation of membranes with anti-p-Akt (Cell signaling Danvers, MA) or anti-β-actin antibodies at 4°C overnight or 1 hour at room temperature, followed by the appropriate secondary antibody conjugated to Horseradish peroxidase. The peroxidase activity was revealed by enhanced chemiluminescence using EZ-ECL (Biological Industries, Israel, Beit-Haemek Ltda.). Finally, the image analysis system, Scion Image 4.0.2 (Center for Information Technology, NIH, Bethesda, MD), was used to quantify the changes in intensity of various bands.
2.8. Statistical Analysis
Data indicate the mean ± s.e.m. of results from at least three independent experiments. Results were analyzed by non-parametric test of Mann-Whitney to compare two groups and post-test of Kruskal-Wallis to compare multiple groups. P value of less than 0.05 was considered to be significantly different.
3. RESULTS
3.1. Cell-cell interaction mediated by endogenous Thy-1 increases matrix adhesion and migration of astrocytes
We have previously shown that cell-cell interaction mediated by the cell adhesion molecule Thy-1 increases adhesion to the extracellular matrix in less than 20 minutes of stimulation and that these events require the combined engagement of integrin and syndecan-4 receptors by Thy-1 [8]. Because these initial events promote strong cell adhesion, which would be predicted to inhibit cell migration, we tested the effect of Thy-1 treatment on cell polarization and movement in a wound-healing assay.
The effect of endogenous Thy-1 on astrocyte migration was studied by co-culturing astrocytes with CAD cells, a neuron-derived cell line of mouse origin that expresses high surface levels of Thy-1, as observed by indirect immunofluorescence analysis (Fig. 1A, top panel). Additionally, CAD cells with reduced levels of Thy-1, obtained by lentiviral transduction of shRNA that specifically target Thy-1 (shThy-1), were employed [27]. Confocal images of shThy-1 CAD cells lacking surface expression of Thy-1 are shown (Fig. 1A, lower panel). Interestingly, the absence of Thy-1 changed the shape of CAD cells decreasing their circularity. This effect was not observed when using shRNA to Luciferase as a control (shLuc) (Fig. 1B).
Figure 1.
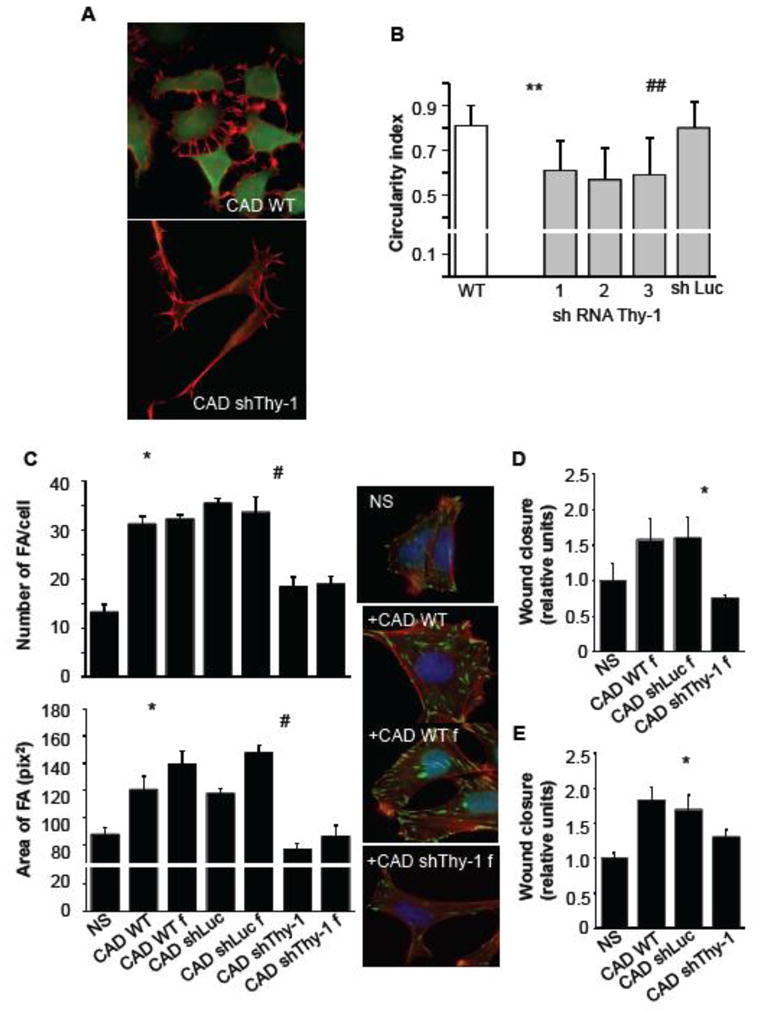
Expression levels of endogenous Thy-1 determine CAD cell morphology as well as stimulation of focal adhesion formation and migration of DI TNC1 astrocytes. A) CAD cells were transduced or not (CAD WT) with lentiviral vectors for shRNA Thy-1 (CAD shThy-1). Pictures are optical sections of cells obtained by confocal microscopy. Cells were stained for Thy-1 (green) and F-actin (red). Scale bar: 20 μm. B) Morphological analyses were performed using ImageJ software. Graph shows the cell circularity index for wild type CAD cells and for cells with Thy-1 silenced by different shRNA to Thy-1 (1, 2 and 3). Control values were obtained for cells transduced with shRNA Luciferase (CAD shLuc). Values are means ± s.e.m. obtained from 3 independent experiments, whereby at least 100 cells were analyzed per experiment. Significant differences **P<0.01 compared to wild type cells, ##P<0.01 compared to control cells. C) DI TNC1 cells were stimulated or not (NS) with CAD cells (CAD WT) or CAD cells fixed with 4% paraformaldehyde (CAD WT f), CAD shLuc or fixed CAD shLuc (CAD shLuc f), CAD shThy-1 or fixed CAD shThy-1 (CAD shThy-1 f), for 10 minutes. Focal adhesion formation was evaluated by immunofluorescence using the anti-vinculin monoclonal antibody followed by Alexa 488 anti-mouse IgG (green). Stress fibers were stained with Rhodamine-conjugated phalloidin (red) and the nuclei with DAPI (blue). Graphs show mean ± s.e.m. of number (upper) and average area (bottom) of focal adhesions. Significant differences compared to NS (*P<0.05), and to the respective CAD shLuc controls (#P<0.05) are indicated. D) Migration was evaluated using the wound-healing assay. DI TNC1 cells were stimulated or not (NS) with fixed wild type CAD cells, fixed CAD shLuc or fixed CAD shThy-1 cells for 24 hours. Significant differences compared to CAD shLuc (*P<0.05) are indicated. E) Same as in D, but CAD cells were live (non-fixed) cells. Statistical significance of difference between shThy-1 compared to control WT, p=0.052.
Unfortunately, after extended incubation periods, live CAD cells adhere strongly to astrocytes and to the plate, making the study of migratory events impossible after 24 hours in co-culture. Therefore, two different approaches were used to perform wound-healing assay stimulating astrocytes with whole CAD cells. First, fixed CAD cells were added to stimulate the wounded monolayer of astrocytes. First, the ability of these fixed CAD cells to induce formation of focal adhesions in astrocytes was corroborated by immunofluorescence analysis. Fixed CAD cells were found to behave like live cells in the cell adhesion assays performed here and reported previously, in that they increased both the number and size of focal adhesions, as well as stress fiber formation compared to non-stimulated control cells (Fig. 1C and [5, 28]). In addition, both CAD WT cells and CAD cells transduced with shRNA targeting Luciferase (CAD shLuc) increased number and the average size of focal adhesions, while no effect was detected when using shRNA to Thy-1 (CAD shThy-1, Fig. 1C). Because the results were comparable, whether using fixed or live cells to stimulate astrocyte adhesion, fixed CAD cells were utilized to induce cell migration in a wound-healing assay. As found for adhesion, both CAD WT and CAD shLuc cells induced cell migration, whereas no effect was detected when using CAD shThy-1 cells (Fig. 1D). The second approach was to add live CAD cells to the astrocytes prior to scratching the cell monolayer for the wound-healing assay. Here, we observed that the wounded area remained free of CAD cells for the period of time during which astrocytes were stimulated to migrate into the cell-free zone (data not shown). Astrocyte migration was similarly induced by either CAD WT or CAD shLuc cells, but for astrocytes stimulated with CAD shThy-1, migration was significantly reduced (Fig. 1E). Thus, both strategies using either fixed or live CAD cells generated similar results indicating that cell surface Thy-1 promoted astrocyte migration.
3.2. Prolonged Thy-1 stimulation decreases astrocyte adhesion while cell protrusions and polarity are established in an αVβ3 integrin and syndecan-4 dependent manner
To study whether the effect of CAD cells on migratory events was due to Thy-1, and to correlate these effects with those previously reported for Thy-1 on astrocyte adhesion, we assessed the effects of purified Thy-1 on astrocytes in serum-free medium at different time points.
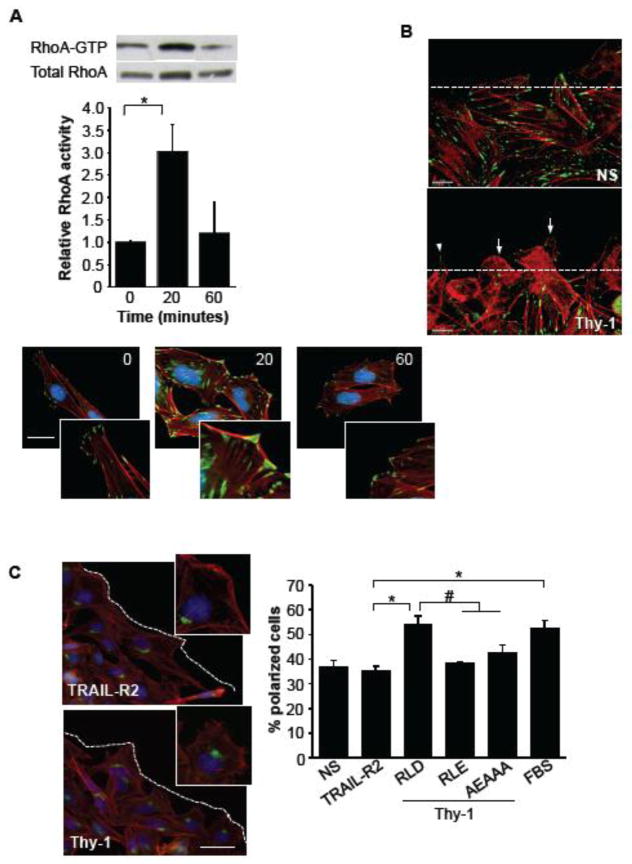
The activation of RhoA, reportedly involved in promoting strong cell adhesion and tension via focal adhesion and stress fiber formation [6], was found enhanced at 20 minutes but decreased at 60-minute incubation (Fig. 2A, graph). These activation-deactivation events were paralleled by assembly-disassembly of focal adhesions and stress fibers, respectively (Fig. 2A, photos).
Figure 2.
Thy-1/αVβ3 integrin and Thy-1/syndecan-4 interactions are required to induce astrocyte polarization. A) DI TNC1 incubated for 16 hours in serum-free medium were stimulated with Thy-1-Fc-Protein A (RLD)-beads, 20 or 60 minutes. The cells were then lysed, and active RhoA was affinity-precipitated using GST-RBD recombinant protein coupled to GSH-Agarose beads. Total RhoA from whole cell lysates and active RhoA were visualized by immunoblotting with anti-RhoA polyclonal antibody. Representative western blot result is shown. The fold-increase in RhoA activity was normalized to total protein present in the input lysate. *P<0.05, compared with time 0. For the immunofluorescence analysis, DI TNC1 cells incubated for 30 minutes in serum-free medium were stimulated with Thy-1-Fc-Protein A (RLD)-beads for 20 or 60 minutes. Focal adhesion formation was evaluated by using the anti-vinculin monoclonal antibody followed by Alexa 488 anti-mouse IgG (green). Stress fibers were stained with Rhodamine-conjugated phalloidin (red) and the nuclei with DAPI (blue). Magnification bar is 20 μm. Digital zoomed pictures are also shown to visualize focal adhesions better. B) Filopodia and lamellipodia formation were evaluated in a wound-healing assay. DI TNC1 cells in a wounded monolayer were stimulated or not (NS) with Thy-1-Fc-Protein A (4 μg/0.4 μg) (Thy-1) for 1 hour. The cells were fixed and stained with Rhodamine-conjugated phalloidin (red) and focal adhesions were visualized with an anti-vinculin antibody (green). Lamellipodia are indicated by arrows and filopodia by an arrowhead. The dashed line corresponds to the edge of the wound. Scale bar: 20 μm. C) Positioning of the Golgi apparatus in DI TNC1 cells within a segment of 120° in front of the nucleus facing the wound was evaluated by immunofluorescence in a wound-healing assay. The golgi was visualized with anti-giantin antibody (green), actin microfilaments with Rhodamine-conjugated phalloidin (red) and nuclei with DAPI (blue). The photos shown at the upper-right side of each image are digital zooms of a non-polarized (TRAIL-R2) or a polarized cell (Thy-1). Larger images show several cells at the border of the cell-free area (dashed line). The percentage of polarized DI TNC1 cells, present in the first two rows of the wound border was evaluated. The cells were incubated or not (NS) with Thy-1-Fc-Protein A (Thy-1, RLD) or Thy-1 with the integrin-binding site mutated (RLE), the HBD mutated (AEAAA) or 3% FBS for 7 hours. TRAIL-R2-Fc-Protein A (TRAIL-R2) was used as a negative control. The percentage of cells with the Golgi oriented towards the wounded area (polarized cells) are shown as mean ± s.e.m.
We then tested the effect of Thy-1 treatment on early events of cell migration, namely the formation of filopodia and lamellipodia, as well as polarization, of cells present in the migrating front of a wound-healing assay. Soluble, purified, Thy-1-Fc fusion protein conjugated to Protein-A or control preparations were added to a wounded astrocyte monolayer. After 60 minutes of exposure to Thy-1-Fc in serum-free medium, filopodia and lamellipodia formation were evident (Fig. 2B). These structures were not observed in cells incubated with medium alone, which instead appeared to increase focal adhesion formation upon prolonged (≥60 minutes) incubation in serum-free medium (Fig. 2B). Additionally, after 7 hours of treatment, cells became polarized as evidenced by positioning of the Golgi within a 120° segment in front of the nucleus facing the leading edge in those cells treated with Thy-1-Fc-Protein-A complexes, but not with the negative control of this fusion protein, TRAIL-R2-Fc-Protein A complex (Fig. 2C, photo and digital zoom for better visualization of Golgi). As described for cell adhesion, polarization required the interaction of Thy-1 with both integrin and syndecan-4 receptors, since the treatment with Thy-1-Fc mutated in the integrin-binding site (RLD to RLE) or in the HBD (REKRK to AEAAA) did not induce cell polarization (Fig. 2C, graph). Medium in the presence of serum (FBS) was used as a positive control (Fig. 2C, graph).
3.3. Sustained treatment with Thy-1 induces astrocyte migration without affecting cell proliferation
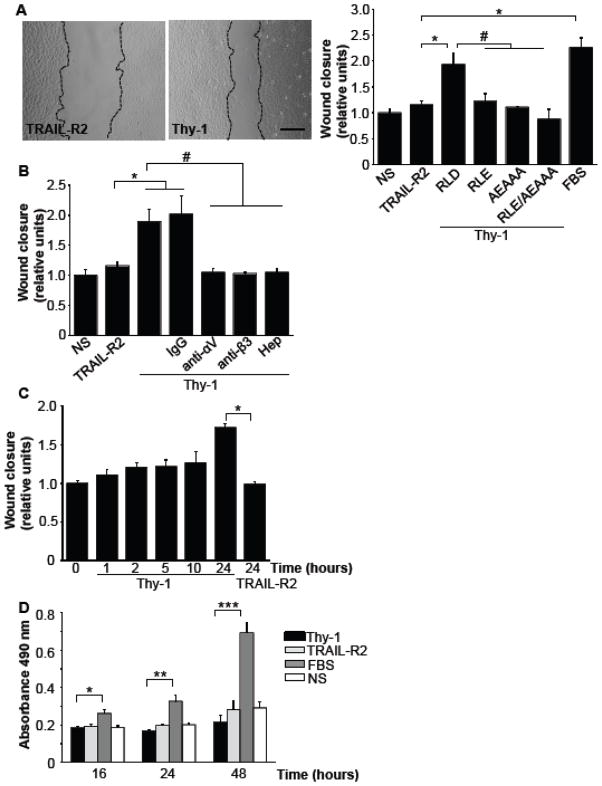
As for cell polarization, cell migration monitored 24 hours post-stimulus was found induced by Thy-1. Cell-free area in the wound-healing assay was smaller for cells treated with Thy-1-Fc-Protein A (Thy-1) than in those treated with TRAIL-R2-Fc-Protein A used as a negative control (Fig. 3A, photo). Quantification of the cell-free area, normalized to the values obtained for non-stimulated controls, indicated that either serum or Thy-1-Fc, possessing intact RLD and REKRK sites, stimulated cells to migrate and populate the cell-free area (Fig. 3A, graph). Alternatively, cell migration was not stimulated by Thy-1 mutated in either integrin-binding site and/or in the HBD (Fig. 3A, graph). The requirement of both integrin and syndecan-4 binding to Thy-1 for cell migration was corroborated by testing Thy-1-induced migration following pre-incubation of astrocytes with either anti-αV- or anti-β3- antibodies, or pre-incubation of Thy-1 with Heparin (Fig. 3B) to block its HBD. Control antibody had no effect on Thy-1 stimulated astrocyte migration (Fig. 3B). Thus, as previously reported for adhesion [8], Thy-1-induced cell polarization and migration are shown here to require interaction of Thy-1 with both αVβ3 integrin and syndecan-4.
Figure 3.
Sustained treatment with Thy-1 stimulates astrocyte migration without affecting cell proliferation. A) The migratory capacity of astrocytes incubated with Thy-1-Fc-Protein A (Thy-1, RLD) or variants of Thy-1 mutated (RLE, AEAAA, double mutant: RLE/AEAAA) during 24 hours is shown. Cells incubated with TRAIL-R2-Fc-Protein A (TRAIL-R2) or serum (FBS) were used as a negative and positive controls, respectively. The wounded area was evaluated with Image J program from photos like those shown at the left for cells migrating in the presence of TRAIL-R2-Fc (TRAIL-R2) or Thy-1-Fc (Thy-1). The relative wound closure was calculated in every sample, with respect to the value obtained for the non-stimulated samples. Values are expressed as means ± s.e.m. B) DI TNC1 astrocyte monolayer was pre-incubated with different antibodies: anti-αV integrin, anti-β3 integrin or control IgG antibody (5 μg each) for 10 minutes. The cells were then stimulated with Thy-1-Fc-Protein A for 24 hours. In other experiments Thy-1-Fc-Protein A (Thy-1) was pre-treated with Heparin (400 μg/ml) for 30 minutes at 4°C. The cells were incubated with this complex and the wounded area evaluated. A significant difference (*P<0.05) is indicated, when comparing cells incubated with TRAIL-R2 and cells incubated with either Thy-1 (RLD) or control IgG. Significant differences are also indicated (#P < 0.05) compared with Thy-1 (RLD). C) DI TNC1 astrocytes incubated with serum-free RPMI medium were stimulated with Thy-1-Fc-Protein A (Thy-1) for different time points. Then, Thy-1 was removed, and the wounded area evaluated at hour 24. Cells stimulated with TRAIL-R2-Fc-Protein A for 24 hours were used as a negative control. D) Proliferation is not affected by Thy-1 binding. DI TNC1 astrocytes were seeded in 96-well plates at a density of 3 × 103 per well and incubated for 24 hours in the absence of serum. The cells were then incubated with Thy-1-Fc-Protein-A complexes for 16, 24, and 48 hours. TRAIL-R2-Fc-Protein A (TRAIL-R2) was used as a negative and FBS as a positive control. Proliferation was measured using the MTS® assay (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium). Results obtained measuring A 490 nm after MTS assays are shown as the average from 3 independent experiments (mean ± s.e.m.). Statistical significance (*P<0.05; **P<0.01; ***P<0.001) is indicated.
Thy-1-Fc-induced migration was dependent on sustained incubation with the stimulus, since the removal of Thy-1 at shorter time periods, led to decreased cell migration observed after 24 hours (Fig. 3C). With respect to the negative control using TRAIL-R2-Fc, the only significant difference was observed when Thy-1 stimulation was maintained the entire 24-hour period (Fig. 3C). Furthermore, the appearance of cells in the wounded area could not be attributed to proliferation, since these experiments were performed in the absence of serum, under conditions where astrocyte proliferation was not detected within 24 hours, as shown using the MTS assay (Fig. 3D, see bars of Thy-1, TRAIL-R2 and NS) and the trypan blue exclusion assay (not shown). On the contrary, in the presence of 3% serum, astrocytes divided every 24 hours (Fig. 3D, FBS), indicating that in this case, wound closure could be attributed to both migration and proliferation. To examine DI TNC1 cells during active migration, we imaged these cells by time-lapse confocal microscopy (Supplementary material, Videos 1–3). Many cells in layers proximal to the wound stimulated with Thy-1-Fc-Protein-A extended protrusions, spread and migrated towards the cell-free area. In contrast, cells treated with TRAIL-R2-Fc-Protein-A extended long protrusions during the first 8 hours, but then, only a few cells in the first layer spread and migrated to the wound. Astrocytes treated with 3% serum extended very long processes. Also in this case, only cells of the first layer spread and moved towards the cell-free area. In addition, cells treated with serum proliferated considerably more than in the other conditions, as revealed also by the MTS assay (Fig. 3D). Noteworthy, cell proliferation in the presence of serum occurred to a large extent in those cells located in the layers far from the wound. Daughter cells inserted themselves in between cells of the monolayer, thereby pushing the cells most proximal to the wound into the cell-free space. Thus, as shown in figure 3, serum-stimulated closure of the cell-free area is the consequence of both migration and proliferation, whereas Thy-1 stimulates cell migration and wound closure in the absence of proliferation.
3.4. Migration requires Thy-1-integrin engagement and integrin-induced FAK-dependent activation of PI3K
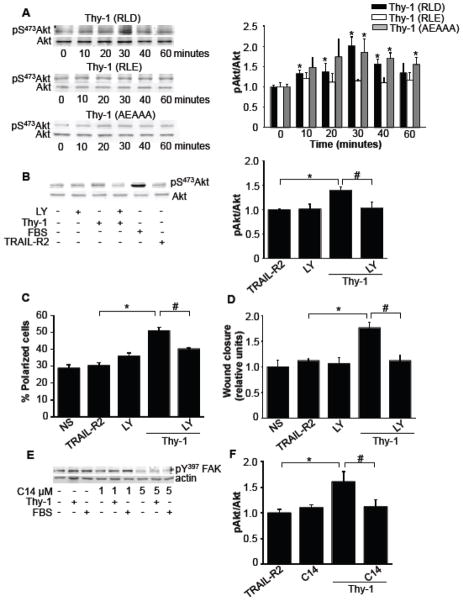
For astrocytes incubated with Thy-1, FAK phosphorylation on tyrosine 397 is enhanced [8]. Therefore, because PI3K reportedly binds to this phosphorylated residue, we wondered whether PI3K, a kinase known to participate in cell migration [30], was activated downstream of FAK. To this end, cells were incubated for different periods of time with Thy-1, and activation of PI3K was monitored by assessing phosphorylation of Akt, an important downstream target in the PI3K pathway. Indeed, Akt phosphorylation increased in the presence of Thy-1 and required binding to integrin, since mutation of the integrin-binding domain (RLE mutant), did not stimulate Akt phosphorylation (Fig. 4A). Noteworthy, Thy-1 mutated in the HBD, which possesses an intact integrin-binding site (RLD) also promoted Akt phosphorylation with kinetics similar to the wild type Thy-1 fusion protein. A peak of phosphorylation was detected after 30 minutes of Thy-1 exposure (Fig. 4A). Thus, integrin but not syndecan-4 engagement triggers PI3K activation. Moreover, Thy-1-induced Akt phosphorylation was inhibited by LY294002 (Fig. 4B), a known inhibitor of PI3K. Interestingly, cell polarization (Fig. 4C) and cell migration (Fig. 4D) were also reduced by this inhibitor at concentrations reported to be specific for PI3K [31, 32]. To test whether PI3K activation occurred downstream of FAK activation, C14, an inhibitor of FAK, was employed. For astrocytes incubated with C14 (5 μM), Y397-pFAK was reduced (Fig. 4E). At this same concentration, C14 inhibited S473-pAkt induced by Thy-1 stimulation for 30 minutes (Fig. 4F). These results suggest that the FAK/PI3K signaling pathway is activated by Thy-1-integrin interaction and that this pathway is required to induce astrocyte polarization and migration.
Figure 4.
The Thy-1/αVβ3 integrin interaction activates the FAK/PI3K signaling pathway. A) Astrocytes were stimulated with Thy-1-Fc-Protein A (RLD), Thy-1 (RLE) or Thy-1 (AEAAA) for 0, 10, 20, 30, 40 and 60 minutes. After rinsing cells with PBS, whole cell lysates were separated on a 10% SDS-PAGE and analyzed by western blotting using polyclonal anti-pS473Akt antibody. Total Akt levels were used as loading controls. Values were compared to normalized time 0 and are presented in the graph as means ± s.e.m. B) The inhibitory capacity of 3 μM LY 294002 (LY) in astrocytes stimulated with Thy-1-Fc-Protein A (Thy-1) was measured by immunoblotting and quantified by densitometryc analysis. Values obtained (mean ± s.e.m.) are indicated in the graph. C) and D) The percentage of polarized cells and the migratory capacity of DI TNC1 astrocytes stimulated with Thy-1 were evaluated, as indicated in figure 2, after inhibiting PI3K with LY 294002 (LY) 3 μM for 30 minutes prior to Thy-1 stimulation. E) Cells were incubated or not with C14 (1 or 5 μM) in serum-free medium and then either stimulated with Thy-1-Fc-Protein A (Thy-1) for 10 minutes or 3% serum (FBS) for 3 minutes. Astrocytes were rinsed with cold PBS and lysed in Laemmli buffer. Cell lysates were analyzed by immunoblotting with anti pY397FAK antibody. Actin was used as a loading control. F) Astrocytes, pre-incubated with C14 (5 μM) for 30 minutes in serum-free medium, were stimulated or not with Thy-1-Fc-Protein A (Thy-1) for 30 minutes. TRAIL-R2-Fc-Protein A (TRAIL-R2) was used as a negative control. Values shown were obtained as in (A) and presented as means ± s.e.m. Statistically significant differences are indicated (*P<0.05 compared to TRAIL-R2 or to time 0; #P<0.05 compared to cells stimulated with Thy-1).
3.5. Thy-1 interaction with its receptors activates Rac1 and is necessary for astrocyte migration
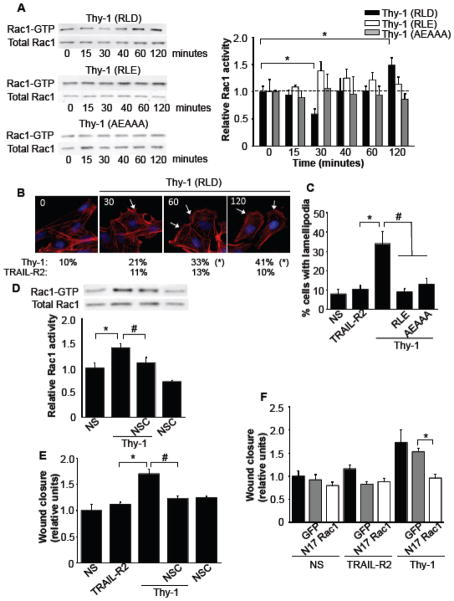
Another well-known participant in cell migration is the small GTPase Rac1. Therefore, the kinetics of Rac1 activation were analyzed after stimulation with Thy-1-Fc or mutated variants of this fusion protein. Thy-1-RLD-Fc-integrin binding was found to reduce Rac1 activation in the first 30 minutes, while longer exposure to Thy-1 increased Rac1 activity as observed at 60 and 120 minutes (Fig. 5A). Rac1 activity found at both 60 and 120 minutes, but not at 40 minutes, was significantly different from values obtained at 30 minutes (p<0.05; not indicated in figure 5A). On the contrary, no significant effect was observed at any time point when using Thy-1-RLE-Fc, or the Thy-1 molecule mutated in the HBD, Thy-1-AEAAA-Fc (Fig. 5A) compared to their respective controls at time zero. These results suggest that Thy-1-engagement of both integrin and syndecan-4 proteins is required to induce the changes in activity of Rac1 GTPase. Since Rac1 is known to promote formation of cell protrusions at the leading edge [33], we analyzed lamellipodia formation upon stimulation with Thy-1-RLD-Fc, Thy-1-RLE-Fc, or Thy-1-AEAAA-Fc at different time points. After 30 minutes of stimulation with Thy-1-RLD-Fc, the number of cells with lamellipodia doubled and continued to increase for up to the 2 hours. Alternatively, mutated Thy-1 did not alter the number of cells with lamellipodia. These results indicate that only wild type Thy-1 induces the formation of these structures (Fig. 5B and 5C). Additionally, the Rac1 inhibitor, NSC, not only precluded Rac1 activation at 120 minutes (Fig. 5D), but also completely abolished Thy-1 enhanced migration (Fig. 5E). In a similar manner, Thy-1-induced astrocyte migration was precluded by the over-expression of a EGFP-N17Rac1, a dominant negative form of Rac1 (Fig. 5F). Thus, astrocyte motility stimulated by Thy-1 requires the activation of the FAK/PI3K/Rac1 signaling pathway.
Figure 5.
Thy-1/αVβ3 integrin and Thy-1/syndecan-4 interactions activate Rac1. A) DI TNC1 incubated for 16 hours in serum-free medium were stimulated with Thy-1-Fc-Protein A (RLD)-beads, Thy-1 (RLE) or Thy-1 (AEAAA) for different periods of time. The cells were then lysed, and active Rac1 was affinity-precipitated using GST-PBD recombinant protein coupled to GSH-Agarose beads. Total Rac1 from whole cell lysates and active Rac1 were visualized by immunoblotting with anti-Rac1 polyclonal antibody. Representative western blots are shown. The fold-increase in Rac1 activity was normalized to total protein present in the input lysate. *P<0.05, compared with time 0. B) Lamellipodia formation was evaluated in wound-healing assay using DI TNC1 cells stimulated with Thy-1-Fc-Protein A (RLD)-beads for 30, 60 or 120 minutes. TRAIL-R2-Fc-Protein A (TRAIL-R2) was used as a negative control. Cells were fixed and actin was labeled with rhodamine-conjugated phalloidin (red), and the nucleus with DAPI (blue). White arrows indicate cells with lamellipodia. Values under the microphotographs correspond to the percentage (%) of cells with lamellipodia averaged from 3 independent experiments. Significant differences (*P<0,05) between results obtained in cells incubated with Thy-1 (RLD) and those incubated TRAIL-R2 at any given time point are indicated. C) Lamellipodia formation was evaluated as in B, but after stimulation with wild-type Thy-1 or mutated Thy-1 (RLE, AEAAA) variants for 120 minutes. Values shown are presented as means ± s.e.m. Statistically significant differences are indicated (*P<0.05 compared to TRAIL-R2; #P<0.05 compared to cells stimulated with Thy-1). D) The effect of pre-incubation with 10 μM of the Rac1 inhibitor NSC 23766 (NSC) was evaluated after 120 minutes in Thy-1-Fc-Protein A (Thy-1)-stimulated astrocytes using the affinity precipitation Rac assay described in (A). Values shown are means ± s.e.m. Significant differences are indicated (*P<0.05, compared with NS, #P<0.05, compared to cells stimulated with Thy-1 in the presence of NSC). E) The effect of the Rac1 inhibitor NSC was also tested in Thy-1-Fc-Protein A (Thy-1)-stimulated astrocytes using the wound-healing assay. Values shown are means ± s.e.m. Significant differences are indicated (*P<0.05, compared with TRAIL-R2, #P<0.05, compared to cells stimulated with Thy-1 in the presence of NSC). F) The effect of the over-expression of a dominant negative form of Rac1 was tested in Thy-1-Fc-Protein A (Thy-1)-stimulated astrocytes using the wound-healing assay (white bars). As a control, cells were either not transfected (black bars) or nucleofected with EGFP vector alone (gray bars). Values shown are means ± s.e.m. Significant difference is indicated (*P<0.05).
4. DISCUSSION
Mechanistic insight to the events that control cell migration is required to comprehend processes including metastasis of cancer cells, tissue formation during development and repair after wounding. In this study, we describe that sustained Thy-1 mediated ligation of integrin and syndecan-4 positively controls 2D cell migration of astrocytes. Interestingly, the neuronal glycoprotein Thy-1 employs binding motifs (RLD and HBD) similar to those reported for ECM proteins and upon binding activates the FAK/PI3K/Rac1 signaling pathway to increase astrocyte polarization and migration.
4.1. Thy-1 in cell adhesion and migration
Interactions of more than 100 components are an essential part of the integrin signaling complexes. Based on these interactions, a hypothetical integrin database named “adhesome” has been created [34]. Importantly, Thy-1 was identified as an adhesion-associated partner of integrins, which is in good agreement with experimental evidence showing that Thy-1 acts as an integrin ligand to mediate cell-cell adhesion/communication between several cell types [3–5]. Very interestingly, Thy-1 is the only identified integrin-binding partner that also requires binding to syndecan-4 to promote cell adhesion and migration (this study and [8, 28]).
It is thought that integrins and syndecans connect the cytoskeleton to the plasma membrane, which transduces mechanical forces from pliable ECM to the cytoskeleton [35]. In a 2D system, like the one used in this study, the addition of Thy-1 containing cells or Thy-1-Fc coupled to Protein-A beads [1, 5] over the astrocyte monolayer may have affected the astrocytes by a mechanotransduction pathway. However, Thy-1−/− cells did not induce focal adhesion formation, while soluble Thy-1-Fc induced cellular responses, including cell adhesion and migration. This can be taken to indicate that most likely Thy-1-mediated ligation of integrin and syndecan-4, rather than mechanical stress, triggered the downstream signaling events.
4.2. Thy-1-triggered cell signaling
Upon activation, PI3K phosphorylates phosphatidylinositol (4,5)-bisphosphate (PIP2) resulting in the production of PIP3, which recruits downstream effectors to the plasma membrane, such as Akt [36]. In our studies, the phosphorylation of Akt at serine 473 was used as a read-out for PI3K activation. Such pS473Akt phosphorylation was dependent on prior FAK autophosphorylation and blocked by incubation with the PI3K-specific inhibitor LY294002. Additionally, this inhibitor also prevented Thy-1-induced cell polarization and migration, suggesting the involvement of FAK and PI3K in this migratory process.
PI3K activation was also shown to depend on Thy-1-integrin, but not Thy-1-syndecan interaction (Fig. 4), since Thy-1 mutated in the integrin-binding site did not induce Akt phosphorylation. Alternatively, Thy-1 containing the RLD tripeptide, but not the HBD, induced phosphorylation of Akt with similar kinetics and intensity as observed for the wild type Thy-1 protein (Fig. 4A). Noteworthy, Akt dephosphorylation occurred faster when induced by the wild-type Thy-1-Fc than with the Thy-1 mutated in the HBD. Interestingly, due to its association with PKCα, syndecan-4 has been described as an integrin regulator, rather than as a co-receptor, and this regulation is related with dynamin- and caveolin-1-dependent integrin endocytosis [37]. Therefore, in the absence of syndecan-4 engagement by Thy-1 mutated in the HBD, integrin desensitization might be impaired, accounting for an elevated pS473Akt phosphorylation found after 60 minutes of stimulation (Fig. 4A).
Another key player of the signaling cascade leading to cell migration is Rac1 [38]. Here, we observed that Rac1 activation becomes detectable after 30 minutes (Fig. 5A). Thus temporally, this event occurs after Akt phosphorylation (Fig. 4A) and therefore, also after PI3K activation and FAK autophosphorylation (see section 4.3.). Rac1 activation is reportedly regulated by syndecan-4 in a PKCα-dependent manner [39]. On the other hand, syndecan-4 has been described as a primary receptor that, once engaged, may alter β1 integrin signaling to produce changes in cell adhesion [37, 40, 41]. Our data suggest that binding of Thy-1 only to integrin in the absence of syndecan-4 ligation leads to phosphorylation of Akt (Fig. 4), but neither to activation of Rac1 (Fig. 5A) nor to cellular adhesion [8], polarization or migration (Fig. 2B and 2C). On the contrary, binding of Thy-1 only to syndecan-4 without integrin engagement [Thy-1(RLE)-Fc], does not lead to activation of any of the molecules or cellular functions analyzed in these studies. Although Rac1 activation shows a tendency to increase at 30 minutes of stimulation with this Thy-1 mutant, the differences were not statistically significant (Fig. 5A). In addition, events known to be caused by Rac1 activation such as lamellipodia formation [33], do not occur upon treatment with Thy-1(RLE)-Fc (Fig. 5C). These results argue that for Thy-1-triggered events ligation of syndecan-4 alone is insufficient to trigger Rac1 activation and lamellipodia formation.
4.3. Working model
We have previously shown that Thy-1 directly interacts with αVβ3 integrin triggering integrin clustering, tyrosine phosphorylation of focal adhesion proteins, increased intracellular Ca2+ levels and PKCα activity, activation of RhoA in astrocytes, thereby inducing focal adhesion formation, cell attachment and spreading. Also, Thy-1 requires interaction with syndecan-4 to stimulate focal adhesion formation, possibly through complex formation of syndecan-4 with PKCα to further activate RhoA and promote morphological changes and cell adhesion (Fig. 6A). All these signaling events have been shown to occur in less than 20 minutes upon addition of Thy-1 [1, 5–8, 28]. In the present study, Thy-1 is shown to stimulate Rac1-dependent migration of astrocytes with distinct kinetics. While RhoA is activated within 20 minutes after Thy-1 stimulation and inactivated at 60 minutes, Rac1 activation shows a drop in activity at 30 minutes and it is activated after 30 minutes of stimulation with Thy-1 (Fig. 6B). We propose that after initial Thy-1-integrin engagement leading to changes in cell adhesion and spreading (Fig. 6A), phosphorylated FAK recruits PI3K, which increases PIP3 levels at the plasma membrane. PH domain-containing proteins, like RacGEFs (not identified in this study), translocate to membrane-bound PIP3 and activate Rac1, inducing cell migration (Fig. 6C). Evidences indicate that Rac1 is the main GTPase activated downstream of syndecan-4 ligation [39]. However, the link between Rac1 activation downstream of either PI3K or syndecan-4 in our model, awaits further investigation.
Figure 6.
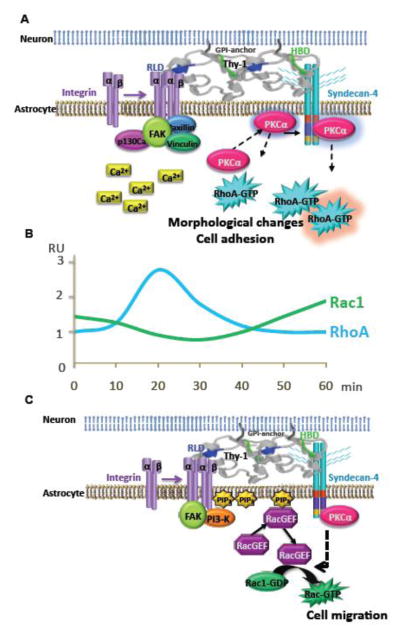
Proposed model: Thy-1/αVβ3 integrin and Thy-1/syndecan-4 interactions induce cell adhesion and migration by sequential activation of RhoA and Rac1. A) αVβ3 integrin and syndecan-4 bind to Thy-1 to induce focal adhesion and stress fiber formation [1]. Signaling events include activation of FAK, increased intracellular Ca2+ levels and translocation of PKCα to the membrane, where it is suggested to activate RhoA [1, 8, 28]. Then, PKCα is depicted bound to syndecan-4, which enhances PKCα activity, further contributing to RhoA activation and cell adhesion [8]. RhoA activation is observed in less than 20 minutes after Thy-1 stimulation [6]. B) Schematic representation of the kinetics of RhoGTPases activation expressed in relative units (RU). Upon Thy-1 stimulation, RhoA is activated at 20 minutes and inactivated at 60 minutes; whereas, Rac1 shows decreased activity at 30 minutes and is activated at 60 minutes. C) αVβ3 integrin and syndecan-4 bind to Thy-1 to induce cell migration. Signaling events here include activation of FAK, and PI3K and translocation of a yet unknown RacGEF to the plasma membrane, which leads to activation of Rac1 and cell migration. Rac1 activation is observed after 30 minutes of Thy-1 stimulation (Fig. 5).
Regulation of the cytoskeleton during cell migration has been thought to involve Rac1-dependent formation of membrane protrusions at the leading edge, Cdc42-mediated filopodia formation and cell polarization, and RhoA-dependent contractility at the rear of the cell [33]. Imaging-techniques utilized to study activation of RhoGTPases have uncovered a more complex scenario and generated considerable controversy in the RhoGTPase field [42]. However, supporting our model of temporally distinct RhoA/Rac1 activation, new probes that permit monitoring Rac1 activation without inducing cellular compensation mechanisms, indicate that Rac1 and RhoA activation are indeed inversely correlated in cells [43].
Cells at the border of a wound-healing assay sense the environmental changes and in the presence of serum, adapt by modifying points of adhesion, changing cellular tension and inducing cell polarization as well as migration to close the cell-free area. In this report, we show that focal adhesion dynamics are also modified, in serum-free medium, by signals generated from dorsally added neuron-like cells, which induce astrocyte polarization and migration. The signals received from neuronal cells need to be persistently present to induce the cellular responses (Figs. 2 and 3), suggesting a requirement for repetitive stimulatory cycles to trigger cell polarization and migration. Thus, exchange of information controlling migration over a matrix, not only occurs as the consequence of interactions between the ECM and the cell surface, but it is controlled by signals received from other cells that contact the migrating cells. How cells integrate these inputs received from different sites of the cell surface (dorsally or ventrally) to generate apparently similar outcomes is an open question that requires further investigation.
Supplementary Material
Videos 1–3: Phase-contrast time-lapse of DI TNC1 cells migrating on a plate. DI TNC1 cells were seeded in 8-well plates (Lab-Tek II, Coverglass System, Nunc) for 18 hours. Upon formation of a sub-confluent monolayer, wounds were made with a sterile pipette tip. After wounding, detached cells were washed twice with PBS. The medium was replaced with serum-free RPMI medium for 30 minutes prior to the addition of Thy-1-Fc-Protein-A (4 μg/0.4 μg) complexes (Video 1). As negative control, TRAIL-R2-Fc-Protein-A at the same concentration was used (Video 2). Astrocytes stimulated with 3% fetal bovine serum in RPMI medium were the positive control (Video 3). Phase images were captured at one frame/10 min with a phase FluoView FV10i (Olympus) for 24 hours under controlled temperature and CO2 conditions. The video plays at 15 frames/s.
Highlights.
Strong cell adhesion is opposite to cell migration.
Neuronal Thy-1 engages integrin and syndecan-4 in astrocytes to greatly increase their adhesion.
Thy-1 stimulation for 1 hour activates Rac1 and inactivates RhoA.
Extended Thy-1 stimulation period triggers the activation of FAK-PI3K-Rac1 signaling pathway.
Neuron-astrocyte binding dorsally engages receptors and signaling molecules to promote cell motility over a substratum.
Acknowledgments
This work was supported by the grants: FONDECYT # 1070699 and 1110149 (LL), #1090071 and 1130250 (AFGQ), # 24080094 (AV); CONICYT #21090323 (AA); Fondecyt-FONDAP # 15010006 (AFGQ); Anillos # ACT1111 (AFGQ); Iniciativas Milenio # P09–015-F (LL and AC); NIH grant #GM029860 (KB); Fogarty International Center-NIH grant # 5R03TW007810–03 (LL, KB); Grants of the Swiss National Science Foundation (PS). RH-M acknowledges fellowships from DAAD, the Journal of Cell Science, and the CAEN International Society for Neurochemistry; support from the COST action ECMNet and the State of Saxony-Anhalt.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leyton L, Schneider P, Labra CV, Rüegg C, Hetz CA, Quest AFG, Bron C. Thy-1 binds to the integrin β3 on astrocytes and triggers formation of focal contact sites. Curr Biol. 2001;11:1028–1038. doi: 10.1016/s0960-9822(01)00262-7. [DOI] [PubMed] [Google Scholar]
- 2.Barker TH, Hagood JS. Getting a grip on Thy-1 signaling. Biochim Biophys Acta. 2009;1793:921–923. doi: 10.1016/j.bbamcr.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi J, Leyton L, Nham SU. Characterization of αX I-domain binding to Thy-1. Biochem Biophys Res Commun. 2005;331:557–561. doi: 10.1016/j.bbrc.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Saalbach A, Wetzel A, Haustein UF, Sticherling M, Simon JC, Anderegg U. Interaction of human Thy-1 (CD 90) with the integrin αVβ3 (CD51/CD61): an important mechanism mediating melanoma cell adhesion to activated endothelium. Oncogene. 2005;24:4710–4720. doi: 10.1038/sj.onc.1208559. [DOI] [PubMed] [Google Scholar]
- 5.Hermosilla T, Muñoz D, Herrera-Molina R, Valdivia A, Muñoz N, Nham SU, Schneider P, Burridge K, Quest AF, Leyton L. Direct Thy-1/αVβ3 integrin interaction mediates neuron to astrocyte communication. Biochim Biophys Acta-Molecular Cell Research. 2008;1783:1111–1120. doi: 10.1016/j.bbamcr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avalos AM, Arthur WT, Schneider P, Quest AF, Burridge K, Leyton L. Aggregation of integrins and RhoA activation are required for Thy-1-induced morphological changes in astrocytes. J Biol Chem. 2004;279:39139–39145. doi: 10.1074/jbc.M403439200. [DOI] [PubMed] [Google Scholar]
- 7.Avalos AM, Labra CV, Quest AF, Leyton L. Signaling triggered by Thy-1 interaction with β3 integrin on astrocytes is an essential step towards unraveling neuronal Thy-1 function. Biol Res. 2002;35:231–238. doi: 10.4067/s0716-97602002000200015. [DOI] [PubMed] [Google Scholar]
- 8.Avalos AM, Valdivia AD, Muñoz N, Herrera-Molina R, Tapia JC, Lavandero S, Chiong M, Burridge K, Schneider P, Quest AF, Leyton L. Neuronal Thy-1 induces astrocyte adhesion by engaging syndecan-4 in a cooperative interaction with αVβ3 integrin that activates PKCα and RhoA. J Cell Sci. 2009;122:3462–3471. doi: 10.1242/jcs.034827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker TH, Grenett HE, MacEwen MW, Tilden SG, Fuller GM, Settleman J, Woods A, Murphy-Ullrich J, Hagood JS. Thy-1 regulates fibroblast focal adhesions, cytoskeletal organization and migration through modulation of p190 RhoGAP and Rho GTPase activity. Exp Cell Res. 2004;295:488–496. doi: 10.1016/j.yexcr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Saalbach A, Haustein UF, Anderegg U. A ligand of human Thy-1 is localized on polymorphonuclear leukocytes and monocytes and mediates the binding to activated Thy-1-positive microvascular endothelial cells and fibroblasts. J Invest Dermatol. 2000;115:882–888. doi: 10.1046/j.1523-1747.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- 11.Saalbach A, Hildebrandt G, Haustein UF, Anderegg U. The Thy-1/Thy-1 ligand interaction is involved in binding of melanoma cells to activated Thy-1- positive microvascular endothelial cells. Microvasc Res. 2002;64:86–93. doi: 10.1006/mvre.2002.2401. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Hagood JS, Lu B, Merryman WD, Murphy-Ullrich JE. Thy-1-integrin αVβ5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-β1 activation and myofibroblast differentiation. J Biol Chem. 2010;285:22382–22393. doi: 10.1074/jbc.M110.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe T, Noritake J, Kaibuchi K. Regulation of microtubules in cell migration. Trends Cell Biol. 2005;15:76–83. doi: 10.1016/j.tcb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Dubash AD, Menold MM, Samson T, Boulter E, Garcia-Mata R, Doughman R, Burridge K. Chapter 1. Focal adhesions: new angles on an old structure. Int Rev Cell Mol Biol. 2009;277:1–65. doi: 10.1016/S1937-6448(09)77001-7. [DOI] [PubMed] [Google Scholar]
- 16.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloom L, Ingham KC, Hynes RO. Fibronectin regulates assembly of actin filaments and focal contacts in cultured cells via the heparin-binding site in repeat III13. Mol Biol Cell. 1999;10:1521–1536. doi: 10.1091/mbc.10.5.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierschbacher MD, Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci U S A. 1984;81:5985–5988. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 21.Woods A, Couchman JR, Johansson S, Hook M. Adhesion and cytoskeletal organisation of fibroblasts in response to fibronectin fragments. Embo J. 1986;5:665–670. doi: 10.1002/j.1460-2075.1986.tb04265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woods A, McCarthy JB, Furcht LT, Couchman JR. A synthetic peptide from the COOH-terminal heparin-binding domain of fibronectin promotes focal adhesion formation. Mol Biol Cell. 1993;4:605–613. doi: 10.1091/mbc.4.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen HC, Appeddu PA, Isoda H, Guan JL. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- 24.Hamelers IH, Olivo C, Mertens AE, Pegtel DM, van der Kammen RA, Sonnenberg A, Collard JG. The Rac activator Tiam1 is required for α3β1-mediated laminin-5 deposition, cell spreading, and cell migration. J Cell Biol. 2005;171:871–881. doi: 10.1083/jcb.200509172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marignani PA, Carpenter CL. Vav2 is required for cell spreading. J Cell Biol. 2001;154:177–186. doi: 10.1083/jcb.200103134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshii S, Tanaka M, Otsuki Y, Wang DY, Guo RJ, Zhu Y, Takeda R, Hanai H, Kaneko E, Sugimura H. αPIX nucleotide exchange factor is activated by interaction with phosphatidylinositol 3-kinase. Oncogene. 1999;18:5680–5690. doi: 10.1038/sj.onc.1202936. [DOI] [PubMed] [Google Scholar]
- 27.Herrera-Molina R, Frischknecht R, Maldonado H, Seidenbecher CI, Gundelfinger ED, Hetz C, de Aylwin LM, Schneider P, Quest AF, Leyton L. Astrocytic αVβ3 Integrin Inhibits Neurite Outgrowth and Promotes Retraction of Neuronal Processes by Clustering Thy-1. PLoS One. 2012;7:e34295. doi: 10.1371/journal.pone.0034295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henriquez M, Herrera-Molina R, Valdivia A, Alvarez A, Kong M, Muñoz N, Eisner V, Jaimovich E, Schneider P, Quest AF, Leyton L. ATP release due to Thy-1-integrin binding induces P2X7-mediated calcium entry required for focal adhesion formation. J Cell Sci. 2011;124:1581–1588. doi: 10.1242/jcs.073171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luescher B, Bron C. Biosynthesis of mouse Thy-1 antigen. J Immunol. 1985;134:1084–1089. [PubMed] [Google Scholar]
- 30.Reiske H, Kao SC, Cary L, Guan JL, Lai JF, Chen HC. Requirement of Phosphatidylinositol 3-Kinase in Focal Adhesion Kinase-promoted Cell Migration. J Biol Chem. 1999;274:12361–12366. doi: 10.1074/jbc.274.18.12361. [DOI] [PubMed] [Google Scholar]
- 31.Casagrande F, Bacqueville D, Pillaire MJ, Malecaze F, Manenti S, Breton-Douillon M, Darbon JM. G1 phase arrest by the phosphatidylinositol 3-kinase inhibitor LY 294002 is correlated to up-regulation of p27Kip1 and inhibition of G1 CDKs in choroidal melanoma cells. FEBS Lett. 1998;422:385–390. doi: 10.1016/s0014-5793(98)00043-x. [DOI] [PubMed] [Google Scholar]
- 32.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 33.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 34.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 36.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 37.Bass MD, Williamson RC, Nunan RD, Humphries JD, Byron A, Morgan MR, Martin P, Humphries MJ. A syndecan-4 hair trigger initiates wound healing through caveolin- and RhoG-regulated integrin endocytosis. Dev Cell. 2011;21:681–693. doi: 10.1016/j.devcel.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barber MA, Welch HC. PI3K and RAC signalling in leukocyte and cancer cell migration. Bull Cancer. 2006;93:E44–52. [PubMed] [Google Scholar]
- 39.Bass MD, Roach KA, Morgan MR, Mostafavi-Pour Z, Schoen T, Muramatsu T, Mayer U, Ballestrem C, Spatz JP, Humphries MJ. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J Cell Biol. 2007;177:527–538. doi: 10.1083/jcb.200610076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito Y, Imazeki H, Miura S, Yoshimura T, Okutsu H, Harada Y, Ohwaki T, Nagao O, Kamiya S, Hayashi R, Kodama H, Handa H, Yoshida T, Fukai F. A peptide derived from tenascin-C induces β1 integrin activation through syndecan-4. J Biol Chem. 2007;282:34929–34937. doi: 10.1074/jbc.M705608200. [DOI] [PubMed] [Google Scholar]
- 41.Thodeti CK, Albrechtsen R, Grauslund M, Asmar M, Larsson C, Takada Y, Mercurio AM, Couchman JR, Wewer UM. ADAM12/syndecan-4 signaling promotes β1 integrin-dependent cell spreading through protein kinase Cα and RhoA. J Biol Chem. 2003;278:9576–9584. doi: 10.1074/jbc.M208937200. [DOI] [PubMed] [Google Scholar]
- 42.Pertz O. Spatio-temporal Rho GTPase signaling - where are we now? J Cell Sci. 2010;123:1841–1850. doi: 10.1242/jcs.064345. [DOI] [PubMed] [Google Scholar]
- 43.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Videos 1–3: Phase-contrast time-lapse of DI TNC1 cells migrating on a plate. DI TNC1 cells were seeded in 8-well plates (Lab-Tek II, Coverglass System, Nunc) for 18 hours. Upon formation of a sub-confluent monolayer, wounds were made with a sterile pipette tip. After wounding, detached cells were washed twice with PBS. The medium was replaced with serum-free RPMI medium for 30 minutes prior to the addition of Thy-1-Fc-Protein-A (4 μg/0.4 μg) complexes (Video 1). As negative control, TRAIL-R2-Fc-Protein-A at the same concentration was used (Video 2). Astrocytes stimulated with 3% fetal bovine serum in RPMI medium were the positive control (Video 3). Phase images were captured at one frame/10 min with a phase FluoView FV10i (Olympus) for 24 hours under controlled temperature and CO2 conditions. The video plays at 15 frames/s.